To the Editor
The Hyper IgM (HIGM) syndromes are a group of rare primary immunodeficiency disorders caused by defects in immunoglobulin class switch recombination (CSR), often including defects of somatic hypermutation (SHM) [1]. X-linked HIGM is the most well-known form of these syndromes, and it is due to mutations in the CD40Ligand gene (CD40LG), located at Xq26.3-27. Mechanistically, the loss of interactions between CD40L, which is expressed on activated CD4+ Tcells, and CD40, which is constitutively expressed on B cells, macrophages, monocytes, and dendritic cells, lead to impaired B cell proliferation, CSR, and SHM, as well as Tcell activation. In addition, functional abnormalities in dendritic cells and macrophages have also been described [2, 3]. The laboratory profile of these patients is therefore notable for normal or elevated serum immunoglobulin M (IgM), usually with low or absent IgG, IgA, and IgE. Clinically, patients present with sinopulmonary infections, but neutropenia, autoimmunity, liver or gastrointestinal inflammatory diseases also occur. Infections due to bacteria (Streptococcus pneumoniae, Pseudomonas aeruginosa) and fungi (Pneumocystis jiroveci and candida species), and liver disease due to Cryptosporidium are common [4, 5], but susceptibility to viral infections such as papilloma virus, is not a hallmark of CD40L deficiency.
Warts are benign human papilloma virus (HPV)-induced cutaneous or mucosal tumors. It is relatively common in the general population, with prevalence up to 5% in adolescents. Recalcitrant warts are typically defined as treatment failure with five different agents over a period of 6 months. Though the exact mechanisms are poorly understood, intact host T cell and natural killer (NK) cell immunity are known to be essential for defense against HPV. Therefore, severe or recalcitrant warts may suggest an underlying immune defect. Widely disseminated cutaneous warts have not been reported in X-linked HIGM.
We report a case of disseminated and recalcitrant cutaneous warts in a 49-year-old male with a life-long history of hypogammaglobulinemia. The patient initially presented at the Boston Children’s hospital with frequent respiratory tract infections and otitis media at 6 months of age. He was diagnosed with a primary antibody deficiency at age 3 and immunoglobulin replacement was initiated at age 5. His initial immunoglobulin levels at age 3 were: IgG 107 (reference range: 700–1600) mg/dL, IgA 70 (reference range: 70–400) mg/dL, and IgM 70 (reference range: 40–230) mg/dL. There was no family history of immunodeficiency, and he was born of a non-consanguineous marriage. There were no male relatives on the maternal side of his family in recent generations. As an adult, he had at least four episodes of pneumonia requiring hospitalization and an episode of empyema requiring chest tube placement. He also had bronchiectasis and chronic sinusitis. On referral to Mount Sinai at age 39 while on immune globulin replacement, his IgG was 679 mg/dL; IgA was 19 mg/dL, and IgM was 205 mg/dL. He had the working diagnosis of common variable immunodeficiency (CVID). His medical history was also notable for HPV-negative urothelial carcinoma at age 42, treated surgically with success.
The patient had first developed scattered cutaneous warts at the age of 5. Since his teenage years, the warts had become increasingly severe and wide-spread. On physical exam, there were hundreds of exophytic papules covering his neck, trunks, and extremities (Fig. 1a). On the palmoplantar surfaces, there were extensive coalescing warts forming thick, hyperkeratotic plaques (Fig. 1b, c). Working with sheet metal at his job, the patient noted that small cuts sustained allowed the warts to spread on his hands. The warts had been recalcitrant over the course of four decades despite various therapy attempts, including salicylic acid, acitretin, cimetidine, intralesional candida immunotherapy, and peg-interferon alpha. Additional laboratory investigations were notable for mild neutropenia, low CD19+ B cells, and modest decrease in mitogen induced lymphocyte proliferation (Supplemental Table 1). B cell analysis by flow cytometry revealed low percentage of CD27+IgM−IgD− class-switched memory B cells and relatively high percentage of CD38hi transitional B cells (Supplemental Table 1).
Fig. 1.
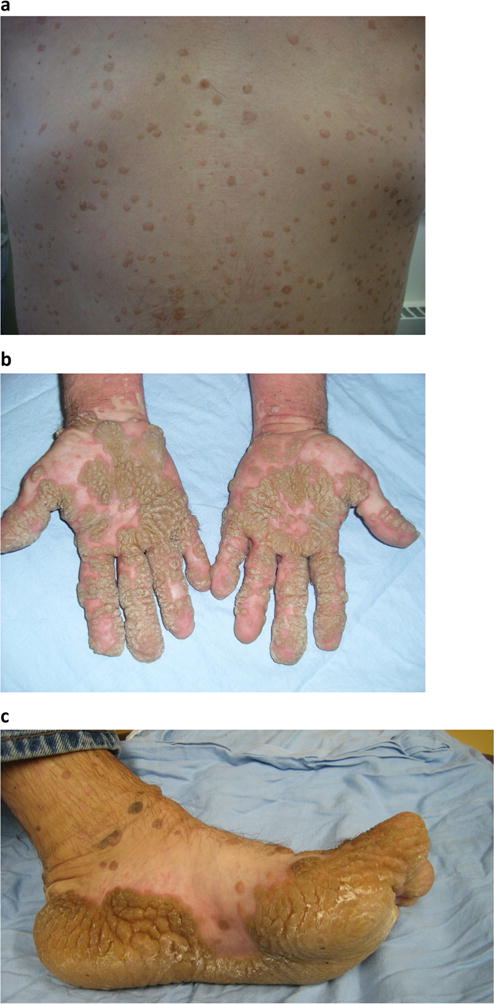
Disseminated cutaneous warts in a 49-year-old male with X-linked HIGM syndrome. a Exophytic papules on trunk. b, c Coalescing warts forming hyperkeratotic plaques on palmoplantar surfaces
Due to his early onset hypogammaglobulinemia and cutaneous warts, WHIM syndrome was first considered; however, the CXCR4 gene sequence was normal. Whole exome sequencing (WES) was performed, which revealed a deleterious novel frameshift mutation p.Ser214fs (c.641delC in NM_000074.2) in his CD40LG and a carrier status in his mother (confirmed with Sanger sequencing, Supplemental Fig. 1). No nonsynonymous mutations in genes more commonly associated with extensive warts (TMC6, TMC8, GATA 2, IKBKG) or other genes plausibly connected to his phenotype [6] were identified, except three common missense variations in DOCK8 (Supplemental Tables 2 and 3). Western blotting analysis demonstrated that DOCK8 protein was present in the patient (Supplemental Fig. 2). Flow cytometry analysis of peripheral blood T cells confirmed a lack of CD40L expression on the surface of activated CD4+ T cells upon in vitro stimulation with ionomycin and PMA, establishing the diagnosis of X-linked HIGM (Supplemental Fig. 3). The patient remains on gamma globulin infusion and clarithromycin prophylaxis with no additional infectious complications in recent years; however, his extensive cutaneous warts have remained recalcitrant.
Susceptibility to viral infections is not a typical feature of HIGM syndrome and noted in only small numbers in several series. Viral infections (e.g., parvovirus B19, n = 13; herpes spp., n = 7; cytomegalovirus, n = 5) were noted in 46 cases in the USIDNET registry [4]; in the LASID registry, cytomegalovirus, hepatitis B virus, herpes simplex, molluscum contagiosum, HPV, and parainfluenza were found in one subject each [5]. To our knowledge, there is one report of a 28-year-old male with HIGM syndrome who had recurrent herpes labialis and recalcitrant warts on the hands, elbows, and knees [7]. There are limited reports of wide-spread and treatment-resistant warts in patients with presumed CVID [8, 9]. Interestingly, these patients were found to have concurrent defects in cellular immunity, raising the possibility that they may in fact carry an alternative more specific genetic diagnosis.
Our patient does have modestly diminished mitogen-induced lymphocyte proliferation (supplemental Table 1). Both normal and reduced in vitro antigen-specific T cell response have previously been reported in XHIGM patients, indicating a degree of variability in T cell functional deficiency in this disorder [10]. While impaired antigen-specific T cell response could have partially contributed to HPV susceptibility in this patient, it may be less likely to be the sole mechanism as viral susceptibility remains uncommon in XHIGM cohort. NK cells can contribute to immune defense against HPV. In the literature, there is one report of a single XHIGM patient with an absence of NK cells, though he did not present with severe HPVor other viral infections [11]. Our patient has normal NK cell counts upon examination. CD40L has pleiotropic biological effects, and the functional consequences of its absence on various immune cell lineages remain an area of active investigation [1–3]. In this context, an additional causal genetic mutation in our patient (e.g., an intronic mutation or an undefined warts-associated gene) may be less likely, especially in the absence of family history and consanguineous birth.
In summary, this case highlights an atypical phenotype for a patient with the X-linked HIGM syndrome. Additionally, it underlines the need for considering alternative genetic diagnoses when a clinical phenotype does not fit the norm for CVID. The host mechanisms to control HPV infections remain poorly understood, and further studies in immune deficient subjects with susceptibility to warts may be revealing.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10875-018-0505-z) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Davies EG, Thrasher AJ. Update on the hyper immunoglobulin M syndromes. Br J Haematol. 2010;149:167–80. doi: 10.1111/j.1365-2141.2010.08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabral-Marques O, Ramos RN, Schimke LF, Khan TA, Amaral EP, Barbosa Bomfim CC, et al. Human CD40 ligand deficiency dysregulates the macrophage transcriptome causing functional defects that are improved by exogenous IFN-γ. J Allergy Clin Immunol. 2017;139:900–12. doi: 10.1016/j.jaci.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Cabral-Marques O, Arslanian C, Ramos RN, Morato M, Schimke L, Soeiro Pereira PV, et al. Dendritic cells from X-linked hyper-IgM patients present impaired responses to Candida albicans and Paracoccidioides brasiliensis. J Allergy Clin Immunol. 2012;129:778–86. doi: 10.1016/j.jaci.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Leven EA, Maffucci P, Ochs HD, Scholl PR, Buckley RH, Fuleihan RL, et al. Hyper IgM syndrome: a report from the USIDNET Registry. J Clin Immunol. 2016;36:490–501. doi: 10.1007/s10875-016-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral-Marques O, Klaver S, Schimke LF, Ascendino ÉH, Khan TA, Pereira PV, et al. First report of the hyper-IgM syndrome registry of the Latin American Society for Immunodeficiencies: novel mutations, unique infections, and outcomes. J Clin Immunol. 2014;34(2):146–56. doi: 10.1007/s10875-013-9980-4. [DOI] [PubMed] [Google Scholar]
- 6.Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immune. 2012;130:1030–48. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang MW, Romero R, Scholl PR, Paller AS. Mucocutaneous manifestations of the hyper-IgM immunodeficiency syndrome. J Am Acad Dermatol. 1998;38:191–6. doi: 10.1016/s0190-9622(98)70239-7. [DOI] [PubMed] [Google Scholar]
- 8.Reid TM, Fraser NG, Kernohan IR. Generalized warts and immune deficiency. Br J Dermatol. 1976;95:559–64. doi: 10.1111/j.1365-2133.1976.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 9.Lynn J, Knight AK, Kamoun M, Levinson AI. A 55-year-old man with hypogammaglobulinemia, lymphopenia, and unrelenting cutaneous warts. J Allergy Clin Immunol. 2004;114:409–14. doi: 10.1016/j.jaci.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Jain A, Atkinson TP, Lipsky PE, Slater JE, Nelson DL, Strober W. Defects of T-cell effector function and post-thymic maturation in X-linked hyper-IgM syndrome. J Clin Invest. 1999;103:1151–8. doi: 10.1172/JCI5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostenstad B, Giliani S, Mellbye OJ, Br N, Abrahamsen T. A boy with X-linked hyper-IgM syndrome and natural killer cell deficiency. Clin Exp Immunol. 1997;107:230–4. doi: 10.1111/j.1365-2249.1997.284-ce1174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


