Abstract
Purpose
Current evidence regarding salvage resection for recurrent retroperitoneal (RP) sarcomas generally lacks detailed histology-specific analyses, but the aggressiveness of these tumors varies widely by histology. We investigated associations between timing and extent of salvage surgery and survival outcomes in patients with recurrent RP well-differentiated liposarcoma (WDLPS).
Methods
The University of Texas MD Anderson Cancer Center Surgical Oncology sarcoma database was reviewed to identify patients with RP WDLPS who underwent surgical resection for 1st recurrent disease (salvage surgery) in 1995–2015. Medical records were retrospectively reviewed to identify factors associated with overall survival (OS) and disease-free survival (DFS).
Results
We identified 52 patients who underwent salvage surgery for RP WDLPS for 1st local recurrence; 28 (54%) underwent salvage surgery within 6 months after recurrence. Concomitant organ resections were performed in 32 (62%) patients, 4 (13%) of whom had pathologic invasion of resected organs. After R0/R1 resections (n=45), 38 (84%) experienced a 2nd local recurrence. Multivariable analyses revealed that organ invasion at the primary surgery (HR 13.08; p=0.005) and disease-free interval <1 year (HR 3.64; p=0.044) were associated with shorter OS. Recurrence-to-salvage interval <6 months was associated with shorter DFS (HR 2.18; p=0.025). Concomitant organ resection was associated with a longer hospital stay, ≥14 days (OR 21.58; p=0.007).
Conclusions
Early salvage surgery may not always be the best approach for recurrent RP WDLPS patients. Since organ invasion is rare among recurrent RP WDLPS patients and concomitant organ resection is associated with a longer hospital stay, preservation of uninvolved organs should be considered.
Keywords: Retroperitoneal sarcoma, well-differentiated liposarcoma, recurrence, salvage surgery, disease-free survival
Introduction
Retroperitoneal (RP) sarcomas account for approximately 15% of soft tissue sarcomas.1 RP sarcomas comprise various histologic subtypes,2 and liposarcoma is the most common among them.3,4 Each histologic subtype has a distinct recurrence pattern5; therefore, reports that do not specify histology may not effectively guide treatment strategy.6 For example, leiomyosarcoma has a high risk of distant metastasis (>50%) and a low risk of local recurrence (10–15%).4,5 In contrast, RP well-differentiated liposarcoma (WDLPS) has a high risk of local recurrence and rarely metastasizes.4 Reports combining these two distinct histologies cannot effectively guide treatment of either. There is accumulating evidence from retrospective studies that supports salvage surgery for recurrent RP sarcomas7–10; however, a specific analysis of the impact of salvage surgery for recurrent RP WDLPS has not been reported.
Patient selection for salvage surgery is critical. Factors reportedly associated with a longer survival after salvage surgery for RP sarcomas in general are a long recurrence-free interval,11 unifocal disease,12 and complete resection.7 Park et al. reported that a slow tumor growth rate after recurrence (<0.9 cm/month) was predictive for longer disease-free survival (DFS) in recurrent RP liposarcomas.13 However, the effect of timing of salvage surgery (or time between diagnosis of recurrent disease and salvage surgery) has not been investigated. Our hypothesis was that watchful waiting to characterize the clinical behavior of the recurrent disease may improve patient selection for salvage surgery for recurrent RP WDLPS. In this study, we investigated factors, including time between recurrence and salvage surgery, associated with improved survival outcomes in patients who underwent salvage resection for recurrent RP WDLPS at The University of Texas MD Anderson Cancer Center. We also sought to investigate the associations between concomitant organ resection at salvage surgery and survival outcomes, incidence of complications, and length of hospital stay.
Methods
After receiving Institutional Review Board approval, with a waiver of the informed consent requirement, we comprehensively searched the tumor registry at MD Anderson to identify patients who, between 1995 and 2015, underwent curative-intent resection of 1st local recurrence of previously resected RP WDLPS. We identified 52 patients, including 35 patients who were analyzed in our previous publication on organ invasion at the time of primary resection, which shares some common methodology (manuscript under review, J Surg Oncol). Clinicopathologic data were retrospectively collected from medical records. Variables included: age, sex, race/ethnicity, size of recurrent tumor (measured at pathologic assessment), focality (unifocal or multifocal as noted in the operative report; multifocal tumor was defined as a tumor completely separated by non-tumorous connective tissue), presence of symptoms at recurrence, macroscopic margin status (R0/R1 or R2), organ invasion status at primary resection and at salvage resection (tumor invasion/infiltration into concomitantly resected solid organs confirmed by pathology; obtained by review of the pathology report), postoperative complications (defined as an event of Clavien-Dindo classification14 grade 3 or higher observed within 30 days after surgical resection), length of hospital stay at salvage resection, and postoperative mortality within 30 days. For patients who had more than two CT scans available to assess change in tumor size after recurrence, tumor growth rates were calculated (change in the largest diameter between the first CT scan that identified recurrent disease and the last CT scan before the salvage surgery [cm/month]). The pathologic evaluation at the time of resection was conducted by pathologists with specialized experience in sarcoma pathology. In general, resected organs were sliced into 5- to 10-mm blocks to make slides for pathologic review to evaluate invasion or infiltration of resected organs. After the primary or salvage resection at MD Anderson, all patients were recommended for follow-up with serial physical examination every 3–6 months and radiographic evaluation with CT of the chest, abdomen, and pelvis every 6–12 months for 5–10 years. Patients who elected to be followed up by local physicians were contacted by the institutional tumor registry annually to update vital and recurrence status and treatment of disease. Disease-free interval was defined as the time from surgical resection of the primary tumor to 1st local recurrence, and recurrence-to-salvage interval was defined as the time from diagnosis of the 1st local recurrence to salvage surgery. Treatments for all patients were determined by a multidisciplinary team, including specialists in surgical oncology, medical oncology, radiation oncology, and pathology.
Categorical variables of patient/tumor characteristics were compared by recurrence-to-salvage interval length (<6 months or ≥6 months) using chi-square or Fisher’s exact test as appropriate. Overall survival (OS) was defined as the time from surgical resection of the recurrent tumor (salvage surgery) to the time of death; DFS was defined as the time from salvage surgery to the time of local recurrence or death. OS and DFS curves were estimated using the Kaplan-Meier method. A log-rank test was performed to assess differences in survival outcomes between groups. Univariable and multivariable Cox proportional hazards regression analyses were performed to identify variables associated with OS and DFS for patients who underwent macroscopically complete (R0/R1) resection. Univariable and multivariable logistic regression analyses were performed to identify variables associated with postoperative complications and length of hospital stay for all study patients. In order that the study results would be applicable for selecting patients and determining the timing of salvage surgery, we only included preoperative factors in the Cox regression models. For both Cox regression and logistic regression analyses, factors with p<0.10 by univariable analyses were included in the primary model for the multivariable model, and a stepwise method with backward elimination was used to build the final model. SAS (version 9.2) statistical software was used to perform all analyses. The p-value was considered significant when it was ≤0.05.
Results
We identified 52 patients with recurrent RP WDLPS who underwent salvage surgery for 1st local recurrence at MD Anderson during the study period. Patient and tumor characteristics are summarized in Table 1. The median age was 58 years (range 34–80), 42% were male, and 75% were white. The mean tumor size was 13.5 cm, and 69% (36/52) of tumors were multifocal. Among 43 patients who had more than two preoperative CT scans, the mean tumor growth rate was 0.58 cm/month (median 0.39 cm/month), and 9 patients (21%) had a 0.9 cm/month or higher tumor growth rate. Of all 52 study patients, 28 (54%) underwent salvage resection within 6 months after the diagnosis of recurrent disease; no preoperative factors or postoperative short-term outcomes were significantly different between groups defined based on the length of this interval (Table 1). Ninety-four percent (49/52) underwent surgical resection alone without pre- or postoperative therapy, and 87% (45/52) underwent macroscopically complete resection (R0/R1). Concomitant organ resections were performed at salvage surgery in 32 (62%) patients (37 organs); the resected organs were kidney (n=9), gastrointestinal tract (n=8; colon [n=6], small bowel [n=3], stomach [n=1]), spleen (n=9), pancreas (n=6), liver (n=2), diaphragm (n=2), and inferior vena cava (n=1). Pathologic evidence of organ invasion was seen in the salvage surgery specimen in 4 patients (8% of entire cohort, 13% of patients who underwent concomitant organ resection); 5 organs were affected (pancreas [n=3], colon [n=2]).
Table 1.
Patient and tumor characteristics
| Characteristic | All (n=52) |
Recurrence-to-salvage interval | P-value | ||||
|---|---|---|---|---|---|---|---|
| ≥6 months (n=24) |
<6 months (n=28) |
||||||
| N | % | N | % | N | % | ||
| Age at diagnosis | 0.458 | ||||||
| <55 yr | 21 | 40 | 11 | 46 | 10 | 36 | |
| ≥55 yr | 31 | 60 | 13 | 54 | 18 | 64 | |
| Sex | 0.634 | ||||||
| Male | 22 | 42 | 11 | 46 | 11 | 39 | |
| Female | 30 | 58 | 13 | 54 | 17 | 61 | |
| Race | 0.395# | ||||||
| White | 39 | 75 | 19 | 79 | 20 | 71 | |
| Black | 2 | 4 | 0 | 0 | 2 | 7 | |
| Hispanic | 9 | 17 | 5 | 21 | 4 | 14 | |
| Asian | 2 | 4 | 0 | 0 | 2 | 7 | |
| Tumor size | 0.477 | ||||||
| <10 cm | 19 | 37 | 10 | 42 | 9 | 32 | |
| ≥10 cm | 33 | 63 | 14 | 58 | 19 | 68 | |
| Focality | 0.817 | ||||||
| Unifocal | 16 | 31 | 7 | 29 | 9 | 32 | |
| Multifocal | 36 | 69 | 17 | 71 | 19 | 68 | |
| Symptoms | 0.324# | ||||||
| No | 48 | 92 | 21 | 88 | 27 | 96 | |
| Yes | 4 | 8 | 3 | 13 | 1 | 4 | |
| Disease-free interval | 0.335 | ||||||
| <1 year | 14 | 27 | 8 | 33 | 6 | 21 | |
| ≥1 year | 38 | 73 | 16 | 67 | 22 | 79 | |
| Organ invasion at primary | 0.240# | ||||||
| No | 49 | 94 | 24 | 100 | 25 | 89 | |
| Yes | 3 | 6 | 0 | 0 | 3 | 11 | |
| Organ resection at salvage | 0.895 | ||||||
| Yes | 32 | 62 | 15 | 62.5 | 17 | 61 | |
| No | 20 | 38 | 9 | 37.5 | 11 | 39 | |
| Organ invasion at salvage | 0.615# | ||||||
| No | 48 | 92 | 23 | 96 | 25 | 89 | |
| Yes | 4 | 8 | 1 | 4 | 3 | 11 | |
| Margin status | 0.227# | ||||||
| R0/R1 | 45 | 87 | 19 | 79 | 26 | 93 | |
| R2 | 7 | 13 | 5 | 21 | 2 | 7 | |
| Complications | 0.711# | ||||||
| No | 44 | 85 | 21 | 88 | 23 | 82 | |
| Yes | 8 | 15 | 3 | 13 | 5 | 18 | |
| Length of hospital stay | 0.480# | ||||||
| <14 days | 43 | 83 | 21 | 88 | 22 | 79 | |
| ≥14 days | 9 | 17 | 3 | 13 | 6 | 21 | |
| Growth rate (n=43) | 0.153# | ||||||
| <0.9 cm/mo | 34 | 79 | 21 | 87.5 | 13 | 68 | |
| ≥0.9 cm/mo | 9 | 21 | 3 | 12.5 | 6 | 32 | |
Fisher’s exact test
Survival outcomes
The median follow-up duration after salvage surgery was 4.5 years. Among the 45 patients who underwent macroscopically complete (R0/R1) resection at the time of the salvage surgery, 38 (84%) experienced a 2nd local recurrence during follow-up. Of all 52 study patients, 22 (42%) died during the follow-up. The estimated median OS duration for the entire cohort was 7.4 years (5-year OS, 68%), and median DFS duration was 1.4 years (5-year DFS, 17%). Sensitivity analyses using different cut-off points for disease-free interval (6 months, 1 and 2 years) and for recurrence-to-salvage interval (6 months, 1 and 2 years) were performed to determine the optimal cutoff. None of these cutoff points improved the prediction of OS and DFS. Therefore, the cutoff of 1 year for disease-free interval and 6 months for recurrence-to-salvage interval were determined based on clinical impressions of what timing constituted early recurrence after resection and what timing constituted a quick return to salvage resection after recurrence; these cutoffs were used in further analyses.
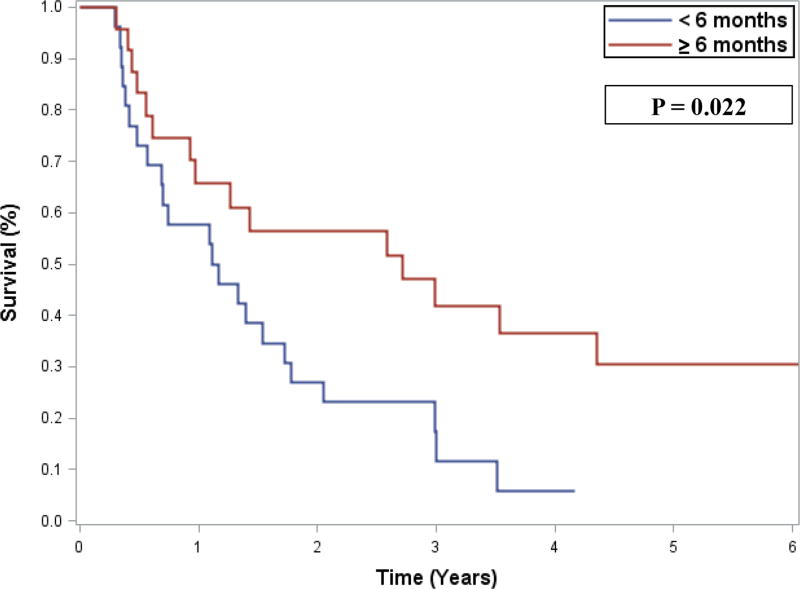
By multivariable analysis of OS, organ invasion at the primary surgery (hazard ratio [HR] 13.08, 95% confidence interval [CI] 2.17–78.96; p=0.005) and disease-free interval <1 year (HR 3.64, 95% CI 1.04–12.80; p=0.044) were associated with shorter OS (Table 2). By multivariable analysis of DFS, recurrence-to-salvage interval of <6 months was associated with shorter DFS (HR 2.18, 95% CI 1.10–4.32; p=0.025) (Table 3, Figure 1). Concomitant organ resection was not associated with improved OS (HR 1.14, 95% CI 0.47–2.78; p=0.775) or DFS (HR 0.96, 95% CI 0.50–1.85; p=0.911) by univariable analyses.
Table 2.
Risk factors for overall survival after salvage surgery
| Risk factors | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Age, <55 vs ≥55 years | 0.54 | 0.23 | 1.29 | 0.166 | ||||
| Sex, Male vs Female | 0.53 | 0.21 | 1.38 | 0.194 | ||||
| Symptoms, No vs Yes | 1.23 | 0.29 | 5.30 | 0.780 | ||||
| Tumor size, <10 vs ≥10 cm | 0.45 | 0.17 | 1.24 | 0.124 | ||||
| Focality, Unifocal vs Multifocal | 0.76 | 0.31 | 1.90 | 0.558 | ||||
| Organ invasion at primary, Yes vs No | 8.29 | 2.13 | 32.25 | 0.002 | 13.08 | 2.17 | 78.96 | 0.005 |
| Growth rate, ≥0.9 vs <0.9 cm/month | 2.74 | 0.92 | 8.20 | 0.071 | 2.09 | 0.60 | 7.25 | 0.246 |
| Disease-free interval, <1 vs ≥1 year | 2.52 | 1.005 | 6.33 | 0.049 | 3.64 | 1.04 | 12.80 | 0.044 |
| Recurrence-to-salvage interval, <6 vs ≥6 months | 1.75 | 0.72 | 4.24 | 0.216 | ||||
HR, hazard ratio of death; CI, confidence interval
P-value ≤0.05 were bolded
Table 3.
Risk factors for disease-free survival after salvage surgery
| Risk factors | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Age, <55 vs ≥55 years | 0.71 | 0.37 | 1.34 | 0.289 | ||||
| Sex, Male vs Female | 1.08 | 0.56 | 2.12 | 0.814 | ||||
| Symptoms, No vs Yes | 1.01 | 0.31 | 3.31 | 0.983 | ||||
| Tumor size, <10 vs ≥10 cm | 0.54 | 0.27 | 1.06 | 0.075 | ||||
| Focality, Unifocal vs Multifocal | 0.54 | 0.27 | 1.11 | 0.092 | ||||
| Organ invasion at primary, Yes vs No | 0.87 | 0.21 | 3.52 | 0.848 | ||||
| Growth rate, ≥0.9 vs <0.9 cm/month | 1.13 | 0.43 | 2.96 | 0.808 | ||||
| Disease-free interval, <1 vs ≥1 year | 1.02 | 0.49 | 2.10 | 0.968 | ||||
| Recurrence-to-salvage interval, <6 vs ≥6 months | 2.18 | 1.10 | 4.32 | 0.025 | 2.18 | 1.10 | 4.32 | 0.025 |
HR, hazard ratio of death; CI, confidence interval
P-value ≤0.05 were bolded
Figure 1.
Disease-free survival after salvage surgery, stratified by the duration of the interval between recurrence and salvage surgery (<6 months, n=28; ≥6 months, n=24)
Postoperative complications and hospital stay
Postoperative complications were identified in 8 (15%) of the study patients. There was no postoperative mortality (≤30 days). Univariable analyses failed to identify factors associated with incidence of postoperative complications, including concomitant organ resection (data not shown).
The median hospital stay for the salvage surgery was 8 days (interquartile range 7–10 days), and 9 (17%) patients stayed for ≥14 days. By multivariable analysis, concomitant organ resection was associated with a longer (≥14 days) hospital stay (odds ratio 21.58, 95% CI 2.29–203.6; p=0.007) (Table 4).
Table 4.
Risk factors for hospital stay of ≥14 days
| Risk factors | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Age, <55 vs ≥55 | 1.91 | 0.45 | 8.15 | 0.381 | ||||
| Sex, Male vs Female | 7.64 | 0.88 | 66.4 | 0.066 | 8.18 | 0.81 | 82.34 | 0.075 |
| Symptoms, No vs Yes | 1.22 | 0.12 | 12.40 | 0.867 | ||||
| Tumor size, <10 vs ≥10 cm | 2.29 | 0.42 | 12.36 | 0.336 | ||||
| Focality, Unifocal vs Multifocal | 1.69 | 0.31 | 9.21 | 0.544 | ||||
| Organ resection, Yes vs No | 20.67 | 2.33 | 183.4 | 0.007 | 21.58 | 2.29 | 203.6 | 0.007 |
OR, odds ratio; CI, confidence interval
P-value ≤0.05 were bolded
Discussion
In this single-institutional retrospective study of patients with recurrent RP WDLPS, salvage surgery frequently achieved macroscopically complete (R0/R1) resection (87%), with a long median OS (7.4 years) after salvage surgery. However, the vast majority of patients experienced a 2nd local recurrence during follow-up with a relatively short median DFS (1.4 years). Among preoperative factors examined in this study, presence of organ invasion identified at the primary resection and short disease-free interval (<1 year) were associated with short OS, and short recurrence-to-salvage interval (<6 months) was associated with shorter DFS. Concomitant organ resection at salvage surgery was not associated with longer OS or DFS and was associated with a longer (≥14 days) hospital stay.
Histology-specific data are critical for determining the treatment strategies in RP sarcomas because each histologic subtype has distinct tumor behavior and treatment outcomes.5,6,15 However, currently available evidence for recurrent RP sarcomas supporting salvage resection generally lacks detailed histology-specific analyses, which make it difficult to apply those data to patients with recurrent RP WDLPS.7–9,11,16–18 The survival impact of salvage surgery must differ between histologic subtypes, but no previous study included interaction (effect modification) terms in multivariable models, or they were underpowered to do so. Park et al. from Memorial Sloan Kettering Cancer Center reported a study of 105 patients with locally recurrent RP liposarcomas (48 WDLPS, 49 de-differentiated, 4 myxoid, and 3 round cell liposarcomas), among whom 61 patients underwent salvage surgery; they showed that local recurrent tumor growth rates of less than 0.9 cm/month were associated with longer OS and DFS.13 Although these results must be interpreted with care because their study included high-grade liposarcomas, the idea of using tumor growth rate as a marker of tumor behavior is clinically plausible. In the current study, tumor growth rate was not associated with OS after adjustment for presence of organ invasion at primary resection and disease-free interval length. However, these three factors are likely interconnected, as all represent aggressiveness of tumor behavior/biology. At MD Anderson, all WDLPSs are categorized as low-grade histology, although we observe some heterogeneity in tumor cellularity among WDLPSs at pathologic assessment. Currently, there is no standardized marker that differentiates tumor biology among WDLPSs; recurrent tumors that previously showed tumor invasion to surrounding organs and/or tumors that recurred with a short disease-free interval merit thoughtful consideration of whether patients would benefit from salvage surgery, since these characteristics represent aggressive tumor behavior.
The current study showed that a recurrence-to-salvage interval ≥6 months was associated with longer DFS. Although a longer interval was not associated with longer OS, preoperative strategic watchful waiting for recurrent RP WDLPS has potential benefits in patient selection and planning for salvage surgery. First, we can estimate the tumor behavior/aggressiveness by establishing the tumor growth rate. Second, we may identify a newly emerging remote focus of recurrent multifocal disease, which may not be visualized on the initial CT scan and may be missed at the salvage surgery if the patient undergoes surgery without a watchful waiting period. If the recurrent tumor is rapidly growing with multiple emerging foci during watchful waiting, a patient likely will not benefit from a salvage surgery attempt. On the other hand, if the recurrent tumor is stable in size and number of foci, the patient may be able to continue observation without surgical intervention. Since salvage surgeries for recurrent RP sarcomas are frequently challenging due to a number of technical considerations, patient selection for salvage surgery needs thoughtful evaluation, and strategic watchful waiting after identification of recurrent disease may help in clinical decision making. Development of effective systemic therapy for RP WDLPS, such as MDM2 antagonists,19 may enable a preoperative therapy approach for recurrent RP WDLPS in the future. The potential disadvantage of a strategic watchful waiting approach is the potential for tumor growth and invasion to surrounding structures, which may prohibit complete resection or may result in more extensive surgery. In the current study, we didn’t find a statistical difference in frequency of R2 resection or concomitant organ resections by recurrence-to-salvage interval duration; however, surgeons should be aware of such potential scenarios and perform careful patient evaluations with frequent imaging (at least every 3–6 months).
The extent of resection for primary RP sarcomas is currently an active subject of debate in the surgical oncology literature.20–22 In contrast to primary RP sarcomas, the benefit of extended resection for recurrent RP sarcomas has not been well discussed. Given the high incidence of 2nd recurrence after salvage surgery for recurrent RP sarcomas, the general consensus among sarcoma surgeons worldwide seems to favor a conservative approach that limits the extent of resection to recurrent tumors and directly invaded organs.17 In the current study, even though our sarcoma surgical group does not advocate organ resection unless actual tumor invasion is suspected, concomitant organ resections were performed in 62% of patients, and only 4 patients (13% of patients who underwent organ resections) had actual organ invasion, which highlights the difficulty of intraoperative assessment of tumor invasion at salvage surgery. Although concomitant organ resection did not affect survival outcomes in the current study, it was associated with a longer hospital stay, as expected. Judicious decision making is recommended when considering concomitant organ resection at salvage surgery for recurrent RP WDLPS.
A limitation of our study was its retrospective design. Interpretation of this retrospective single-institution study requires careful consideration because of inherent selection bias, which includes the multiple oncologic, as well as non-oncologic/technical reasons which can lead to the resection of adjacent organs. Although organ resection in this study was not associated with improved outcomes, there are patients with advanced disease which requires concomitant organ resection in order to achieve a complete tumor extirpation which results in a similar survival compared to surgery in patients who didn’t require organ resection. Potential risk factors for poor survival outcomes assessed in the current study, such as short disease-free interval and tumor growth rate, were likely already taken into account in patient selection and in determining the timing of salvage surgery. Such selection bias also may have affected our finding of longer DFS in patients who underwent salvage surgery with a longer recurrence-to-salvage interval. We set a cutoff of 14 days to define a long hospital stay based on our clinical impression that patients with severe complications or an eventful recovery may be associated with a longer than 2-week hospital stay; a different cutoff may have resulted in different findings.
The current study also has several strengths. To our knowledge, this is the largest case series that specifically evaluated surgical patients with recurrent RP WDLPS, although it was still a small cohort with limited statistical power to detect factors associated with survival outcomes. The concept of recurrence-to-salvage interval as a predictive variable for survival outcomes was novel in the current study. A final strength was that our patients represent a standardized treatment strategy carried out at a high-volume sarcoma center with consistent quality. We believe that our findings, which are specific to recurrent RP WDLPS, will help guide treatment strategies in this population.
Conclusions
In patients with resected recurrent RP WDLPS, early salvage surgery may not always be the most beneficial approach, and strategic watchful waiting may improve patient selection for salvage surgery. Since organ invasion is a rare event among recurrent RP WDLPS patients and concomitant organ resection is associated with increased length of stay, preservation of clinically uninvolved organs should be considered.
Synopsis.
Among 52 patients who underwent salvage surgery for RP WDLPS for 1st local recurrence, organ invasion at the primary surgery and disease-free interval <1 year were associated with shorter overall survival, and recurrence-to-salvage interval <6 months was associated with shorter disease-free survival. Early salvage surgery may not always be the most beneficial approach for recurrent RP WDLPS patients.
Acknowledgments
Supported in part by the National Institutes of Health/National Cancer Institute under award number P30CA016672 (used the Clinical Trials Support Resource).
Sunita Patterson, Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editorial assistance.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.Kotilingam D, Lev DC, Lazar AJ, Pollock RE. Staging soft tissue sarcoma: evolution and change. CA: a cancer journal for clinicians. 2006 Sep-Oct;56(5):282–291. doi: 10.3322/canjclin.56.5.282. quiz 314-285. [DOI] [PubMed] [Google Scholar]
- 2.Crago AM, Brennan MF. Principles in Management of Soft Tissue Sarcoma. Advances in surgery. 2015;49:107–122. doi: 10.1016/j.yasu.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng W, Martinez SR, Tamurian RM, Borys D, Canter RJ. Histologic type predicts survival in patients with retroperitoneal soft tissue sarcoma. The Journal of surgical research. 2012 Jan;172(1):123–130. doi: 10.1016/j.jss.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 4.Gronchi A, Strauss DC, Miceli R, et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Annals of surgery. 2016 May;263(5):1002–1009. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 5.Tan MC, Brennan MF, Kuk D, et al. Histology-based Classification Predicts Pattern of Recurrence and Improves Risk Stratification in Primary Retroperitoneal Sarcoma. Annals of surgery. 2016 Mar;263(3):593–600. doi: 10.1097/SLA.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anaya DA, Lahat G, Wang X, et al. Establishing prognosis in retroperitoneal sarcoma: a new histology-based paradigm. Annals of surgical oncology. 2009 Mar;16(3):667–675. doi: 10.1245/s10434-008-0250-2. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Annals of surgery. 1998 Sep;228(3):355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton TD, Cannell AJ, Kim M, et al. Results of Resection for Recurrent or Residual Retroperitoneal Sarcoma After Failed Primary Treatment. Annals of surgical oncology. 2017 Jan;24(1):211–218. doi: 10.1245/s10434-016-5523-6. [DOI] [PubMed] [Google Scholar]
- 9.van Dalen T, Hoekstra HJ, van Geel AN, et al. Locoregional recurrence of retroperitoneal soft tissue sarcoma: second chance of cure for selected patients. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2001 Sep;27(6):564–568. doi: 10.1053/ejso.2001.1166. [DOI] [PubMed] [Google Scholar]
- 10.Hassan I, Park SZ, Donohue JH, et al. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Annals of surgery. 2004 Feb;239(2):244–250. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gronchi A, Miceli R, Allard MA, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and postrelapse outcome after primary extended resection. Annals of surgical oncology. 2015 May;22(5):1447–1454. doi: 10.1245/s10434-014-4130-7. [DOI] [PubMed] [Google Scholar]
- 12.Anaya DA, Lahat G, Liu J, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Annals of surgery. 2009 Jan;249(1):137–142. doi: 10.1097/SLA.0b013e3181928f2f. [DOI] [PubMed] [Google Scholar]
- 13.Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Annals of surgery. 2009 Dec;250(6):977–982. doi: 10.1097/sla.0b013e3181b2468b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery. 2004 Aug;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feig B, Benjamin R. Guidelines for the Treatment of Recurrent Retroperitoneal Sarcoma: Are we Trying to Fit a Square Peg into a Round Hole? Annals of surgical oncology. 2016 Jun 22; doi: 10.1245/s10434-016-5344-7. [DOI] [PubMed] [Google Scholar]
- 16.Lochan R, French JJ, Manas DM. Surgery for retroperitoneal soft tissue sarcomas: aggressive re-resection of recurrent disease is possible. Annals of the Royal College of Surgeons of England. 2011 Jan;93(1):39–43. doi: 10.1308/003588410X12771863936729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacNeill AJ, Miceli R, Strauss DC, et al. Post-relapse outcomes after primary extended resection of retroperitoneal sarcoma: A report from the Trans-Atlantic RPS Working Group. Cancer. 2017 Feb 02; doi: 10.1002/cncr.30572. [DOI] [PubMed] [Google Scholar]
- 18.Grobmyer SR, Wilson JP, Apel B, et al. Recurrent retroperitoneal sarcoma: impact of biology and therapy on outcomes. Journal of the American College of Surgeons. 2010 May;210(5):602–608. 608–610. doi: 10.1016/j.jamcollsurg.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Laroche A, Chaire V, Algeo MP, Karanian M, Fourneaux B, Italiano A. MDM2 antagonists synergize with PI3K/mTOR inhibition in well-differentiated/dedifferentiated liposarcomas. Oncotarget. 2017 Mar 17; doi: 10.18632/oncotarget.16345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Jan 01;27(1):24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 21.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Jan 01;27(1):31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 22.Pisters PW. Resection of some -- but not all -- clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Jan 01;27(1):6–8. doi: 10.1200/JCO.2008.18.7138. [DOI] [PubMed] [Google Scholar]