Abstract
Natural Killer (NK) cells are capable of fighting viral infections and cancer. However, these responses are inhibited by immune suppressor cells in the tumor microenvironment. Tumor progression promotes the recruitment and generation of intratumoral regulatory T cells (Tregs), associated with a poor prognosis in cancer patients. Here, we show that canonical NK cells are highly susceptible to Treg-mediated suppression, in contrast to highly resistant CD57+ FcεRγ−NKG2C+ adaptive (CD56+CD3−) NK cells that expand in cytomegalovirus (CMV)-exposed individuals. Specifically, Tregs suppressed canonical but not adaptive NK-cell proliferation, IFNγ production, degranulation, and cytotoxicity. Treg-mediated suppression was associated with canonical NK-cell downregulation of TIM3, a receptor that activates NK-cell IFNγ production upon ligand engagement, and upregulation of the NK-cell inhibitory receptors PD-1 and the IL1 receptor family member, IL1R8 (SIGIRR or TIR8). Treg production of the IL1R8 ligand, IL37, contributed to the phenotypic changes and diminished function in Treg-suppressed canonical NK cells. Blocking PD-1, IL1R8, or IL37 abrogated Treg suppression of canonical NK cells while maintaining NK-cell TIM3 expression. Our data uncover new mechanisms of Treg-mediated suppression of canonical NK cells and identify that adaptive NK cells are inherently resistant to Treg suppression. Strategies to enhance the frequency of adaptive NK cells in the tumor microenvironment or to blunt Treg suppression of canonical NK cells will enhance the efficacy of NK-cell cancer immunotherapy.
Keywords: Adaptive NK cells, Treg, TIM3, PD-1, IL1R8
Introduction
NK cells are innate immune effectors capable of fighting viral infections and cancer (1). Although NK cells are expected to target malignantly transformed cells and play an important role in the immune surveillance against tumors, it is now appreciated that tumor-induced immune suppression may dampen NK-cell efficacy. Despite the promise of NK-cell therapy in hematological malignancies (2,3), the suppressive components in the tumor microenvironment, including regulatory T cells (Tregs), may limit NK-cell efficacy (4). In cancer patients, infiltration of tumors by Tregs is associated with poor prognosis (5–7). In vitro studies have revealed a central role for Tregs in suppressing NK and conventional T-cell responses (8,9). Tregs can secrete immune suppressive cytokines [e.g. transforming growth factor beta (TGFβ), IL10, IL35, and IL37], and express inhibitory proteins [e.g., cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-ligand 1 (PD-L1)] on their cell surfaces (9). Therefore, strategies to resist or overcome the suppressive pressure by Tregs on NK cells may enhance antitumor responses.
NK-cell function is tuned as a result of a balance between inhibitory and activating receptor signaling (10). NK cells that arise in response to cytomegalovirus (CMV) infection exhibit properties of immunological memory (11,12). A subset of NK cells, termed “adaptive” NK cells, is characterized by a downregulation of the transcription factor PLZF and the proximal signaling molecules SYK, EAT-2, and FcεRγ. Additionally, adaptive NK cells exhibit elevated expression of the activating receptor NKG2C and the terminal maturation marker CD57 (13). Adaptive NK cells exhibit enhanced secretion of IFNγ relative to canonical NK cells after exposure to tumor targets and in response to stimulation through CD16 (a low affinity receptor for the Fc portion of IgG) (13).
We have shown that adaptive NK cells are inherently resistant to myeloid-derived suppressor cells (MDSC) suppression in patients with cancer (14) and hypothesized that adaptive NK cells may also be resistant to Treg-mediated suppression. We found that, compared to canonical NK cells, adaptive NK cells expressed a higher density of T-cell immunoglobulin and mucin-domain containing-3 (TIM3), which is an inhibitory receptor on T cells but an activating receptor on NK cells, resulting in heightened IFNγ production. Additionally, adaptive NK cells expressed a density inhibitory receptors PD-1 and IL1R8. ILR8 belongs to the IL1 receptor family, with IL37 as a ligand (15). IL37 is produced by Tregs and contributes to their suppressive function (16). IL1R-IL37-IL18Rα tripartite complex formation results in negative regulation of IL18R and inhibits IL18 activity (15,17). Signaling through IL18 contributes to NK-cell function, as IL18R-deficient NK cells are unable to secrete IFNγ in response to ex vivo stimulation with IL12 (18). Our findings demonstrate the mechanisms of how adaptive, unlike canonical, NK cells resist Treg suppression and implicate IL37 as a major mediator of NK-cell suppression through downregulation of TIM3.
Material and Methods
Healthy donors
Peripheral blood mononuclear cells (PBMCs) from healthy CMV seropositive donors were obtained from Memorial Blood Bank (Memorial Blood Center, St Paul, MN, USA). All samples were de-identified before receipt. The University of Minnesota institutional review board, in accordance with the Declaration of Helsinki, approved use.
Treg sorting and expansion
A previously established Treg expansion protocol was utilized in this study (19). Briefly, PBMC from healthy blood donors were collected after Ficoll gradient centrifugation (Ficoll-Paque Plus). Tregs (CD4+CD127−CD25highCD45RA+) were sorted to > 95% purity on a FACS Aria II (Becton Dickinson) following CD25 microbead enrichment following the manufacturer’s instructions (Miltenyi Biotech). Tregs were expanded by coculture with a K562 feeder cell line (KT) engineered to express CD86 and CD64 (KT64/86) (fresh, 1:2 KT:Treg, cell density: 0.25 × 106 – 0.5 × 106 cells/ml) and medium supplemented with IL2 (300 IU/ml) and rapamycin (100 ng/ml (109 nM)) (Rapammune, Wyeth-Ayerst, Princeton, NJ) (20). Cells were cultured for 14 days at 0.25 × 106 – 0.5×106 cells/ml and then restimulated with anti-CD3/28 activation beads (3 Dynabeads/cell, Thermofisher) and expanded an additional 7–10 days before use in experiments. Tregs expanded for 14 or 21 days were 90 ± 3% Foxp3+CD127− and suppressed CD8+ T cell proliferation 65 ± 4% in a standard CFSE assay (21).
Suppression assay
PBMCs were isolated from whole blood by density gradient centrifugation using Ficoll-Paque Premium (GE Healthcare). Untouched CD3−CD56+ NK cells were isolated using negative depletion kits (StemCell Technologies). NK-cell suppression was assessed in a mixed-lymphocyte reaction (MLR) (22). Blood circulating HLA-DR+ antigen-presenting cells (APCs, > 85% monocytes) were isolated (purity > 93%) from PBMC using HLA-DR positive selection microbeads following the manufacturer’s instructions (Miltenyi Biotech). NK cells (0.5 × 105 – 1 × 105/well in a 96-well plate) and APCs were cocultured at a 1:1 ratio in the presence or absence of Tregs (Treg:NK cells: 0:1–1:8) in RPMI supplemented with IL12 (50 IU/ml) for 6 days.
Cytokine stimulation
To induce TIM3 expression in TIM3low/negative NK cells, purified NK cells (0.5 × 105 – 1 × 105/well in 96-well plate) were cocultured with APCs (1:1) and stimulated overnight with IL12 (5 ng/ml) and IL18 (50 ng/ml) (R&D Systems) or left unstimulated (23). Following overnight stimulation, cells were washed and cocultured with Tregs (2:1) for 5 days in the presence of IL2 (50 IU/ml) and antagonistic mAb to TIM3 (10 μg/ml, Biolegend) prior to evaluation of NK-cell function. NK cells were phenotyped for TIM3 expression before and after coculture as described below.
NK-cell function and flow cytometry analysis
NK cells were cocultured with APCs ± Tregs for 6 days in the presence of IL2. NK-cell degranulation and cytokine production were then evaluated following stimulation with agonistic anti-CD16 (1 μg/ml), IL12 (5 ng/ml), and IL18 (50 ng/ml) for 6 hours prior to staining. Cells were stained with fluorochrome-conjugated antibodies as described in Supplementary Table 1S. Detection of CD107a, Ki67, and IFNγ was performed following fixation and permeabilization (eBioscience), according to the manufacturer’s instructions. In some experiments, NK cells and Tregs were cultured in the presence of blocking antibodies (Supplementary Table S1), including anti-IL37 (10 μg/ml, R&D systems), anti-IL1R8 (10 μg/ml, R&D systems), anti-PD-1 scFv, anti-TIM3 (10 μg/ml, Biolegend) or control IgG (10 μg/ml). In the absence of Tregs, NK cells were cultured in the presence of recombinant IL37 (3 μg/ml, R&D Systems) and PD-L1/Fc chimera protein (10 μg/ml, R&D Systems) for 6 days prior staining. In addition, cryopreserved, expanded Tregs were thawed and stimulated with IL2 (300 IU/ml) overnight and then evaluated for the expression of Gal-9 (a TIM3 ligand), PD-L1, and IL37 (Supplementary Table S1). Cells were treated with the protein transport inhibitors Golgistop and Golgiplug (BD Biosciences) 6 hours prior staining. All FACS data was acquired using an LSRII (BD Biosciences) and analyzed by FlowJo 10.0.
Phosflow
NK cells from healthy blood donors were cocultured with APCs ± Tregs for 6 days in the presence of IL2 (50 IU/ml). Cells were then washed and stimulated with recombinant Gal-9 (50 nM) for 20 min. before analysis of NF-κB and Akt phosphorylation. Cells were fixed, permeabilized (eBiosciences), and stained for pNF-κB (pS529) and pAkt (pS473) (BD Biosciences).
Live kinetic analysis of tumor cell killing
K562 (chronic myelogenous leukemia), THP1 (acute monocytic leukemia), and DU145 (prostate cancer) (authenticated from ATCC, used within three months of the first passage) were labeled with red fluorescent CellTracker (5 μM, Invitrogen). For analysis of tumor cell killing. K562, THP-1, and DU-145 cells were plated at a concentration of 1 × 104 cells per well in 96-well flat bottom plates and incubated with the Caspase 3/7 green dye (Essen BioScience) to detect killing. The following day, precultured, FACS-sorted, adaptive (CD3−CD56dimCD57+NKG2C+) or canonical (CD3−CD56dimCD57+ NKG2C−) NK cells along with APCS ± Tregs were added at a 3:1 ratio to the target cells. The number of killed target cells was monitored by hourly fluorescence imaging over 48 hours using an IncuCyte Live Cell Analysis System (Essen BioScience). Dead cell numbers were quantified using IncuCyte Zoom software (Essen BioScience) and normalized to the number of dead cells remaining in the target cell only control group (% killing = (overlap counts/red counts) × 100).
Statistical analysis
Data were aggregated as mean ± standard error of the mean (SEM). Paired t tests or the mixed effects model was used for comparisons, maintaining internal paring within each donor sample depending on whether the paired data were completely observed or not. P values for multiple pairwise comparisons were adjusted using the method of Hommel (24). TIM3 and PD-1 were correlated with IFNγ production using linear regression. Mean relative target cell killing over time was analyzed using the mixed effects model with fixed effects of time and group, and a random intercept. All tests were two sided. Statistical analyses were carried out with SAS 9.4 (SAS Institute, Cary, NC), and graphs were made with Prism 6 software (GraphPad).
Results
Tregs inhibit proliferation and function of canonical, but not adaptive, NK cells
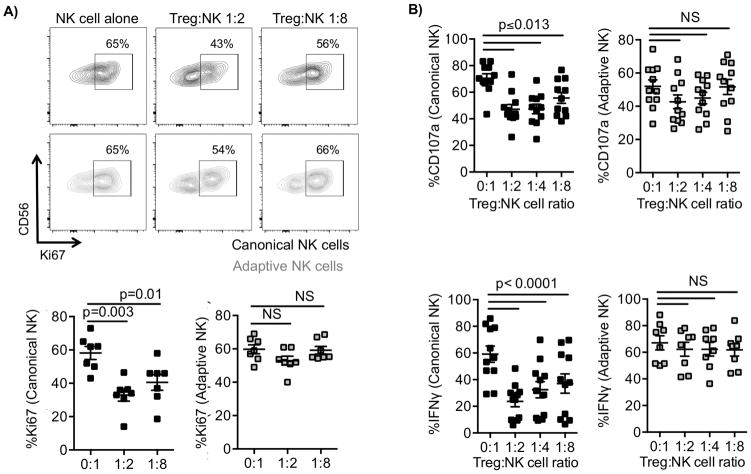
To test our hypothesis that adaptive NK cells can resist suppression mediated by Tregs, we isolated NK cells from the peripheral blood of healthy CMV seropositive donors. Given the variability of adaptive NK cells in CMV seropositive donors, to ensure a sufficiently large population of cells for analysis, donors were screened and selected for study based on having at least 6% CD57+NKG2C+ CD56dim NK cells. Enriched bulk NK-cell proliferation was assessed in a mixed lymphocyte reaction (MLR) following coculture with autologous APCs (1:1), with or without expanded allogeneic Tregs, at different Treg:NK-cell ratios (1:2–1:8). Allogeneic APCs were used instead of anti-CD3/CD28 stimulation beads to avoid artificial stimulation of Tregs. Cells were cocultured for 6 days in the presence of IL2 (50 IU/ml). Canonical NK cells were defined as CD3−CD56dimCD57+FcεRγ+NKG2C− and adaptive NK cells as CD3−CD56dimCD57+FcεRγ−NKG2C+. We observed a profound inhibition of canonical NK-cell proliferation, as assessed by Ki67 staining, compared with no significant suppression of adaptive NK-cell proliferation (Fig. 1A).
Figure 1. Canonical but not adaptive NK-cell function is suppressed by Tregs.
NK cells were cocultured with IL2 (50 IU/ml) and autologous HLA-DR+ cells (APCs, 1:1) ± allogeneic Tregs at different Treg:NK ratios (1:1–1:8) for 6 days. CD3−CD56+CD57+FCεRγ+NKG2C− gated canonical and CD3−CD56+CD57+FCεRγ−NKG2C+ gated adaptive NK-cell proliferation (as determined by Ki67 activation) was evaluated by flow cytometry. A) Scatter plots of Ki67 against CD56 for one representative donor and cumulative data from 7 donors shown as mean ± SEM. B) NK cells were cultured with IL2 (50 IU/ml) and APCs ± Tregs at different Treg:NK ratios (1:2–1:8) for 6 days and then stimulated with anti-CD16 antibody (1 μg/ml), IL12 (5 ng/ml), and IL18 (50 ng/ml) 6 hours prior to analysis. Canonical and adaptive NK-cell degranulation (CD107a) and IFNγ production were evaluated by flow cytometry. Results from 4 independent experiments, and pooled data (n = 8–11) are shown as mean ± SEM and statistical analyses were performed using a paired t test.
To further characterize NK-cell susceptibility to Treg-mediated suppression, NK cells were cultured with APCs ± Tregs at different Treg:NK ratios in the presence of IL2 for 6 days and then stimulated with anti-CD16, IL12, and IL18 for 6 hours prior to analysis. Adaptive NK cells are less responsive to IL12 and IL18 stimulation, likely due to low IL12/IL18 receptor expression, but they are more sensitive to stimulation by CD16 ligation compared to canonical NK cells (13). Therefore, to stimulate both canonical and adaptive NK cells, an agonistic antibody to CD16, in addition to IL12/IL18, was used to study function. We performed a FACS-based assay to measure NK-cell degranulation by surface CD107a expression and intracellular IFNγ production. We observed a potent inhibition of canonical, but not adaptive, NK-cell degranulation (P ≤ 0.013) and IFNγ production (P < 0.0001, Fig. 1B). Our data demonstrate that adaptive NK cells, in contrast to canonical NK cells, are resistant to immune suppression mediated by Tregs, as measured by CD107a degranulation and cytokine production.
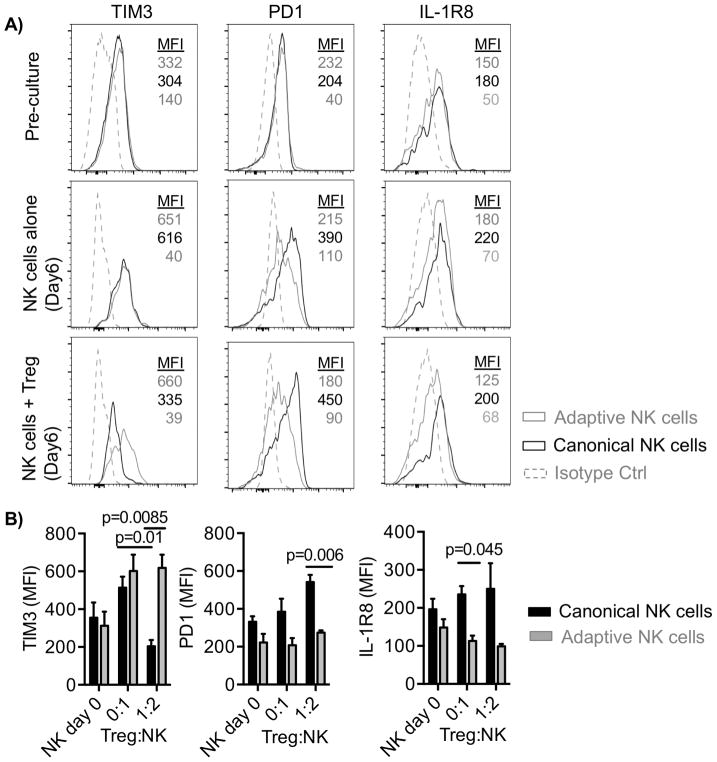
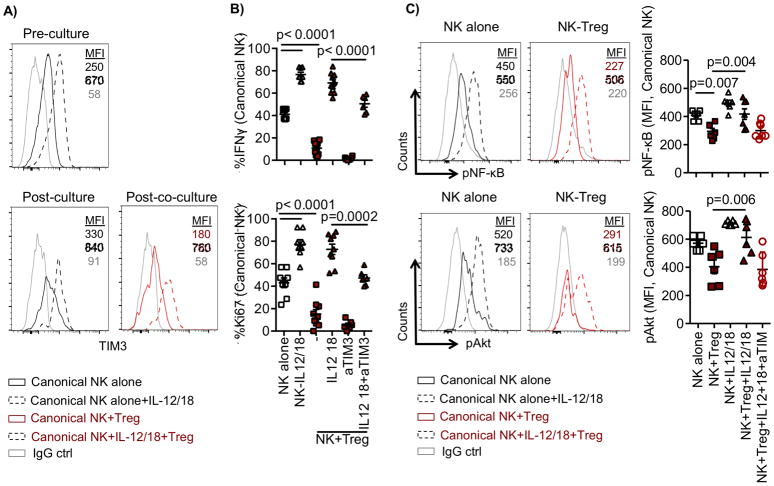
Tregs inhibit activation-induced TIM3 expression only in canonical NK cells
To determine whether specific receptor expression on adaptive NK cells was associated with resistance to Treg suppression, we analyzed the surface expression of inhibitory and activating receptors (NKp44, NKp46, DNAM-1, NKG2A, TIGIT, PD1, TIM3, and IL1R8) on canonical and adaptive NK cells following 6 days of culture with APCs ± Tregs. Among the investigated receptors, the expression of the inhibitory receptors PD-1 and IL1R8 and the activating receptor TIM3 on resting NK cells prior to culture was not significantly different between the two subsets of NK cells (Fig. 2A). However, activation-induced TIM3 expression was observed in canonical and adaptive NK cells following 6 days of culture in the absence of Tregs (TIM3 MFI: 534.17 ± 71 and 630 ± 94, respectively) (Fig. 2A and B). Although adaptive NK cells maintained high expression of TIM3, 70% downregulation of TIM3 expression was observed on canonical NK cells following coculture with Tregs (MFI: 645 ± 73 vs. 204 ± 33, P = 0.01). In addition, adaptive NK cells expressed less PD-1 and IL1R8, compared with canonical NK cells cultured in the presence of Tregs (PD-1 MFI: 274 ± 12 vs. 542 ± 37, P = 0.006; IL1R8 MFI: 99 ± 6 vs. 250 ± 67, P > 0.05) (Fig. 2A and B). We excluded other receptors (NKp44, NKp46, DNAM-1, NKG2A, TIGIT) from further analysis due to the lack of differences between NK-cell subsets in the presence of Tregs. Together, our results show that, relative to canonical NK cells, adaptive NK cells have a sustained active phenotype through high expression of the activating receptor TIM3 and lower expression of inhibitory PD-1 and IL1R8 after coculture with Tregs.
Figure 2. Adaptive NK cells express high levels of TIM3 and low levels of PD-1 and IL1R8.
NK cells were cocultured with IL2 (50 IU/ml) and APCs ± Tregs and evaluated for TIM3, PD-1, and IL1R8 expression before culture and 6 days post-culture. A) Representative histogram plots of receptor expression on gated subsets of NK cells prior to coculture, after culture in the absence of Tregs, or after culture with Tregs. B) Pooled data (n = 6) are shown as mean ± SEM. Data are shown from 3 independent experiments statistical analysis was performed using a paired t test.
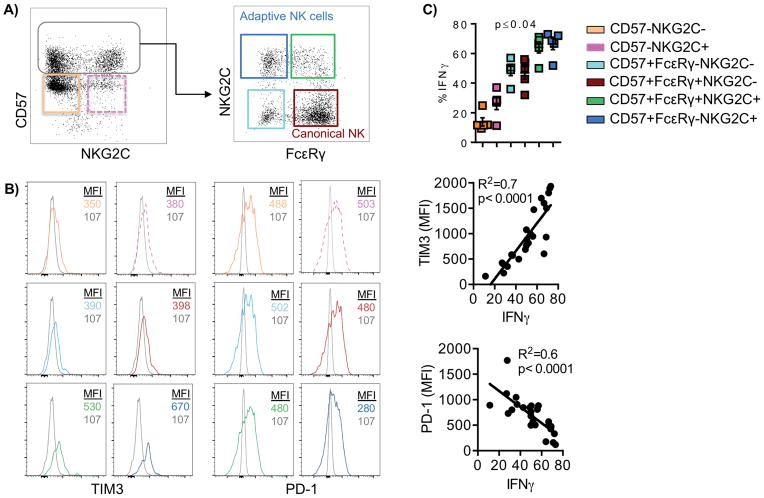
TIM3- and PD-1–dependent regulation of NK cells during culture with Tregs
Expression of PD-1 on NK cells is associated with diminished NK-cell responsiveness against multiple myeloma plasma cells (25). However, conflicting studies have shown both inhibitory and activating function of TIM3 engagement in NK cells (23,26). Here, we investigated the correlation between PD-1 or TIM3 expression and IFNγ production by different NK-cell subsets. Purified NK cells were cocultured with APCs and Tregs for 6 days. NK cells were then stimulated with anti-CD16 and IL12 and IL18 for 6 hours prior to analysis. Early mature NK cells characterized by the lack of CD57 expression were segregated by their expression of NKG2C and gated as CD57− NKG2C− or CD57−NKG2C+. In addition, CD57+ late mature NK cells were subgated as CD57+ FcεRγ+NKG2C− (canonical NK cells) and, CD57+ FcεRγ+NKG2C+, or CD57+ FcεRγ−NKG2C+ (adaptive NK cells) (Fig. 3A). These gating strategies were selected based on the methylation signatures defining adaptive versus canonical NK cells (13). We sought to determine how each marker associated with adaptive NK cells (CD57, FcεRγ, and NKG2C) influenced the NK-cell activation state. Comparing immature to mature NK cells, mature NK cells had increased TIM-3 expression with an opposing decrease in PD-1 expression (Fig. 3B). This phenotype (TIM3highPD-1low) correlated well with increased IFNγ production (TIM3 vs. IFNγ, R2 = 0.7, P < 0.0001; PD-1 vs. IFNγ, R2 = 0.6, P < 0.0001), with adaptive NK cells displaying the highest IFNγ production (all pairwise P values ≤ 0.04) (Fig. 3B, C). Together, these results show that high TIM3 and low PD-1 expression correlates with enhanced function in different NK-cell subpopulations and supports an activating role for TIM3 with regard to NK-cell function.
Figure 3. Canonical and adaptive NK-cell respond differently to Treg-mediated suppression.
NK cells were cocultured with APCs + Tregs for 6 days in the presence of IL2 (50 IU/ml) and stimulated with anti-CD16, IL12, and IL18 6 hours prior to analysis. A) The gating strategy is shown for different NK cells subsets. B) Histogram plots showing the expression of TIM3 and PD-1 from one representative donor. C) IFNγ production and correlation analyses were done by evaluating IFNγ production of different NK-cell subsets (n = 5 donors) and the expression of TIM3 and PD-1 following 6 days of coculture with IL2, APCs and Tregs after terminal stimulation with anti-CD16 antibody, IL12, and IL18. Pooled data (n = 5) are shown from two independent experiments as mean ± SEM and statistical analyses were performed using paired t test and Mixed effects model.
Effects of blocking the IL37 pathway on NK-cell phenotype and function
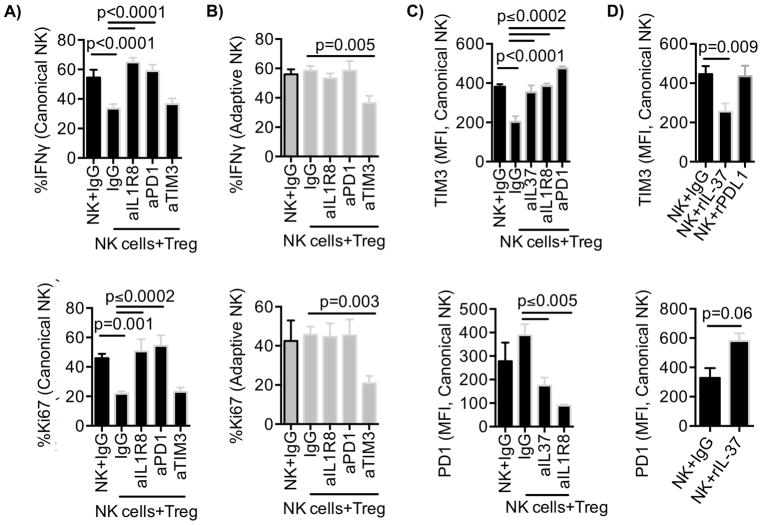
Our data suggest that adaptive NK-cell resistance to Tregs is associated with high TIM3 and low PD-1 expression. To better understand this phenomenon, we performed blocking experiments to evaluate the functional role of TIM3 and PD-1 individually in adaptive versus canonical NK cells. Purified NK cells were cocultured with APCs ± Tregs in the presence of blocking antibodies against PD-1, TIM3, or an IgG isotype control antibody for 6 days and analyzed for function. IL1R8 expression was significantly lower on activated adaptive, compared to canonical, NK cells (Fig. 2). To explore this, we blocked IL1R8, the receptor for IL37, on canonical NK cells to inhibit IL18 signaling (15,17) necessary for NK cells to display full effector activity (18).
The addition of blocking antibodies against IL1R8, PD-1, or TIM3 to canonical and adaptive NK cells alone, or in coculture with APCs in the absence of Tregs, did not affect IFNγ production compared to IgG isotype control antibody conditions (Supplementary Fig. S1A, B). However, in conditions where NK cells were cultured with APCs + Tregs and IgG isotype control antibody, we observed significantly reduced canonical NK-cell responses (Fig. 4A). By adding blocking antibodies against IL1R8 or PD-1 during coculture, we were able to restore canonical NK-cell IFNγ production and proliferation (Fig. 4A). We found no IL1R8 expression on Tregs, supporting the conclusion that blocking IL1R8 predominantly affects NK-cell activity. Canonical NK-cell degranulation was also significantly increased in the presence of blocking PD-1 in Treg cocultures (% CD107a: 21 ± 1% vs. 30 ± 1%, p = 0.02, Supplementary Fig. S1C). Conversely, blocking antibodies against TIM3 significantly inhibited adaptive NK-cell function and proliferation in Treg cocultures (CD107a: 32 ± 3% vs. 20 ± 2%, P = 0.04; % IFNγ: 58 ± 3% vs. 36 ± 5%, P = 0.005; % Ki67: 46 ± 4% vs. 21 ± 5%, P = 0.003) (Fig. 4B and Supplementary Fig. S1C), confirming an activating role of TIM3 on NK-cell function.
Figure 4. Blocking IL1R8 and PD-1 abrogates Treg-mediated suppression of canonical NK cells.
A) Canonical and B) adaptive NK cells cultured with IL2 (50 IU/ml) and APCs ± Tregs (Treg:NK, 1:2) in the presence of blocking antibodies against IL1R8, PD-1, TIM3, or control IgG for 6 days. NK-cell cultures were then stimulated with anti-CD16 antibody, and IL12, and IL18 for 6 hours prior analysis of IFNγ production and proliferation (Ki67). Cumulative data (n = 7) are shown from three independent experiments as mean ± SEM. C) Canonical NK-cell TIM3 (upper panel) and PD-1 (lower panel) expression were analyzed following culture with APCs ± Tregs and in the presence of blocking antibodies against IL37, IL1R8, and PD-1 or IgG. D) Canonical NK cells in culture with APCs were treated with recombinant IL37 (3 μg/ml) or PD-L1 Fc chimera protein (10 μg/ml) and evaluated for the expression of TIM3 and PD-1 expression after 6 days of culture. Cumulative data (n = 6–8) are shown from three independent experiments as mean ± SEM and statistical analyses were performed using pairwise comparison mixed-effects model.
Next, we confirmed the expression of IL37, PD-L1, and a TIM3 ligand, Gal9, in expanded Tregs. We found that the expanded Tregs expressed PD-L1, IL37, and Gal9 (Supplementary Fig. S1D). Although APCs expressed little IL37, the level expressed on Tregs was nearly double that of APCs (P = 0.002, Supplementary Fig. S2A). Subsequently, we analyzed the relationship between PD-1, IL1R8, and TIM3 on NK cells and IL37 produced by Tregs. Blocking PD-1 or IL1R8, or neutralizing IL37, during 6 days of culture in the presence of Tregs, restored TIM3 expression in canonical NK cells (P ≤ 0.0002, Fig. 4C, upper panel). Blocking IL37 and IL1R8 in NK-cell cocultures with Tregs led to a significant decrease in PD-1 expression on canonical NK cells (P ≤ 0.005, Fig. 4C, lower panel). Our data suggest that PD-1, IL1R8, and IL37 are involved in the regulation of TIM3 expression on canonical NK cells.
To rule out the involvement of additional suppressive mediators we tested whether recombinant IL37 alone or a PD-L1 fusion protein alone could directly regulate PD-1 or TIM3 expression on NK cells cultured with APCs in the absence of Tregs. Addition of IL37 led to a downregulation of TIM3 and upregulation of PD-1 expression (TIM3 (MFI): 446 ± 41 vs. 255 ± 41, P = 0.009; PD-1 (MFI): 329 ± 66 vs. 580 ± 52, P = 0.06) (Fig. 4D). Recombinant IL37 significantly reduced proliferation and IFNγ production by NK cells cultured in the absence of Tregs (Supplementary Fig. S2B). However, engaging PD-1 with an PD-L1/Fc protein did not reduce TIM3 expression on NK cells (Fig. 4D) and did not affect NK-cell function. This suggests that canonical NK cells expressed little PD-1 in the absence of Tregs and engagement by PD-L1 protein was not enough to suppress NK cells under the activation conditions tested.
To confirm that the antibody to IL1R8 used in this study is a blocking antibody, we cultured NK cells with rIL37 alone or with the addition of anti-IL1R8 antibody. We found that rIL37 decreased TIM3 expression on NK cells, and this decrease was abrogated with the anti-IL1R8 antibody, confirming the blocking nature of the anti-IL1R8 reagent used (Supplementary Fig. S2C). Adaptive NK cells exhibited no change in PD-1 or TIM3 receptor expression after blocking IL37, IL1R8, or PD-1 in the presence of Tregs or with recombinant IL37 or PD-L1 in the absence of Tregs (Supplementary Fig. S2D). This demonstrates the inherent resistance of adaptive NK cells to Treg suppressive mechanisms. Collectively, our data demonstrate that IL37 produced by Tregs modulates TIM3 and PD-1 expression on canonical NK cells and suppresses their function.
Induced TIM3 expression reverses Treg-mediated suppression
Given the substantial downregulation of TIM3 on canonical NK cells following culture with Tregs, we sought to manipulate TIM3 expression to determine whether function could be restored. Purified NK cells were cultured with APCs and primed with IL12 (5 ng/ml) and IL18 (50 ng/ml) overnight to induce TIM3 expression prior coculture with Tregs. Cells were then washed to eliminate residual cytokines and subsequently cultured with or without Tregs for 6 days. NK cells were subsequently stimulated with anti-CD16 and IL12/IL18 6 hours prior to functional analysis. TIM3 expression was examined before and after coculture with Tregs. There was a clear increase in TIM3 expression on canonical NK cells following IL12 and IL18 overnight priming (Fig. 5A). Six days later, canonical NK cells primed with IL12 and IL18 maintained high expression levels of TIM3 that were similar to those observed on adaptive NK cells, despite the presence of Tregs (Fig. 5A, Supplementary Fig. S3A). Overnight treatment with IL12/IL18 significantly increased NK-cell proliferation (shown by Ki67) and IFNγ production in the absence of Tregs (Fig. 5B). As predicted, Tregs were not able to suppress canonical NK cells that were overnight-primed with IL12/IL18 prior to coculture. Tregs retained their ability to suppress IL12/IL18-primed canonical NK cells in the presence of blocking antibodies against TIM3, showing the specificity of the IL12/IL18 priming effect (Fig. 5B).
Figure 5. Induction of TIM3 on canonical NK cells restores function in cocultures with Tregs.
Purified NK cells were cocultured with APCs (1:1) overnight and stimulated with IL12 (5 ng/ml) and IL18 (50 ng/ml) to increase TIM3 or left unstimulated. Following overnight stimulation, cells were washed and cultured with IL2 (50 IU/ml) ± Tregs (Treg:NK, 1:2) for 6 days in the presence of anti-TIM3 or control IgG. (A) Representative histogram of TIM3 expression is shown before and after coculture. B) NK-cell function was evaluated following stimulation with anti-CD16 antibody and IL2 and IL18 6 hours prior staining. C) Following coculture, NK cells were stimulated with soluble recombinant Gal9 (50 nM) for 20 min. prior to analysis for the phosphorylation of NF-κB (pNF-κB) and Akt (pAkt). Representative and cumulative data are shown from 2 independent experiments. Cumulative data (n = 6–12) are shown as mean ± SEM and statistical analyses were performed using pairwise comparisons and mixed effect models.
Engagement of TIM3 with one of its cognate ligands, Gal9, can result in phosphorylation of downstream proteins NF-κB and Akt (23,27). Here, we evaluated the phosphorylation of NF-κB and Akt in NK cells cultured with APCs ± Tregs and stimulation with or without recombinant Gal9. Phosphorylation of NF-κB and Akt was not different between unstimulated NK cells alone and NK cells cultured with Tregs at the baseline levels (Supplementary Fig. S3B). However, compared to unstimulated, stimulation with recombinant Gal9 increased the phosphorylation of NF-κB (MFI: 46 ± 6 vs. 418 ± 37) and Akt (314 ± 34 vs. 612 ± 56) in NK cells cultured without Tregs. In contrast, we found a persistent lack of stimulation of NF-κB and Akt phosphorylation in canonical, but not adaptive, NK cells cultured with Tregs. Yet, overnight priming of canonical NK cells with IL12/IL18 resulted in sustained NF-κB and Akt phosphorylation levels (Fig. 5C and Supplementary Fig. S3C). However, blocking TIM3 abolished the positive effect generated by the IL12/18 pretreatment of NK cells (Fig. 5C). Together, our data show a strong TIM3-dependent regulation of NK-cell function in Treg cocultures.
Adaptive NK-cell function is resistant to Treg-mediated suppression
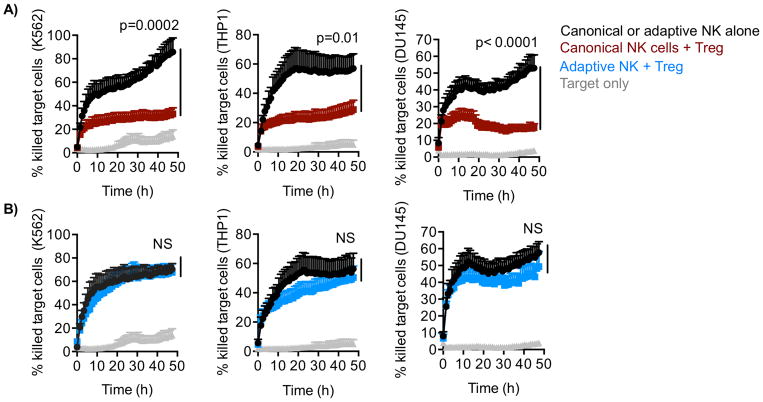
Since Tregs are frequently recruited to the tumor site in many cancers, we studied how adaptive and canonical NK cells perform in an in vitro tumor model. We performed 48-hour cytotoxicity analyses of canonical and adaptive NK cells against various tumor cell lines in the presence or absence of Tregs.
Sorted adaptive and canonical NK cells were separately cultured with APCs ± Tregs in the presence of IL2 for 6 days. Various target cell lines were first analyzed for the expression of PD-L1 and Gal9. We selected three cell lines based on high expression of Gal9 but different expression levels of PD-L1. The chronic myelogenous leukemia cell line K562 was Gal9highPD-L1absent, the acute monocytic leukemia cell line THP1 was Gal9highPD-L1low, and the prostate cancer line DU145 was Gal9highPD-L1high (Supplementary Fig. S3D). To evaluate NK-cell cytotoxicity, we incubated adaptive or canonical NK cells at a 3:1 E:T ratio with the different tumor cell lines pre-labeled with red Celltracker dye (live) and Caspase 3/7 green dye (apoptotic). Target cell killing was measured hourly over the course of 48 hours by imaging using the IncuCyte Live Cell Analysis System. Labeled target cells incubated alone were used in this assay as a control. Target cell killing by adaptive and canonical NK cells was similar in the absence of Treg. In agreement with 6-hour functional readouts, canonical NK-cell cytotoxicity against all three cell lines was significantly inhibited by the presence of Tregs (P < 0.0001, Fig. 6A). In contrast, adaptive NK-cell cytotoxicity was similar in the presence or absence of Treg (Fig. 6B). Together, these results demonstrate that, in contrast to canonical NK cells, adaptive NK cells are inherently resistant to functional suppression mediated by Tregs.
Figure 6. Adaptive NK cells resist immunosuppression in the tumor microenvironment.
FACS-sorted NK cells were cultured with IL2 (50 IU/ml) and APCs ± Tregs (Treg:NK, 1:2) for 6 days. A) Canonical and B) adaptive NK cells were separately co-incubated with dye-labeled tumor cell lines, K562, THP-1, DU-145 at a 3:1 effector to target cell ratio, and NK-cell cytotoxicity was evaluated over 48-hours by live imaging. Cumulative data (n = 6) of quantified relative killing are shown from two independent experiments as mean ± SEM and statistical analyses were performed using mixed effect models to compare slopes between groups.
Discussion
Cancer progression is generally associated with impaired antitumor immunity and recruitment of regulatory cell populations to the tumor microenvironment. A common feature of several cancers is the frequent infiltration of Tregs (28,29). Studies have shown that Tregs frequencies are highly correlative with impaired NK-cell function in patients with cancer and in murine tumor models (30,31). Our results show that CD3−CD56dimCD57+FcεRγ−NKG2C+ adaptive NK cells are highly resistant to Treg-mediated suppression through mechanisms involving IL37, PD-1, and TIM3, adding IL37 as a regulator of PD-1 and TIM3 expression. Luo, et al. showed that IL37-expressing transgenic mice have a marked reduction in CD8+ T cells but an increased frequency of Tregs that was associated with impaired conventional T cell responses (32)., IL37 is described as a fundamental inhibitor of the innate immune responses. IL37-expressing transgenic mice have reduced concentrations of circulating inflammatory cytokines, including IFNγ (33). IL37 binds to the IL18 receptor, leading to recruitment of the inhibitory receptor component IL1R8, in order to induce anti-inflammatory activity (15). However, one study reported that overexpression of IL37 in immunohistochemical sections of 163 primary hepatocellular carcinoma (HCC) clinical specimens was positively associated with tumor-infiltrating CD57+ natural killer (NK) cells and better overall survival and disease-free survival (34). Discrepancies in the earlier reports might be explained by differences in IL37 concentrations produced by different cell types. Li, et al. found that low, rather than high, concentrations of IL37 resulted in suppression of cytokines production in vitro (35).
In our study, we found that IL37 produced by Tregs led to downregulation of TIM3 expression on canonical, but not adaptive, NK cells. TIM3 downregulation resulted in decreased function of canonical NK cells that was restored by blocking the IL37 receptor IL1R8. IL1R8 blockade of Treg IL37 restored IFNγ production and proliferation but not the lower signal threshold function defined by degranulation of NK cells. Our data are consistent with studies showing that IL1R8 inhibits inflammatory cytokine production (e.g. IFNγ, IL6, and MIP-2) (36). IL1R8 is extensively expressed in NK cells compared to other immune cells (37). The IL1R8 subunit functions as an immune checkpoint for NK-cell mediated antitumor and viral activity. In a hepatocellular carcinoma model using IL1R8−/− transgenic mice, the authors found an NK cell–dependent decrease in liver and lung metastasis. In addition, they found lower virus titers after infection with cytomegalovirus in IL1R8−/− compared to wild-type mice (37). These studies support our current findings that IL1R8 serves as an inhibitory immune checkpoint for human NK cells.
Differences in canonical and adaptive NK-cell responses to Treg inhibition could be explained by the significantly lower induction of IL1R8 in adaptive NK cells as shown here. We found that treating canonical NK cells with recombinant IL37 in the absence of Tregs induced higher expression of PD-1 and downregulated TIM3. Thus, our data show that IL37 regulates PD-1 and TIM3 expression and inhibits canonical NK-cell responses (Supplementary Fig. S4).
We have previously shown that IL12 and IL18 stimulation of NK cells induced TIM3 expression, which increased NK-cell IFNγ production (23). Hence, we pre-stimulated NK cells with IL12/IL18 prior coculture with Tregs. TIM3 induction preserved normal function in canonical NK cells in the presence of Tregs. Our data implicate TIM3 as a critical activating receptor on NK cells in the context of interactions with Tregs. TIM3 can have different functions on T and NK cells. Blockade of T-cell TIM3 in combination with anti-PD1/PD-L1 or anti-CTLA-4 reverses their exhaustion and enhances their antitumor activity in murine models, suggesting that TIM3 is an inhibitory receptor (38,39). A multi-center clinical trial is now open and recruiting patients with solid tumors to test this treatment clinically (TSR-022), but results are not yet available. Based on our studies with IL12/IL18 priming to increase TIM3 function as an activating receptor, the effect of cytokine priming on TIM3 expression may explain the robust IFNγ production of “cytokine-induced memory like” NK cells in patients with AML treated with HLA-haploidentical NK-cell infusions (40).
We found that blocking PD-1 restored canonical NK-cell function. However, neither blocking PD-1 nor engaging PD-1 by PD-L1 fusion protein affected TIM3 expression on canonical NK cells, indicating no direct role of PD-1/PDL-1 in regulating TIM-3 expression. Our data is consistent with studies that have shown PD-1/PD-L1 interactions are associated with impaired NK-cell activity (25,41). Our data suggest that downregulation of TIM3, perhaps in combination with signaling through PD-1, results in a net suppression of NK cells.
Here we show that adaptive NK cells are resistant to Treg-mediated suppression and have sustained cytotoxicity in long-term cytotoxicity analyses against a number of tumor cell lines. This data adds to our study showing that adaptive NK cells resist MDSC suppression in patients with cancer (14). In the previous study, we found that adaptive NK cells, in contrast to canonical NK cells, expressed less of the inhibitory receptor TIGIT. Moreover, MDSC highly expressed the TIGIT ligand CD155 compared to normal monocytes. Lack of engagement of TIGIT with CD155 enabled the interaction between the activating receptor DNAM-1 and CD155 resulting in an activation of adaptive NK cells with resistance to MDSC-mediated suppression. In this current study, we found no difference in TIGIT expression between adaptive and canonical NK cells following coculture with Tregs, indicating different resistance mechanisms. Taken together, our data suggests that adaptive NK cells have the capacity to maintain normal function in the tumor microenvironment even when suppressor cells are present.
Over the last decade, the field of cancer immunotherapy has seen considerable progress. However, numerous studies suggest that suppressive immune cells within the tumor microenvironment, including Tregs, negatively affect efficacy of cancer immunotherapy. New approaches to resist suppression are necessary to achieve objective clinical responses. Our findings have immediate translational value and support the rationale for expanding adaptive NK cells to treat cancer patients. Alternatively, developing approaches to block the suppressive mechanisms of Tregs on canonical NK cells will enhance antitumor responses and increase the efficacy of immunotherapy using NK cells.
Supplementary Material
Acknowledgments
We wish to acknowledge the flow cytometry core at the University of Minnesota for excellent service and technical support.
Financial support: This work was supported by a fellowship to D. Sarhan from Karolinska Institutet, Sweden and the following NIH grants: K99HL123638 (F. Cichocki), P01 CA111412 (J.S. Miller, X. Luo, S. Cooley), P01 CA65493 (J.S. Miller, B.R. Blazar, K.L. Hippen, X. Luo, S. Cooley), R35 CA197292 (J.S. Miller.), and R01 HL11879 and HL56067 (B.R. Blazar).
Footnotes
Conflict of interest statement: Dr. JS Miller serves on the Scientific Advisory Board of Celgene, Fate Therapeutics, and GT Biopharma and has received research funds and/or clinical trials support from Fate Therapeutics and GT Biopharma. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest polices. These relationships had no role in funding this research, which has been funded by the NIH grants. Dr. BR Blazar declares a financial conflict with Tmunity and Kadmon Corp. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest polices. None of these relationships had any role in this research. The other authors have no conflict of interest to declare.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–9. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 4.Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris JH, Noman MZ, et al. Critical Role of Tumor Microenvironment in Shaping NK Cell Functions: Implication of Hypoxic Stress. Frontiers in immunology. 2015;6:482. doi: 10.3389/fimmu.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liotta F, Gacci M, Frosali F, Querci V, Vittori G, Lapini A, et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU international. 2011;107(9):1500–6. doi: 10.1111/j.1464-410X.2010.09555.x. [DOI] [PubMed] [Google Scholar]
- 6.Vacchelli E, Semeraro M, Enot DP, Chaba K, Poirier Colame V, Dartigues P, et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget. 2015;6(25):20840–50. doi: 10.18632/oncotarget.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fialova A, Partlova S, Sojka L, Hromadkova H, Brtnicky T, Fucikova J, et al. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. International journal of cancer. 2013;132(5):1070–9. doi: 10.1002/ijc.27759. [DOI] [PubMed] [Google Scholar]
- 8.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunological reviews. 2006;214:229–38. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell research. 2017;27(1):109–18. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual review of immunology. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nature reviews Immunology. 2016;16(2):112–23. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 12.Pal M, Schwab L, Yermakova A, Mace EM, Claus R, Krahl AC, et al. Tumor-priming converts NK cells to memory-like NK cells. Oncoimmunology. 2017;6(6):e1317411. doi: 10.1080/2162402X.2017.1317411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–56. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, et al. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer research. 2016;76(19):5696–706. doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nature immunology. 2015;16(4):354–65. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 16.Shuai X, Wei-min L, Tong YL, Dong N, Sheng ZY, Yao YM. Expression of IL-37 contributes to the immunosuppressive property of human CD4+CD25+ regulatory T cells. Scientific reports. 2015;5:14478. doi: 10.1038/srep14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bufler P, Azam T, Gamboni-Robertson F, Reznikov LL, Kumar S, Dinarello CA, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13723–8. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, et al. Cutting edge: Priming of NK cells by IL-18. Journal of immunology. 2008;181(3):1627–31. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(6):1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Science Translational Medicine. 2011;3(78):78ra33. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hippen KL, O’Connor RS, Lemire AM, Saha A, Hanse EA, Tennis NC, et al. In Vitro Induction of Human Regulatory T Cells Using Conditions of Low Tryptophan Plus Kynurenines. Am J Transplant. 2017 doi: 10.1111/ajt.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran DQ. In vitro suppression assay for functional assessment of human regulatory T cells. Methods Mol Biol. 2013;979:199–212. doi: 10.1007/978-1-62703-290-2_16. [DOI] [PubMed] [Google Scholar]
- 23.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–72. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hommel G. A Stagewise Rejective Multiple Test Procedure Based on a Modified Bonferroni Test. Biometrika. 1988;75(2):383–6. doi: 10.1093/Biomet/75.2.383. [DOI] [Google Scholar]
- 25.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119(16):3734–43. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikushige Y, Miyamoto T, Yuda J, Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, et al. A TIM-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression. Cell stem cell. 2015;17(3):341–52. doi: 10.1016/j.stem.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Advances in cancer research. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 29.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125(9):3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. The Journal of experimental medicine. 2005;202(8):1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. Journal of immunology. 2006;176(3):1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y, Cai X, Liu S, Wang S, Nold-Petry CA, Nold MF, et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(42):15178–83. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nature immunology. 2010;11(11):1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Scientific reports. 2014;4:5177. doi: 10.1038/srep05177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(8):2497–502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nold C, Lo C, Rudloff I, Elgass K, Li S, Gantier M, et al. IL-37 requires the receptors IL-18Ra and IL-1R8 (SIGIRR) to carry out its multi-faceted anti-inflammatory program on innate signal transduction. European Journal of Immunology. 2016;46:866. [Google Scholar]
- 37.Molgora M, Bonavita E, Ponzetta A, Riva F, Barbagallo M, Jaillon S, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551(7678):110-+. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL, Smyth MJ. Anti-TIM3 Antibody Promotes T Cell IFN-gamma-Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011;71(10):3540–51. doi: 10.1158/0008-5472.Can-11-0096. [DOI] [PubMed] [Google Scholar]
- 39.Xie DY, Lin BL, Xie JQ, Zheng YB, Liu Q, Gao ZL. Co-expression of PD-1 and Tim-3 on HBV-specific CD8+T Cells is Associated with Liver Disease Severity in Chronic Hepatitis B Virus Infection. Hepatology. 2012;56:1054a-a. [Google Scholar]
- 40.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Science translational medicine. 2016;8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Della Chiesa M, Pesce S, Muccio L, Carlomagno S, Sivori S, Moretta A, et al. Features of Memory-Like and PD-1(+) Human NK Cell Subsets. Frontiers in immunology. 2016;7:351. doi: 10.3389/fimmu.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.