Abstract
Objective
We aimed to clarify the impact of Extracorporeal Membrane Oxygenation (ECMO) as a platform to drive hemodialysis (HD) for ammonia clearance on outcomes of neonates with severe hyperammonemia.
Study Design
All neonates treated for hyperammonemia at a single children’s hospital between 1992 and 2016 were identified. Patient characteristics and outcomes were compared between those receiving medical management or ECMO/HD.
Result
Twenty-five neonates were treated for hyperammonemia, of which 13 (52%) received ECMO/HD. Peak ammonia levels among neonates treated with ECMO/HD were significantly higher than those medically-managed (1041 [IQR 902–1581] μmol/L versus 212 [IQR 110–410] μmol/L; p=0.009). Serum ammonia levels in the ECMO/HD cohort declined to the median of medically managed within 4.5 (IQR 2.9–7.0) hours and normalized within 7.3 (IQR 3.6–13.5) hours. All neonates survived ECMO/HD, and 9 (69.2%) survived to discharge.
Conclusion
ECMO/HD is an effective adjunct to rapidly clear severe hyperammonemia in newborns, reducing potential neurodevelopmental morbidity.
INTRODUCTION
Hyperammonemia of the newborn is a rare, but potentially lethal and neurologically debilitating condition characterized by high ammonia levels and excess glutamine accumulation in astrocytes, leading to cellular swelling and cerebral edema.(1) The most common etiology of neonatal hyperammonemia involves urea cycle defects that result from a deficiency of key metabolic enzymes, such as carbamoyl phosphate synthetase 1 (CPS1), ornithine transcarbamylase (OTC), arginosuccinate synthetase (ASS), and arginosuccinate lyase (ASL).(2–4) Organic acidurias, in which there is intramitochondrial accumulation of acyl-CoA esters and a block of urea cycles enzymes, is another less frequent etiology.(5) The absence of these critical enzymes blocks conversion of ammonia to urea, its excretory by-product.
Neonates having urea cycle disorders typically appear normal immediately after birth, but the rapid accumulation of ammonia and progressive cerebral swelling lead to neurologic symptoms, such as somnolence, lethargy, and eventually coma.(2) A direct relationship between the duration of hyperammonemic coma and neurological outcome has been established.(6–8) Rapid ammonia clearance provides neonates with the best chance at intact survival. Thus, the optimal strategy for prevention of high morbidity and mortality involves early recognition followed by prompt therapeutic intervention.(9) Dialysis is the primary method to clear ammonia most rapidly.(1, 2, 10–12) Peritoneal dialysis (PD) is effective in reducing serum ammonia levels but often takes several days because of slower clearance rates.(10, 11, 13, 14). Direct hemodialysis (HD), while successful at rapid clearance of ammonia, is fraught with potential complications in the newborn principally related to catheter size and an increased risk of vascular instability.(6) While HD has shown success in neonates under 5 kilograms, a significant proportion develop hypotension due to small intravascular volumes, limiting its efficacy.(15, 16) Summar et al. reported in 1996 the first successful cases of treating severe neonatal hyperammonemia with the use of an extracorporeal membrane oxygenation pump (ECMO) to support hemodynamic stability during HD.(6) Since then, there has been little research on the outcomes of children treated with traditional HD and medical management versus ECMO/HD, with only two additional case reports.(17, 18) Here, we aimed to clarify the impact of ECMO as a platform to drive HD on outcomes of neonates presenting with severe hyperammonemia.
METHODS
We reviewed retrospectively the outcomes of all neonates treated for hyperammonemia at a tertiary-referral children’s hospital between 1992 and 2016 to compare patient characteristics, neurological morbidity, treatment complications, and mortality between those receiving medical management or ECMO/HD.
Subjects and Setting
The study population consisted of all children treated for neonatal hyperammonemia at the Monroe Carell, Jr. Children’s Hospital of the Vanderbilt University Medical Center (VUMC) in Nashville, TN from January 1, 1992 to December 31, 2016. All neonates treated for hyperammonemia greater than 70 μmol/L at the institution were prospectively recorded in a database. Normal range for ammonia level is typically considered less than 50 μmol/L in the newborn period, although reference ranges vary slightly. Charts were reviewed manually and individually by two reviewers (JRR, PCC) with a third reviewer (HNL) involved to address uncertainty. Children were excluded if initial presentation with hyperammonemia was either not at the study institution or not within the first 60 days of life. The institutional review board at VUMC approved the study and waived requirement for obtaining informed consent.
Presenting Characteristics
All patient records were reviewed to ascertain demographic data, including age at presentation, sex, race, gestational age, and birthweight. Records were also reviewed to determine if neonatal screening was performed at birth and, if so, whether an inborn error of metabolism was recognized. Each case was reviewed to determine if the neonate presented with symptoms, such as lethargy, irritability, seizures, or respiratory distress. Each neonate’s ammonia level at presentation was extracted from the medical record. If subsequent genetic testing was performed, the enzyme deficiency was noted.
Hyperammonemia Management
Treatment of the hyperammonemia, either with medical therapy alone, PD, HD, or ECMO/HD was noted. If the child was treated with any form of dialysis, the time of dialysis initiation and length of the run-time on dialysis was noted. All ammonia levels during the management of hyperammonemia were recorded. Time to normalization of ammonia level was determined for each neonate. Any missing data was categorized as unknown.
Assessment of Clinical Outcomes
The main outcomes of interest included time to ammonia normalization, neurological morbidity at last date of follow-up, adverse events attributable to ECMO or dialysis, survival to discharge, and later need for liver transplantation. Adverse events were predefined as bleeding secondary to either catheterization, anticoagulation, or catheter-related morbidities. All adverse events documented in the medical record during the index admission were included in analysis. Categorical outcomes were compared using Fisher exact test and continuous variables with generalized linear regression models. Logistic regression, adjusted for gender and race, was used to determine the association of initial and peak ammonia levels with mortality. All analyses were conducted in R version 3.3.3.(19)
RESULTS
Demographic Characteristics of Neonates with Hyperammonemia
During the 25-year period, 25 neonates were treated for hyperammonemia (Table 1). The slight majority of neonates were female (15, 60%) and predominantly white (22, 88%). All neonates were over 35 weeks’ gestational age at birth except for one born at 26 weeks, 2 days. Median gestational age was 38.1 (IQR 37.0–39.0) weeks and median birthweight was 2.92 (2.54–3.38) kilograms. Newborn screening for inborn errors of metabolism was performed in 17 of the 25 neonates, 10 (58.8%) of which detected a possible enzyme deficiency. On more detailed genetic analysis, urea cycle disorders (n=16, 64%) predominated, and most commonly were deficiencies of ASL (n=6, 37.5%), CPS1 (n=5, 31.3%), and OTC (n=2, 12.5%). Organic acidemia was present in 2 children (8%), one presenting with isovaleric acid CoA dehydrogenase deficiency and the other with propionyl CoA carboxylase deficiency.
Table 1.
Demographics and Clinical Characteristics of Hyperammonemic Neonates Treated with ECMO versus Traditional Medical Management
| Non-ECMO, n (%) (n = 12) | ECMO, n (%) (n = 13) | All, n (%) (n = 25) | P-value* | |
|---|---|---|---|---|
|
| ||||
| Gender | 0.688 | |||
| Male | 4 (33.3%) | 6 (46.2%) | 10 (40%) | |
| Female | 8 (66.7%) | 7 (53.8%) | 15 (60%) | |
|
| ||||
| Race | 0.723 | |||
| White | 10 (83.3%) | 12 (92.3%) | 22 (88%) | |
| Black | 1 (8.3%) | 1 (7.7%) | 2 (8%) | |
| Other | 1 (8.3%) | 0 | 1 (4%) | |
|
| ||||
| Gestational age, median weeks (IQR) | 38.6 (37.3–39.1) | 37.3 (36.7–38.8) | 38.1 (37.0–39.0) | 0.716 |
|
| ||||
| Birthweight, median grams (IQR) | 3359 (2765–3775) | 2750 (2234–3079) | 2928 (2545–3384) | 0.255 |
|
| ||||
| Symptoms at presentation | 0.015 | |||
| Somnolence, lethargy, seizures | 4 (33.3%) | 9 (69.2%) | 13 (52%) | |
| Other | 3 (25.0%) | 4 (30.8%) | 7 (28%) | |
| None | 5 (41.7%) | 0 | 5 (20%) | |
|
| ||||
| Newborn screen | 0.320 | |||
| Positive | 6 (50%) | 4 (30.8%) | 10 (40%) | |
| Negative | 4 (33.3%) | 3 (23.1%) | 7 (28%) | |
| Not performed | 2 (16.7%) | 6 (46.2%) | 8 (32%) | |
|
| ||||
| Hyperammonemia etiology | 0.340 | |||
| Urea cycle disorder | 6 (50.0%) | 10 (76.9%) | 16 (64%) | |
| Organic acidemia | 1 (8.3%) | 1 (7.7%) | 2 (8%) | |
| Unknown | 5 (41.7%) | 2 (15.4%) | 7 (28%) | |
|
| ||||
| Age at presentation, median days (IQR) | 5 (3–26) | 3 (2–5) | 4 (2–6) | 0.113 |
|
| ||||
| First Ammonia Level, μmol/L, median (IQR) | 159 (93–244) | 998 (902–1476) | 591 (205–1041) | 0.006 |
|
| ||||
| Peak Ammonia Level, μmol/L, median (IQR) | 212 (110–410) | 1041 (902–1581) | 591 (219–1121) | 0.009 |
|
| ||||
| Time to normalization of ammonia level, median hours (IQR) | 28.8 (6.0–43.9) | 7.3 (3.6–13.5) | 9.1 (4.7–22.0) | 0.052 |
|
| ||||
| Liver Transplantation | 0.096 | |||
| Yes | 0 | 4 (30.8%) | 4 (16%) | |
| No | 0 | 9 (69.2%) | 9 (36%) | |
Fisher’s Exact Test for categorical variables and generalized linear regression models for linear variables with comparison groups of Non-ECMO versus ECMO
Comparison of Presenting Characteristics of Neonates Treated Medically versus ECMO/HD
Of the 25 hyperammonemic neonates, 13 (52%) received ECMO/HD, and 12 were treated with medical management alone. No neonates were treated with PD or with HD without the utilization of ECMO. No significant differences in demographics or genetic etiology were detected between neonates managed medically-only or with ECMO/HD (Table 1).
All neonates who were treated with ECMO/HD were profoundly symptomatic with either lethargy, seizures, or respiratory distress at presentation (Table 1). Neonates treated with medical management were significantly less likely to be symptomatic at presentation (p = 0.015). First ammonia level among neonates who were treated with ECMO/HD was also significantly higher than among those treated with medical management alone (998 [IQR 902–1476] μmol/L versus 159 [IQR 93–244] μmol/L, p = 0.006).
Clinical Outcomes of Medically-Managed Cohort
Of the 12 neonates who received medical-management alone of their hyperammonemia, one (8.3%) presented with an ammonia level of 1121 μmol/L in the context of a CPS1 deficiency and died less than 24 hours after admission. No children who had medical management only subsequently required liver transplantation.
Clinical Outcomes of ECMO/HD Cohort
Peak ammonia levels among neonates treated with ECMO/HD were significantly higher than those medically-managed (1041 [IQR 902–1581] μmol/L versus 212 [IQR 110–410] μmol/L, p=0.009). Venovenous ECMO was used in 9 neonates (69.2%) and venoarterial in 4 (30.8%). No significant difference in initial (973 [IQR 902–1400] μmol/L versus 1237 [IQR 931–1964] μmol/L; p = 0.494) or peak (1041 [IQR 902–1581] μmol/L versus 1265 [IQR 943–2067] μmol/L; p = 0.151) serum ammonia levels were observed between neonates treated with VV versus VA ECMO/HD, respectively.
Serum ammonia levels in the ECMO/HD cohort declined to under 300 μmol/L within a median of 4.5 (IQR 2.9–7.0) hours and normalized within 7.3 (IQR 3.6–13.5) hours. Median ECMO run-time was 50.3 (IQR 33.1–70.3) hours. All neonates cannulated on ECMO survived the run; however, complications occurred in three neonates. Coagulopathy was common (10 neonates, 76.9%), but only two neonates (15.4%) had bleeding complications secondary to ECMO/HD. One bleeding complication was a grade 4 intraventricular hemorrhage in a 2.0-kg neonate who underwent VV ECMO and the other was mild pulmonary hemorrhage (Table 2). One neonate (7.7%) developed a non-occlusive thrombus and stenosis of the internal carotid artery secondary to VA ECMO cannulation.
Table 2.
Clinical Outcomes of Neonates with Hyperammonemia Treated with ECMO
| ECMO Cohort, n (%) (n = 13) |
|
|---|---|
|
| |
| ECMO Cannulation | |
| Venovenous | 9 (69.2%) |
| Venoarterial | 4 (30.8%) |
|
| |
| Time to Ammonia Normalization, Hours | |
| Median (IQR) | 7.3 (3.6–13.5) |
|
| |
| ECMO Complications | |
| Coagulopathy | 10 (76.9%) |
| Bleeding | 2 (15.4%) |
| Thrombosis | 1 (7.7%) |
|
| |
| Liver Transplantation | |
| Yes | 4 (30.8%) |
| No | 9 (69.2%) |
|
| |
| Mortality* | |
| Yes | 4 (30.8%) |
| No | 9 (69.2%) |
all neonates survived entirety of ECMO run
Nine neonates (69.2%) treated with ECMO/HD survived to discharge. Four of the survivors were neurologically normal, 2 showed mild developmental delay, and 3 had moderate or severe developmental delay. Four survivors later received liver transplantation. Follow-up time for survivors treated with ECMO/HD was 4.1 [IQR 3.6–9.4] years.
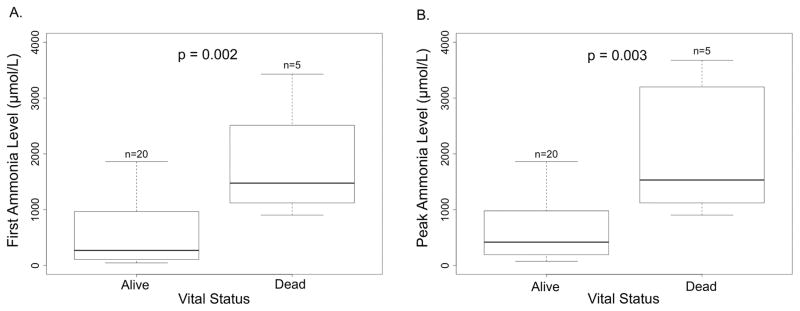
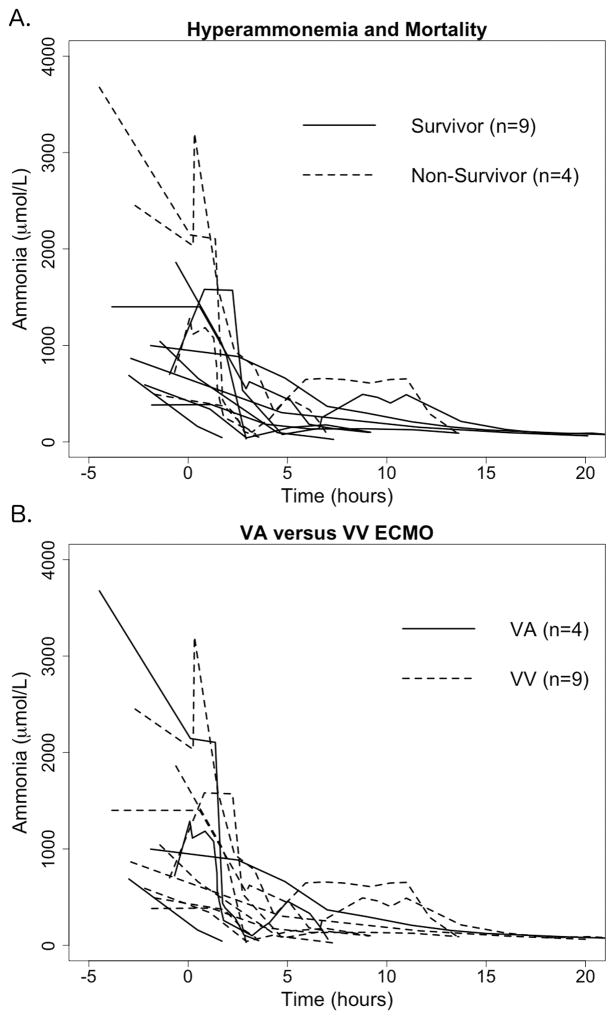
Among all neonates, including those who received medical management alone and those who received ECMO/HD, higher initial (p = 0.002) and peak (p = 0.003) ammonia levels were associated with increased mortality (Figure 1). In the ECMO/HD cohort, a non-significant trend toward increased mortality with higher initial ammonia levels (973 [IQR 728–1041] μmol/L in survivors compared to 1995 [IQR 1333–2742] μmol/L in non-survivors, p = 0.193) and higher peak ammonia levels (998 [IQR 776–1400] μmol/L in survivors compared to 2366 [IQR 1374–3319] μmol/L in non-survivors, p = 0.221) was observed. No neonate with an initial or peak ammonia level over 1860 μmol/L survived to discharge (Figure 2). The rapid decline of ammonia levels in neonates during treatment with ECMO/HD is demonstrated in Figure 2. Of the 4 neonates that died, all with profound hyperammonemic encephalopathy, 1 had CPS1 deficiency, 2 had OTC deficiency, and one had an unknown cause for hyperammonemia. No deaths were directly attributable to ECMO/HD.
Figure 1.
Initial and peak ammonia levels in all neonates with hyperammonemia. Horizontal line represents median, box represents interquartile range, and whiskers represent minimum and maximum. A. First ammonia level in neonates stratified by survival. B. Peak ammonia level in neonates stratified by survival.
Figure 2.
Presenting ammonia levels and time to clearance in ECMO/HD cohort. Time 0 represents ECMO cannulation. A. Neonates treated with ECMO/HD stratified by survival. Presenting ammonia levels in non-survivors appeared higher than survivors (1995 μmol/L [IQR 1333–2742] versus 973 μmol/L [IQR 728–1041], although not statistically significant (p = 0.193). B. Mode of ECMO/HD cannulation, venovenous (VV) or venoarterial (VA), showed no difference in mortality (p = 0.530) or initial ammonia levels (p = 0.494).
A total of 8 (32%) neonates did not receive neonatal screening for amino acid and organic acid disorders. No significant difference between peak ammonia levels at presentation in neonates before (885 [IQR 223–1572] μmol/L) and after (401 [IQR 205–973] μmol/L) state neonatal screening was introduced was demonstrated (p = 0.296). In the 12 years prior to neonatal screening, 6 neonates were placed on ECMO/HD. In the 13 years since neonatal screening to identify urea cycle disorders commenced, 7 neonates have been placed on ECMO/HD for severe hyperammonemia.
DISCUSSION
This review summarizes the largest known cohort of neonates who presented with life-threatening hyperammonemia and were supported with ECMO as a platform to drive HD. Here, we show that ECMO/HD can be an effective adjunct to rapidly clear severe hyperammonemia in neonates having inborn errors of metabolism. The best chance for reducing long-term neurodevelopmental morbidity in neonates with severe hyperammonemia is to rapidly clear the blood of toxic ammonia levels, thus decreasing cerebral swelling and long term brain damage.(16) In this study, the use of ECMO/HD allowed for ammonia level reduction to within the range of medically-managed in under 5 hours and for ammonia normalization in 7.5 hours.
Wen et al. reported in 2016 on preterm twin infants with propionic academia who were treated with ECMO/HD, one of which survived with delayed neurodevelopmental milestones.(17) The preterm twins in this case had low birthweights with small sizes that limited the ability for rapid solute removal with traditional HD. In the current study, all infants who were placed on ECMO/HD were over 35 weeks’ gestational age. The decision to utilize ECMO was based upon the premise that increasing the total blood volumes provided hemodynamic stability with ECMO, allowing for safe application of HD with greater flow rates, greater surface area, and subsequently more rapid ammonia elimination.(6, 17)
While ECMO/HD allowed for the rapid clearance of extremely high levels of ammonia, despite treatment, no infant presenting with an ammonia level greater than 2000 μmol/L survived to discharge despite treatment. Prior studies are consistent with this finding with few reports of neonates surviving with ammonia levels exceeding 2000 μmol/L.(11, 20) This observation may be secondary to coma duration preceding the initiation of dialysis in these neonates, as suggested in prior studies to be an accurate predictor of prognosis.(8, 16) Thus, early recognition of hyperammonemia is necessary for rapid, successful treatment.
One method of early recognition could be through newborn screening. Prior to 2004, newborn screening for amino acid and organic acid disorders was not performed in state in which the institution resides (21); therefore, 8 neonates treated prior to this time did not have neonatal screen results. Routine neonatal screening has not significantly decreased the levels of ammonia at presentation or reduced the need for ECMO/HD in this population; however, it has increased the number of children who are identified with hyperammonemia in the neonatal period, resulting in a non-significant trend in reduced ammonia levels over time. Of the 17 neonates with neonatal screening performed, 10 (59%) had a positive screen. Four of these neonates presented only for the positive screen results with no symptoms and each with ammonia levels under 300 μmol/L. The remainder of the neonates presented with symptoms prior to the return of the positive screening results. Of the 7 neonates with a negative screen, 43% were later found to have a genetically determined urea cycle disorder (2 neonates with CPS1 deficiency and 1 neonate with ASL deficiency). All of these neonates with negative neonatal screening results presented to the institution with symptoms. Newborn screening tests are known to have false negatives as we have identified in this study (22, 23); therefore, a negative screening test should not rule-out the possibility of a urea cycle disorder in a neonate presenting with symptoms. Historically, only two urea cycle disorders, ASS and ASL deficiency, are reliably detected and reported by tandem mass spectroscopy-based newborn screening in the United States.(22) While expanding neonatal screening could improve its accuracy, neonates with severe hyperammonemia and symptoms often present before the return of newborn screen results, thus, screen results are rarely available to the clinician when making a diagnosis in a symptomatic neonate.
Several limitations to this study are to be acknowledged that temper drawing rigorous conclusions. Foremost, the study design was retrospective, and the sample size was small and from a single institution. For example, neonates treated with medical management had lower ammonia levels and less symptoms, subsequently with improved outcomes, in comparison to those neonates treated with ECMO/HD who presented with markedly higher ammonia levels. An obvious patient selection bias existed for those neonates having the most severe presentations of hyperammonemia to be placed on ECMO to drive HD. The reasons for this patient selection likely stem from the known potential risks of ECMO, encouraging providers to weigh the benefits and risks of its use. Due to the significant differences between the two treatment groups, comparisons must be made with these considerations in mind.
Another limitation of this study is that it was performed at a single institution with a well-developed pediatric ECMO program. Ability to obtain success with the use of ECMO to drive HD requires expertise by many different teams and support personnel. The study institution has been using ECMO to support hemodynamic stability in neonates with a wide range of clinical diagnoses for nearly 3 decades and over 1000 ECMO cannulations.(24) The study institution was also the first to report the use of ECMO as a support platform for HD in 1996.(6) With ECMO resources and expertise at the institution, no neonates with hyperammonemia were treated with HD alone. While this strategy has also shown success in several studies (15, 16), we anticipate that expertise with ECMO, along with the presence or concern for neonatal hypotension, were the main factors driving this practice management. We show that survival rates of almost 70% with minimal to no neurological morbidity in two-thirds of the neonates can be achieved through a coordinated and multidisciplinary approach comprised of neonatologists, pediatric nephrologists, surgeons, geneticists, and highly-skilled nursing.
In summary, ECMO/HD appears to be an effective adjunct to rapidly clear severe hyperammonemia in newborns, potentially improving mortality and reducing long-term neurodevelopmental morbidity. The use of ECMO/HD in hyperammonemic neonates should be limited to those with life-threatening elevation of ammonia levels for which rapid clearance of ammonia with hemodynamic stability is needed and neurologic sequelae are not too far advanced. Overall, higher ammonia levels at both presentation and peak are associated with increased risk for mortality, regardless of treatment modality, likely related to the duration of hyperammonemia prior to presentation. Rapid diagnosis and adequate resolution of hyperammonemia provide these neonates with the best opportunity for survival with the least neurological morbidity.
Acknowledgments
JR Robinson receives salary and tuition support from the National Institutes of Health National Library of Medicine (T15 LM007450).
Funding: Supported by the National Institutes of Health National Library of Medicine (T15 LM007450 to JRR).
Footnotes
Conflict of Interest:
The authors declare no conflicts of interest.
References
- 1.Picca S, Dionisi-Vici C, Bartuli A, De Palo T, Papadia F, Montini G, et al. Short-term survival of hyperammonemic neonates treated with dialysis. Pediatr Nephrol (Berlin, Germany) 2015;30(5):839–47. doi: 10.1007/s00467-014-2945-x. [DOI] [PubMed] [Google Scholar]
- 2.Summar M. Current strategies for the management of neonatal urea cycle disorders. The J Pediatr. 2001;138(1 Suppl):S30–9. doi: 10.1067/mpd.2001.111834. [DOI] [PubMed] [Google Scholar]
- 3.Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94(4):397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell AG, Rosenberg LE, Snodgrass PJ, Nuzum CT. Ornithine transcarbamylase deficiency: a cause of lethal neonatal hyperammonemia in males. N Engl J Med. 1973;288(1):1–6. doi: 10.1056/NEJM197301042880101. [DOI] [PubMed] [Google Scholar]
- 5.Kolker S, Garcia-Cazorla A, Valayannopoulos V, Lund AM, Burlina AB, Sykut-Cegielska J, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis. 2015;38(6):1041–57. doi: 10.1007/s10545-015-9839-3. [DOI] [PubMed] [Google Scholar]
- 6.Summar M, Pietsch J, Deshpande J, Schulman G. Effective hemodialysis and hemofiltration driven by an extracorporeal membrane oxygenation pump in infants with hyperammonemia. J Pediatr. 1996;128(3):379–82. doi: 10.1016/s0022-3476(96)70287-1. [DOI] [PubMed] [Google Scholar]
- 7.Batshaw ML. Inborn errors of urea synthesis. Ann Neurol. 1994;35(2):133–41. doi: 10.1002/ana.410350204. [DOI] [PubMed] [Google Scholar]
- 8.Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310(23):1500–5. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- 9.Deodato F, Boenzi S, Rizzo C, Abeni D, Caviglia S, Picca S, et al. Inborn errors of metabolism: an update on epidemiology and on neonatal-onset hyperammonemia. Acta Paediatr Suppl. 2004;93(445):18–21. doi: 10.1111/j.1651-2227.2004.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong KY, Wong SN, Lam SY, Tam S, Tsoi NS. Ammonia clearance by peritoneal dialysis and continuous arteriovenous hemodiafiltration. Pediatr Nephrol (Berlin, Germany) 1998;12(7):589–91. doi: 10.1007/s004670050511. [DOI] [PubMed] [Google Scholar]
- 11.Arbeiter AK, Kranz B, Wingen AM, Bonzel KE, Dohna-Schwake C, Hanssler L, et al. Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant. 2010;25(4):1257–65. doi: 10.1093/ndt/gfp595. [DOI] [PubMed] [Google Scholar]
- 12.Donn SM, Swartz RD, Thoene JG. Comparison of exchange transfusion, peritoneal dialysis, and hemodialysis for the treatment of hyperammonemia in an anuric newborn infant. J Pediatr. 1979;95(1):67–70. doi: 10.1016/s0022-3476(79)80085-2. [DOI] [PubMed] [Google Scholar]
- 13.Snyderman SE, Sansaricq C, Phansalkar SV, Schacht RC, Norton PM. The therapy of hyperammonemia due to ornithine transcarbamylase defiency in a male neonate. Pediatrics. 1975;56(1):65–73. [PubMed] [Google Scholar]
- 14.Wiegand C, Thompson T, Bock GH, Mathis RK, Kjellstrand CM, Mauer SM. The management of life-threatening hyperammonemia: a comparison of several therapeutic modalities. J Pediatr. 1980;96(1):142–4. doi: 10.1016/s0022-3476(80)80352-0. [DOI] [PubMed] [Google Scholar]
- 15.Sadowski RH, Harmon WE, Jabs K. Acute hemodialysis of infants weighing less than five kilograms. Kidney Int. 1994;45(3):903–6. doi: 10.1038/ki.1994.119. [DOI] [PubMed] [Google Scholar]
- 16.Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, et al. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol (Berlin, Germany) 2001;16(11):862–7. doi: 10.1007/s004670100702. [DOI] [PubMed] [Google Scholar]
- 17.Wen JX, Feldenberg LR, Abraham E, Sadiq F, Christensen KM, Braddock SR. Continuous venovenous hemodialysis via extracorporeal membrane oxygenation pump for treatment of hyperammonemia secondary to propionic acidemia in monochorionic diamniotic twin boys. J Pediatr. 2016;175:231–2. doi: 10.1016/j.jpeds.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Gander JW, Rhone ET, Wilson WG, Barcia JP, Sacco MJ. Veno-venous extracorporeal membrane oxygenation for continuous renal replacement in a neonate with propionic acidemia. J Extra Corpor Technol. 2017;49(1):64–6. [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. http://www.r-project.org/ [Google Scholar]
- 20.Schwahn BC, Pieterse L, Bisset WM, Galloway PG, Robinson PH. Biochemical efficacy of N-carbamylglutamate in neonatal severe hyperammonaemia due to propionic acidaemia. Eur J Pediatr. 2010;169(1):133–4. doi: 10.1007/s00431-009-1036-7. [DOI] [PubMed] [Google Scholar]
- 21.State of Tennessee Department of Health Family Health and Wellness. [Accessed November 13, 2017];Tennessee Newborn Screening Program. 2011 http://www.tn.gov/assets/entities/health/attachments/NBS_Guide_Practitioners.pdf.
- 22.Naylor EW. Newborn screening for urea cycle disorders. Adv Exp Med Biol. 1982;153:9–18. doi: 10.1007/978-1-4757-6903-6_3. [DOI] [PubMed] [Google Scholar]
- 23.Summar ML, Koelker S, Freedenberg D, Le Mons C, Haberle J, Lee HS, et al. The incidence of urea cycle disorders. Mol Genet Metab. 2013;110(1–2):179–80. doi: 10.1016/j.ymgme.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovvorn HN, 3rd, Hardison DC, Chen H, Westrick AC, Danko ME, Bridges BC, et al. Review of 1,000 consecutive extracorporeal membrane oxygenation runs as a quality initiative. Surgery. 2017 doi: 10.1016/j.surg.2017.03.020. [DOI] [PubMed] [Google Scholar]