Abstract
Since its discovery in 2012, ferroptosis has been well-characterized by the accumulation of lipid peroxides due to the failure of glutathione-dependent antioxidant defenses. It is known as an iron-dependent form of programmed cell death, which is distinct from other forms of cell death such as apoptosis and necrosis. Nonetheless, little is known about the ferroptotic agent-induced endoplasmic reticulum (ER) stress response and its role in cell death. Recent studies reveal that the ferroptotic agent-induced ER stress response plays an important role in the crosstalk between ferroptosis and other types of cell death. Ferroptotic agents induce the unfolded protein response and subsequently ER stress-mediated activation of the PERK-eIF2α-ATF4-CHOP pathway. CHOP (C/EBP homologous protein) signaling pathway-mediated p53-independent PUMA (p53 upregulated modulator of apoptosis) expression is involved in the synergistic interaction between ferroptosis and apoptosis. This review highlights the recent literature on ferroptotic and apoptotic agent interactions through the ER stress-mediated PERK-eIF2α-ATF4-CHOP-PUMA pathway and implicates combined treatment to effectively enhance tumoricidal efficacy as a novel therapeutic strategy for cancer.
Introduction
Ferroptosis
Ferroptosis was coined in 2012 by the lab of Dr. Brent R. Stockwell [1]. It is a unique iron-dependent form of non-apoptotic regulated cell death [1]. Ferroptosis is considered different from other types of cell death in various aspects. For example, ferroptosis does not result in morphological changes like the loss of plasma membrane integrity that occurs during necrosis, the formation of double membrane-layered autophagic vacuoles that occurs during autophagy, or the chromatin condensation that occurs during apoptosis; instead, it manifests primarily as increased mitochondrial membrane density and mitochondrial shrinkage. Nevertheless, few studies have reported an interrelationship between ferroptosis and apoptosis: switching apoptosis to ferroptosis [2] and ferroptotic agent-mediated sensitization of apoptosis [3].
Synthetic lethal screening studies have identified several genes responsible for ferroptosis, including those involved in lipid and amino acid metabolism [4–6]. Chemical compounds inhibiting these genes trigger ferroptosis: the glutathione S-transferase inhibitor artesunate (ART), the glutathione-dependent antioxidant enzyme glutathione peroxidase 4 (GPX4) inhibitor (1S, 3R)-RSL3, the glutathione (GSH) synthesis inhibitor buthionine sulfoximine (BSO), and the Na+-independent cystine–glutamate Xc− antiporter inhibitors sorafenib and erastin [1, 7–11]. In the presence of ferroptosis-inducing agents, the iron storage protein ferritin and/or the ferritinophagy cargo receptor NCOA4 (nuclear receptor coactivator 4) are degraded via ferritinophagic degradation and release ferrous iron, which generates reactive oxygen species (ROS) through the Fenton reaction and subsequently induces lipid peroxidation [12, 13]. The accumulation of lipid peroxidation and depletion of plasma membrane polyunsaturated fatty acids have been well known to result in this lethal event [1, 4, 14, 15]. Genetic variation and complexity of cancer cells affect the pharmacodynamic response of ferroptosis-inducing agents. Functional p53 expression or high-level RAS-RAF-MEK pathway activity may elevate the generation of ROS through inhibition of cystine uptake or involvement of mitochondrial voltage-dependent anion channel 2/3 (VDAC2/3), respectively, and sensitize cancer cells to ferroptosis [16–21]. Conversely, iron chelators (e.g., desferrioxamine mesylate and deferoxamine) and lipid peroxidation inhibitors (e.g., zileuton, ferrostatin, and liproxstatin) are known to suppress ferroptosis and block pathological cell death events in the brain, kidney, and other tissues [10, 22–25].
Ferroptosis-induced ER stress
When endoplasmic reticulum (ER) lumenal conditions are altered or chaperone capacity is overwhelmed due to alterations in redox state, calcium levels, or failure to post-translationally modify secretory proteins, the cells activate the unfolded protein response (UPR) to these ER stresses [26]. ER stress is sensed by three upstream signaling proteins: IRE1 (inositol requiring protein-1), ATF6 (activating transcription factor-6), and PERK (protein kinase RNA (PKR)-like ER kinase). The activation of these three signaling pathways induces apoptosis [26, 27].
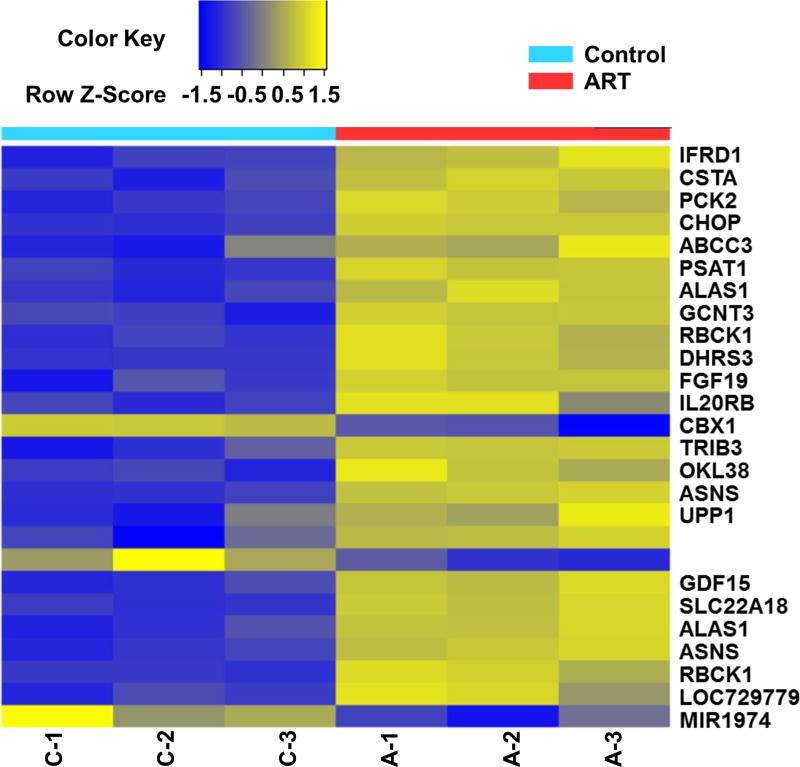
System Xc− is an amino acid antiporter that typically mediates the exchange of extracellular cystine for intracellular glutamate [28]. Previous studies have shown that inhibition of cystine-glutamate exchange by ferroptotic agents leads to activation of an ER stress response and upregulation of the CHAC1 (glutathione-specific gamma-glutamylcyclotransferase 1) gene [29, 30]. The ER stress indicator ATF4 (activating transcription factor 4) is known to be a basic leucine zipper transcription factor that regulates several UPR target genes [31]. It is well known that the ER stress response mediated by the PERK-eIF2α (eukaryotic initiation factor 2α)-ATF4 pathway is involved in the regulation of the expression of several target genes such as CHOP (C/EBP [CCAAT-enhancer-binding protein] homologous protein) [32]. Data from microassay studies reveal that the ferroptotic agent ART promotes the expression of ATF4-dependent genes such as CHOP, TRIB3, and ASNS (Fig. 1). Previous studies show that CHOP binds to the promoter of the pro-apoptotic protein PUMA (p53 upregulated modulator of apoptosis) during ER stress and induces PUMA expression [33]. CHOP also induces several other pro-apoptotic proteins such as GADD34 (growth arrest and DNA damage-inducible protein), ERO1α (endoplasmic reticulum, oxidoreductin-1α), Bim (Bcl-2 [B-cell lymphoma 2]-like protein 11), and NOXA (Latin for damage) [34]. The ferroptotic agent ART induces PUMA expression, but not NOXA or BIM expression [3]. Interestingly, ferroptotic agent-induced PUMA expression does not induce apoptosis [3]. These studies suggest that ferroptosis and apoptosis are antagonistic.
Figure 1. Microarray assay for detection of ART-induced gene expression.
Human colon cancer HCT116 cells were treated with 50 µM ART for 24 h and triplicate Illumina gene expression microarrays were performed with BeadArray microarray technology.
When ferroptosis meets apoptosis: TRAIL-induced apoptosis and synergistic interaction between ferroptotic agent and TRAIL
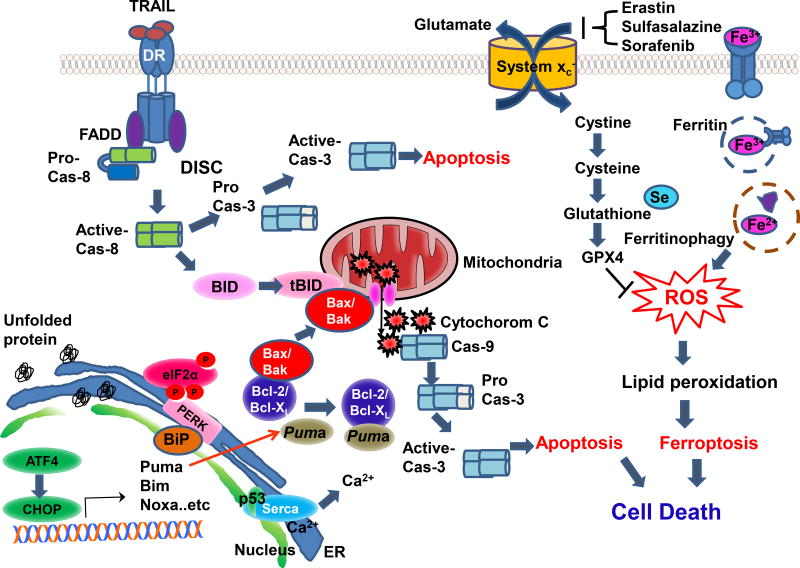
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis through initiating the extrinsic pathway by binding to its respective death receptors (DRs) such as DR4 and DR5 (Fig. 2). Ligation of TRAIL to DRs results in trimerization of DRs and leads to the recruitment of Fas-associating protein with death domain (FADD) and procaspase-8 and then the formation of the death-inducing signaling complex (DISC) [35]. Procaspase-8 is activated through two cleavage events at the DISC [36]. Activated caspase-8 leads to further activation of downstream executioner caspase-3, -6, and -7, which culminates in apoptotic death [37]. Activated caspase-8 also cleaves a pro-apoptotic Bcl-2 homology (BH3) interacting-domain death agonist (Bid) into truncated Bid (tBid), which translocates to the mitochondria and induces insertion and oligomerization of Bax (Bcl-2–associated X protein) and Bak (Bcl-2 homologous antagonist killer) [38, 39]. Insertion of homo-/hetero-oligomerized Bax and Bak into the mitochondrial outer membrane culminates in pore formation, membrane permeabilization, and depolarization of the mitochondria, which leads to cytochrome c release [40, 41]. Released cytochrome c binds to Apaf1 (apoptosis signal-regulating kinase) and facilitates the formation of the apoptosome, which activates caspase-9 and subsequently caspase-3 [42]. Recent studies reveal that TRAIL-induced cytotoxicity can be modulated by various agents - not only chemotherapeutic drugs [43–45], ionizing radiation [46], other cytokines [47], and matrix metalloprotease inhibitors [48], but also ferroptotic agents [3]. Synergistic interaction between ferroptotic agents and the apoptotic agent TRAIL may be mediated through ER stress-induced p53-independent PUMA expression [3] (Fig. 2). Previous biochemical studies indicate that PUMA induces apoptosis by activating the multidomain proapoptotic protein Bax and/or Bak through its interaction with antiapoptotic Bcl-2 family members such as Bcl-2 (B-cell lymphoma 2) and Bcl-xL (B-cell lymphoma-extra large), thereby triggering mitochondrial dysfunction, cytochrome c release, and caspase activation [49].
Figure 2. Schematic diagram of ferroptotic agent-induced ER stress and its role in the interplay between ferroptosis and apoptosis.
Schematic diagram of ferroptotic agent-induced ER stress and its role in the interplay between ferroptosis and apoptosis
Conclusion
Ferroptosis is a recently recognized form of programmed cell death that is dependent on iron and characterized by the accumulation of lipid peroxidation through generation of ROS by the Fenton reaction. It is considered genetically and biochemically distinct from other forms of regulated cell death. However, emerging evidence suggests that ferroptosis often shares common pathways with other types of cell death. In light of recent studies, ferroptotic agents induce ER stress and elevate expression of the proapoptotic molecule PUMA through the ER stress-mediated PERK-eIF2α-ATF4-CHOP pathway without inducing apoptosis. Ferroptotic agent-induced PUMA plays an important role in the crosstalk between ferroptosis and apoptosis. Much work is still needed to understand how ferroptotic agent-induced PUMA sustains a biochemically inactive state during treatment with ferroptotic agent alone. Furthermore, such studies should examine how PUMA switches from an inactive to an activate state during combinatorial treatment with ferroptotic agent and the apoptotic agent TRAIL.
Acknowledgments
We thank Christine Heiner (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript.
Grant Support
This work was supported by the following grants: NCI grants R03 CA205267 and R03 CA212125 (to Y.J. Lee). This project used the UPCI Core Facility and was supported in part by award P30CA047904 (to Y.S. Lee, H.A. Choudry, D.L. Bartlett, and Y.J. Lee).
Abbreviations used in this paper
- Apaf1
apoptosis signal-regulating kinase
- ART
artesunate
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor-6
- Bak
Bcl-2 homologous antagonist killer
- Bax
Bcl-2–associated X protein
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- BH3
Bcl-2 homology
- Bim
Bcl-2-like protein 11
- Bid
BH3 interacting-domain death agonist
- BSO
buthionine sulfoximine
- C/EBP
CCAAT-enhancer-binding proteins
- CHAC1
glutathione-specific gamma-glutamylcyclotransferase 1
- CHOP
CCAAT-enhancer-binding protein homologous protein
- DISC
death-inducing signaling complex
- DR
death receptor
- DR4
death receptor 4
- DR5
death receptor 5
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- ERO1α
endoplasmic reticulum, oxidoreductin-1α
- FADD
Fas-associating protein with death domain
- GADD34
growth arrest and DNA damage-inducible protein
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- IRE1
inositol requiring protein-1
- NCOA4
nuclear receptor coactivator 4
- PERK
protein kinase RNA (PKR)-like ER kinase
- PUMA
p53 upregulated modulator of apoptosis
- ROS
reactive oxygen species
- tBid
truncated Bid
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- UPR
unfolded protein response
- VDAC2/3
voltage-dependent anion channel 2/3
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng DW, Lei Q, Zhu JY, Fan JX, Li CX, Li C, et al. Switching Apoptosis to Ferroptosis: Metal-Organic Network for High-Efficiency Anticancer Therapy. Nano Lett. 2017;17:284–91. doi: 10.1021/acs.nanolett.6b04060. [DOI] [PubMed] [Google Scholar]
- 3.Hong SH, Lee DH, Lee YS, Jo MJ, Jeong YA, Kwon WT, et al. Molecular crosstalk between ferroptosis and apoptosis: Emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget. 2017;8:115164–78. doi: 10.18632/oncotarget.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magtanong L, Ko PJ, Dixon SJ. Emerging roles for lipids in non-apoptotic cell death. Cell Death Differ. 2016;23:1099–1109. doi: 10.1038/cdd.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad M, Friedmann Angeli JP. Glutathione peroxidase 4 (Gpx4) and ferroptosis: what's so special about it? Mol Cell Oncol. 2015;2:e995047. doi: 10.4161/23723556.2014.995047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisewski AM, Quiros JP, Ng CL, Adikesavan AK, Miura K, Putluri N, et al. (2014) Supergenomic network compression and the discovery of EXP1 as a glutathione transferase inhibited by artesunate. Cell. 2014;158:916–28. doi: 10.1016/j.cell.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braughler JM, Duncan LA, Chase RL. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986;261:10282–9. [PubMed] [Google Scholar]
- 13.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–75. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165–76. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schott C, Graab U, Cuvelier N, Hahn H, Fulda S. Oncogenic RAS Mutants Confer Resistance of RMS13 Rhabdomyosarcoma Cells to Oxidative Stress-Induced Ferroptotic Cell Death. Front Oncol. 2015;5:131. doi: 10.3389/fonc.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 1015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao Y, et al. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016;17:366–73. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy ME. Ironing out how p53 regulates ferroptosis. Proc Natl Acad Sci U S A. 2016;113:12350–2. doi: 10.1073/pnas.1615159113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–8. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–6. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Wang W, Li Y, Xiao Y, Cheng J, Jia J. The 5-Lipoxygenase Inhibitor Zileuton Confers Neuroprotection against Glutamate Oxidative Damage by Inhibiting Ferroptosis. Biol Pharm Bull. 2015;38:1234–9. doi: 10.1248/bpb.b15-00048. [DOI] [PubMed] [Google Scholar]
- 25.Dächert J, Schoeneberger H, Rohde K, Fulda S. RSL3 and Erastin differentially regulate redox signaling to promote Smac mimetic-induced cell death. Oncotarget. 2016;7:63779–92. doi: 10.18632/oncotarget.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishima N, Nakanishi K, Nakano A. Activating transcription factor-6 (ATF6) mediates apoptosis with reduction of myeloid cell leukemia sequence 1 (Mcl-1) protein via induction of WW domain binding protein. J Biol Chem. 2011;286:35227–35. doi: 10.1074/jbc.M111.233502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridges RJ, Natale NR, Patel SA. System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, et al. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol. 2007;27:5499–513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem. 2008;283:35106–17. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh AP, Klocke BJ, Ballestas ME, Roth KA. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One. 2012;7:e39586. doi: 10.1371/journal.pone.0039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim Biophys Acta. 2013;1833:3507–17. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11(Suppl 1):S86–96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- 36.Chang DW, Xing Z, Capacio VL, Peter ME, Yang X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–42. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 38.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–71. [PMC free article] [PubMed] [Google Scholar]
- 39.Grinberg M, Sarig R, Zaltsman Y, Frumkin D, Grammatikakis N, Reuveny E, et al. tBID Homooligomerizes in the mitochondrial membrane to induce apoptosis. J Biol Chem. 2002;277:12237–45. doi: 10.1074/jbc.M104893200. [DOI] [PubMed] [Google Scholar]
- 40.Logue SE, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–46. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 41.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–35. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baliga B, Kumar S. Apaf-1/cytochrome c apoptosome: an essential initiator of caspase activation or just a sideshow? Cell Death Differ. 2003;10:16–8. doi: 10.1038/sj.cdd.4401166. [DOI] [PubMed] [Google Scholar]
- 43.Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–41. [PubMed] [Google Scholar]
- 44.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–53. [PubMed] [Google Scholar]
- 45.Kim YH, Lee YJ. Time sequence of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and cisplatin treatment is responsible for a complex pattern of synergistic cytotoxicity. J Cell Biochem. 2006;98:1284–95. doi: 10.1002/jcb.20844. [DOI] [PubMed] [Google Scholar]
- 46.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Nat Acad Sci USA. 2000;97:1754–59. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SY, Billiar TR, Seol DW. IFN-gamma inhibition of TRAIL-induced IAP-2 upregulation, a possible mechanism of IFN-gamma-enhanced TRAIL-induced apoptosis. Biochem Biophys Res Commun. 2002;291:233–6. doi: 10.1006/bbrc.2002.6452. [DOI] [PubMed] [Google Scholar]
- 48.Nyormoi O, Mills L, Bar-Eli M. An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ. 2003;10:558–69. doi: 10.1038/sj.cdd.4401209. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]