Abstract
We report that PTEN-deficient prostate cancer cells use macropinocytosis to survive and proliferate under nutrient stress. PTEN loss increased macropinocytosis only in the context of AMPK activation revealing a general requirement for AMPK in macropinocytosis and a novel mechanism by which AMPK promotes survival under stress. In prostate cancer cells, albumin uptake did not require macropinocytosis, but necrotic cell debris proved a specific macropinocytic cargo. Isotopic labeling confirmed that macropinocytosed necrotic cell proteins fueled new protein synthesis in prostate cancer cells. Supplementation with necrotic debris, but not albumin, also maintained lipid stores suggesting that macropinocytosis can supply nutrients other than amino acids. Non-transformed prostatic epithelial cells were not macropinocytic, but patient-derived prostate cancer organoids and xenografts and autochthonous prostate tumors all exhibited constitutive macropinocytosis, and blocking macropinocytosis limited prostate tumor growth. Macropinocytosis of extracellular material by prostate cancer cells is a previously unappreciated tumor-microenvironment interaction that could be targeted therapeutically.
Keywords: macropinocytosis, prostate cancer, scavenging, AMPK, PTEN, cancer metabolism, necrosis
INTRODUCTION
Cancer cells up-regulate nutrient acquisition pathways to fuel oncogene-driven anabolism and proliferation (1). However, as tumors grow, their abnormal vasculature leads to the development of areas of extracellular nutrient limitation. Nutrient import pathways become substrate-limited and fail to meet nutrient demand, leading to tumor necrosis (2). Macropinocytosis, a process by which cells non-selectively engulf extracellular material via plasma membrane ruffling (3–5), allows cancer cells with activating mutations in RAS to use extracellular proteins such as albumin as fuel when amino acids are limiting (6–9). Downstream of RAS, PI(3,4,5)P3 (PIP3) and RAC1-GTP are both necessary for macropinosome formation. RAC1 activation induces the actin remodeling and membrane ruffling necessary to form macropinosomes by activating PAK kinases (10, 11). PIP3 produced by type I PI3Ks is required for macropinosome closure; in some cell types, PIP3 is also required for membrane ruffling (12, 13). The lipid phosphatase PTEN opposes PI3K pathway signaling by converting PIP3 to PI(4,5)P2 (14). PTEN is the most frequently deleted tumor suppressor gene in prostate cancer (15, 16); monoallelic PTEN deletion occurs in up to 60% of localized prostate cancers and complete loss of PTEN is commonly associated with increased risk of metastasis and the development of lethal, castration-resistant disease (16, 17). Consistent with the central role of PIP3 in macropinocytosis, we report that PTEN-deficient prostate cancer cells use macropinocytosis to support anabolism and survival in nutrient-limiting environments. Interestingly, PTEN loss was not sufficient to trigger macropinocytosis in all contexts, revealing a previously unappreciated requirement for AMPK activation to support RAC1 activation and macropinosome formation. Robust macropinocytosis-independent albumin uptake in prostate cancer cells also led to the discovery that necrotic cell debris is a specific macropinocytic cargo suggesting that macropinocytosis can supply many nutrients, not just amino acids. Taken together, these studies provide critical insights into how nutrient-responsive signaling pathways coordinate the adaptive response to nutrient limitation and suggest a novel mechanism by which the microenvironment impacts prostate tumor growth.
RESULTS
PTEN loss promotes macropinocytosis in fibroblasts under nutrient-limiting conditions
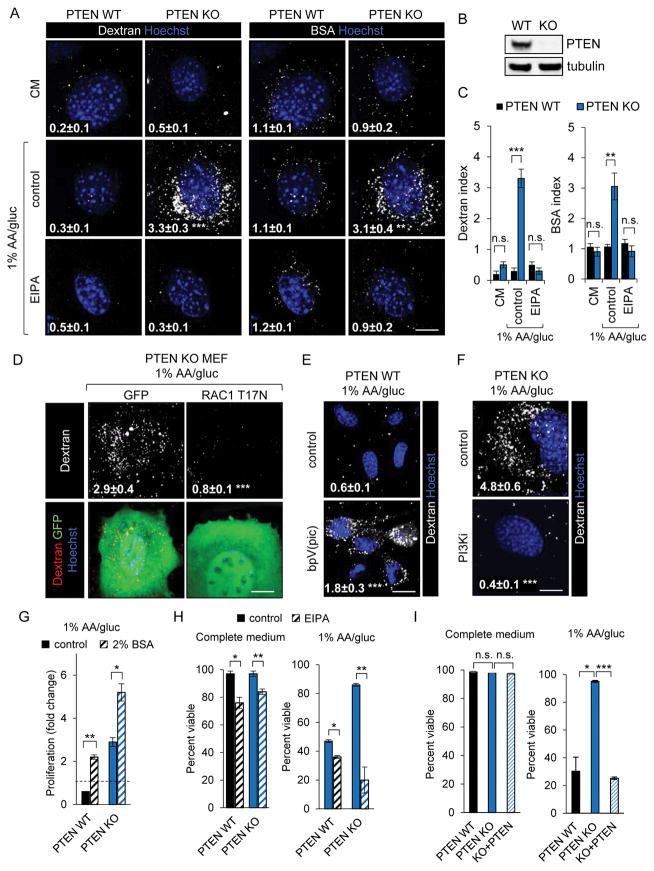
Oncogenic mutations constitutively drive anabolism and limit adaptive metabolic changes under nutrient stress (18). Nevertheless, tumor cells with activating mutations in RAS are paradoxically resistant to amino acid deprivation because they can degrade macropinocytosed proteins in the lysosome to produce amino acids (6–8). PI3K pathway activation is essential for macropinocytosis downstream of RAS (4, 5, 19). To assess whether activating the PI3K pathway would be sufficient to drive macropinocytosis and confer resistance to nutrient stress, uptake of the macropinocytic cargos 70 kD dextran and bovine serum albumin (BSA) was measured in PTEN null and wild type murine embryonic fibroblasts (PTEN KO and WT MEFs, respectively) in the presence or absence of the NHE inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA). The dextran or BSA index (percent of cell area that contains dextran or BSA) was calculated using Image J and established protocols (Supplemental Methods and Supplementary Fig. S1A) (20). EIPA inhibits RAC1 activation indirectly by reducing the submembranous pH (21). While EIPA has pleiotropic effects on cells (22), it is selective for macropinocytosis among endocytic pathways and does not inhibit the clathrin-mediated endocytosis of the EGFR or the transferrin receptor (23, 24). Neither PTEN WT nor PTEN KO MEFs took up dextran or BSA in complete medium (Fig. 1A–C). However, incubation in medium containing only 1% the amount of glucose and amino acids present in standard DMEM (1% AA/gluc) dramatically enhanced both dextran and BSA uptake selectively in PTEN KO MEFs. This increased uptake was sensitive to EIPA, the RAC inhibitor EHT1864, the PAK inhibitor FRAX597, and to dominant-negative RAC1 T17N expression (Fig. 1A,C,D and Supplementary Fig. S1B) consistent with internalization via macropinocytosis. Chemical inhibitors of PI3K and PTEN confirmed that PTEN’s catalytic activity suppresses macropinocytosis. Inhibiting PTEN with bpV(pic) was sufficient to stimulate macropinocytosis in PTEN WT MEFs in low-nutrient medium (Fig. 1E) but not complete medium. Conversely, inhibiting Class I PI3K with a combination of the α or β isoform-selective inhibitors BYL719 and AZD8186 prevented macropinocytosis in PTEN KO MEFs in 1% AA/gluc (Fig. 1F). Together, these results suggest that PI3K pathway activation by PTEN loss is sufficient to promote macropinosome formation selectively under nutrient-limiting conditions.
Figure 1. PTEN loss promotes growth and survival in nutrient-limiting conditions by promoting macropinocytosis.
A) 70 kD Dextran or BSA uptake in complete medium (CM) or 1% AA/gluc ± EIPA (50 μM). B) Western blot demonstrating loss of PTEN in PTEN KO MEFs. C) Quantification of dextran uptake (dextran index) and BSA uptake (BSA index) from panel (A). D) Dextran uptake in GFP-positive PTEN KO MEFs in 1% AA/gluc 24 h after transfection with plasmids encoding GFP or GFP and dominant-negative RAC1 T17N. E) Dextran uptake in PTEN WT MEFs in 1% AA/gluc ± 1 h pretreatment with the PTEN inhibitor bpV(pic) (10 μM). F) Dextran uptake in PTEN KO MEFs in 1% AA/gluc ± 1 h pretreatment with the PI3K inhibitors (PI3Ki) BYL719 (2.5 μM) and AZD8186 (250 nM). G) Proliferation of PTEN WT or KO MEFs after 72 h in 1% AA/gluc ± 2% BSA. H) Viability of PTEN WT and KO MEFs in CM or 1% AA/gluc ± EIPA. I) Viability of PTEN WT, PTEN KO, or PTEN KO MEFs reconstituted with PTEN in CM or 1% AA/gluc after 48 hr. In all panels, means ± SEM shown, n ≥ 3. Using a paired, two-tailed t test, *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001, n.s. not significant. Uptake index (dextran or BSA) is indicated in images in white; ≥ 25 cells were examined. Scale bar, 20 μm. See also Supplementary Figures S1 and S2.
When macropinocytic cells are subjected to amino acid limitation, albumin supplementation (2–5%) stimulates proliferation (6–8). A caveat to this approach is that albumin also enters cells through receptor-mediated endocytosis (RME), and supplementation with BSA promotes survival and proliferation even in non-macropinocytic cells, albeit to a lesser degree (7). Similar to published data from control LSL and KRAS G12D MEFs (7), supplementation with 2% BSA increased proliferation of both PTEN WT and KO MEFs in 1% AA/gluc medium, although macropinocytic PTEN KO MEFs proliferated more (Fig. 1G). The value of macropinocytosis was much more apparent in unsupplemented 1% AA/gluc medium as macropinocytic PTEN KO MEFs were able to proliferate while non-macropinocytic PTEN WT MEFs died. Albumin is the principal protein in fetal calf serum (Supplementary Fig. S2A). Because BSA uptake by macropinocytosis was much more efficient than uptake by RME (Fig. 1A,C), the 0.3% albumin contributed by the serum in the 1% AA/gluc medium was likely sufficient to support survival only in macropinocytic PTEN KO MEFs. Macropinocytic KRAS G12D-expressing MEFs, but not matched non-macropinocytic LSL MEFs, also survived in unsupplemented 1% AA/gluc medium (Supplementary Fig. S2B). In both KRAS G12D MEFs and PTEN KO MEFs, this survival advantage was fully reversed by EIPA (Fig. 1H and Supplementary Fig. S2B,C). Importantly, EIPA was minimally and equally toxic to macropinocytic and non-macropinocytic MEFs in complete medium (Fig. 1H and Supplementary Fig. S2B). Similar to EIPA, EHT1864 (allosteric RAC inhibitor) and FRAX597 (PAK inhibitor) were selectively toxic to PTEN KO MEFs relying on macropinocytosis for nutrients (Supplementary Fig. S2D,E). Furthermore, reconstitution with PTEN blocked macropinocytosis and eliminated the survival advantage of PTEN KO MEFs in low nutrient medium (Fig. 1I and Supplementary Fig. S2F). Taken together, these results demonstrate that macropinocytosis confers a survival and proliferative advantage on PTEN KO MEFs in low-nutrient medium.
AMPK activation is necessary for macropinocytosis
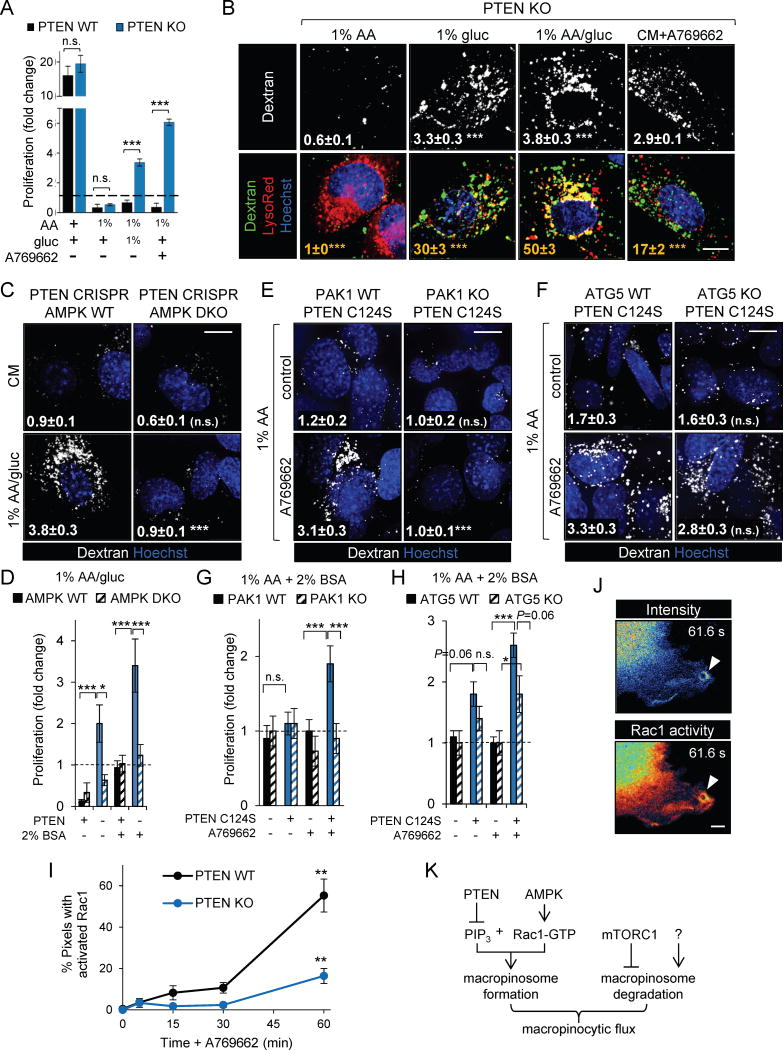
Unexpectedly, PTEN KO MEFs that proliferated in 1% AA/gluc medium (Fig. 1G and 2A) died when deprived of only amino acids (Fig. 2A and Supplementary Fig. S3A). This result suggested that glucose withdrawal stimulated growth. Cells sense and respond to glucose depletion by activating AMPK (25). Strikingly, the allosteric AMPK activator A769662 stimulated robust proliferation in 1% AA medium in PTEN KO MEFs but not in PTEN WT MEFs (Fig. 2A). AMPK promotes the macropinocytosis-dependent entry of Ebola and vaccinia viruses (26, 27). Either glucose depletion or A769662 was sufficient to stimulate dextran uptake in PTEN KO but not WT MEFs (Fig. 2B and Supplementary Fig. S3B). In contrast, amino acid depletion failed to trigger macropinocytosis in PTEN KO MEFs (Fig. 2B). These results suggest that PTEN loss is not sufficient to drive macropinocytosis; AMPK activation is also necessary. Consistent with this model, PTEN deletion from MEFs lacking both AMPK catalytic subunit isoforms (28) failed to trigger macropinocytosis in 1% AA/gluc medium (Fig. 2C and Supplementary Fig. S3C,D). The expression of a dominant-negative AMPK mutant or treatment with the AMPK inhibitor Compound C also blocked macropinocytosis in PTEN KO MEFs in 1% AA/gluc (Supplementary Fig. S3E,F). Although glucose deprivation or A769662 was sufficient to stimulate dextran uptake in PTEN KO MEFs in the presence of normal amino acid levels, co-localization of dextran and Lysotracker Red was reduced relative to 1% AA/gluc (Fig. 2B). This result is consistent with previous reports that mTORC1 inactivation is necessary for efficient macropinosome-lysosome fusion in MEFs (7, 29). In keeping with its role in macropinocytosis, AMPK was necessary for PTEN null cells to proliferate in 1% AA/gluc medium (Fig. 2D). Taken together, these results demonstrate that AMPK activation is necessary for PTEN-deficient MEFs to form macropinosomes and proliferate under nutrient-limiting conditions.
Figure 2. AMPK activation is necessary for macropinocytosis in PTEN-deficient cells.
A) Proliferation of PTEN WT or KO MEFs after 72 h in complete or nutrient-deficient medium ± A769662 (10 μM). Where indicated, amino acids and/or glucose were reduced to 1% the level in complete medium. B) Dextran uptake (white, statistics relative to CM control) or dextran-lysosome co-localization (yellow, statistics relative to 1%AA/gluc) in PTEN KO MEFs in the indicated media ± A769662 (50 μM). C) Dextran uptake in AMPK WT or DKO MEFs ± CRISPR/Cas9-mediated PTEN deletion in CM or 1% AA/gluc; statistics relative to AMPK replete cells. D) Proliferation of MEFs null for AMPK and/or PTEN as indicated after 96 h in 1% AA/gluc medium ± 2% BSA. E,F) Dextran uptake in PAK1 WT or KO (E) or ATG5 WT or KO (F) MEFs expressing dominant-negative PTEN C124S in 1% AA ± A769662; statistics relative to PAK1 (E) or ATG5 (F) replete cells. G,H) Proliferation of PAK1 WT or KO MEFs (G) or ATG5 WT or KO MEFs (H) ± PTEN C124S after 72 h in 1% AA medium supplemented with 2% BSA ± A769662 (10 μM). I) FLIM-FRET-phasor analysis of RAC1 activation in PTEN WT or KO MEFs in CM ± A769662 (50 μM); statistics relative to T=0. J) Single frames from Supplementary Videos 1 and 2 showing ratiometric GP-FRET (RAC1 activity) and RAC1-CyPet intensity in the periphery of a PTEN KO MEF stimulated with A769662 for 2 h. Arrowheads indicate macropinosomes. Scale bar, 5 μm. K) Summary of signaling pathways regulating macropinocytic flux. Means ± SEM shown, n ≥ 3 in all panels. In panels D, G, and H, a two-way ANOVA was performed with Tukey’s correction for multiple comparisons. In all other panels, a paired two-tailed t test was employed. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n.s., not significant. Scale bar, 20 μm except in (J). For imaging, ≥ 25 cells were examined. See also Supplementary Figures S3–5 and Supplementary Videos S1–2.
AMPK activation drives autophagy by activating ULK1 and inactivating mTORC1 (30–32). Autophagy is often necessary for maximal tumor growth (33). To dissect the relative contribution of autophagy and macropinocytosis to AMPK-driven cell proliferation in low nutrients (Fig. 2A), dominant-negative PTEN C124S was introduced into MEFs that were either capable of autophagy but deficient in macropinocytosis due to PAK1 deletion or capable of macropinocytosis but deficient in autophagy due to ATG5 deletion. PTEN C124S expression in either PAK1 WT or ATG5 WT MEFs led to macropinocytosis upon AMPK activation with A769662 similar to results in PTEN null MEFs (Fig. 2E,F and Supplementary Fig. S4A–D). Deletion of PAK1, but not ATG5, eliminated macropinocytosis. Conversely, deletion of ATG5, but not PAK1, blocked autophagy (Supplementary Fig. S4E–G). Consistent with their effect on macropinocytosis (Fig. 2E), PTEN C124S expression combined with A769662 drove proliferation in PAK1 WT, but not PAK1 KO, MEFs in amino acid deficient medium (Fig. 2G). Compensatory up-regulation of autophagy was not observed when macropinocytosis was blocked by PAK1 deletion, however, autophagy was slightly elevated basally in PAK1 KO MEFs relative to PAK1 WT controls (Supplementary Fig. S4E). Intriguingly, A769662 stimulated proliferation in both ATG5 WT and KO MEFs expressing PTEN C124S in low amino acids (Fig. 2H). Autophagy-deficient PTEN C124S ATG5 KO MEFs may have proliferated less than autophagy-competent PTEN C124S ATG5 WT MEFs, but autophagy was not necessary for proliferation in amino acid deficient medium. Taken together, these studies demonstrate that AMPK drives proliferation in nutrient-stressed cells by inducing macropinocytosis. While autophagy may promote proliferation by sparing macropinocytosis-derived nutrients for anabolic processes, cell-autonomous autophagy is not sufficient to support cell division.
How AMPK stimulates macropinosome formation was next investigated. AMPK activates RAC1 in certain contexts (34), and RAC1 activation is required for membrane ruffling during macropinocytosis (4, 5, 19). We measured RAC1-GTP levels and localization using a dual-chain RAC1 Förster resonance energy transfer (FRET) biosensor and fluorescence lifetime imaging microscopy (FLIM) (35). When the biosensor (PAK1 effector domain-YPET fusion) binds CyPet-RAC1-GTP, the resulting FRET quenches the fluorescence lifetime of the donor (CyPET). Detecting FRET by monitoring donor lifetime rather than following the ratio of acceptor:donor fluorescence intensity has the key advantage that measurements are independent of protein concentration, while the phasor approach to fluorescence lifetime data analysis can provide a global view of the RAC1 activation state in an image by transforming the histogram of time delays in each pixel into a phasor (Supplementary Fig. S5A). When the AMPK activator A769662 was added to PTEN KO MEFs in complete medium, RAC1-GTP levels increased, particularly in the cell periphery (Fig. 2I and Supplementary Fig. S5B,C). A769662 also stimulated robust AMPK and RAC1 activation and membrane ruffling in PTEN WT MEFs indicating that PTEN deficiency was not required for RAC1 activation. Macropinosome closure leads to RAC1 inactivation (36). Thus, productive macropinosome formation in PTEN KO MEFs (Fig. 1A) may lead to reduced total RAC1-GTP levels relative to PTEN WT MEFs. Confirming the specificity of the assay, RAC1-GTP levels did not increase in the absence of A769662, and adding the RAC inhibitor EHT1864 60 min after A769662 restored donor lifetime to basal levels (Supplementary Fig. S5D,E). Using standard ratiometric techniques to monitor the localization and dynamics of RAC1 activation in real time, dynamic waves of RAC1-GTP were seen in the periphery of A769662-stimulated PTEN WT and KO cells; consistent with dextran uptake results, circular macropinosome-like structures bounded by activated RAC1 were observed only in PTEN KO MEFs (Fig. 2J and Supplementary Video S1–2). Thus, AMPK activation promotes macropinocytosis by increasing RAC1 activation (Fig. 2I), but RAC1 activation is not sufficient to trigger macropinosome formation in the presence of PTEN (Fig. 2I,K and Supplementary Fig. S3B).
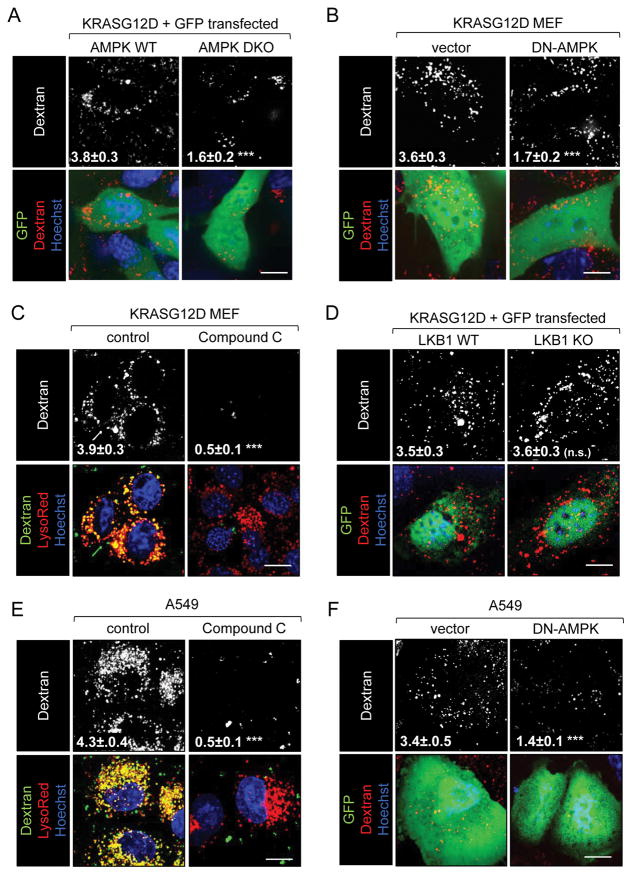
Whether AMPK activation was a general requirement for macropinocytosis or selectively required in PTEN-deficient cells was not clear. The LKB1 tumor suppressor is mutated in up to 30% of NSCLC, including tumor cells with activating mutations in KRAS (37). LKB1 is reported to be the major AMPK-activating kinase under metabolic stress (38). It was therefore of interest to determine whether LKB1 and AMPK are required for RAS-driven macropinocytosis. Introduction of KRAS G12D drove macropinocytosis in AMPK WT but not AMPK DKO MEFs (Fig. 3A). Consistent with these results, DN-AMPK expression or Compound C blocked dextran uptake in KRAS G12D MEFs (Fig. 3B,C). Thus, AMPK activity is also necessary for KRAS-driven macropinocytosis. In contrast, KRAS G12D expression stimulated equally robust macropinocytosis in LKB1 WT and KO MEFs (Fig. 3D) consistent with studies showing that LKB1 loss reduces but does not eliminate AMPK activity. Moreover, the LKB1-deficient, KRAS G12S-expressing NSCLC cell line A549 exhibited a high macropinocytic index that was dramatically reduced by Compound C or DN-AMPK expression (Fig. 3E,F). These results suggest that AMPK activation is a general requirement for macropinosome formation.
Figure 3. AMPK activity is necessary for KRAS-driven macropinocytosis.
A) Dextran uptake in GFP-positive AMPK WT or DKO MEFs 48 h after co-transfection with KRAS G12D and GFP plasmids. B) Dextran uptake in GFP-positive KRAS G12D MEFs 72 h after transfection with plasmids expressing GFP or DN-AMPK-IRES-GFP. C) Dextran uptake in KRAS G12D MEFs ± Compound C (10 μM). D) As in (A) but in LKB1 WT or KO MEF. E) Dextran uptake in A549 (LKB1−/−, KRAS G12S) ± Compound C (10 μM). F) As in (B) but in A549 cells. Scale bars, 20 μm. All experiments were performed in complete medium. Dextran index ± SEM indicated in white with statistics relative to untreated, vector, or wildtype control; ≥ 25 cells were examined. Using a paired, two-tailed t test, ***, P ≤ 0.001; n.s., not significant.
PTEN-deficient prostate cancer cells exhibit constitutive macropinocytosis
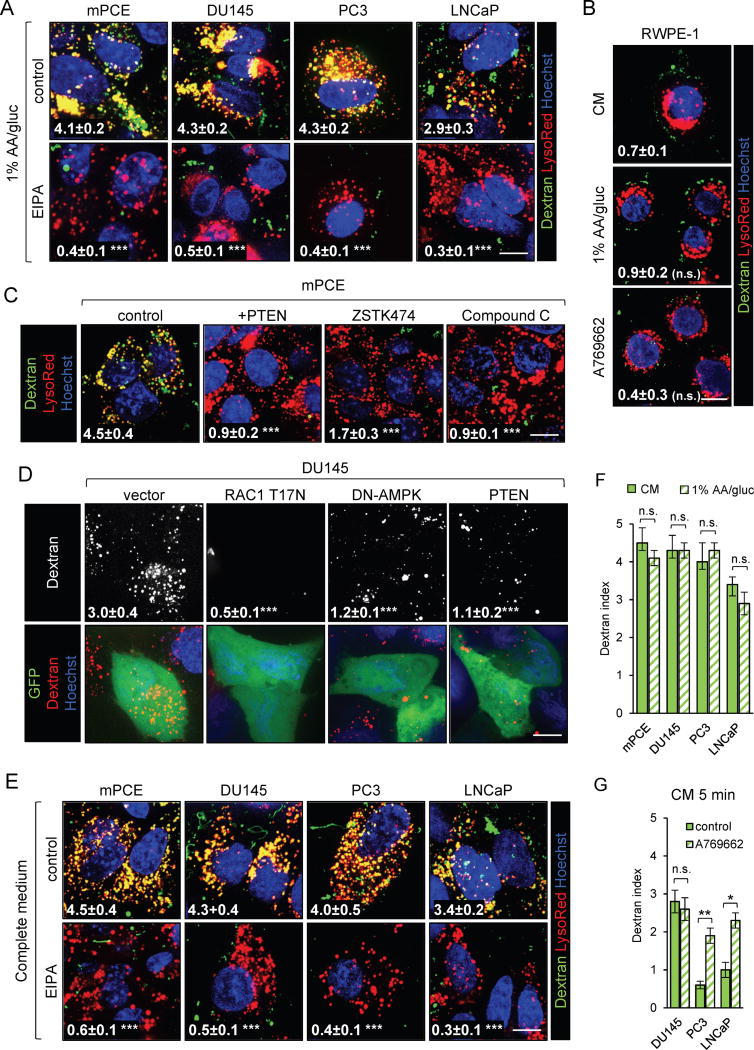
At diagnosis, the majority of prostate tumors exhibit PTEN deficiency or mutation and complete loss of PTEN is closely linked to the castration resistance and metastasis that render prostate cancer a lethal disease (16). Our results in MEFs suggest that macropinocytosis may supply PTEN-deficient prostate cancers with fuel for biosynthesis and growth. For initial in vitro studies, human prostate cancer cells with PTEN deletion (PC3, LNCaP) or deficiency (DU145) and mouse prostate cancer epithelial (mPCE) cells derived from a tumor in a Ptenflox/flox;tp53flox/flox;PB-Cre4 mouse, an established model for castration-resistant prostate cancer (CRPC), were utilized (18, 39). All of these PTEN-deficient prostate cancer cell lines exhibited robust, EIPA-sensitive dextran uptake in 1% AA/gluc medium (Fig. 4A). In contrast, the immortalized but non-transformed PTEN-replete prostate epithelial cell line RWPE-1 did not exhibit macropinocytosis under nutrient deprivation or in the presence of A769662 (Fig. 4B). These results suggests that prostatic epithelial cells are not normally macropinocytic and that macropinocytosis is a cancer-associated phenotype that stems from the loss of PTEN function. In keeping with this model, PTEN reconstitution or the pan-PI3K inhibitor ZSTK474 blocked dextran uptake in prostate cancer cells (Fig. 4C,D). As observed in MEFs (Fig. 2C, 3A–C, and Supplementary Fig. S3E–F), AMPK activation was also necessary for macropinocytosis in prostate cancer cells, as Compound C or expression of DN-AMPK blocked macropinocytosis to a similar extent as RAC inhibition (Fig. 4C,D and Supplementary Figure S6A). Given this requirement for AMPK activation, it was somewhat surprising that prostate cancer cells exhibited equally robust dextran uptake in complete and nutrient-deficient media (Fig. 4E,F). Neither nutrient deprivation nor A769662 increased the amount of dextran taken up by prostate cancer cells over 30 min, a time point when steady state was reached (Fig. 4F and Supplementary Fig. S6B,C). However, when the dextran pulse was shortened to 5 min, A769662 increased uptake in both PC3 and LNCaP prostate cancer cells (Fig. 4G and Supplementary Fig. S6D). These results are consistent with studies demonstrating that AMPK activity is basally elevated in prostate tumors relative to normal tissue (40). AMPK also stimulates autophagy, and blocking macropinocytosis in prostate cancer cells did not further increase autophagic flux (Supplementary Fig. S6E). Together, these results confirm that PTEN loss and AMPK activation promote macropinosome formation in prostate cancer cells even in complete medium.
Figure 4. PTEN-deficient prostate cancer cells exhibit constitutive macropinocytosis.
A) Dextran uptake in prostate cancer in 1% AA/gluc ± EIPA (50–75 μM). B) Dextran uptake in RWPE-1 cells in CM or 1% AA/gluc ± A769662 (50 μM). C) Dextran uptake in mPCE cells ± PTEN reconstitution or a 1 h pretreatment with the pan-PI3Ki ZSTK474 (200 nM) or Compound C (10 μM). D) Dextran uptake in GFP-positive DU145 cells 48 h after transfection with plasmids expressing GFP or GFP and RAC1 T17N, DN-AMPK, or wild type PTEN. E,F) Dextran uptake in prostate cancer cell lines in CM ± EIPA (50–75 μM), compared to results in 1% AA/gluc in (F). G) Dextran index in prostate cancer cells incubated with dextran for 5 min in CM ± A769662 (50 μM). All panels except (A) and part of (B) were conducted in complete medium. Scale bar, 20 μm. Dextran index in white, means ± SEM shown with statistics relative to control, CM, or vector; ≥ 25 cells were examined. Using a paired two-tailed t test, *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n.s., not significant. See also Supplementary Figure S6.
Albumin uptake is independent of macropinocytosis in prostate cancer cells
Having established that PTEN-deficient prostate cancer cells exhibit robust macropinocytosis (Fig. 4), whether prostate cancer cells could use macropinocytosis to support growth and survival in low nutrients was evaluated. BSA is routinely used as a fuel in assays designed to measure whether macropinocytosis supports proliferation and survival in low nutrients (6–8). However, BSA is taken up by multiple mechanisms (41). Efficient BSA uptake in MEFs required macropinocytosis (Fig. 1A,C), and thus this cargo could be used to dissect the role of macropinocytosis in resistance to nutrient stress in MEFs (Figs. 1G–I and 2A,D,G,H). In contrast, prostate cancer cells took up similar amounts of BSA in the presence or absence of macropinocytosis (Supplementary Fig. S7A–C). As expected, LDL and transferrin, two classic RME cargos, were taken up with equal efficiency in the presence or absence of EIPA suggesting that BSA entry via RME would also be EIPA-resistant (Supplementary Fig. S7D,E). Consistent with this model, dominant-negative dynamin1 K44A expression inhibited uptake of transferrin and BSA, but not dextran (Supplementary Fig. S7F); RME but not macropinocytosis is dynamin-dependent (5). Because these experiments indicated that albumin efficiently enters prostate cancer cells through macropinocytosis-independent pathways, an alternative, physiologically relevant and purely macropinocytic cargo was sought to determine whether macropinocytosis can support prostate cancer anabolism.
Necrotic cell debris is taken up by prostate cancer cells solely by macropinocytosis
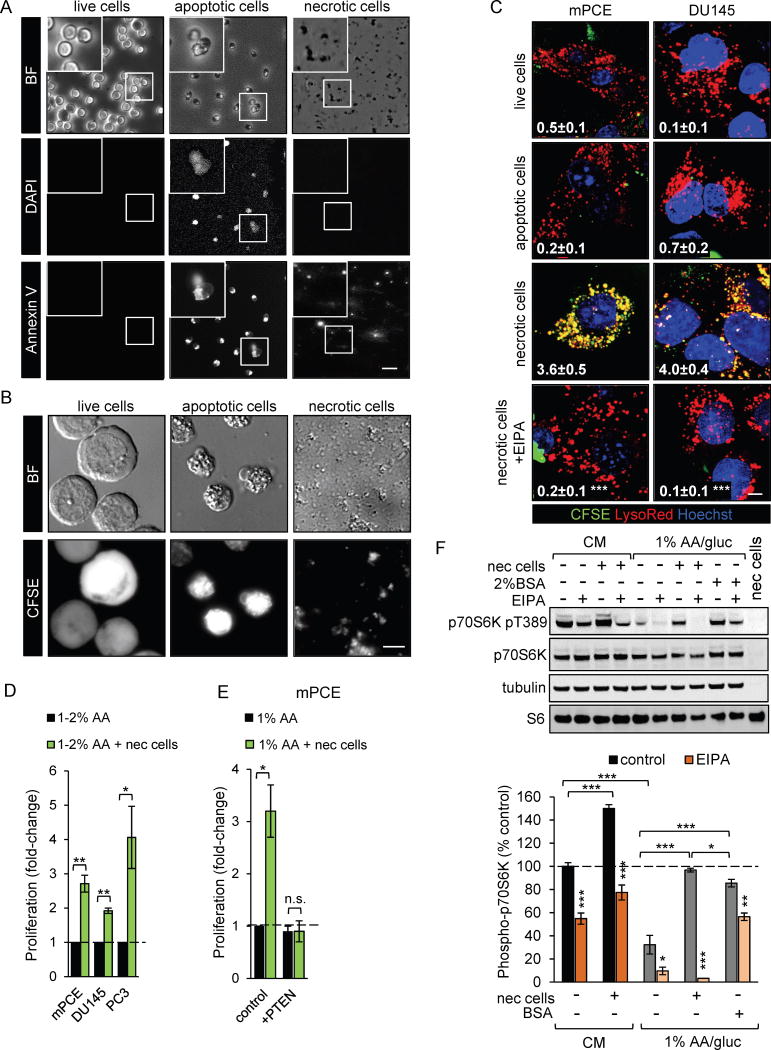
As tumors grow, tortuous and poorly formed vasculature leads to necrosis in regions where oxygen and nutrient delivery are inadequate to meet tumor cell demand (2). Necrosis is present in many aggressive, high-grade tumors, including prostate cancers and RAS-driven tumors, and correlates with negative patient outcomes and resistance to radiation and chemotherapy (42–48). Macropinosomes are large structures, ranging from 0.2 to 5 μM in diameter. Necrotic cell debris could be small enough to be engulfed via macropinocytosis, while live or apoptotic cells should be too large to enter via this mechanism. To test this idea, murine hematopoietic FL5.12 cells were fluorescently labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and then killed using different protocols. FL5.12 cells were ideal for these studies because apoptotic death upon withdrawal of the cytokine IL-3 avoids the need to remove a cytotoxic drug before supplying the corpses as fuel for prostate cancer cells. A necrotic FL5.12 cell preparation was prepared by allowing sufficient time after IL-3 withdrawal for secondary necrosis to occur (48–72 h). As shown in Fig. 5A and B, freshly killed apoptotic FL5.12 cells are intact, Annexin V- and CFSE-positive, and label with the fluorescent vital dye DAPI. In contrast, necrotic FL5.12 cell preparations contain only cell fragments that retain CFSE- and Annexin V-positivity. As expected, live FL5.12 cells are CFSE-positive but do not stain with DAPI or Annexin V. Consistent with their relative sizes, CFSE-labeled necrotic debris but not apoptotic or live FL5.12 cells were engulfed by macropinocytic mPCE and DU145 cells (Fig. 5C). Importantly, uptake of necrotic debris was was fully EIPA-sensitive, only observed in macropinocytic cells, and green necrotic debris fully co-localized with red dextran in macropinosomes (Fig. 5C and Supplementary Fig. S8A,B). The selective uptake of necrotic debris but not intact cell corpses or live cells clearly differentiates this process from efferocytosis (phagocytosis of apoptotic cells) (49) or entosis (engulfment of viable cells by cancer cells)(50). To confirm that necrotic debris could be accommodated within macropinosomes, co-labeling studies were performed. When prostate cancer cells were fed both red and green dextrans, macropinosomes were uniformly yellow in the merged image as expected (Supplementary Fig. S8C,D). In contrast, when red and green necrotic debris were added simultaneously, macropinosomes were either red or green demonstrating that large cell fragments in the necrotic cell preparation were taken up via macropinocytosis (Supplementary Fig. S8D,E). Taken together, these experiments demonstrate that necrotic debris enters cells solely via macropinocytosis.
Figure 5. PTEN-deficient prostate cancer cells consume necrotic debris via macropinocytosis to fuel growth.
A) FL5.12 cells were killed by IL-3 withdrawal for 24 h at low density density (25,000 cells/ml) to produce apoptotic cells or 48 h at high density (100 million cells/ml) to trigger primary and secondary necrosis and stained as indicated. B) CFSE-labeled FL5.12 cells were killed as in (A). Statistics comparing necrotic cells ± EIPA. C) Prostate cancer cells were fed 1 million live, apoptotic, or necrotic CFSE-labeled FL5.12 cells or cell equivalents for 1 h prior to imaging. Where indicated, EIPA (50 μM) was added 1 h prior to necrotic cells; statistics test control versus EIPA. D) Prostate cancer cell proliferation after 72 h in 1% (mPCE, PC3) or 2% (DU145) AA medium ± necrotic debris (0.1% protein). DU145 cells did not proliferate in 1% AA medium. E) mPCE cells treated as in (D) ± PTEN reconstitution. In (D) and (E), results are expressed relative to the low nutrient control. F) mTORC1 signaling in mPCE cells after 4 h in CM or 1% AA/gluc ± necrotic cells (0.05% protein), 2% BSA, and/or EIPA (75 μM) as indicated. The far right lane is necrotic cells only. Scale bars, 10 μm. Means ± SEM shown, n ≥ 3 in all panels. Using a paired, two-tailed t test, *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n.s., not significant. Tukey’s method was used to correct for multiple comparisons in (F). Uptake index indicated in white in (C); ≥ 25 cells were examined. See also Supplementary Figure S7–8.
Whether macropinocytosis could drive prostate cancer cell proliferation in low-nutrient medium was measured using necrotic debris as fuel. Because macropinocytosis in prostate cancer cells did not depend on glucose depletion (Fig. 4E,F), only amino acids were limited to allow maximal macropinocytosis-driven proliferation. The addition of necrotic cell debris significantly stimulated the proliferation of mPCE, PC3, and DU145 cells in amino acid-deficient medium (Fig. 5D). Macropinocytosis was required to derive a benefit from necrotic debris as PTEN reconstitution eliminated both macropinocytosis (Fig. 4C) and the enhanced prostate cancer cell proliferation (Fig. 5E). Necrotic debris also supported the proliferation of nutrient-deprived macropinocytic KRAS G12D MEFs and PANC-1 pancreatic cancer cells but not non-macropinocytic LSL MEFs or BxPC3 pancreatic cancer cells (Supplementary Fig. S8F,G). In fact, adding necrotic debris increased death in nutrient-deprived non-macropinocytic LSL MEFs or BxPC3 cells confirming that the necrotic cell preparation did not contain sufficient soluble amino acids to support growth. In summary, macropinocytic cells can use necrotic cell debris to support anabolism under nutrient stress.
To confirm that macropinocytosed material fueled prostate cancer anabolism, we assessed amino acid-sensitive mTORC1 signaling. Amino acids produced by the degradation of macropinosomes can re-activate mTORC1 (7). As expected, shifting prostate cancer cells to amino acid-deficient medium reduced mTORC1-dependent phosphorylation of p70S6 kinase at Thr389 (Fig. 5F). Supplementation with necrotic debris at a concentration of 0.05% protein restored mTORC1 signaling in an EIPA-sensitive manner. BSA supplementation (2%) also restored Thr389 phosphorylation, but this effect was incompletely reversed by EIPA as expected given that BSA uptake is macropinocytosis-independent in prostate cancer cells (Fig. 5F and Supplementary Fig. S7A,B). Interestingly, necrotic debris increased mTORC1 signaling in an EIPA-sensitive manner even in complete medium suggesting that macropinocytosis also fuels prostate cancer growth in nutrient-replete conditions (Fig. 5F). Importantly, necrotic cell debris did not itself contribute to the pThr389 p70S6K signal. These results suggest that necrotic debris supplies amino acids to nutrient-deprived macropinocytic prostate cancer cells.
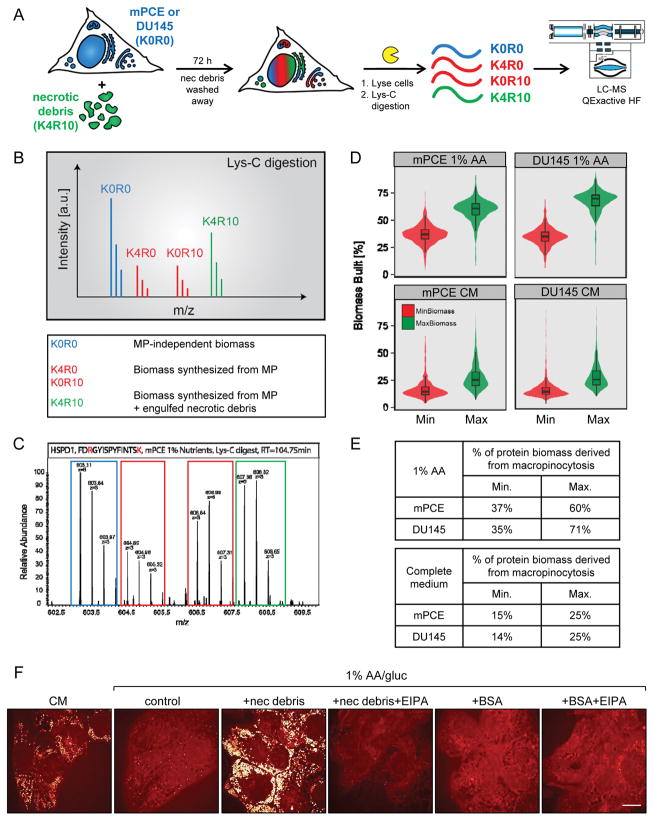
To directly test whether proteins scavenged via macropinocytosis are broken down into amino acids to build biomass, we developed a novel isotopic labeling strategy (Fig. 6A). To label the macropinocytic cargo, FL5.12 cells were grown in stable isotope labeling with amino acids in cell culture (SILAC) medium containing 13C3,15N1 lysine (K4) and 13C6,15N4 arginine (R10) for more than 10 generations. Complete labeling of the FL5.12 proteome was confirmed by LC-MS/MS. Necrotic debris containing only fully-labeled (K4R10, heavy, 14 Da mass shift) peptides, depicted in green in Fig. 6A–C, was produced from these FL5.12 cells and fed to macropinocytic prostate cancer cells whose proteins contained only unlabeled amino acids (K0R0, light, depicted in blue). Proliferation assays were conducted in medium containing only unlabeled amino acids, and thus the only source of isotopically labeled amino acids was the proteins in the necrotic debris. When prostate cancer cells fed necrotic debris are harvested and their proteins digested with LysC, peptides that contain both lysine and arginine can be present in four different isotopic forms (Fig. 6A–C). Peptides produced independently of macropinocytosis will be unlabeled (K0R0, light, blue). Peptides that contain one unlabeled and one labeled amino acid (K4R0 producing a 4 Da shift or K0R10 producing a 10 Da shift, mixed peaks, red) must have been synthesized in prostate cancer cells using both a labeled amino acid obtained via macropinocytosis and an unlabeled amino acid obtained from the medium. Fully isotopically labeled peptides (K4R10 producing a 14 Da shift, heavy, green) could come from either undigested, heavy-labeled FL5.12 proteins or represent proteins synthesized in prostate cancer cells using both heavy-labeled arginine and lysine obtained via macropinocytosis. An example isotopic profile for the LysC peptide 219FDRGYISPYFINTSK233 from the mitochondrial heat shock protein HSPD1 exhibits these four classes of peptides (Fig. 6C). To summarize, peptides that are K0R0 were generated independent of macropinocytosis, K4R0 and K0R10 peptides can only be generated if macropinocytosis supplies amino acids, and peptides that are K4R10 are either from engulfed but undigested FL5.12 proteins or proteins synthesized in prostate cancer cells using two labeled amino acids derived from macropinocytosis.
Figure 6. Necrotic debris consumed by macropinocytosis is used to build biomass.
A) Proteomics workflow. Unlabeled mouse mPCE or human DU145 prostate cancer cells were fed K4R10-labeled necrotic FL5.12 cells for 72 h in 1% AA or CM, washed with PBS 3 times, then digested with Lys-C for LC-MS peptide analysis. B) Hypothetical predicted mass spectra of one peptide from the experiment in (A). Light peaks (blue) do not contain lysine or arginine derived from macropinocytosed proteins. Medium peaks (red) correspond to newly synthesized protein containing both unlabeled (MP independent) and labeled (MP dependent) lysine and arginine. Heavy peaks (green) correspond to newly synthesized peptides containing both heavy labeled lysine and arginine (MP dependent) or peptides from engulfed undigested necrotic debris. C) Actual peptide spectra of a triply charged peptide (amino acid sequence FDRGYISPYFNTSK from the HSPD1 protein) from Lys-C digested mPCE cells fed necrotic debris. D,E) Maximum and minimum protein biomass derived from macropinocytosed debris calculated as depicted graphically using violin plots (D) or described in the text and tabulated in (E). F) DU145 cells were cultured for 24 h in 1% AA/gluc ± necrotic debris, 2% BSA, ± EIPA (50 μM) then lipid droplets were imaged by CARS. Scale bar, 20 μm. See also Supplementary Figure S9.
To calculate the maximum biomass derived from macropinocytosis, we assumed that all K4R10 peptides (heavy, green) were synthesized in prostate cancer cells using lysine and arginine derived from macropinocytosis (see Supplementary Methods for details). The minimum amount of protein biomass derived from macropinocytosis was calculated by assuming all of these K4R10 peptides were derived from engulfed but undigested FL5.12 proteins. Using this approach, we found that in amino acid-deficient medium, at least 35–37% of proteins and as much as 60–71% of prostate cancer cell protein biomass was derived from macropinocytosed protein (Fig. 6D,E and Supplementary Fig. S9A). Moreover, even in complete medium where free, unlabeled amino acids were abundant, between 14% and 25% of the prostate cancer cell protein biomass was derived from macropinocytosed protein. To complement the LysC approach, we performed tryptic digests of proteins isolated from human DU145 prostate cancer cells fed heavy-labeled necrotic debris generated from murine FL5.12 cells. In this scenario, uniquely human peptides that contain an isotopically-labeled amino acid (K4 or R10) must have been synthesized using amino acids acquired via macropinocytosis. This approach produced similar estimates of the contribution of macropinocytosis to the amino acid pool for protein synthesis (Supplementary Fig. S9B). In summary, isotopic labeling conclusively demonstrates that macropinocytosed protein contributes to prostate cancer biomass even in nutrient-replete conditions.
Cellular corpses are rich sources of other building blocks besides amino acids. Lipids and cholesterol in particular are key drivers of prostate cancer growth (51, 52). Fatty acids and cholesterol are stored in lipid droplets, and depleting these lipid pools suppresses prostate cancer proliferation (51–53). Lysosomal degradation of lipid droplets increases in response to amino acid or glucose depletion as triglycerides in these droplets are utilized to fuel mitochondrial metabolism (53, 54). Using label-free Coherent Anti-stokes Raman Spectroscopy (CARS) to detect C-H stretching vibration signals from lipids (52), lipid droplet content declined significantly in glucose- and amino acid-restricted prostate cancer cells as expected (Fig. 6F and Supplementary Fig. S9C). Supplying necrotic cell debris completely restored lipid droplet content in an EIPA-sensitive manner. As noted earlier (Supplementary Fig. S7D), EIPA did not interfere with LDL uptake and it did not decrease lipid droplet content when added in complete medium (Supplementary Fig. S9D). Interestingly, supplementation with 2% BSA did not restore lipid droplet content in nutrient-deprived prostate cancer cells (Fig. 6F). This suggests that the membranes and lipids present in necrotic debris rather than proteins are responsible for maintaining lipid stores and that prostate cancer cells can scavenge multiple nutrients from cell corpses.
Prostate cancer cells exhibit macropinocytosis under physiologic conditions
To assess whether normal and transformed prostate epithelial cells perform macropinocytosis under more physiologic conditions, we evaluated dextran uptake in prostate organoids derived from C57BL/6 mice and from Ptenflox/flox;tp53flox/flox;PB-Cre4 mice that develop autochthonous prostate tumors. PTEN-deficient prostate cancer spheroids but not PTEN-replete normal prostate epithelial organoids exhibited EIPA-sensitive macropinocytosis in nutrient-replete standard 3D culture medium (Fig. 7A) (55). Macropinocytosis in prostate cancer organoids was sensitive to Compound C suggesting that AMPK activation was also required in this context (Supplementary Fig. 10A). Primary, metastatic tumor cells from CRPC patients can also be propagated in 3D culture where they form tumor organoids that exhibit histological features that mimic the original patient tumor (56). A patient sample deficient in both PTEN and p53, MSK-PCa1, also exhibited constitutive macropinocytosis in 3D culture (Fig. 7B). Patient-derived xenografts (PDX) were also evaluated in situ. Subcutaneous PTEN- and p53-deficient PDX prostate tumors (Jackson Laboratory PDX model TM00298) also exhibited EIPA-sensitive dextran uptake in vivo following intra-tumoral injection of dextran (Fig. 7C). Similarly, autochthonous tumors in Pten flox/flox;tp53flox/flox;PB-Cre4 mice exhibited robust macropinocytosis of intravenously delivered 70 kD FITC-Ficoll; uptake was again sensitive to systemic EIPA administration (Fig. 7D). Macropinocytosis was not observed in the prostates of normal mice given 70 kD FITC-Ficoll intravenously (Fig. 7E). Importantly, in all cases where macropinocytic uptake was not detected (e.g. in EIPA-treated and wild-type animals), Evan’s Blue dye that was co-injected with the dextran or Ficoll was present in the tumor or tissue confirming successful delivery of dextran or Ficoll and EIPA. These results demonstrate that PTEN-deficient prostate cancer cells, but not normal prostate epithelial cells, exhibit robust macropinocytosis in 3D culture and in vivo.
Figure 7. Prostate cancer cells exhibit macropinocytosis in 3D and in vivo.
A,B) Dextran uptake in organoid cultures generated from prostate epithelial cells from C57BL/6 or Pten flox/flox;tp53flox/flox;PB-Cre4 mice (A) or PTEN- and p53-deficient MSK-PCa1 metastatic human CRPC organoids (B). In (A), mouse prostate organoid media (55) or mPCE medium (67) containing 5% growth factor reduced Matrigel was utilized. In (B), human prostate organoid medium was utilized (56). C) Dextran uptake (2 mg intratumorally) in subcutaneous PTEN- and p53-deficient JAX PDX TM00298 tumors ± EIPA (10 mg/kg i.p.). D,E) 70 kD FITC-Ficoll (250 mg/kg i.v.) uptake in autochthonous tumors in Pten flox/flox;tp53flox/flox;PB-Cre4 mice ± EIPA (10 mg/kg i.p.) (D) or normal prostate in C57BL/6 mice (E). F,G) Tumor volume in C57BL/6 mice bearing subcutaneous mPCE isografts after 28 d of treatment with vehicle or EIPA (7.5 mg/kg s.c. every other day) once tumors reached 100 mm3. In G, means ± SD shown, n = 10–11. Using an unpaired, two-tailed t test, ***, P ≤ 0.001. H) FITC-Ficoll (250 mg/kg i.v.) uptake in tumors in mice in (F,G) 2 h after treatment with vehicle or EIPA. Dextran or Ficoll index (mean ± SEM) shown in white; statistics not performed for normal prostate, otherwise are relative to control. Scale bar, 20 μm. See also Supplementary Figure S10–11.
To evaluate whether prostate tumors rely on macropinocytosis for growth, C57BL/6 mice bearing subcutaneous prostate cancer isografts were treated with EIPA. Consistent with a role for macropinocytosis in driving prostate tumor growth in vivo, alternate day subcutaneous injections of EIPA inhibited both 70 kD FITC-Ficoll uptake and prostate tumor growth, even producing some regressions, without affecting body weight (Fig. 7F–H and Supplementary Fig. S10B–E). Again, Evan’s Blue dye co-injected with Ficoll robustly labeled tumors that failed to take up FITC-Ficoll in the presence of EIPA confirming successful IV injection and that EIPA delivered with this dosing protocol inhibits macropinocytosis (Supplementary Fig. S10E). While all prostate tumor cells we evaluated exhibited macropinocytosis, xenograft growth of a non-macropinocytic pancreatic cancer cell line is not affected by EIPA (6). Taken together, these results in cell lines, organoid cultures, and mouse models support the conclusion that prostate cancers use macropinocytosis to acquire extracellular nutrients to fuel growth and proliferation both in vitro and in vivo.
DISCUSSION
This study defines macropinocytosis as a previously unrecognized fuel source in prostate cancer, a tumor class known for its enigmatic nutrient dependencies. This discovery could lead to new therapeutic approaches to this lethal disease. Despite the introduction of second-generation androgen inhibitors such as enzalutamide and abiraterone acetate, CRPC patients still develop resistance to all available targeted drugs; chemotherapy with docetaxel affords limited clinical benefit and produces significant toxicity (57, 58). Our finding that prostate cancer cells use macropinocytosis to support anabolism suggests that inhibiting this pathway could provide a novel, safe, and effective strategy to target metabolism in late-stage prostate cancer. Macropinocytosis inhibition could synergize with inhibitors of androgen signaling. Androgen deprivation therapy triggers tumor cell death, but castration-resistant disease eventually emerges. The growth of PTEN-deficient tumor cells that survive androgen deprivation therapy may be fueled in part by the macropinocytic catabolism of the corpses of their deceased, androgen-dependent brethren. Similarly, cell death resulting from chemotherapy or radiation therapy may paradoxically increase the nutrient pool available to the surviving PTEN-deficient tumor cells. Inhibitors of lysosomal function such as chloroquine that are often used as autophagy inhibitors may limit prostate cancer cell growth in part by blocking macropinosome degradation. The synthetic sphingolipid SH-BC-893 works upstream from chloroquine, preventing macropinosome-lysosome fusion; SH-BC-893’s anti-neoplastic activity in prostate cancer models may stem in part from its ability to block macropinocytosis (18). While EIPA is generally considered to have poor pharmacological properties, it inhibited both macropinocytosis and tumor growth in mice following systemic administration without obvious toxicity (Fig. 7F–H, and Supplementary Fig. S10B–E). It will be important to evaluate the sensitivity of autochthonous and metastatic prostate tumors to macropinocytosis inhibitors alone and in combination with standard of care therapies in future studies to provide a more complete picture of their potential clinical value.
The discovery that AMPK activation is a general requirement for macropinosome formation in cancer cells dramatically extends our understanding of the regulation of this process. By increasing RAC1-GTP levels, AMPK activation stimulates macropinosome formation (Fig. 2I–K and Supplementary Fig. S5). The relatively slow kinetics of RAC1 activation by A769662 (Fig. 2I), while somewhat unexpected, are consistent with prior work (59). Our finding that A769662 activates RAC1-GTP even more efficiently in PTEN WT MEFs than in PTEN KO cells (Fig. 2I) without stimulating dextran uptake (Supplementary Fig. S3B) seems at first contradictory. However, macropinosome maturation leads to RAC1 inactivation (36), and RAC1 may be trapped in the GTP-bound state in PTEN WT MEFs. Closure of macropinosomes in the presence of elevated PIP3 may prevent the accumulation of RAC1-GTP in PTEN KO MEFs. Intriguingly, the RAC GEFs ARHGEF6 and ARHGEF7 may be substrates for AMPK (60). Additional studies will be required for a full mechanistic understanding of how macropinocytosis is regulated by signal transduction cascades.
While previous studies evaluating the role of macropinocytosis in cancer deprived cells of individual or classes of amino acids (6–8), we utilized media that was either deficient in all amino acids or in both amino acids and glucose. It is difficult to accurately model the nutrient stress that tumors will experience in their normal microenvironment, but inadequate perfusion is likely to restrict access to multiple nutrients simultaneously. The 1% nutrient condition was selected because nutrient titration experiments indicated that this was the least severe reduction in nutrients that killed the majority of wild type MEFs within 48 h. The specific nutrient conditions employed can influence conclusions regarding the importance of macropinocytosis. When PTEN KO MEFs are deprived of only amino acids, they do not exhibit macropinocytosis (Fig. 2B). Amino acid limitation would only be relieved by autophagy, a catabolic process that cannot by itself drive cell-autonomous growth (Fig. 2F). In contrast, combined glucose and amino acid stress (1% AA/gluc) activates AMPK and stimulates macropinocytosis (Fig. 2B) thereby providing amino acids from the degradation of albumin in the medium. That AMPK-driven macropinocytosis is responsible for the growth-stimulatory effects of glucose deprivation is supported by the observations that: 1) A769662 enhances proliferation in macropinocytosis-competent amino acid-deprived PTEN KO MEFs but not non-macropinocytic PTEN WT MEFs (Fig. 2A), 2) PAK1 is necessary for A769662 to drive macropinocytosis and proliferation in PTEN-deficient MEFs in low nutrients (Fig. 2F), and 3) A769662 can drive proliferation in PTEN-deficient MEFs in low nutrients even in the absence of autophagy (Fig. 2H). Thus, it is not glucose limitation per say but rather AMPK-induced macropinocytosis that stimulates proliferation in Fig. 2A. Indeed, direct AMPK activation in full glucose (1% AA medium + A769662) permits almost twice as much proliferation as AMPK activation by glucose limitation (1% AA/gluc). It is worth noting that, while autophagy is not sufficient to fuel proliferation in low nutrient medium, autophagy is necessary for maximal macropinocytosis-driven proliferation (Fig. 2H), most likely because it spares amino acids derived from macropinocytosis for anabolism. In conclusion, the seemingly paradoxical effect of glucose deprivation on the proliferation of amino acid-deprived MEFs is readily explained by the stimulation of macropinocytosis.
An intriguing implication of this study is that AMPK inhibitors could be deployed against cancer cells as macropinocytosis inhibitors. AMPK is a negative regulator of macromolecular synthesis and often classified as a tumor suppressor based on its ability to limit anabolism and stimulate catabolic processes (25). On the other hand, multiple studies suggest that AMPK plays a supportive role in established tumors similar to the reciprocal role of autophagy as a suppressor of tumor initiation and a driver of tumor progression (38, 61–64). Indeed, many studies indicate that AMPK promotes prostate cancer progression (40). AMPK stimulates autophagy, and autophagy inhibitors have been incorporated into combination therapies based on the premise that blocking the stress response will sensitize tumor cells to other drugs. Like autophagy, macropinocytosis may provide a bypass system that could rescue tumor cells from metabolic stress induced by other agents. Our finding that AMPK promotes not just autophagy but also macropinocytosis suggests that AMPK inhibitors could be effective against macropinocytic tumors alone or as a part of drug combinations.
Histologic evidence of tumor necrosis is a negative prognostic indicator that is correlated with recurrence and aggressive, metastatic disease in multiple solid tumors, including prostate cancer and RAS-driven malignancies (42–46). Our observations that necrotic cell corpses are a rich source of proteins and lipids capable of driving both PTEN-deficient prostate cancer cell and KRAS-driven pancreatic cancer cell growth (Fig. 5D,E and Supplementary Figs. S8F,G) may partially explain this correlation. Necrosis may enhance tumor growth by triggering inflammation (65) while at the same time providing a fuel source for the cytokine-driven growth of macropinocytic tumor cells. The correlation between inflammatory markers and necrosis is imperfect (47), and in some tumors necrosis may promote cancer cell anabolism primarily by providing metabolic substrates rather than by stimulating a pro-tumorigenic immune response. It is important to recognize that any extracellular material small enough to fit in a macropinosome could be consumed and catabolized through this pathway; cargo that are taken up by selective pathways, for example albumin, would still be taken up non-selectively by macropinocytosis. It is also significant that necrotic cell debris was supplied here at only 0.05 – 0.1% protein, a 20–40 fold lower concentration than BSA, suggesting that relatively small amounts of necrosis could have a significant impact on tumor growth. Rather than attempting to define the relative anabolic value of different macropinocytic cargos in vivo, the most critical next step is to uncover more specific ways to disrupt macropinocytosis than the chemical tools currently available in order to better define the importance of this nutrient scavenging pathway for tumor initiation and progression. Therapeutically, any inhibitor of macropinocytosis would simultaneously block the uptake of BSA, necrotic debris, and any other extracellular cargos. While the contribution that dead cell catabolism makes to tumor cell growth is difficult to quantify, the uptake of necrotic debris through macropinocytosis (necrocytosis) defines a new way the microenvironment likely supports tumor cell anabolism.
These studies also highlight that caution is required when using BSA as a tool to evaluate the contribution of macropinocytosis to anabolism and growth as there are significant, cell line-specific differences in the relative amount of BSA that is taken up through macropinocytosis and dynamin-dependent processes like RME (Fig. 1A,B and Supplementary Fig. S7A,B,F). In cell lines that take up BSA efficiently in the absence of macropinocytosis (Supplementary Fig. S7A,B), necrotic cell debris provides an alternative, physiologically-relevant substrate that is taken up solely by macropinocytosis (Fig. 5C). Moreover, necrotic cells are likely a more complete diet than extracellular protein. The observation that necrotic debris, but not extracellular protein, can maintain lipid droplets in nutrient-restricted cells (Fig. 6F) is strong evidence that the membrane component of dead cells can be efficiently recycled via macropinocytosis. From a technical perspective, using necrotic cell debris as macropinocytic cargo has the advantage of facile fluorescent (Fig. 5A–C and Supplementary Fig. S8A–E) or isotopic (Fig. 6A–E) labeling, permitting both tracing of the engulfed material and analysis of its ultimate fate. Importantly, the novel SILAC labeling approach we developed to show that macropinocytosed proteins contribute amino acids to protein synthesis in nutrient-replete as well as -limited media is readily adoptable by other research groups. We anticipate that the use of necrotic cells as cargo will facilitate future studies defining the role of macropinocytosis in tumor cell growth.
In conclusion, while the anabolic value of macropinocytosis in tumor cells is now well accepted, there remains an unmet need for specific agents that block only macropinosome formation or degradation to better define the importance of this process in tumor growth and progression, particularly in vivo. While their pleiotropic actions somewhat complicate the dissection of the role of macropinocytosis in cancer, NHE inhibitors, AMPK inhibitors, and SH-BC-893 may actually prove to be superior therapeutic agents for the very reason that they possess multifaceted and complementary anti-neoplastic effects (18).
METHODS
Cell culture
DMEM or RPMI containing 1% of the normal amount of amino acids and/or glucose was generated by preparing DMEM or RPMI lacking amino acids and/or glucose from chemical components and mixing it 99:1 with complete medium. In all experiments, the medium was supplemented with 10% standard fetal calf serum which supplies amino acids, glucose, and albumin. LNCaP (2016), PC3 (2011), DU145 (2011), A549 (2013), PANC-1 (2013), BxPC3 (2017), and RWPE-1 (2012) were obtained from the ATCC in indicated years. mPCE cells and PTEN WT/KO MEFs were generated in the Edinger lab (2015); other MEF lines were obtained from collaborating labs in 2003 (LSL/KRAS G12D), 2007 (AMPK WT/DKO and ATG5 WT/KO), or 2017 (PAK1 WT/KO). Cell lines were cultured for ≤ 3 wk at which point a frozen low passage (≤ 4 wk after receipt) stock was thawed. Cell lines were authenticated by evaluating the expression patterns of androgen receptor (PC3, DU145, LNCaP, mPCE) and gene deletions (MEFs, LNCaP, PC3, mPCE) at least every 6 months. Cells were tested for Mycoplasma at least every 4 months (VENOR GeM PCR kit, Sigma). Before they were used in experiments, PAK1 WT and KO MEFs were cured of Mycoplasma by culture in ciprofloxacin for 2 months (PCR confirmed); all other cell lines tested negative. Additional details in Supplementary Methods.
Light Microscopy
Unless otherwise indicated, fluorescent dextran (1 mg/mL), Alexa 488 BSA (0.5 mg/ml), or Alexa 488 transferrin (0.5 mg/ml) were added in combination with Lysotracker Red (1:10,000 dilution) and Hoechst 33342 for 30 min, cells were washed three times with PBS, and live cells evaluated on a spinning disc confocal microscope. Dextran index was calculated using ImageJ software as detailed in (20) and Supplementary Fig. S1A. When nutrient-deficient medium was used, 70 kD dextran or BSA uptake was measured after a 16 h incubation; cells were pre-incubated with 50 μM A769662 for 2 h. EIPA was used at 50–75 μM and added 1 h prior to dextran addition. In Dil-LDL uptake assays, cells were incubated in media with 10% charcoal-stripped serum for 24 h then 20 μg/ml Dil-LDL was added ± EIPA for 2 h.
SILAC labeling
FL5.12 cells were incubated in SILAC media containing “heavy” 13C- or 15N-labeled arginine (R10) and lysine (K4) for >10 divisions. FL5.12 cells were then killed by IL-3 withdrawal; cells were maintained at high density and 72 h allowed for secondary necrosis to produce necrotic debris. Supernatant was fully aspirated from necrotic cell pellets which were used directly or stored at −80°C. In isotopic labeling experiments, prostate cancer cells were washed with PBS after 72 h, lysed, and proteins digested with trypsin or Lys-C and analyzed by mass spectrometry as described in the Supplementary Methods.
RAC1 activity assays
MEFs were transfected with biosensors generously provided by the Hahn Laboratory (University of North Carolina): (1) RAC1 FLARE dual-chain biosensor (CyPet-RAC1 and YPet-PBD); (2) RAC1 constitutively-active dual-chain biosensor (CyPet-RAC1-Q61L and YPet-PBD); or (3) CyPet-RAC1 donor alone. Cells were imaged 24 h after transfection and stimulated with 50 μM A769662 where indicated. FLIM-FRET measurements of the RAC1 FLARE biosensors were acquired and processed using the SimFCS software package developed at the Laboratory for Fluorescence Dynamics (www.lfd.uci.edu) as described in the Supplementary Methods.
Lipid droplet content measurement by CARS
The CARS imaging system is described in detail in (66). Cells were fixed with 4% formaldehyde and imaged with a 60× water objective. The laser power on the sample was at 10 mW with 10 ms pixel dwell time. The lipid droplet area was estimated from CARS images using a customized Matlab program. Four components Otsu thresholding method was used to separate the lipid droplets, cell cytoplasm, cell nucleus and the background. The lipid droplet area was defined as the number of pixels covered by lipid droplets over the number of pixels covered by cytoplasm.
In vivo experiments
Experiments conducted in mice were performed in accordance with the Institutional Animal Care and Use Committee of University of California, Irvine. Prostate isografts were produced by injecting 5 million mPCE cells subcutaneously in the flank of 5 wk old C57BL/6 mice. Once tumors reached 100 mm3, animals were randomly assigned to either the vehicle (1% DMSO in PBS) or EIPA (7.5 mg/kg subcutaneously every other day) group (n=10–11). Tumor volume was calculated using the formula volume (mm3) = length [mm] × (width [mm])2 × 0.52. For in vivo dextran uptake analysis, JAX PDX TM00298 tumors were intratumorally injected with 2 mg Oregon Green dextran dissolved in 1% Evan’s Blue Dye 1 h after intraperitoneal (i.p.) injection with vehicle or 10 mg/kg EIPA. Ten wk old male C57BL/6, Ptenflox/flox;p53flox/flox;PB-Cre4,or C57BL/6 mice with or without mPCE isografts were intravenously injected with 250 mg/kg FITC-Ficoll dissolved in 1% Evan’s Blue Dye 1 h after i.p. injection of vehicle or 10 mg/kg EIPA or 1.5 h after subcutaneous injection of 7.5 mg/kg EIPA as indicated.
Statistical methods and data analysis
Significance was determined using two-tailed t-tests. *, P <0.05; **, P <0.01; ***, P <0.001; n.s., not significant (P >0.05). Tukey’s method was used to correct for multiple comparisons.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
As PTEN-deficient prostate cancer cells proliferate in low nutrient environments by scavenging necrotic debris and extracellular protein via macropinocytosis, blocking macropinocytosis by inhibiting AMPK, RAC1, or PI3 kinase may have therapeutic value, particularly in necrotic tumors and in combination with therapies that cause nutrient stress.
Acknowledgments
Financial Support: This work was supported by grants to ALE from the NIH (R01 GM089919), CDMRP (W81XWH-11-1-0535), the American Cancer Society (RSG-11-111-01-CDD), and UCI Applied Innovation. BJT, EOP, JH were supported by NIH/NIBIB Biomedical Technology Research Center LAMMP: P41EB015890 (Laser Microbeam and Medical Program, LAMMP). SMK was supported by GAANN P200A120207. LM and MAD were supported in part by Grants NIH-P41-RR03155 and NIH-P50-GM076516. PK is supported by a Vanier scholarship.
The authors thank Yu Chen (MSKCC) for generously providing MSK-PCa1 and David Fruman (UC Irvine) for comments on the manuscript. This work was supported by grants to ALE from the NIH (R01 GM089919), CDMRP (W81XWH-11-1-0535), the American Cancer Society (RSG-11-111-01-CDD), University of California Cancer Research Coordinating Committee (CRR-17-426826), and UCI Applied Innovation. BJT, EOP, JH were supported by NIH/NIBIB Biomedical Technology Research Center LAMMP: P41EB015890 (Laser Microbeam and Medical Program, LAMMP). SMK was supported by GAANN P200A120207. LM and MAD were supported in part by Grants NIH-P41-RR03155 and NIH-P50-GM076516. Core facilities at UCI were supported by Cancer Center Support grant P30 CA62203. Proteomics work was supported by Canada Research Chairs in Proteomics and Bioanalytical Mass Spectrometry (PT), the Genome Canada Genomics Innovation Network (PT), and the National Science and Engineering Research Council (311598, PT). PK is supported by a Vanier scholarship.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Selwan EM, Finicle BT, Kim SM, Edinger AL. Attacking the supply wagons to starve cancer cells to death. FEBS Lett. 2016;590:885–907. doi: 10.1002/1873-3468.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 3.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–71. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 4.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunology and cell biology. 2011;89:836–43. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 5.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–49. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–7. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, Thompson CB. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259–70. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–53. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson SM, Jonas O, Keibler MA, Hou HW, Luengo A, Mayers JR, et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat Med. 2017;23:235–41. doi: 10.1038/nm.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 11.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–52. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–67. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–60. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillon LM, Miller TW. Therapeutic targeting of cancers with loss of PTEN function. Current drug targets. 2014;15:65–79. doi: 10.2174/1389450114666140106100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phin S, Moore MW, Cotter PD. Genomic Rearrangements of PTEN in Prostate Cancer. Frontiers in oncology. 2013;3:240. doi: 10.3389/fonc.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SM, Roy SG, Chen B, Nguyen TM, McMonigle RJ, McCracken AN, et al. Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways. J Clin Invest. 2016;126:4088–102. doi: 10.1172/JCI87148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egami Y, Taguchi T, Maekawa M, Arai H, Araki N. Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Frontiers in physiology. 2014;5:374. doi: 10.3389/fphys.2014.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Commisso C, Flinn RJ, Bar-Sagi D. Determining the macropinocytic index of cells through a quantitative image-based assay. Nat Protoc. 2014;9:182–92. doi: 10.1038/nprot.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188:547–63. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews H, Ranson M, Kelso MJ. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int J Cancer. 2011;129:2051–61. doi: 10.1002/ijc.26156. [DOI] [PubMed] [Google Scholar]
- 23.Gekle M, Freudinger R, Mildenberger S. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol. 2001;531:619–29. doi: 10.1111/j.1469-7793.2001.0619h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–9. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondratowicz AS, Hunt CL, Davey RA, Cherry S, Maury WJ. AMP-activated protein kinase is required for the macropinocytic internalization of ebolavirus. J Virol. 2013;87:746–55. doi: 10.1128/JVI.01634-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS pathogens. 2010;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palm W, Araki J, King B, DeMatteo RG, Thompson CB. Critical role for PI3-kinase in regulating the use of proteins as an amino acid source. Proc Natl Acad Sci U S A. 2017;114:E8628–E36. doi: 10.1073/pnas.1712726114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–42. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae HB, Zmijewski JW, Deshane JS, Tadie JM, Chaplin DD, Takashima S, et al. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. Faseb J. 2011;25:4358–68. doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinde E, Digman MA, Hahn KM, Gratton E. Millisecond spatiotemporal dynamics of FRET biosensors by the pair correlation function and the phasor approach to FLIM. Proc Natl Acad Sci U S A. 2013;110:135–40. doi: 10.1073/pnas.1211882110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii M, Kawai K, Egami Y, Araki N. Dissecting the roles of Rac1 activation and deactivation in macropinocytosis using microscopic photo-manipulation. Scientific reports. 2013;3:2385. doi: 10.1038/srep02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 38.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan AS, Frigo DE. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nature reviews Urology. 2017;14:164–80. doi: 10.1038/nrurol.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Frontiers in physiology. 2014;5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gkogkou C, Frangia K, Saif MW, Trigidou R, Syrigos K. Necrosis and apoptotic index as prognostic factors in non-small cell lung carcinoma: a review. SpringerPlus. 2014;3:120. doi: 10.1186/2193-1801-3-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiraoka N, Ino Y, Sekine S, Tsuda H, Shimada K, Kosuge T, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer. 2010;103:1057–65. doi: 10.1038/sj.bjc.6605854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, et al. Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. Am J Clin Pathol. 2012;137:283–9. doi: 10.1309/AJCPLBK9L9KDYQZP. [DOI] [PubMed] [Google Scholar]
- 45.Ali TZ, Epstein JI. Basal cell carcinoma of the prostate: a clinicopathologic study of 29 cases. The American journal of surgical pathology. 2007;31:697–705. doi: 10.1097/01.pas.0000213395.42075.86. [DOI] [PubMed] [Google Scholar]
- 46.Magers M, Kunju LP, Wu A. Intraductal Carcinoma of the Prostate: Morphologic Features, Differential Diagnoses, Significance, and Reporting Practices. Archives of pathology & laboratory medicine. 2015;139:1234–41. doi: 10.5858/arpa.2015-0206-RA. [DOI] [PubMed] [Google Scholar]
- 47.Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2004;17:292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]
- 48.Caruso RA, Branca G, Fedele F, Irato E, Finocchiaro G, Parisi A, et al. Mechanisms of coagulative necrosis in malignant epithelial tumors (Review) Oncology letters. 2014;8:1397–402. doi: 10.3892/ol.2014.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DR, Oguin TH, Martinez J. The clearance of dying cells: table for two. Cell Death Differ. 2016;23:915–26. doi: 10.1038/cdd.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Daniels G, Lee P, Monaco ME. Lipid metabolism in prostate cancer. American journal of clinical and experimental urology. 2014;2:111–20. [PMC free article] [PubMed] [Google Scholar]
- 52.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaini RR, Sillerud LO, Zhaorigetu S, Hu CA. Autophagy regulates lipolysis and cell survival through lipid droplet degradation in androgen-sensitive prostate cancer cells. The Prostate. 2012;72:1412–22. doi: 10.1002/pros.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell. 2015;32:678–92. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chua CW, Shibata M, Lei M, Toivanen R, Barlow L, Shen M. Culture of mouse prostate organoids. Protoc Exch. 2014 doi: 10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–87. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Current oncology. 2010;17(Suppl 2):S72–9. doi: 10.3747/co.v17i0.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nussbaum N, George DJ, Abernethy AP, Dolan CM, Oestreicher N, Flanders S, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science. Prostate cancer and prostatic diseases. 2016;19:111–21. doi: 10.1038/pcan.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee YM, Lee JO, Jung JH, Kim JH, Park SH, Park JM, et al. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J Biol Chem. 2008;283:33969–74. doi: 10.1074/jbc.M804469200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–41. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito Y, Chapple RH, Lin A, Kitano A, Nakada D. AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell stem cell. 2015;17:585–96. doi: 10.1016/j.stem.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zadra G, Batista JL, Loda M. Dissecting the Dual Role of AMPK in Cancer: From Experimental to Human Studies. Molecular cancer research : MCR. 2015;13:1059–72. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–35. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–10. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–77. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suhalim JL, Chung CY, Lilledahl MB, Lim RS, Levi M, Tromberg BJ, et al. Characterization of cholesterol crystals in atherosclerotic plaques using stimulated Raman scattering and second-harmonic generation microscopy. Biophys J. 2012;102:1988–95. doi: 10.1016/j.bpj.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–91. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.