Abstract
Emerging evidence demonstrates that autophagy and apoptosis are interconnected and their interplay greatly affects cell death. However, the key regulators in this crosstalk remain elusive. Therefore, the role of N-terminal arginylated BiP (R-BiP)/Beclin-1/p62 complex was examined in the crosstalk between apoptosis and autophagy during combination chemotherapy with mitomycin C and bortezomib using immunoblot, immunoprecipitation, and cellular imaging assays in wild-type and genetically engineered colorectal cancer cells. In addition, the tumoricidal efficacy of the combinatorial treatment in a nude mouse tumor xenograft model of colorectal cancer was assessed. Bortezomib combined with mitomycin C synergistically induced cytotoxicity and apoptosis rather than autophagy. Mechanistically, this combination inactivated Akt and subsequently induced Beclin-1 (BECN1) dephosphorylation at Ser 234/295. Dephosphorylation of Beclin-1 resulted in increased cleavage of Beclin-1 and disruption of the R-BiP/Beclin-1/p62 complex, which led to switching autophagy to the synergistic induction of apoptosis. Importantly, the combination significantly suppressed LS174T intraperitoneal xenograft tumor growth, induced Akt inactivation and Beclin-1 cleavage, and decreased autophagy in vivo. Moreover, the tumoricidal efficacy of the combinatorial treatment was less effective, in vitro and in vivo, in HCT116 tumors harboring a Beclin-1 caspase 8 cleavage site mutant knock-in.
Keywords: apoptosis, autophagy, BiP, Beclin-1, p62, Akt
Introduction
Peritoneal carcinomatosis (PC) is regarded as the most common secondary cancerous disease to affect the peritoneal cavity after colorectal, appendiceal, ovarian, and gastric cancer, or diffuse malignant peritoneal mesothelioma [1, 2]. Current standard treatments involve cytoreductive surgery and perioperative chemotherapy [3]. However, PC is generally considered to be an untreatable condition, causing clinicians to relinquish further aggressive treatment. Therefore, improved regimens for PC patients are needed.
Mitomycin C is an alkylating chemotherapy agent that is widely used to treat PC [4, 5]. It causes DNA crosslinks in the cells and leads to the inhibition of DNA replication and transcription and the induction of apoptosis, predominantly through the mitochondria-dependent pathway [6–9]. Nevertheless, mitomycin C alone is not potent enough to generate sustained tumor regression and may also cause unexpected side effects [10, 11]. Therefore, we and other researchers have been investigating the development of multidrug therapies to promote enhanced cytotoxicity or reduce side-effects [12–15]. After screening synergistic drug combinations for colorectal cancer treatment, we succeeded in developing a combination treatment of mitomycin C and the proteasome inhibitor bortezomib for PC.
Bortezomib (PS-341; Velcade), a highly selective and reversible inhibitor of the 26S proteasome, is frequently used to treat multiple myeloma and mantle cell lymphoma [16, 17]. The ubiquitin-proteasome pathway is considered a biomarker, indicating a poor prognosis for peritoneal mesothelioma [18]; thus, it is reasonable to employ bortezomib to treat PC. Bortezomib has been well-documented to disrupt the equilibrium between protein biosynthesis and protein degradation, including the endoplasmic reticulum (ER)-associated degradation system. The accumulation of unfolded or misfolded proteins in the ER induces an unfolded protein response (UPR), which ultimately results in apoptosis and autophagy [19–23]. One major concern is that bortezomib resistance and the mechanisms involved are still being investigated [24, 25]. The upregulation of the ER chaperone binding immunoglobulin protein (BiP), a central regulator of ER, indicated poor cancer survival and might be related to bortezomib resistance [26–28]; however, the underlying mechanisms regarding the role of BiP in the crosstalk between apoptosis and autophagy are largely unknown.
In this study, we observed that bortezomib induced BiP arginylation through ATE1-encoded Arg-tRNA transferase. We and others previously reported that the most investigated crosstalk in the cell death and autophagy regulatory pathways is involved in the Beclin-1 network. Here, we found that Beclin-1 bound with R-BiP and p62, but not non-arginylated-BiP during treatment with bortezomib, which resulted in accumulated autophagy, thus dramatically limiting the tumoricidal efficacy of bortezomib. Strikingly, we found that the combinatorial treatment of mitomycin C and bortezomib dephosphorylation at Serine 234/295 by Akt inactivation induced Beclin-1 cleavage, disrupted Beclin-1/R-BiP/p62 complex formation, and switched autophagy to apoptosis in peritoneal or colorectal cancer in vitro and in vivo. Specifically, tumoricidal efficacy of combinatorial treatment was less effective in Beclin-1 caspase 8 cleavage site mutant knock-in (KI) HCT116 tumors in vitro and in vivo.
Materials and Methods
Cell cultures and transfection
Human colorectal carcinoma CX-1 cells were obtained from Dr. J.M. Jessup (National Institutes of Health, Bethesda, MD, USA) (29) in 1999 and no authentification was performed by the authors. The cells were cultured in RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA). Human colon cancer HCT116 and LS174T cells were purchased from American Tissue Type Culture Collection (ATCC) (Manassas, VA, USA) in 2014, and no authentification was performed by the authors. These cell lines were cultured in McCoy’s 5A medium or Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL) containing 10% FBS. ATE1+/+ and ATE1−/− mouse embryo fibroblasts (MEFs) were obtained from Dr. Y.T. Kwon (Seoul National University) in 2014, and no authentification was performed by the authors. They were maintained in DMEM medium with the addition of 55 μM β-mercaptoethanol (Invitrogen, Carlsbad, CA, USA). Cells were kept in a 37°C humidified incubator with 5% CO2. For transient transfection, cells were transfected with Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) and treated 48 h after transfection.
Reagents and antibodies
Bortezomib and mitomycin C were obtained from Santa Cruz (Dallas, TX, USA). Akt inhibitor (LY294002), S6K1 inhibitor (PF-4708671), and protease inhibitor cocktail were purchased from Sigma Aldrich (St. Louis, MO, USA). Flag-Beclin-1 constructs (wild-type (WT) and S234A/S295A (AA)) were provided by Dr. Beth Levine (University of Texas Southwestern Medical Center, Dallas, TX, USA). Anti-hemagglutinin (HA), anti-caspase 8, anti-caspase 9, anti-caspase 3, anti-phosphorylated Akt (S473)/Akt, anti-autophagy-related protein (ATG)7, anti-BiP, anti-activating transcription factor 4 (ATF4), anti-CCAAT-enhancer-binding protein homologous protein (CHOP), and anti-poly (ADP-ribose) polymerase (PARP) antibody were purchased from Cell Signaling (Danvers, MA, USA). Anti-microtubule-associated protein 1A/1B-light chain 3 (LC3) and anti-actin antibody were purchased from Sigma Aldrich. Anti-Beclin-1 and anti-cytochrome c antibody were purchased from BD PharMingen (San Jose, CA, USA). Phospho-Beclin-1 S234 and S295 antibodies were purchased from PhosphoSolutions (Aurora, CO, USA). Mouse monoclonal anti-ATE1, anti-green fluorescent protein (GFP), and anti-p62 came from Santa Cruz.
Production of anti-R-BiP antibody
Production of rabbit polyclonal anti-R-BiP antibody specific for the arginylated form of BiP was described previously (Cha-Molstad et al., 2015). In brief, the peptide sequence REEEDKKEDVGC (the last C was introduced to conjugate with keyhole limpet hemocyanin) corresponding to the N-terminal sequence of R-BiP was injected into rabbit and immunization was boosted with incomplete Freund’s adjuvant. IgG was purified using immobilized Protein A followed by two-step affinity chromatography. The specificity of anti-R-BiP antibody was confirmed using a peptide binding assay.
Plasmids, cloning, and stable transfection
Beclin-1 WT and S234A/S295A (AA) were sub-cloned into pENTER-D-TOPO (Invitrogen) with HA tag and sequenced for verification. The expression vector pLenti CMV/HA-Beclin-1 WT and pLenti CMV/HA-Beclin-1 AA were generated through LR recombination between pENTR/HA-Beclin-1 WT/AA and pLenti CMV Puro DEST (Addgene, Cambridge, MA, USA) with Gateway LR Clonase II enzyme mix (Invitrogen). Lentiviral particles were generated by co-transfecting 293T cells with pMD2.g (VSVG), pVSV-REV, PMDLg/pRRE, and pLenti Beclin-1 WT/AA and lentivirus containing supernatant was collected and passed through 0.45 μM filters to isolate the viral particles, in accordance with procedures optimized by the University of Pittsburgh Cancer Center (UPCI) Lentiviral Facility. After lentiviral transduction, cells were selected with puromycin (5 μg/ml, Invitrogen). The expression of the puromycin-resistant clones was then analyzed using western blotting.
pcDNA3.1-BiP-HA was also cloned. Cells were transfected with pcDNA3.1-BiP-HA or mock vector using Lipofectamine 2000 (Invitrogen). Cells were selected with 500 μg/ml G418 for four weeks and five clones were pooled.
Recombinant proteins and in vitro caspase cleavage assays
To generate glutathione-S-transferase (GST) fusion proteins, WT and mutant Beclin-1 cDNA fragments were sub-cloned into pGEX-4T1 vector (GE Healthcare). GST-fused Beclin-1 WT/AA was expressed in BL21 (DE3) E. coli cells by induction with 0.5 mM IPTG (isopropyl-β-D-1-thiogalactopyranoside) for 3 h at 37°C. GST fusion proteins were affinity purified with Glutathione-Sepharose resin (GE Healthcare, Waukesha, WI, USA) according to the manufacturer’s protocol. Protein samples were analyzed using gel electrophoresis and visualized by staining the gels with Gel Code Blue Stain Reagent (Pierce, Rockford, IL, USA).
1 μg Beclin-1 WT-GST and Beclin-1 AA-GST proteins were incubated with 100 ng active Akt1 (sigma) in 1X kinase buffer supplemented with ATP (Cell Signaling) for 30 min at 30°C followed by incubation with two units of caspase 8 (Millipore, Billerica, MA, USA) in the buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 10% sucrose, 1 mM EDTA, 0.1% CHAPS, and 10 mM DTT) at 37°C for 2 h. The reaction was stopped by adding 2× Laemmli sample buffer. Samples were heated at 95°C for 10 min and subsequently analyzed using gel electrophoresis, followed by western blotting.
Small interfering RNA (siRNA)
Cells were transfected with BiP siRNA and negative control siRNA (Santa Cruz) using Lipofectamine RNAi Max (Invitrogen) according to the manufacturer’s instructions.
MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assays
MTS studies were carried out using the Promega CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) [13].
Combination index (CI) analysis
CIs were calculated using the CompuSyn software program (ComboSyn, Inc., Paramus, NJ, USA). Briefly, CI values of 0.9-1.10 indicated additive effects, those between 0.9-0.85 indicated slight synergy, those in the range of 0.7-0.3 suggested moderate synergy, and those less than 0.3 indicated strong synergy.13
Annexin V binding
Cells were harvested using trypsinization, suspended in binding buffer (Annexin V-FITC Staining Kit, BD PharMingen, San Diego, CA, USA), stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI), and then analyzed by flow cytometry.
Immunoprecipitation
Briefly, cells were lysed in cell lysis buffer (Cell Signaling) with protease inhibitor cocktail. 0.3-1 mg of lysate was incubated with 1 μg of anti-HA/Beclin-1 antibody or rabbit/mouse IgG (Santa Cruz) at 4°C overnight; protein G PLUS-agarose beads (Santa Cruz) were added and rotated at 4°C for 1.5 h, followed by immunoblot analysis.
Immunoblot analysis
The protein content of the cells was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to nitrocellulose membrane, which was blocked with nonfat dry milk for 0.5 h and incubated with primary antibody at 4°C overnight. Horseradish peroxidase-conjugated anti-rabbit or mouse IgG was incubated at room temperature for 1 h and then visualized using the chemiluminescence protocol. Actin was used as a loading control.
Analysis of GFP-LC3 puncta formation
Stably-transduced HCT116/HCT116 Beclin-1 KI (DM; D133A/D146A), ATE1+/+, and ATE1−/− MEF cells expressing the GFP-LC3 gene were generated via infection with the pCT-Autophagosome-GFP lentivirus (UPCI Lentiviral Facility) and selected with puromycin. Cells were fixed in 2% paraformaldehyde for 10 min. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Cell Signaling), mounted, and visualized using an OlympusFluoview 1000 confocal microscope and the companion software FV10-ASW2.1 under a 63× oil immersion objective. GFP-LC3 puncta formation in HCT116 cells was quantified by counting at least 300 cells and plotted as mean ± SD of three independent experiments.
Animal model
Nude mice (six weeks old, Taconic, Germantown, NY, USA) were intraperitoneally (i.p.) challenged with 5×105 LS174T cells or HCT116 Beclin-1 wild-type (WT)/HCT116 Beclin-1 KI cells expressing firefly luciferase, which was stably transduced via lentiviral transfection of the pGL4 Luciferase Reporter Vector (Promega, Madison, WI, USA) and selected with puromycin. The luciferase signal was monitored by injecting the luciferase substrate luciferin (150 mg/kg, i.p.; GoldBio, St Louis, MO, USA) 5 min after anesthesia with 2% isoflurane prior to imaging on an IVIS200 system (Perkin Elmer, Waltham, MA, USA). Bioluminescence signal was quantified using the LivingImage software (Perkin Elmer). For LS174T xenografts, mice with similar levels of bioluminescence were divided into four groups (five per group) including sham, bortezomib (0.5mg/kg, i.p.) alone, mitomycin C (1.5 mg/kg, i.p.) alone, or bortezomib + mitomycin C combination. For HCT116 Beclin-1 WT/HCT116 Beclin-1 KI xenografts, mice with similar levels of bioluminescence were divided into four groups (five per group) including sham, bortezomib (0.5mg/kg, i.p.) + mitomycin C (1.5 mg/kg, i.p.) combination. Each group of mice was treated every other day. The tumor load was checked from week 1 to week 5 via the IVIS bioluminescent Imaging System. Mice were fed ad libitum and maintained in environments with a controlled temperature of 22–24°C and 12 h light and dark cycles. All animal experiments were carried out at the University of Pittsburgh in accordance with the Guide for the Care and Use of Laboratory Animals.
TUNEL assay
An in situ cell apoptosis detection kit (Trevigen, Gaithersburg, MD, USA) was used to detect apoptosis in paraffin-embedded tissues. An in situ cell death detection kit, TMR red (Roche, Indianapolis, USA) was used to detect apoptotic cell death in adherent cells. The staining was performed according to the manufacturer’s instructions. Briefly, sections of paraffin-embedded tissues were deparaffinized and permeabilized with proteinase K. Adherent cells were fixed before permeabilization. DNA strand breaks were end-labeled with terminal transferase and visualized using fluorescence microscopy.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Two group comparisons were performed with the Student’s t test. Comparisons among more than two groups were assessed using ANOVA with post hoc testing.
Results
The combination of mitomycin C and bortezomib synergistically induces cytotoxicity and apoptosis
To investigate the effect of the combination of mitomycin C and bortezomib on cell viability, human colorectal carcinoma HCT116, LS174T, and CX-1 cells were treated with different doses of mitomycin C and bortezomib for 24 h. MTS assay was used to determine cell viability. Figs. 1A, 1B, and 1C show the synergistic cytotoxic effect in the group that received the combination compared with the group that received single treatments (P <0.01). Confirmation was obtained by CI analysis as shown in Fig. 1D; there was strong or moderate synergy of mitomycin C in combination with bortezomib, especially in the low dose group in the three cell lines. In Fig. 1E, apoptotic cells detected by annexin V binding assay were observed in the lower right (early apoptosis) and upper right (late apoptosis) quadrant of each plot. Our data clearly show that treatment with mitomycin C and bortezomib enhanced the synergistic induction of apoptotic death. These synergistic effects were due to an increased activation of caspases and thus, the hallmark of apoptosis, PARP cleavage (Figs. 1F, 1G, and 1H).
Figure 1. The combination of mitomycin C and bortezomib synergistically induces cytotoxicity and apoptosis.

(A, B, C) HCT116 (A), LS174T (B), and CX-1 (C) cells were treated with mitomycin C and bortezomib for 24 h. Cell viability was analyzed using MTS assay. Error bars represent SD from triplicate experiments. Asterisk * or ** represents a statistically significant difference compared with the control (P <0.05 or P <0.01). # or ## represents a statistically significant difference compared with the single treatment (P <0.05 or P <0.01). (D) Combination index (CI) for mitomycin C and bortezomib calculated by CompuSyn software for HCT116, CX-1, and LS174T colon cancer cells. (E) LS174T cells were treated with mitomycin C and bortezomib for 24 h and cells were stained with fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI). Apoptosis was detected by flow cytometric assay. (F-H) After drug treatment, the cleavage of caspase 8, caspase 9, caspase 3, and PARP was detected with immunoblotting in LS174T (F), HCT116 (G), and CX-1 (H) cells. Actin was used to confirm an equal amount of proteins loaded in each lane.
Bortezomib induces ER stress and autophagy
Emerging evidence shows significant communication between apoptosis and autophagy. The UPR is a cellular stress response related to the ER, known as ER stress. ER stress has been reported to induce autophagy. Autophagy can promote cell survival by adapting cells to the stress conditions.
In the present study, we investigated the role of ER stress in the combination of mitomycin C and bortezomib-induced autophagy and apoptosis. We observed that the ER stress markers BiP, Chop, and ATF4 were increased with bortezomib (30 nM) alone and with the combination treatment in LS174T cells (Fig. 2A). Interestingly, the level of N-terminal arginylated BiP (R-BiP), which was detected by a specific anti-R-BiP antibody [30], was increased during treatment with bortezomib alone but decreased with the combination treatment. The hallmark of autophagy, microtubule-associated protein 1A/1B-light chain 3 (LC3)-II, increased dramatically in bortezomib-treated cells. Notably, a significant decrease of LC3-II was observed with the combination treatment compared with bortezomib treatment alone. We also observed in HCT116 cells that BiP, Chop, and ATF4 were increased with bortezomib (50 nM) alone as well as the combinatorial treatment. LC3-II was increased with bortezomib but decreased with the combination in HCT116 cells (Fig. 2B). We then examined GFP-LC3 puncta formation in HCT116 cells. We observed that LC3 puncta formation (Figs. 2C and 2D) significantly increased with bortezomib alone but decreased with the combination of mitomycin C and bortezomib. These results indicated that bortezomib alone induced ER stress and autophagy. The combinatorial treatment produced similar or decreased ER stress and decreased autophagy compared with bortezomib alone. To further investigate the effect of bortezomib on ER stress and autophagy, we treated LS174T and HCT116 cells with increased doses of bortezomib. We observed that PARP cleavage was not increased at the dose of 25-1000 nM and was dramatically enhanced only at a very high dose (5000 nM) (Figs. 2E and 2F), indicating that these two cell lines are resistant to bortezomib treatment. Both or at least one of the ER stress markers (Chop, ATF4, p-eIF2α, BiP, and R-BiP) and the autophagy marker (LC3-II) were increased at 25 nM bortezomib in both cell lines. The ubiquitin-binding protein p62 can function as a molecular adaptor between the autophagic machinery and its substrates. p62 is normally degraded in autophagy; however, the p62 level was increased by bortezomib as the degradation of p62 was specifically inhibited. Collectively, our data show that bortezomib alone induced ER stress and autophagy; the combinatorial treatment decreased autophagy.
Figure 2. Bortezomib induces ER stress and autophagy.

(A, B) LS174T (A) and HCT116 (B) cells were treated with mitomycin C and bortezomib for 24 h; BiP/R-BiP, Chop, ATF4, and LC3 were detected with immunoblotting. (C) After treatment, LC3 puncta formation was examined using confocal microscopy in HCT116 cells. (D) Quantification of GFP-LC3 puncta formations. Asterisk ** represents a statistically significant difference (P <0.01). (E, F) LS174T (E) and HCT116 (F) cells were treated with different doses (0-5000 nM) of bortezomib for 24 h, PARP, p-eIF-2α, p62, BiP, R-BiP, Chop, ATF4, and LC3 were detected using immunoblotting.
The role of BiP in the crosstalk between apoptosis and autophagy during the combinatorial treatment of mitomycin C and bortezomib
BiP, a well-studied ER chaperone also referred to as GRP78 or HSPA5, is critical in ER protein quality control and in ER-transmembrane signaling. In the present study, we investigated the role of BiP in bortezomib alone or in the combinatorial treatment of bortezomib and mitomycin C-induced apoptosis and autophagy. As shown in Figures 3A and 3B, knockdown (KD) of BiP increased combinatorial treatment-induced apoptosis (as measured by PARP cleavage) and decreased bortezomib-induced autophagosome formation (as measured by LC3 puncta formation) in HCT116 cells. These results suggest that BiP has anti-apoptotic properties and acts as an autophagy inducer.
Figure 3. The role of BiP in the crosstalk between apoptosis and autophagy during the combinatorial treatment of mitomycin C and bortezomib.

(A) HCT116 cells were transfected with control or BiP siRNA. After 48 h, cells were treated with mitomycin C and bortezomib for 24 h. PARP and LC3 were detected using immunoblotting. The levels of BiP were detected with immunoblotting 72 h after transfection. Actin was used as loading control. (B) HCT116-LC3-GFP cells were transfected with control or BiP siRNA and treated with 2 μM mitomycin C and 25 nM bortezomib for 24 h. LC3 puncta formation was examined using confocal microscopy. (C) HCT116-Beclin-1-HA cells were transfected with R-BiP-GFP or V-BiP GFP. After 48 h, cells were treated with bortezomib for 24 h and cell lysates were immunoprecipitated with anti-HA antibody or IgG and then immunoblotted with anti-GFP or anti-HA antibody (upper panels). The presence of GFP and HA in the lysates was examined (lower panels). (D) MEF ATE1+/+ and MEF ATE1−/− cells were treated with mitomycin C and bortezomib for 24 h; PARP and ATE1 were detected using immunoblotting. Actin was used to confirm an equal amount of proteins loaded in each lane. (E) MEF ATE1+/+-LC3-GFP and MEF ATE1−/−-LC3-GFP cells were treated with mitomycin C and bortezomib for 24 h and subjected to TUNEL assay. Cell nuclei were stained with DAPI. LC3 puncta formation was also examined using confocal microscopy. Representative images are shown (magnification ×1200). (F) MEF ATE1+/+ and MEF ATE1−/− cells were treated with mitomycin C and bortezomib for 24 h and cell lysates were immunoprecipitated with anti-Beclin-1 antibody or IgG and then immunoblotted with anti-Beclin-1, anti-p62, or anti-BiP antibody (upper panels). The presence of Beclin-1, p62 and BiP in the lysates was examined (lower panels). (G) HCT116-BiP-HA cells were treated with mitomycin C and bortezomib for 24 h and cell lysates were immunoprecipitated with anti-HA antibody or IgG and then immunoblotted with anti-Beclin-1, anti-p62, anti-R-BiP, or anti-HA antibody (upper panels). The presence of Beclin-1, BiP-HA, p62, LC3, and R-BiP in the lysates was examined (lower panels). (H) HCT116 shRNA scramble and HCT116 shRNA targeting of Atg7 cells were treated with mitomycin C and bortezomib for 24 h. PARP, ATG7, and LC3 were detected with immunoblotting.
Next, we sought to explore further mechanisms of how BiP is involved in autophagy during treatment with bortezomib alone or with the combination of bortezomib and mitomycin C. For this study, expression plasmid containing R-BiP-GFP (Arg-Glu19-BiP-GFP) or V-BiP-GFP (Val-Glu19-BiP-GFP) was transduced into Beclin-1-HA cDNA stably transfected HCT116 cells. Cells were treated with bortezomib and Beclin-1-HA was immunoprecipitated. Figure 3C clearly demonstrates that Beclin-1-HA bound with R (arginylated)-BiP-GFP, but not V (non-arginylated)-BiP-GFP. ATE1-encoded Arg-tRNA transferase is known to arginylate BiP (EEEDKKE →REEEDKKE of BiP) [30]. Moreover, we observed that ATE1−/− MEFs showed more sensitivity towards the treatment of mitomycin C and bortezomib in terms of PARP cleavage compared with ATE1+/+ MEFs, indicating that R-BiP has an anti-apoptotic function in the combination treatment (Fig. 3D). Figure 3E shows that ATE1−/− MEFs had more apoptotic cell death towards the treatment of mitomycin C and bortezomib. Interestingly, autophagosome formation was decreased (as measured by LC3 puncta formation) in the bortezomib-treated ATE1−/− MEFs. More importantly, we observed that BiP bound with Belcin-1 as well as p62 in the bortezomib treatment but not with p62 in the combination treatment in ATE1+/+ MEFs, and no binding was observed among BiP/Beclin-1/p62 in ATE1−/− MEFs (Fig. 3F). These observations were confirmed in BiP-HA cDNA stably transfected HCT116-BiP-HA cells. BiP bound with Belcin-1 and p62 in the bortezomib treatment, but not with p62 in the combinatorial treatment (Fig. 3G). Notably, p62 bound with R-BiP in the bortezomib treatment, but not in the combinatorial treatment, which might explain why autophagy was decreased in the combinatorial treatment (as measured by LC3). These results also highlight the role of arginylated BiP/Beclin-1/p62 in the combination-induced apoptosis and autophagy.
The classic autophagy pathway is known to depend on markers such as Beclin-1 and ATG7. Figure 3H shows that consistently, the combinatorial treatment-induced apoptosis was enhanced in HCT116 ATG7 KD cells, suggesting that inhibition of autophagy increased apoptosis in the combination-induced apoptosis.
The role of Akt in the combinatorial treatment of mitomycin C and bortezomib-induced apoptosis
Next, we studied the possible upstream signaling of Beclin-1, the key molecule in the crosstalk between apoptosis and autophagy. We observed that phosphorylation of Akt and S6K (S6 kinase) were decreased in the combination of mitomycin C and bortezomib; Beclin-1 phosphorylation at Ser 295 was decreased and Beclin-1 cleavage was increased (Fig. 4A). We then added a specific S6K inhibitor PF-4708671 or Akt inhibitor Ly294002 in the absence or presence of mitomycin C and bortezomib. Figure 4B shows that the combination-induced apoptosis as well as Beclin-1 cleavage were significantly enhanced even with mitomycin C or bortezomib alone by the addition of Akt inhibitor, but not S6K inhibitor, which clearly indicates that Akt played an important role in the combination-induced apoptosis. Similar results were obtained in HCT116 and CX-1 cells (Figs. 4C and 4D). We also infected LS174T cells with adenovirus expressing Akt-WT-HA and the constitutively active form of myr-Akt-HA. We observed that the constitutively active form of Akt abrogated the combination-induced apoptosis with Akt phosphorylation and Beclin-1 phosphorylation at 295 (Fig. 4E). Notably, Beclin-1 cleavage was also blocked with the active form of Akt. These results implied that Akt might be upstream of Beclin-1 and contribute to Beclin-1 cleavage. Interestingly, we also observed that Akt inactivation was actually due to Akt cleavage (band is around 40 kD). Using the caspases pan-inhibitor Z-Vad-FMK, we observed abrogation of Akt cleavage, Akt inactivation, Beclin-1 cleavage, and PARP cleavage in the combination treatment (Fig. 4F). These results suggest that Akt inactivation dramatically enhances the apoptosis of the combination treatment and also indicates correlations between Akt and Beclin-1 in the combinatorial treatment-induced apoptosis.
Figure 4. The role of Akt in mitomycin C and bortezomib-induced apoptosis.

(A) LS174T cells were treated with mitomycin C and bortezomib at the indicated doses for 24 h; p-Akt/Akt, p-S6K/S6K, and p-Beclin-1(S295)/Beclin-1 were detected with immunoblotting. (B) LS174T cells were pretreated with 10 μM S6K inhibitor PF-4708671 or Akt inhibitor Ly294002 for 30 min followed by mitomycin C and/or bortezomib for 24 h. PARP, p-Akt/Akt and Beclin-1 were detected using immunoblotting. (C, D) HCT116 (C) and CX-1 (D) were treated with mitomycin C and bortezomib at the indicated doses for 24 h; PARP, p-Akt/Akt, and p-Beclin-1(S295)/Beclin-1 were detected with immunoblotting. (E) LS174T cells were infected with adenoviral vector containing HA-tagged wild-type Akt (Ad-Akt-WT) or constitutively active Akt (Ad-Akt-myr). After 48 h, cells were treated with mitomycin C and bortezomib for 24 h. PARP, p-Akt, HA-Akt, pBeclin-1 (S295/S234), and Beclin-1 were detected with immunoblotting. (F) HCT116 cells were pretreated with 10 μM pan-caspases inhibitor Z-VAD-FMK for 30 min, followed by mitomycin C and/or bortezomib for 24 h. PARP, caspase 3, p-Akt/Akt, and Beclin-1 were detected by immunoblotting. Actin was used to confirm an equal amount of proteins loaded in each lane.
Akt inactivation induced Beclin-1 dephosphorylation and dissociation from R-BiP and p62, as well as the switch from autophagy to apoptosis
To further investigate the relationship between Akt and Beclin-1 and their role in the crosstalk between apoptosis and autophagy, we investigated the kinetics of the combination-induced apoptosis and autophagy in LS174T and CX-1 cells (Figs. 5A and 5B). We observed that Akt cleavage/inactivation, Beclin-1 dephosphorylation and cleavage, and PARP cleavage started at 16 h, while LC3-II (autophagy) was increased during 6-12 h, reached a maximum at 16 h, and then decreased, indicating autophagy may switch to apoptosis through Akt/Beclin-1. Cleavage of Beclin-1 was accompanied by caspase activation. We observed that endogenous Beclin-1, Akt, and caspase 8 bound with each other; then Akt dissociated from the complex during the combinatorial treatment (Fig. 5C).
Figure 5. Inactivation of Akt induces Beclin-1 dephosphorylation and dissociation from R-BiP and p62, and the switch from autophagy to apoptosis.

(A) LS174T cells were treated with mitomycin C (2 μg/ml) and bortezomib (30 nM) for various times (3-44 h). PARP, p-Akt/Akt, p-Beclin-1(S295)/Beclin-1, and LC3 were detected with immunoblotting. (B) CX-1 cells were treated with mitomycin C (5 μg/ml) and bortezomib (50 nM) for various times (3-44 h). PARP, p-Akt/Akt, p-Beclin-1(S295)/Beclin-1, and LC3 were detected with immunoblotting. (C) HCT116 cells were treated with mitomycin C and bortezomib for 24 h and cell lysates were immunoprecipitated with anti-Beclin-1 antibody or IgG and then immunoblotted with anti-caspase-8, anti-Akt, or anti-Beclin-1 antibody (upper panels). The presence of caspase-8, Akt, and Beclin-1 in the lysates was examined (lower panels). (D) HCT116 cells stably expressed with the vector control (GFP), HA-Beclin-1 WT (Beclin-1-WT), or HA-Beclin-1 S234/295A (Beclin-1 AA) were treated with mitomycin C and bortezomib for 24 h. PARP, HA-Beclin-1, and LC3 were detected using immunoblotting. (E) Left panel: SDS-PAGE analysis of recombinant proteins. Right panel: in vitro translated WT and mutant Beclin-1 proteins were incubated with Akt at 30°C for 30 min and then incubated with active caspase 8 at 37°C for 2 h, and then analyzed for p-Beclin-1(S295)/Beclin-1, and p-Akt with western blotting. (F) HCT116 cells stably expressed with the vector control, HA-Beclin-1 WT, and HA-Beclin-1 S234/295A were treated with mitomycin C and bortezomib for 24 h. Cell lysates were immunoprecipitated with anti-HA antibody or IgG and then immunoblotted with anti-caspase-8/3, anti-Akt, or anti-HA antibody (upper panels). The presence of caspase-8/3, Akt, and HA-Beclin-1 in the lysates was examined (lower panels). (G) HCT116 cells stably expressing HA-Beclin-1 WT or HA-Beclin-1 S234/295A were treated with bortezomib for 24 h. Cell lysates were immunoprecipitated with anti-HA antibody or IgG and then immunoblotted with anti-BiP, anti-R-BiP, anti-p62, or anti-HA antibody (upper panels). The presence of BiP, R-BiP, p62, HA-Beclin-1, and LC3 in the lysates was examined (lower panels). (H) Parental HCT116 (HCT116 Beclin-1 WT) and HCT116 Beclin-1 knock-in (KI) (double mutant; D133A/D146A) cells were treated with mitomycin C and bortezomib for 24 h and immunoblotted with anti-PARP, anti-Beclin-1, or anti-LC3 antibody. (I) HCT116-LC3-GFP and HCT116 Beclin-1 KI-LC3-GFP cells were treated with mitomycin C and bortezomib for 24 h and subjected to TUNEL assay. Cell nuclei were stained with DAPI. LC3 puncta formation was also examined using confocal microscopy. Representative images are shown (magnification ×600). (J) Parental HCT116 and HCT116 Beclin-1 KI cells were treated with mitomycin C and bortezomib for 24 h. Cell lysates were immunoprecipitated with anti-Beclin-1 antibody or IgG and then immunoblotted with anti-BiP, anti-p62, or anti-Beclin-1 antibody (upper panels). The presence of BiP, p62, Beclin-1, and LC3 in the lysates was examined (lower panels). Actin was used to confirm an equal amount of proteins loaded in each lane.
Next, we evaluated the effect of Beclin-1 phosphorylation at Ser 234/295 on its cleavage; we established stably expressing Beclin-1-WT and S234/295A (residues 234 and 295 serine were replaced by alanine), and treated these cells with mitomycin C and bortezomib for 24 h. As shown in Fig. 5D, Beclin-1 S234/295A enhanced the combination-induced PARP cleavage as well as the cleavage of HA-tagged Beclin-1. In addition, LC3II was decreased in Beclin-1 S234/295A compared with that in Beclin-1 WT. These results indicate that Beclin-1 phosphorylation at Ser 234/295 may inhibit its cleavage by the combinatorial treatment.
To confirm the role of Beclin-1 phosphorylation at Ser 234/295 on its cleavage, we generated and purified the GST fusion proteins Beclin-1 WT and Beclin-1 S234/295A. We performed in vitro Akt kinase assay, followed by caspase 8 cleavage assay as shown in Fig. 5E. We observed that caspase 8 was able to cleave both Beclin-1 WT and Beclin-1 S234/295A GST fusion proteins. However, Beclin-1 was resistant to cleavage by caspase 8 when Beclin was phosphorylated by active Akt. These results confirm our hypothesis that Beclin-1 phosphorylation at Ser 234/295 inhibits its cleavage by caspase 8 (Fig. 5E).
As shown in Fig. 5F, wild-type Beclin-1 (Beclin-1 WT), as well as its mutant type Beclin-1 S234/295A, bound with caspase 8, whereas binding with caspase 3 was hardly detected. Notably, the binding ability of caspase 8 with Beclin-1 S234/295A was slightly stronger than that with Beclin-1 WT. We also observed that the binding ability of Akt with Beclin-1 was decreased after treatment of mitomycin C and bortezomib in both WT Beclin-1 and the mutant type.
Next, we studied whether Beclin-1 phosphorylation at Ser 234/295 affects bortezomib-induced BiP/Beclin-1/p62 complex formation. HCT116-HA-Beclin-1 WT and HA-Beclin-1 S234/295A cells were treated with bortezomib and Beclin-1 was immunoprecipitated with anti-HA antibody. We observed that Beclin-1 WT bound with BiP and p62 in bortezomib treatment and that decreased binding among Beclin-1 S234/295A, BiP, and p62 reduced autophagy (LC3-II) (Fig. 5G). We also observed an interaction between R-BiP, p62, and Beclin-1 during treatment with bortezomib and a decrease in R-BiP/p62/Beclin-1 complex formation in Beclin-1 S234/295A cells. Since Beclin-1 bound with only R-BiP (Fig. 3C), these results suggest that the status of Beclin-1 phosphorylation at Ser 234/295 plays an important role in the formation of R-BiP/Beclin-1/p62 complex, which induces autophagy.
It is known that D133 and D146 of Beclin-1 are cleaved by caspase 8 during apoptosis. The HCT116 Beclin-1 knock-in (KI) cell line with cleavage-site mutant was generated [31]. Figure 5H shows that the combination treatment-induced Beclin-1 cleavage was eliminated in Beclin-1 KI cells, suppressing mitomycin C and bortezomib-induced apoptosis. Figure 5I shows that HCT116 Beclin-1 KI cells had less apoptotic cell death after treatment with mitomycin C and bortezomib, with increased autophagosome formation (as measured by LC3 puncta formation) compared with that of HCT116 Beclin-1 WT cells.
Beclin-1 was then immunoprecipitated; increased binding between Beclin-1 KI and p62 were observed with bortezomib alone and with the combinatorial treatment compared with that of Beclin-1 WT (Fig. 5J). These indicate that the non-cleaved form of Beclin-1 promotes the formation of BiP/Beclin-1/p62 complex, which will induce autophagy.
The combination of mitomycin C and bortezomib induced apoptosis, Akt inactivation, and Beclin-1 cleavage and decreased autophagy in vivo
Next, we performed in vivo studies to assess the effect of the combinatorial treatment with mitomycin C and bortezomib on LS174T intraperitoneal tumor growth. Mice were treated with mitomycin C and bortezomib every other day at doses of 0.5 mg/kg. We did not observe autoimmune signs such as loss of weight (Fig. 6A) or hair, lymphopenia, or other toxicities during the observation period. Figures 6B and 6C show that administration of bortezomib significantly decreased tumor growth compared to the control group (P < 0.01). Mitomycin C alone caused a decrease in tumor growth (P < 0.01). Of note, the combinatorial treatment with mitomycin C and bortezomib was more effective at inhibiting tumor growth compared with the single groups (P < 0.01).
Figure 6. The combination of mitomycin C and bortezomib induces apoptosis, Akt inactivation, and Beclin-1 cleavage and decreases autophagy in vivo.
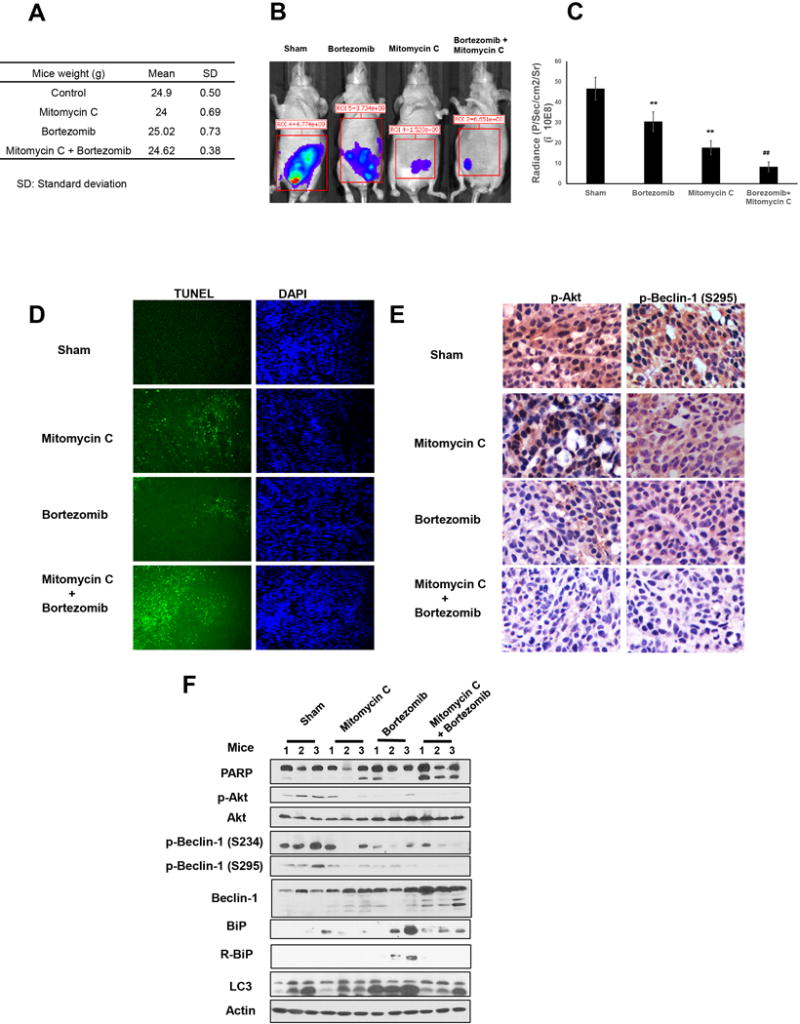
(A) Nude mice were intraperitoneally inoculated with 5×105 LS174T-luc cells/mouse on day 0. Four days after tumor inoculation, all the tumor-bearing mice (five mice/group) were treated with either PBS alone (sham), bortezomib alone at a dose of (0.5 mg/kg body weight), mitomycin C (1.5 mg/kg body weight) alone, or a combination of mitomycin C and bortezomib every other day from day 4 to day 28. Body weight of the mice in each group was measured on day 28. (B) Mice were imaged using the IVIS Imaging System Series 200. (C) Histogram illustrating the luciferase activity (photons/second) in LS174T tumor-bearing mice (five mice/group) treated with PBS alone, bortezomib alone, mitomycin C alone or a combination of bortezomib with mitomycin C on day 28. ** represents a statistically significant difference compared with the control group (P <0.01). ## represents a statistically significant difference compared with the single treatment group (P <0.01). (D) Tumor tissues were harvested at day 28 and subjected to TUNEL assay. Cell nuclei were stained with DAPI. Representative images are shown (magnification ×100). (E) Tumor tissues were harvested at day 28 and subjected to immunohistochemistry staining with anti-Akt and p-Beclin-1 primary antibodies. Representative images are shown (magnification ×400). (F) Tumor tissues were harvested at day 28. PARP, p-Akt/Akt, p-Beclin-1(S234, S295)/Beclin-1, R-BiP/BiP, and LC3 were detected with immunoblotting from three mice from each treatment group.
TUNEL assay showed more apoptotic death in tumor tissues in the combinatorial treatment group compared with other groups, as shown in Fig. 6D. We also observed a decrease in p-Akt and p-Beclin-1 levels with the combinatorial treatment of mitomycin C and bortezomib (Fig. 6E). We also observed that PARP cleavage and Beclin-1 cleavage were significantly increased, while the level of p-Akt and p-Beclin-1(234, 295) were decreased in the tumor tissue with the combination treatment (Fig. 6F). Notably, expression of BiP was decreased, especially R-BiP and LC3-II, with bortezomib treatment. R-BiP and LC3-II were decreased with the combinatorial treatment compared with treatment with bortezomib alone.
Next, we utilized the HCT116 Beclin-1 KI cells in vivo to see if combinatorial treatment was less effective in tumoricidal efficacy. Figures 7A and 7B show that Beclin-1 KI tumors were significantly less responsive to the combinatorial treatment with mitomycin C and bortezomib treatment compared with WT tumors (P < 0.05). TUNEL assay showed less apoptotic death with the combinatorial treatment in Beclin-1 KI tumor tissues compared with WT tumors (Fig. 7C), even though similar levels of p-Akt and p-Beclin-1 were observed in both groups (Fig. 7D).
Figure 7. Comparison of tumoricidal efficacy of mitomycin C and bortezomib on Beclin-1 WT and Beclin-1 KI.

Nude mice were intraperitoneally inoculated with 5×105 HCT116 Beclin-1 WT-luc or HCT116 Beclin-1 KI-luc cells/mouse on day 0. One week after tumor inoculation, all the tumor-bearing mice (five mice/group) were treated with either PBS alone (sham) or a combination of bortezomib (0.5 mg/kg body weight) and mitomycin C (1.5 mg/kg body weight) every other day from week 1 to week 4. (A) Mice were imaged using the IVIS Imaging System Series 200 at week 5. (B) Luciferase activity (photons/second) in each group was measured up to week 5 and plotted. ** represents a statistically significant difference compared with the sham group (P <0.01). # represents a statistically significant difference compared with Beclin-1 WT vs. Beclin-1 KI group (P <0.05). (C) Tumor tissues were harvested at week 5 and subjected to TUNEL assay. Cell nuclei were stained with DAPI. Representative images are shown (magnification ×100). (D) Tumor tissues were harvested at week 5 and subjected to immunohistochemistry staining with anti-Akt and p-Beclin-1 primary antibodies. Representative images are shown (magnification ×400). (E) A schematic diagram of the role of R-BiP/Beclin-1/p62 and Akt in the crosstalk between apoptosis and autophagy.
Finally, we summarized our observations in Fig. 7E. Bortezomib treatment caused ER stress and formed R-BiP/Beclin-1/p62 complex, which induced autophagy. The combination of mitomycin C and bortezomib induced activation of caspases, which inactivated Akt and mediated Beclin-1 dephosphorylation at 234/295, resulting in Beclin-1 cleavage and causing dissociation of the R-BiP/Beclin-1/p62 complex and the switch from autophagy to apoptosis.
Discussion
The mechanisms of ER stress, autophagy, and apoptosis are of fundamental importance to the development of cancer therapy. However, the link between ER stress, autophagy, and apoptosis is not well understood. Previously, we observed that the combinatorial treatment of sublethal chemotherapy agents with autophagy inducers shift autophagy to apoptosis through cleavage of the key molecule Beclin-1 [13, 15]. In the present study, we demonstrated for the first time that the induction of apoptosis by the combination of mitomycin C and bortezomib is associated with the disruption of the R-BiP/Beclin-1/p62 complex through inactivating Akt, leading to a switch from autophagy to a synergistic induction of apoptosis.
ER stress is activated in response to an accumulation of unfolded or misfolded proteins in the ER to restore normal cell function by inhibiting protein translation, degrading misfolded proteins, activating the signaling pathway, and increasing the production of molecular chaperones such as BiP [26, 32–34]. Under non-stressed conditions, ER-stress sensors such as PERK, IRE1, and ATF6 interact with BiP to form an inactive complex, while under ER stress conditions, BiP dissociates from these sensors and misfolded proteins form [35]. Knockdown BiP sensitized the combination of mitomycin C and bortezomib-induced apoptosis and decreased autophagosome formation, indicating that BiP acted as an anti-apoptotic protein and an autophagy inducer (Fig. 3A and 3B), which agrees with previous reports [34, 36]. But the detailed mechanisms of how the ER chaperone BiP inhibits apoptosis and induces autophagy are poorly understood. We reported here a novel function of BiP in the crosstalk between apoptosis and autophagy through the arginylated BiP/Beclin-1/p62 complex.
Autophagy is an evolutionary-conserved cell survival process for the degradation of long-lived proteins that can prevent the accumulation of unfolded and aggregated proteins, damaged organelles, and protein aggregates [37]. Our colleagues showed that ATE1-encoded Arg-transfer RNA transferase (R-transferase) of the N-end rule pathway mediates N-terminal arginylation of multiple ER-residing chaperones, leading to their cytosolic relocalization and turnover. Among them, R-BiP binds with the mammalian protein p62, which functions as a cargo receptor or adaptor for the autophagic degradation of ubiquitinated substrates, regulating non-ER processes [30, 38–40]. In this study, we observed that N-terminal arginylation of BiP occurs by ATE1-encoded Arg-tRNA transferase, in particular in the bortezomib treatment (Figs. 2A and 2B). Our data show that Beclin-1 bound only with R-BiP, but not V (non-arginylated)-BiP, with the treatment of bortezomib (Fig. 3C). ATE1-deficient MEFs enhanced the combination-induced apoptosis along with the cancellation of autophagosome formation, indicating that R-BiP but not V-BiP acts as an anti-apoptotic protein and an autophagy inducer (Figs. 3D, 3E, and 3F).
Whether autophagy plays a pro-survival or pro-death role depends on the type and extent of the stress, cell context, etc [41, 42]. In this study, the fact that the combination of mitomycin C and bortezomib treatment-induced apoptosis was enhanced in ATG7 knockdown cells indicates that autophagy plays a pro-survival role in this combination treatment (Fig. 3H). We also observed that Beclin-1 binds with R-BiP/P62 and is crucial for the function of the complex R-BiP/Beclin-1/P62 regarding the process of autophagy. Strikingly, the R-BiP/Beclin-1/p62 complex was disrupted by the combination of mitomycin C and bortezomib (Fig. 3E), which might explain why autophagosome formation was increased with bortezomib treatment but decreased with the combination treatment (Figs. 2C and 2D). These findings suggest that it may be possible to overcome the prosurvival pathway of autophagy through switching and “pushing” autophagic tumor cells into apoptosis by dissociating the R-BiP/Beclin-1/p62 complex.
Beclin-1 (also termed ATG6 in yeast) has long been considered a key player in autophagy through the activation of class III phosphatidylinositol-3 kinase (PI3K) complex [43, 44]. In addition to binding to core components or regulators of PI3K complex (e.g., phosphatidylinositol 3-kinase catalytic subunit type 3 [PIK3C3, also known as VPS34 in yeast], ATG14L, and UV radiation resistance associated [UVRAG]), BECN1 can interact with additional protein partners to regulate various cell processes (e.g., endocytosis and phagocytosis) [45, 46]. It is widely reported that the crosstalk between apoptosis and autophagy is associated with Beclin-1 cleavage [31, 44, 47–49], but how Beclin-1 cleavage is regulated in different settings remains elusive.
It is well documented that Beclin-1 post-translation modification sites are Thr 119 [50], Ser 14 [51], Ser 93/96 [13, 52], Thr388 [53], and Ser 234/295 [54]. Akt was reported to mediate the phosphorylation of Beclin-1 function in oncogenesis [54]. We observed that the Akt inhibitor Ly294002 sensitized mitomycin C- or bortezomib-induced apoptosis (Fig. 4B), whereas the Akt constitutively active form myr-Akt-HA abrogated the combination-induced apoptosis (Fig. 4E). We also observed that Akt inactivation in the combination treatment was due to Akt cleavage (Fig. 4F). We also reported that dephosphorylation of Beclin-1 at Ser 234 and 295 by inactivation of Akt enhanced the combination-induced apoptosis and Beclin-1 cleavage (Figs. 5D and 5E). These results highlight the role of Akt/Beclin-1 phosphorylation/Beclin-1 cleavage in the combination treatment-induced apoptosis. Consistently, the HCT116 Beclin-1 KI (D133A/D146A) cell line, which is resistant to cleavage by caspase 8, suppressed mitomycin C and bortezomib-induced apoptosis, increased autophagy, and increased the binding affinity among BiP/Beclin-1 KI/p62 in the bortezomib and the combinatorial treatments (Figs. 5H, 5I and 5J). Notably, no difference was found in the affinity of Beclin-1 WT or Beclin-1 S234/295A binding with caspase 8 or Akt (Figs. 5C and 5F). But the complex BiP/Beclin-1 S234/295A/p62 interaction was weaker compared with the complex BiP/Beclin-1 WT/p62, indicating that phosphorylation of Beclin-1 at Ser 234/295 by Akt is important for the formation of BiP/Beclin-1/p62 complex in the bortezomib treatment (Fig. 5G).
It is well known that the ER stress response mediated by the PERK-eIF2α-ATF4 pathway is involved in the regulation of the expression of several target genes such as CHOP [55]. Previous studies show that CHOP binds to the promoter of the pro-apoptotic protein PUMA (p53 upregulated modulator of apoptosis) during ER stress and induces PUMA expression [56, 57]. CHOP also induces several other pro-apoptotic proteins such as GADD34 (growth arrest and DNA damage-inducible protein), ERO1α (endoplasmic reticulum, oxidoreductin-1α), Bim (Bcl-2-like protein 11), and NOXA (Latin for damage) [58]. It is possible that synergistic interaction between bortezomib and mitomycin C is mediated through ER stress signal-induced these pro-apoptotic proteins. This possibility needs to be clarified in the near future.
In summary, we investigated the effects of mitomycin C and bortezomib at physiologic doses on peritoneal/colon cancer cells in vitro and tumor xenografts in vivo. We present here that novel mechanisms, autophagy and apoptosis, are linked to ER stress. We defined here a regulatory signaling pathway mediated by R-BiP/Beclin-1/p62 complex that positively controls autophagy. The Akt-Beclin-1-(R-BiP/Beclin-1/p62) pathway represents a potential target for drug development in the treatment of PC. Mitomycin C and bortezomib are commonly used drugs that are approved by the U.S. Food and Drug Administration and could be considered a treatment option for PC patients. R-Bip expression may also be used to select patients that would benefit from the addition of mitomycin C to bortezomib.
Implications.
This study uncovers that the R-BiP/Beclin-1/p62 complex has an important role in the crosstalk between apoptosis and autophagy. The results also propose how mono drug resistance can be overcome using potent combinations to improve anticancer therapy.
Acknowledgments
We thank Christine Heiner (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript. This work was supported by the following grants: NCI grant CA205267 (to Y.J. Lee), CA212125 (to Y.J. Lee), National Research Foundation of Korea Program NRF-2013R1A2A2A01014170 (to Y.T. Kwon), NRF-2016R1A2B3011389 (to Y.T. Kwon) and Nobel Laureates Invitation Program of Seoul National University (to Y.T. Kwon). This project used the UPCI Core Facility and was supported in part by award P30CA047904 (to University of Pittsburgh Cancer Institute).
Abbreviations used in this paper
- ATF4
activating transcription factor 4
- ATG
autophagy-related protein
- BiP
binding immunoglobulin protein
- Chop
CCAAT-enhancer-binding protein homologous protein
- CI
combination index
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- HA
hemagglutinin
- i.p
intraperitoneally
- KD
knockdown
- KI
knock-in
- LC3
microtubule-associated protein 1A/1B-light chain 3
- MEFs
mouse embryonic fibroblasts
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PARP
poly (ADP-ribose) polymerase
- PI
propidium iodide
- PUMA
p53 upregulated modulator of apoptosis
- R-BiP
Arg-tRNA transferase arginylated BiP
- PC
peritoneal carcinomatosis
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- siRNA
small interfering RNA
- UPR
unfolded protein response
- WT
wild-type
Footnotes
Competing interests
The authors declare no competing financial interests.
Availability of data and materials
Please contact the corresponding author for all data requests.
Ethics approval and consent to participate
The research protocol was reviewed and approved by the University of Pittsburgh IACUC Committee.
References
- 1.Seidl C, Essler M. Radioimmunotherapy for peritoneal cancers. Immunotherapy. 2013;5:395–405. doi: 10.2217/imt.13.20. [DOI] [PubMed] [Google Scholar]
- 2.Knorr C, Reingruber B, Meyer T, Hohenberger W, Stremmel C. Peritoneal carcinomatosis of colorectal cancer: incidence, prognosis, and treatment modalities. Int J Colorectal Dis. 2004;19:181–7. doi: 10.1007/s00384-003-0524-x. [DOI] [PubMed] [Google Scholar]
- 3.Yan TD, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg. 2008;248:829–35. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PubMed] [Google Scholar]
- 4.Van der Speeten K, Stuart OA, Chang D, Mahteme H, Sugarbaker PH. Changes induced by surgical and clinical factors in the pharmacology of intraperitoneal mitomycin C in 145 patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2011;68:147–56. doi: 10.1007/s00280-010-1460-4. [DOI] [PubMed] [Google Scholar]
- 5.Vahrmeijer AL, van Dierendonck JH, Keizer HJ, Beijnen JH, Tollenaar RA, Pijl ME, et al. Increased local cytostatic drug exposure by isolated hepatic perfusion: a phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer. 2000;82:1539–46. doi: 10.1054/bjoc.2000.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer VN, Szybalski W. Mitomycins and Porfiromycin: Chemical Mechanism of Activation and Cross-Linking of DNA. Science. 1964;145:55–8. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- 7.Iyer VN, Szybalski W. A Molecular Mechanism of Mitomycin Action: Linking of Complementary DNA Strands. Proc Natl Acad Sci U S A. 1963;50:355–62. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirnia F, Schneider E, Betticher DC, Borner MM. Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and Fas-independent pathway. Cell Death Differ. 2002;9:905–14. doi: 10.1038/sj.cdd.4401062. [DOI] [PubMed] [Google Scholar]
- 9.Wu KY, Wang HZ, Hong SJ. Mechanism of mitomycin-induced apoptosis in cultured corneal endothelial cells. Mol Vis. 2008;14:1705–12. [PMC free article] [PubMed] [Google Scholar]
- 10.Thakur JS, Chauhan CG, Diwana VK, Chauhan DC, Thakur A. Extravasational side effects of cytotoxic drugs: A preventable catastrophe. Indian J Plast Surg. 2008;41:145–50. doi: 10.4103/0970-0358.44923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Kondoh K, et al. Survival time and prevention of side effects of intraperitoneal hyperthermic perfusion with mitomycin C combined with surgery for patients with advanced gastric cancer. Cancer Treat Res. 1996;81:169–76. doi: 10.1007/978-1-4613-1245-1_14. [DOI] [PubMed] [Google Scholar]
- 12.Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song X, Kim SY, Zhang L, Tang D, Bartlett DL, Kwon YT, et al. Role of AMP-activated protein kinase in cross-talk between apoptosis and autophagy in human colon cancer. Cell Death Dis. 2014;5:e1504. doi: 10.1038/cddis.2014.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X, Dilly AK, Kim SY, Choudry HA, Lee YJ. Rapamycin-enhanced mitomycin C-induced apoptotic death is mediated through the S6K1-Bad-Bak pathway in peritoneal carcinomatosis. Cell Death Dis. 2014;5:e1281. doi: 10.1038/cddis.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Song X, Zhang L, Bartlett DL, Lee YJ. Role of Bcl-xL/Beclin-1 in interplay between apoptosis and autophagy in oxaliplatin and bortezomib-induced cell death. Biochemical pharmacology. 2014;88:178–88. doi: 10.1016/j.bcp.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, et al. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci U S A. 2007;104:19488–93. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borczuk AC, Cappellini GC, Kim HK, Hesdorffer M, Taub RN, Powell CA. Molecular profiling of malignant peritoneal mesothelioma identifies the ubiquitin-proteasome pathway as a therapeutic target in poor prognosis tumors. Oncogene. 2007;26:610–7. doi: 10.1038/sj.onc.1209809. [DOI] [PubMed] [Google Scholar]
- 19.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–51. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawrocki ST, Carew JS, Dunner K, Jr, Boise LH, Chiao PJ, Huang P, et al. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res. 2005;65:11510–9. doi: 10.1158/0008-5472.CAN-05-2394. [DOI] [PubMed] [Google Scholar]
- 21.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 2008;68:9323–30. doi: 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mimura N, Hideshima T, Shimomura T, Suzuki R, Ohguchi H, Rizq O, et al. Selective and potent Akt inhibition triggers anti-myeloma activities and enhances fatal endoplasmic reticulum stress induced by proteasome inhibition. Cancer Res. 2014;74:4458–69. doi: 10.1158/0008-5472.CAN-13-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Rajkumar SV. Many facets of bortezomib resistance/susceptibility. Blood. 2008;112:2177–8. doi: 10.1182/blood-2008-07-167767. [DOI] [PubMed] [Google Scholar]
- 25.Lu S, Wang J. The resistance mechanisms of proteasome inhibitor bortezomib. Biomark Res. 2013;1:13. doi: 10.1186/2050-7771-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 28.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–16. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Galoforo SS, Battle P, Lee H, Corry PM, Jessup JM. Replicating adenoviral vector-mediated transfer of a heat-inducible double suicide gene for gene therapy. Cancer Gene Ther. 2001;8:397–404. doi: 10.1038/sj.cgt.7700310. [DOI] [PubMed] [Google Scholar]
- 30.Cha-Molstad H, Sung KS, Hwang J, Kim KA, Yu JE, Yoo YD, et al. Amino-terminal arginylation targets endoplasmic reticulum chaperone BiP for autophagy through p62 binding. Nat Cell Biol. 2015;17:917–29. doi: 10.1038/ncb3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011;71:3625–34. doi: 10.1158/0008-5472.CAN-10-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician’s perspective. J Hematol Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–71. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jian B, Hsieh CH, Chen J, Choudhry M, Bland K, Chaudry I, et al. Activation of endoplasmic reticulum stress response following trauma-hemorrhage. Biochim Biophys Acta. 2008;1782:621–6. doi: 10.1016/j.bbadis.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong JL, Corazzari M, Martin S, Pagliarini V, Falasca L, Hill DS, et al. Oncogenic B-RAF signaling in melanoma impairs the therapeutic advantage of autophagy inhibition. Clin Cancer Res. 2011;17:2216–26. doi: 10.1158/1078-0432.CCR-10-3003. [DOI] [PubMed] [Google Scholar]
- 37.Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB., 3rd Autophagy: regulation and role in development. Autophagy. 2013;9:951–72. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–90. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- 39.Ciuffa R, Lamark T, Tarafder AK, Guesdon A, Rybina S, Hagen WJ, et al. The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep. 2015;11:748–58. doi: 10.1016/j.celrep.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 40.Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2:397–413. [PMC free article] [PubMed] [Google Scholar]
- 41.Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, Vandenabeele P. Autophagy: for better or for worse. Cell Res. 2012;22:43–61. doi: 10.1038/cr.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Kang R, Zeh HJ, 3rd, Lotze MT, Tang D. DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy. 2013;9:451–8. doi: 10.4161/auto.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–80. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–72. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–96. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 47.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang X, et al. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1:468–77. doi: 10.1007/s13238-010-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–92. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–50. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang D, Wang W, Sun X, Xu D, Wang C, Zhang Q, et al. AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy. 2016;12:1447–59. doi: 10.1080/15548627.2016.1185576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338:956–9. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem. 2008;283:35106–17. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh AP, Klocke BJ, Ballestas ME, Roth KA. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One. 2012;7:e39586. doi: 10.1371/journal.pone.0039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong SH, Lee DH, Lee YS, Jo MJ, Jeong YA, Kwon WT, et al. Molecular crosstalk between ferroptosis and apoptosis: Emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget. 2017;8:115164–78. doi: 10.18632/oncotarget.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim Biophys Acta. 2013;1833:3507–17. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]


