1. Introduction
Our recent coalescent estimate for the time of colonization of Devil's Hole by Cyprinodon pupfish challenged deeply held and widespread assumptions about this species based only on a poor fossil record and a geological calibration from the Middle East [1,2]. Our genome-wide analyses estimated a recent age for the Devil's Hole pupfish (DHP), Cyprinodon diabolis, within the past 105–830 years and frequent gene flow among Death Valley populations [1]. This estimate depends, in turn, on a good estimate of the pupfish mutation rate. We estimated this indirectly from the Cyprinodon substitution rate, and vertebrate mutation rates are known to vary over at least an order of magnitude [3,4]. However, our initial approach using a time-calibrated phylogenetic concatenated analysis cannot overestimate the rate by more than twofold relative to a gene tree analysis [5]. Furthermore, the unique natural history of DHP—including its miniscule population size, short lifespan, small adult size, high metabolic rate, high environmental temperature and high environmental stressors—all predict a higher mutation rate in this species. Except for their larger effective population sizes [6], this likely applies to Cyprinodon pupfishes in general [3–5,7]. We argue that DHP is a prime candidate for exhibiting one of the highest vertebrate mutation rates known [5] and should be further investigated using the gold standard of pedigree sequencing [8,9]. We further argue that a young age for this species does not decrease its conservation value but enhances it due to the evolution of such a unique life history and phenotype in a remarkably short time period.
Knott et al. (KEA) [10] do not dispute this young age for DHP, but rather the resulting divergence time estimates for Cyprinodon as a whole and its dispersal across North America in the past 25 thousand years (kya) [1]. These ages were based on our internal calibration of the stem age of the Laguna Chichancanab pupfish adaptive radiation to 8 kya, based on fossil evidence and isotopic analyses of multiple cores that indicated that the lake basin was dry prior to that time point [11]. One concern is that this very recent calibration is only appropriate for estimating recent divergent events on the same timescale [12]. Owing to the long-term effects of purifying selection and potentially other forces, the substitution rate over longer time periods (tens to hundreds of thousands of years) is almost certainly slower than the spontaneous mutation rate in each generation [13,14]. Thus, the age of Cyprinodon as a whole is almost certainly older than estimated by our time-calibrated phylogenetic analysis based on a recent geological event. This relationship should scale with effective population size as well as other demographic factors (e.g. population bottlenecks [6]) through time, so it is difficult to say exactly how much older Cyprinodon may be [15]. We also cannot rule out the possibility that the Chichancanab stem lineage is much older than the endemic basin in which it occurs. However, we originally argued that this is unlikely given the inability of these trophic specialist species to coexist with widespread native Yucatan species such as Astyanax fasciatus [1,16]. There are also no other isolated brackish lakes known from this region to provide a suitable habitat.
Instead of discussing these legitimate concerns, KEA take the position that all fish require a waterway to disperse, no matter the timescale. They assert that pupfish required a waterway in the last 25 kya connecting the Gulf of Mexico with the Great Basin of California to disperse this far inland. This was specifically questioned by Echelle [17] based on available phylogenetic evidence consistent with our recent study; geological hypotheses that no connections existed between these basins should also be examined (see Discussion below). The broader position that fish dispersal over land is impossible has been refuted countless times, beginning with Darwin, who conducted famous experiments on the ability of aquatic organisms (snails, seeds and plants) to be transported over land by bird vectors. For example, his most famous experiment involved dipping dried duck's feet into an aquarium containing aquatic snails and documenting that they survived out of water for up to 20 h. In the Origin, he concluded that ‘a duck or heron might fly at least 600 or 700 miles, and would be sure to alight on a pool or rivulet' [18,19]. He did not personally conduct experiments with fish eggs, but corresponded with Sir Humphry Davy who experimented with how long fertilized salmon eggs remained viable after exposure to air, and reported that even a small fish (char) could survive for 72 h barely covered with water [18]. Darwin considered this so important that he made sure it was published by the Royal Society [18,20]. He also collected observations of aquatic animals found on bird's feet in nature throughout his later career, resulting in his final publication [21]. Indeed, given the effort he and colleagues obviously expended on these studies, Darwin certainly would have been quite surprised by KEA's claim that Darwin ‘concluded' that aquatic organisms never dispersed long distances over land.
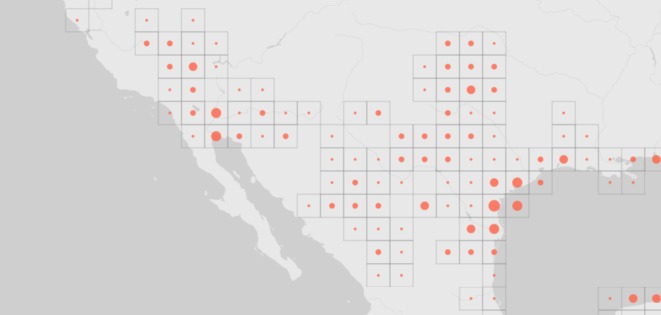
KEA's emphasis on the 3000 km distance from the Gulf of Mexico to Death Valley is misleading. The relevant distance is between drainage basins; it should be obvious that fish can swim up rivers because pupfishes have done this throughout their range (figure 1) [22]. For example, the distance between tributaries for one of the proposed routes from the Rio Grande to the Gila River basin is less than 130 km. These mountain passes lie within the flyways of numerous aquatic birds and well within Darwin's proposed range of 600 miles. Furthermore, pupfish eggs are 1–2 mm in diameter and are repeatedly deposited by the female in aquatic plants, algae mats or fine sand/silt [23–25]. They adhere to these plants or fine mud and are desiccation resistant, like those of most cyprinodontiform fishes, a group in which drought-resistant embryonic diapause has repeatedly evolved [26]. 25 kya is a long time; it is wondrous, though not difficult, for us to imagine rare millennial events such as a duck transporting a few eggs stuck to its webbed feet, which maintain a humid environment when tucked into its body during flight [18].
Figure 1.
Global Biological Information Facility data for Cyprinodon species occurrences within each grid throughout the North American southwest, from the Gulf of Mexico to Death Valley. Note the current widespread occurrences from the Rio Grande to the Gila River and Death Valley drainage basins.
The geological record of ancient inter-basin connections cannot completely account for the observed phylogenetic relationships among Western pupfishes. This is not a novel conclusion of our paper, but has been evident for some time. For example, in a detailed reanalysis of mtDNA sequence divergences, Echelle (pg. 28) noted that ‘there is a general paucity of geological support for the inter-basin connections inferred from this study. This indicates that dispersal across basin divides might have played a greater role than anticipated for the historical biogeography of the group' (emphasis added). The discrepancy is important because pupfishes ‘primarily occupy springs and low-gradient streams on valley floors,’ habitats that ‘are more likely to reflect geological history than are [those] of most other fish groups in the region’ [17]. Thus, ‘either our knowledge of (Neogene surface-water connections) is incomplete or pupfish dispersal across basin divides via small, relatively transient, surface-water connections have been more common than expected based on…habitats generally occupied by this group’ [17]. ‘Dispersal could have been facilitated by headwater stream captures or transient flow across basin divides during extreme rainfall episodes' [17, p. 34]. While Echelle did not consider overland dispersal, the potential explanation we propose, nothing in his analysis precludes this possibility. In any case, KEA to the contrary, there appears to be little argument that non-hydrographic factors likely have been involved in shaping the historical distribution of Western pupfishes.
There are, in fact, numerous examples of isolated water bodies colonized by fishes through long-distance dispersal over land, including nearly a hundred volcanic crater lake fish species flocks [27–34], all bolide craters [35] and thousands of endorheic lake basins (e.g. [22,36,37]). For example, 18% of the world's landmass drains into endorheic lake basins [38,39]. The most relevant of these is Devil's Hole itself, which was apparently never connected to the surrounding Amargosa River basin in its entire 60 kya history [40]. Similarly, Laguna Chichancanab is an endorheic basin which was never connected to other water bodies; this is also the case for the habitats of several other desert pupfish species [36,41]. We have also personally searched over 30 hypersaline endorheic lake basins across eight islands in the Bahamian archipelago and found pupfish in nearly every lake with salinities below 50 ppt (CH Martin, personal observations 2011, 2013, 2018; [42]). There is also strong circumstantial evidence of trans-oceanic dispersal by cichlid and cyprinodontiform fishes [42–46], which is perhaps more remarkable than over land dispersal across kilometres of desert. Finally, several alternative mechanisms of fish dispersal have been directly documented, including human introductions, stream capture events and water spouts [47]. Indeed, just a few months ago it rained fishes in Mexico [48]. We agree that fish primarily colonize lakes and rivers via waterways; our point is that there are rare exceptions to this rule.
Finally, we disagree with KEA's claim that a process must be directly observed by humans to be invoked as a potential explanation. Instead, we argue that long-distance dispersal of fishes and their eggs over land is plausible and has repeatedly occurred all over the world.
Footnotes
The accompanying comment can be viewed at http://dx.doi.org/10.1098/rspb.2017.1648.
Data accessibility
This study contains no associated data.
Authors' contributions
C.M. wrote the manuscript. B.J.T. revised and commented on it. Both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Martin CH, Crawford JE, Turner BJ, Simons LH. 2016. Diabolical survival in death valley: recent pupfish colonization, gene flow, and genetic assimilation in the smallest species range on earth. Proc. R. Soc. B 283, 20152334 ( 10.1098/rspb.2015.2334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed J, Stockwell C. 2014. Evaluating an icon of population persistence: the Devil's Hole pupfish. Proc. R. Soc. B 281, 1648–1658. ( 10.1098/rspb.2014.1648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch M. 2010. Evolution of the mutation rate. Trends Genet. 26, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromham L. 2009. Why do species vary in their rate of molecular evolution? Biol. Lett. 5, 401–404. ( 10.1098/rsbl.2009.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin C, Hohna S, Crawford J, Turner B, Richards E, Simons L. 2017. The complex effects of demographic history on the estimation of substitution rate: concatenated gene analysis results in no more than twofold overestimation. Proc. R. Soc. B 284, 20170537 ( 10.1098/rspb.2017.0537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGirr JA, Martin CH. 2016. Novel candidate genes underlying extreme trophic specialization in Caribbean pupfishes. Mol. Biol. Evol. 34, 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin C, Hohna S.. 2017. New evidence for the recent divergence of Devil's Hole pupfish and the plausibility of elevated mutation rates in endangered taxa. Mol. Ecol. 27, 831–838. [DOI] [PubMed] [Google Scholar]
- 8.Harris K. 2015. Evidence for recent, population-specific evolution of the human mutation rate. Proc. Natl Acad. Sci. USA 112, 3439–3444. ( 10.1073/pnas.1418652112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smeds L, Qvarnstrom A, Ellegren H. 2016. Direct estimate of the rate of germline mutation in a bird. Genome Res. 26, 1211–1218. ( 10.1101/gr.204669.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knott J, Phillips F, Eheis M, Sada D, Jayko A, Axen G. 2018. Geologic and hydrologic concerns about pupfish divergence during the last glacial maximum. Proc. R. Soc. B 285, 20171648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodell D, Curtis J, Brenner M. 1995. Possible role of climate in the collapse of Classic Maya civilization. Nature 375, 391–394. ( 10.1038/375391a0) [DOI] [Google Scholar]
- 12.Ho SYW, Phillips MJ. 2009. Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Syst. Biol. 58, 367–380. ( 10.1093/sysbio/syp035) [DOI] [PubMed] [Google Scholar]
- 13.Ho SYW, Phillips MJ, Cooper A, Drummond AJ. 2005. Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol. Biol. Evol. 22, 1561–1568. ( 10.1093/molbev/msi145) [DOI] [PubMed] [Google Scholar]
- 14.Ho SYW, Lanfear R, Bromham L, Phillips MJ, Soubrier J, Rodrigo AG, Cooper A. 2011. Time-dependent rates of molecular evolution. Mol. Ecol. 20, 3087–3101. ( 10.1111/j.1365-294X.2011.05178.x) [DOI] [PubMed] [Google Scholar]
- 15.Woodhams M. 2006. Can deleterious mutations explain the time dependency of molecular rate estimates? Mol. Biol. Evol. 23, 2271–2273. ( 10.1093/molbev/msl107) [DOI] [PubMed] [Google Scholar]
- 16.Strecker U. 2006. The impact of invasive fish on an endemic Cyprinodon species flock (Teleostei) from Laguna Chichancanab, Yucatan, Mexico. Ecol. Freshw. Fish 15, 408–418. ( 10.1111/j.1600-0633.2006.00159.x) [DOI] [Google Scholar]
- 17.Echelle A. 2008. The western North American pupfish clade (Cyprinodontidae: Cyprinodon): mitochondrial DNA divergence and drainage history. Geol. Soc. Am. Spec. Pap. 439, 27–38. [Google Scholar]
- 18.Costa J. 2017. Darwin's backyard: how small experiments led to a big theory. New York, NY: Norton and Company, Inc. [Google Scholar]
- 19.Darwin C. 1859. The origin of species by means of natural selection: or, the preservation of favored races in the struggle for life. London, UK: Murray. [Google Scholar]
- 20.Davy H. 1856. On the ova of salmon. Phil. Trans. R. Soc. Lond. B 146, 21–29. ( 10.1098/rstl.1856.0005) [DOI] [Google Scholar]
- 21.Darwin C. 1882. On the dispersal of freshwater bivalves. Nature 25, 529–530. ( 10.1038/025529f0) [DOI] [Google Scholar]
- 22.Martin CH, Wainwright PC. 2011. Trophic novelty is linked to exceptional rates of morphological diversification in two adaptive radiations of Cyprinodon pupfishes. Evolution 65, 2197–2212. ( 10.1111/j.1558-5646.2011.01294.x) [DOI] [PubMed] [Google Scholar]
- 23.Martin CH, Wainwright PC. 2013. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339, 208–211. ( 10.1126/science.1227710) [DOI] [PubMed] [Google Scholar]
- 24.Martin CH, Wainwright PC. 2013. A remarkable species flock of Cyprinodon pupfishes endemic to San Salvador Island, Bahamas. Bull. Peabody Museum Nat. Hist. 54, 231–240. ( 10.3374/014.054.0201) [DOI] [Google Scholar]
- 25.Martin CH. 2016. Context-dependence in complex adaptive landscapes: frequency and trait-dependent selection surfaces within an adaptive radiation of Caribbean pupfishes. Evolution 70, 1265–1282. [DOI] [PubMed] [Google Scholar]
- 26.Furness AI, Reznick DN, Springer MS, Meredith RW. 2015. Convergent evolution of alternative developmental trajectories associated with diapause in African and South American killifish. Proc. R. Soc. B 282, 20142189 ( 10.1098/rspb.2014.2189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin CH. 2012. Weak disruptive selection and incomplete phenotypic divergence in two classic examples of sympatric speciation: Cameroon crater lake cichlids. Am. Nat. 180, E90–E109. ( 10.1086/667586) [DOI] [PubMed] [Google Scholar]
- 28.Martin CH. 2013. Strong assortative mating by diet, color, size, and morphology but limited progress toward sympatric speciation in a classic example: Cameroon crater lake cichlids. Evolution 67, 2114–2123. [DOI] [PubMed] [Google Scholar]
- 29.Martin CH, Cutler JS, Friel JP, Dening T, Coop G, Wainwright PC. 2015. Complex histories of repeated colonization and hybridization cast doubt on the clearest examples of sympatric speciation in the wild. Evolution 69, 1406–1422. [DOI] [PubMed] [Google Scholar]
- 30.Kautt AF, Machado-Schiaffino G, Meyer A. 2016. Multispecies outcomes of sympatric speciation after admixture with the source population in two radiations of Nicaraguan Crater Lake Cichlids. PLoS Genet. 12, e1006157 ( 10.1371/journal.pgen.1006157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malinsky M, et al. 2015. Genomic islands of speciation separate cichlid ecomorphs in an East African crater lake. Science 350, 1493–1498. ( 10.1126/science.aac9927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner CE, Harmon LJ, Seehausen O. 2012. Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487, 366–370. [DOI] [PubMed] [Google Scholar]
- 33.Poelstra J, Richards E, Martin C. 2018. Speciation in sympatry with ongoing secondary gene flow and an olfactory trigger in a radiation of Cameroon cichlids. bioRxiv. ( 10.1101/229864) [DOI] [PubMed] [Google Scholar]
- 34.Richards E, Poelstra J, Martin C. 2017. Don't throw out the sympatric species with the crater lake water: fine-scale investigation of introgression provides weak support for functional role of secondary gene flow in one of the clearest examples of sympatric speciation. bioRxiv. ( 10.1101/217984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stager J, Alton K, Martin C, King D, Petruny L, Wiltse B, Livingstone D. 2017. On the age and origin of Lake Ejagham, Cameroon, and its endemic fishes. Quat. Res. 89, 1–12. [Google Scholar]
- 36.Humphries J, Miller RR. 1981. A remarkable species flock of pupfishes, genus Cyprinodon, from Yucatan, Mexico. Copeia 1981, 52–64. ( 10.2307/1444040) [DOI] [Google Scholar]
- 37.Kalk MJ, Schulten-Senden CM. 1977. Zooplankton in a tropical endorheic lake (Lake Chilwa, Malawi) during drying and recovery phases. J. Limnol. Soc. South. Africa 3, 1–7. ( 10.1080/03779688.1977.9632922) [DOI] [Google Scholar]
- 38.2008. Endorheic Lakes: Waterbodies that don't flow to the sea. United Nations Environ. Program.
- 39.Hammer U. 1986. Saline lake ecosystems of the world. Berlin, Germany: Springer. [Google Scholar]
- 40.Riggs A, Deacon J. 2002. Connectivity in desert aquatic ecosystems: the devils hole story. Spring-fed Wetl. Important Sci. Cult. Resour. Intermt. Reg. 11, 1–38. [Google Scholar]
- 41.Contreras-Balderas S, Almada-Villela P.. 1996. Cyprinodon alvarezi. The IUCN Red List of Threatened Species. See www.iucnredlist.org.
- 42.Martin CH. 2016. The cryptic origins of evolutionary novelty: 1000-fold faster trophic diversification rates without increased ecological opportunity or hybrid swarm. Evolution 70, 2504–2519. [DOI] [PubMed] [Google Scholar]
- 43.Friedman M, Keck BP, Dornburg A, Eytan RI, Martin CH, Hulsey CD, Wainwright PC, Near TJ. 2013. Molecular and fossil evidence place the origin of cichlid fishes long after Gondwanan rifting. Proc. R. Soc. B 280, 20131733 ( 10.1098/rspb.2013.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards E, Martin C. 2017. Adaptive introgression from distant Caribbean islands contributed to the diversification of a microendemic radiation of trophic specialist pupfishes. PLoS Genet. 13, e1006919 ( 10.1371/journal.pgen.1006919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin CH, Feinstein LC. 2014. Novel trophic niches drive variable progress towards ecological speciation within an adaptive radiation of pupfishes. Mol. Ecol. 23, 1846–1862. ( 10.1111/mec.12658) [DOI] [PubMed] [Google Scholar]
- 46.Haney RA, Turner BJ, Rand DM. 2009. A cryptic lineage within the pupfish Cyprinodon dearborni suggests multiple colonizations of South America. J. Fish Biol. 75, 1108–1114. ( 10.1111/j.1095-8649.2009.02378.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strange RM. 1998. Mitochondrial DNA variation in johnny darters (Pisces: Percidae) from eastern Kentucky supports stream capture for the origin of upper Cumberland river fishes. Am. Midl. Nat. 140, 96–102. ( 10.1674/0003-0031(1998)140%5B0096:MDVIJD%5D2.0.CO;2) [DOI] [Google Scholar]
- 48.2017. Fish fall from sky with rain in northern Mexico. CBC News., The Associated Press. See http://www.cbc.ca/news/world/its-raining-fish-1.4310791.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study contains no associated data.