Abstract
Increasing body size is accompanied by facial elongation across a number of mammalian taxa. This trend forms the basis of a proposed evolutionary rule, cranial evolutionary allometry (CREA). However, facial length has also been widely associated with the varying mechanical resistance of foods. Here, we combine geometric morphometrics and computational biomechanical analyses to determine whether evolutionary allometry or feeding ecology have been dominant influences on facial elongation across 16 species of kangaroos and relatives (Macropodiformes). We found no support for an allometric trend. Nor was craniofacial morphology strictly defined by dietary categories, but rather associated with a combination of the mechanical properties of vegetation types and cropping behaviours used to access them. Among species examined here, shorter muzzles coincided with known diets of tough, resistant plant tissues, accessed via active slicing by the anterior dentition. This morphology consistently resulted in increased mechanical efficiency and decreased bone deformation during incisor biting. Longer muzzles, by contrast, aligned with softer foods or feeding behaviours invoking cervical musculature that circumvent the need for hard biting. These findings point to a potential for craniofacial morphology to predict feeding ecology in macropodiforms, which may be useful for species management planning and for inferring palaeoecology.
Keywords: herbivory, finite-element analysis, geometric morphometrics, Macropodiformes, marsupials, macroevolution
1. Introduction
The mammalian skull has been shown to exhibit facial elongation with increasing body size for a range of taxa [1,2]. It has been proposed that this trend might be considered an evolutionary rule and has been named craniofacial evolutionary allometry (CREA) [2]. However, biomechanical principles also dictate that behaviours involving biting and the processing of foods are important predictors of mammalian craniofacial morphology [3,4]. A shorter, more robust muzzle (the projecting part of the face, including the nose and mouth) increases leverage during biting [5], and has accordingly been linked with an ability to access and process harder, more resistant foods across many mammalian lineages [5–7]. This relationship between the physical properties of preferred foods, their required feeding behaviours and associated craniofacial morphology might, therefore, be expected to transcend any morphological diversification defined by evolutionary allometry (the common growth relationships between shape and size maintained across species) [8]. A recent study of 12 macropodine kangaroos and wallabies supported CREA [2]. However, these findings were not considered in the context of feeding ecology. In the present study, we predicted that kangaroos and their relatives, a largely herbivorous group, would express craniofacial trends consistent with the diverse physical properties of consumed vegetation.
For herbivores, there is an inverse relationship between forage quality and the abundance of representative foods [9,10]. Smaller species will seek out nutrient-rich foods, such as fruits, invertebrates, fungi and fibrous or tuberous roots. These foods differ in physical properties, with plant roots, in particular, exhibiting greater resistance to breakage. Larger herbivores must consume more abundant plant matter, such as leaves and stems, to meet their metabolic demands. These more abundant foods are typically of lower quality [11,12] and can be broadly separated into two categories: graze, comprising the leaves and stems of monocot grasses; and browse, consisting of dicot leaves, stems and branches. These vegetation types also have differing physical properties that will influence their accessibility and resistance to mechanical breakdown [13,14]. In contrast to the largely homogeneous mechanical properties of grasses, browse vegetation is more heterogeneous in composition, often including woody, lignified tissues, which can be larger in diameter and more resistant to breakage [15–17].
Browsing herbivores have a shorter cranium and jaw, relative to grazing species, among both placental mammals and marsupials [18]. It is likely that a shorter muzzle is beneficial in the obtaining and processing of more resistant vegetation. Very few detailed biomechanical analyses regarding feeding have been conducted on mammalian herbivores to date; especially using advanced computational methods [19,20]. A recent comparison between the feeding biomechanics of four marsupial herbivores showed that the longer muzzle of the grazing red kangaroo (Macropus rufus) experienced greater stress and led to lower mechanical efficiency during biting simulations than that of the swamp wallaby (Wallabia bicolor); a mixed feeder with a relatively shorter muzzle [20]. This suggests that the biomechanical performance of biting behaviours may be linked with muzzle morphology in this group.
The Macropodiformes are a sub-order of herbivorous diprotodont marsupials that includes kangaroos and wallabies (Macropodidae), bettongs and potoroos (Potoroidae), and the musky rat-kangaroo (Hypsiprymnodontidae). The species range in size from less than 1 kg, to over 90 kg [21] and display a diverse range of diets. For this study, we analysed the cranial shape and biomechanical performance of 16 macropodiform species, from the smallest (musky rat-kangaroo), to the largest (red kangaroo), to identify the respective roles that allometry and feeding behaviours play in their cranial morphology. We hypothesized that, while allometric trends in cranial shape may play a part in the craniofacial form of some macropodiforms, feeding ecology has a more distinct influence on face length across this taxon.
2. Material and methods
(a). Shape analysis (geometric morphometrics)
Sixteen species of macropodiforms were sampled for three-dimensional coordinate data. The dataset included 236 intact crania from the Australian Museum, the Queensland Museum and the Natural History Museum of the University of New England. Landmarks were digitized on the crania using a G2X Microscribe (Immersion Corporation, San Jose, CA) by one researcher (DRM). Thirty-two landmarks were digitized at homologous locations on the cranium, including teeth, foramina and suture junctions [22] (electronic supplementary material, figure S1 and table S1).
Each species was categorized according to the dominant preferred vegetation type for each within its natural environment [23] (table 1). Allocations were via consensus of the literature [24] (provided in the electronic supplementary material).
Table 1.
List of species under study, with sample sizes and diet allocations.
| species | specimens | diet |
|---|---|---|
| Aepyprymnus rufescens | 15 | roots |
| Bettongia penicillata | 16 | fungi |
| Dendrolagus dorianus | 19 | browse |
| Dendrolagus lumholtzi | 16 | browse |
| Hypsiprymnodon moschatus | 7 | fruit |
| Lagorchestes hirsutus | 13 | mixed |
| Macropus dorsalis | 10 | graze |
| Macropus giganteus | 15 | graze |
| Macropus robustus | 15 | graze |
| Macropus rufogriseus | 15 | graze |
| Macropus rufus | 15 | graze |
| Onychogalea fraenata | 14 | mixed |
| Petrogale penicillata | 15 | mixed |
| Potorous tridactylus | 15 | fungi |
| Thylogale stigmatica | 21 | mixed |
| Wallabia bicolor | 15 | mixed |
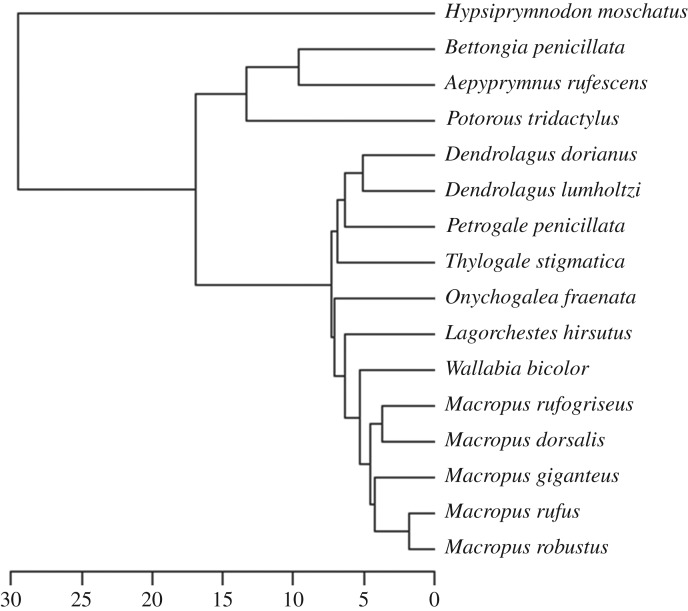
All analyses were conducted in R v. 3.2.5 [25] using the geomorph [26] and nlme [27] packages (details in electronic supplementary material). The data were subjected to a generalized Procrustes superimposition to remove non-shape variation [28]. As most landmarks were paired, the shape variables for the symmetric component of shape were extracted [29]. Centroid size was used as a proxy for body mass (BM) in the morphometric analyses, following a previous study on kangaroos and wallabies [2]. Firstly, we assessed the influence of evolutionary allometry and diet, while accounting for phylogenetic relationships, by conducting a phylogenetic generalized least-squares (PGLS) analysis on shape data, using species average shape and log-transformed centroid size and a phylogenetic tree generated from time-calibrated molecular data [30,31] (figure 1).
Figure 1.
Phylogenetic tree of species in this study. Scale = million years.
A principal component analysis (PCA) of the Procrustes coordinates was then performed to examine shape variation among specimens and distributions of the species in a morphospace. PC axes were explained in relation to morphology by performing correlations on PC scores and ratios (indices) generated from linear measurements between landmarks of interest (for example, muzzle length/total cranium length). Since the muzzle morphology is a functionally relevant trait, we calculated its relative length from the landmark data, then conducted separate PGLS analyses for muzzle length, with size and diet as factors.
To visualize the shape variation associated with the main PC axes, we used a 3D warping approach. A surface mesh (triangular isosurface) of a morphologically average specimen (Thylogale stigmatica), was created in Mimics (v. 17) by thresholding the tomograph for bone, generated by X-ray computed tomography (CT). The mesh was then warped using a thin-plate spline approach to the average shape of the data and this average mesh was then warped to the shapes representing the minima and maxima of each respective PC axis for a visual interpretation of the principal components.
(b). Biomechanical analysis (finite-element analysis)
A morphologically average specimen of each species, in good condition for CT scanning and modelling, was selected from the shape analysis (see electronic supplementary material for scanning details). Finite-element models (FEMs) were composed of approximately 1.7 million tetrahedral elements and were assigned homogeneous and isotropic material properties, a method considered appropriate for comparative studies that aim to identify the influence of shape, rather than predict absolute values [32]. Average material property values for mammalian bone were allocated to the models (Young's modulus: E = 20 GPa; Poisson's ratio: v = 0.3) [20]. Despite previous analyses identifying that homogeneous material properties influence absolute strain magnitudes, they have little effect on relative strain distributions, and thus are acceptable for comparative contexts [33]. The results from this methodology, therefore, represent relative, rather than absolute predictions and should be interpreted as parameters, estimated from cranial geometry alone, that do not reflect actual in vivo bite forces or resulting strain [34].
To predict bite forces and quantify mechanical efficiency, seven masticatory muscle divisions [35] were allocated respective origin plates on the FEMs. Muscle cross-sectional area was determined via dissection of a red-necked wallaby (Macropus rufogriseus) as a reference specimen (electronic supplementary material, table S3) and scaled to each species using cranial volume to the 2/3rd power, following previous protocols [32] (electronic supplementary material, table S4). Forces were applied using Boneload in Strand 7 v. 2.4.4 [36]. A bilateral incisor bite was simulated by constraining a node at each I1 incisor against the dorsoventral axis and a single node at each temporo-mandibular joint in all axis of translation. We used von Mises (VM) strain to represent the magnitude and distribution of bone deformation [37].
The BM was estimated for each specimen using a formula for craniodental measurements [38]. Bite reaction force (BRF) was obtained for each loading from the constrained nodes at the incisors. The correlation between logBM and logBRF was plotted across species. The regression line represented the expected BRF for a given size across all species examined. A mechanical efficiency quotient (MEQ) was generated from the residuals of the regression (see electronic supplementary material) for each species in order to compare the mechanical efficiency across species of different BM. The MEQ thus offers an indication of the disparity between expected BRF for a given BM (arbitrary MEQ value of 100), versus the observed value, represented by an increase (greater than 100) or a decrease (less than 100) in BRF. We then performed a linear regression between muzzle length and MEQ to identify any relationship between the two variables.
In order to more directly compare the influence of muzzle morphology on mechanical performance during biting, muscle forces were adjusted to generate equivalent bite forces for each species. Bite force values were predicted on the basis of BM for each model [39], i.e. by the regression line (MEQ = 100) (electronic supplementary material, figure S3). This method more meaningfully addresses our hypothesis than observing strain magnitudes from different relative output forces due to mechanical advantage caused by muzzle length. To quantify the influence of muzzle morphology on cranial deformation, 13 landmarks were positioned along the mid-sagittal plane of each species [20] using Landmark Editor (IDAV, v. 3.6), which included equidistant semi-landmarks, partitioned by several homologous fixed landmarks (figure 2). Strain magnitudes were then automatically retrieved from these landmarks using purpose-written code (see electronic supplementary material).
Figure 2.
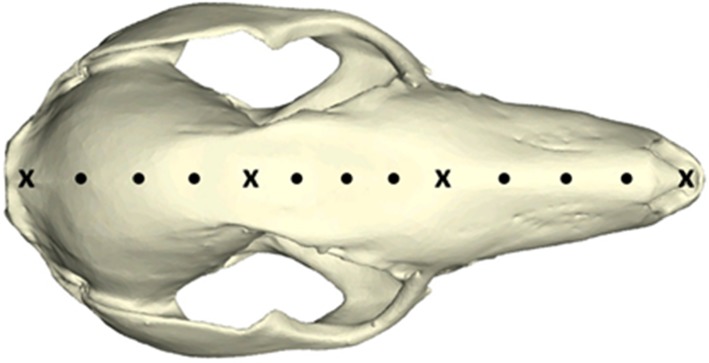
Fixed landmarks (cross symbols) and semi-landmarks (dots) from which strain data were collected. (Online version in colour.)
3. Results
(a). Shape analysis
The initial PGLS analyses examining evolutionary allometry in cranial shape and diet revealed that evolutionary changes in size along the branches of the phylogenetic tree accounted for 19.5% of the shape variation among species (p = 0.001), while diet represented 34.8% of the shape variation when size variation is accounted for (p = 0.001).
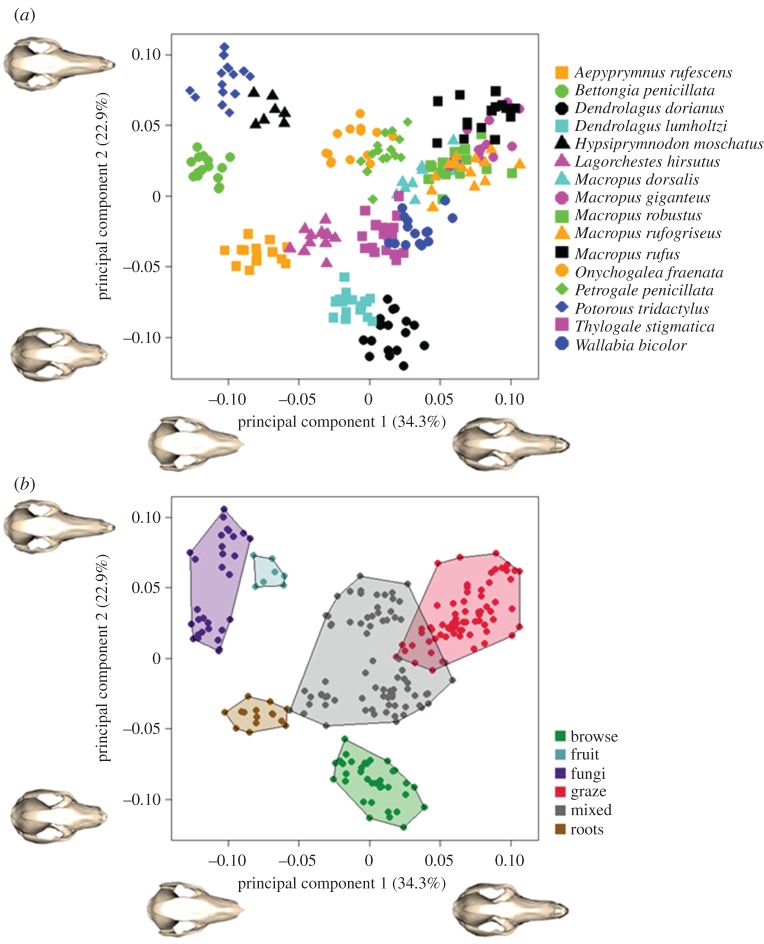
The PCA revealed discrete distributions of species as well as diet groups in shape space (figure 3). The first principal component (PC1: 34.3% of shape variation) was highly correlated with centroid size (p < 0.001), revealing a clear allometric influence on cranial shape within the group (figure 3a). An increase in body size (represented by the centroid size of the cranium) was correlated with a decrease in the size of the braincase, an increase in the dorsoventral breadth of the zygomatic arch, and a corresponding anterior extension of the incisors beyond the base of the muzzle. The length of the muzzle showed no correlation with this component.
Figure 3.
Principal components 1 and 2 of cranial shape for (a) all species sampled and (b) diet preference of these species.
The second principal component (PC2: 22.9% of shape variation) was almost entirely defined by the relative lengths of the muzzle and zygomatic arch. The minimum of this component represented a relatively long zygomatic arch and relatively short muzzle, while the maximum represented the reverse (figure 3a). PC2 was not correlated with size across all species (p = 0.330), however, a post hoc regression solely within the genus Macropus identified an allometric relationship between size and muzzle length within the grazing kangaroos and wallabies of this genus (p = 0.001, R2 = 0.34).
The species distributions across PC1 and PC2 are depicted as their allocated diets in figure 3b. From the trends outlined above, the fruit and fungi specialists have a larger braincase, longer muzzle, and more posteriorly located incisors; the root specialists have a larger braincase, a shorter muzzle, and more posteriorly located incisors; exclusive browsers are found at the mid-range of PC1, but have the shortest muzzle and longest zygomatic arch; grazers have the smallest braincase, a long muzzle, and procumbent incisors (note the correlation between PC1 and PC2 for grazers). The mixed feeders occupy the mid-range of both PC1 and PC2.
The results of a PGLS examining the influence of cranial size and diet on muzzle length are presented in table 2. Diet had a significant effect on muzzle length (F5 = 11.17, p < 0.001), where browsers were significantly different from grazers and frugivores, frugivores were significantly different from all other diets, and grazers were significantly different to root specialists. When including size in the model, diet still had a significant effect (F5 = 20.58, p < 0.001), however, grazers were not significantly different from browsers, mixed feeders or root specialists, respectively, while all other groups differed from each other.
Table 2.
Pairwise significance (p-values) from phylogenetic generalized least-squares of muzzle length versus size, and muzzle length versus size and diet. Italicized values indicate significance at α = 0.05.
| muzzle length ∼ diet diet: F5 = 11.17, p < 0.001 |
|||||
| browse | fruit | fungi | graze | mixed | |
| fruit | 0.0002 | ||||
| fungi | 0.245 | 0.0267 | |||
| graze | 0.0193 | 0.0293 | 0.9093 | ||
| mixed | 0.1811 | 0.0042 | 0.2461 | 0.1282 | |
| roots | 0.3926 | 0.0003 | 0.001 | 0.0079 | 0.0554 |
| muzzle length ∼ log(size) + diet size: F1 = 3.72, p = 0.086 diet: F5 = 20.58, p = 0.0001 |
|||||
| browse | fruit | fungi | graze | mixed | |
| fruit | 0.0001 | ||||
| fungi | 0.0015 | 0.0038 | |||
| graze | 0.1653 | 0.0022 | 0.0364 | ||
| mixed | 0.0084 | 0.0003 | 0.0382 | 0.1589 | |
| roots | 0.0108 | 0.0001 | 0.0001 | 0.6314 | 0.0108 |
(b). Biomechanical analysis
There was a positive correlation between BRF and BM across all species. The regression line (see electronic supplementary material, figure S3) is represented by the horizontal midline, at MEQ = 100 (figure 4). Among the smaller species, Hypsiprymnodon moschatus and Potorous tridactylus have a weaker bite (lower mechanical efficiency) than would be predicted from the regression (MEQ < 100). The root specialist, Aepyprymnus rufescens, lies above the expected value for its size, indicating a stronger bite (higher mechanical efficiency) than expected (MEQ > 100). Of the larger species, all considered mixed feeders lie within close proximity to the regression line, as do most of the grazing species. The exceptions are Macropus dorsalis and M. giganteus, which have the lowest MEQ of all medium to large species. The two browsing Dendrolagus spp. have the highest MEQ, indicating greatest mechanical efficiency for their size. A post hoc linear model evaluated using generalized least-squares revealed that muzzle length is correlated with MEQ values (p < 0.005), with shorter muzzles resulting in significantly greater mechanical efficiency.
Figure 4.
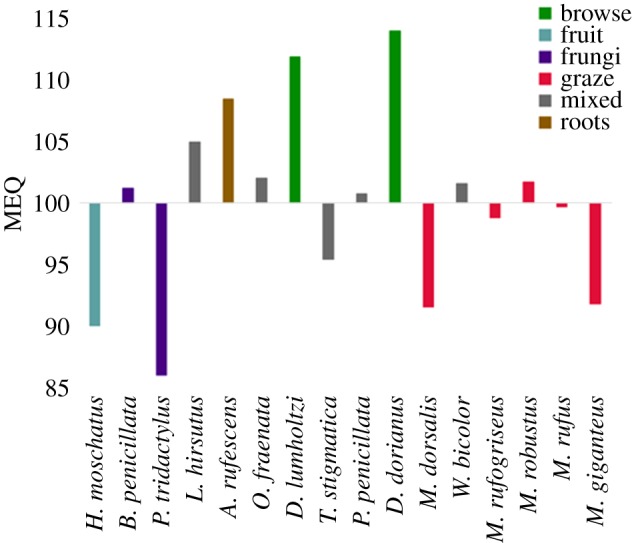
Mechanical efficiency quotients for each species during an incisor bite simulation. Species are ordered by increasing BM estimates.
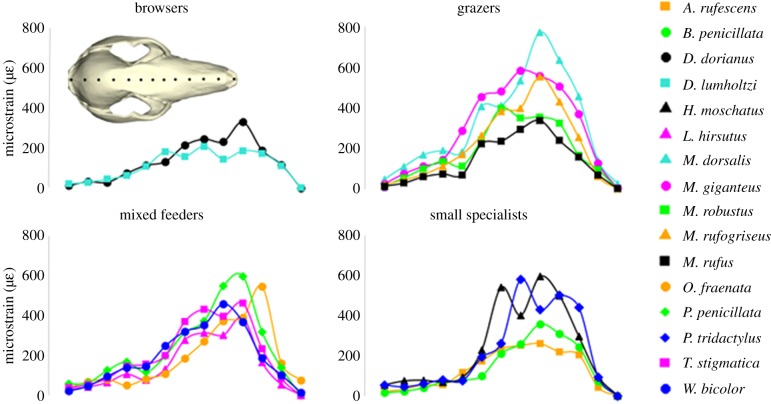
von Mises strain magnitudes represent the amount of deformation undergone along the muzzle for each species when performing the same incisor bite, relative to their BM. Small selective feeders are presented together, due to the low number of representative species (figure 5). Among larger species, browsing Dendrolagus spp. experience lower strain magnitudes. Grazers and mixed feeders tend to experience more deformation. Among the small specialists, the root specialist, A. rufescens, displays low strain magnitudes comparable to the browsers, while fruit and fungi specialists, H. moschatus and P. tridactylus, experience higher deformation, similar to most grazers and mixed feeders. Each FEM is presented in increasing BM (figure 6), indicating that shorter, more robust muzzles consistently experience less deformation when biting at the incisors across all species tested. Although most species with longer, more gracile muzzles show greater strain magnitudes, there is notably less deformation in the sexually dimorphic, particularly robust cranium of the male M. rufus.
Figure 5.
von Mises microstrain magnitudes at 13 landmarks along the dorsal midline of the cranium. Fruit (H. moschatus), fungi (Bettongia penicillata and P. tridactylus) and root specialists (A. rufescens) have been presented together as small specialists. The inset shows the location and direction of landmarks on the crania.
Figure 6.
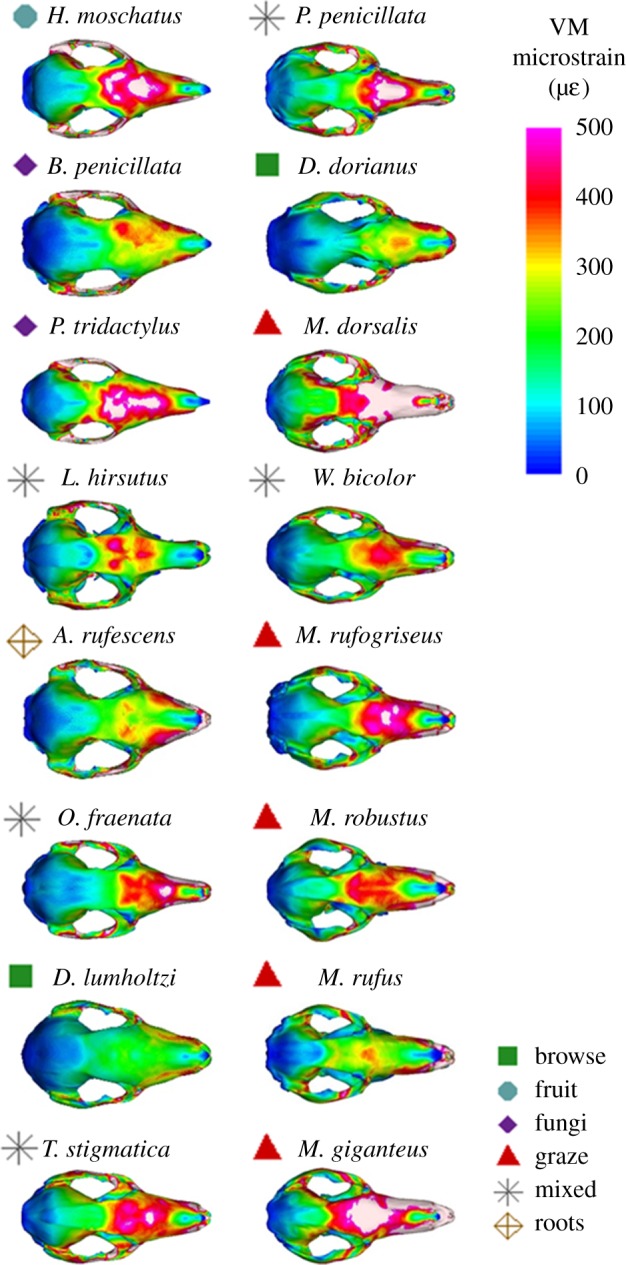
Dorsal view of all 16 FEMs, with VM microstrain (µɛ) distributions. Species arranged in increasing BM; from top to bottom, left then right columns.
4. Discussion
We have found that, across their entire size range, face length of kangaroos and their relatives contributes a major component of variation that is not associated with BM, thus clearly impacting the viability of CREA as a biological generality or rule. The correlation we have identified between muzzle length and mechanical efficiency indicates that a shorter muzzle, coupled with a longer zygomatic arch, results in a greater bite force than expected from the same scaled muscle force. Biomechanically, decreasing muzzle length shortens the out-lever, contributing to higher bite forces [7,40]; while the corresponding longer zygomatic arches offer an increase in leverage for both the temporalis and masseteric musculature, thus permitting greater adducting force. This is typically seen in mammals that bite harder from a wider gape angle [41]. The strain magnitudes along the cranial midline of each species show that a shorter muzzle is also capable of withstanding greater forces from harder biting. Our findings suggest that species that obtain more resistant foods, such as thick, lignified browse or fibrous roots, have a shorter, more robust muzzle and longer zygomatic arches. Alternatively, the weakest performance was found in specialists of soft foods, such as fruits and fungi, and in two of the obligate grazers. Our cranial shape distributions (figure 3a) align well with the results of independent dietary data on many of the same species [24], lending further weight to a clear association between diet and craniofacial morphology among the Macropodiformes.
The differences in muzzle morphology between the species studied here closely correspond with respective cropping behaviours for food procurement and initial processing. In general, larger grazers break blades of grass and low-growing forbs by gripping the item between the incisors and pulling back with their cervical musculature [16,42]. This breaks the tissues via tension, requiring less effort to obtain greater quantities; and means that, despite the fibrous, resistant nature of graze, large grazers do not need to bite hard to obtain grasses. Our findings of incisor orientation along PC1 support this; as more anteriorly projected incisors, also seen in placental herbivores, are more efficient for gripping onto graze [43]. There is little need for larger grazers to have strong bite forces, once they reach a body size that places the mouth at a greater height than the vegetation; plucking vegetation is essentially a less strenuous activity. By contrast, browsers must slice through plant matter using either their incisors or sectorial premolars [16,23,44]. The vertical orientation of the incisors in browsing species studied here matches that observed in browsing placental herbivores [43] and suggests that vegetation is obtained by slicing for these species. Even though most macropodiform species use their forepaws to some extent in the manipulation of their food [45], which could lead to some food acquisition via tension in browsers, the heavily lignified tissues of tougher browse would still require a stronger bite to sever larger food items.
Among the smaller bettongs, potoroos and rat-kangaroos, fruit and fungi specialists have longer, more gracile muzzles that are distributed similarly to grazers in the positive region of PC2 (figure 3b), while the root specialist, A. rufescens, is situated with the browsers towards the negative region of PC2, where animals have a shorter muzzle. Craniofacial morphology among these smaller species aligns closely with the mechanical properties of their respective diets: harder or more resistant items, such as nuts, seeds and fibrous and tuberous roots, require stronger bite forces. A shorter, more robust craniofacial morphology has been associated with the more resistant diets of bettongs from arid to semi-arid zones that focus on such foods [46]. Our findings identify a morphological continuum from harder foods to softer foods for these smaller species that agrees with this association. Furthermore, the largest potoroid species, A. rufescens, has the shortest muzzle relative to BM, indicating that positive allometry in muzzle length is not present within the Potoroidae. Instead, we find that across all species examined, there is a repetitive pattern of shorter muzzles accompanying feeding behaviours that require high jaw adducting forces to slice through more resistant vegetation; and that shorter muzzles result in less deformation during biting (figure 6), supporting the notion that this morphology is associated with feeding biomechanics. Possibly, when freed from the constraints of hard biting, the muzzle can increase in length for other purposes, such as digging in smaller species, or enhanced visual range while open-field grazing in larger species.
We also find that cropping behaviours are not obviously separable in accordance with discrete dietary grouping. In examining the variation in muzzle length among dietary groups, we found no significant difference between grazers and the browsers, mixed feeders or root specialists (table 2). These observations can be attributed to the allometric trend in muzzle length found across grazers (genus Macropus), as browsers, mixed feeders and root specialists are distributed within the potential range of the extrapolated correlation for grazing species and are, therefore, not significantly different from grazers when size variation is accounted for (figure 3b). This relationship suggests that, with decreasing size, grazers express a reduction in muzzle length increasingly consistent with actively biting resistant foods. Such cropping behaviours are common in small grazers, which must also slice through vegetation as browsers do. Acquiring blades of grass via tension becomes less feasible when the height of the vegetation becomes comparable to the height of the grazer and smaller species instead tend to nibble the ends of individual grass blades rather than plucking them [16,42]. Thus, the smallest grazing species, such as the nabarlek (Petrogale concinna) [47], possess a very short muzzle [48] in order to enhance bite force and reduce deformation when slicing through resistant grass fibres. Together, the relationships we identify here show that craniofacial morphology across the Macropodiformes is influenced by a combination of the physical properties of preferred vegetation and cropping actions performed to obtain them.
The monotonous nature of graze can explain the positive allometry in muzzle length observed across larger grazers in this study. Provided that the physical properties of the food remain constant across all species in question, an increase in body size will naturally result in a corresponding increase in muzzle length. While the physical properties of grasses remain largely constant for grazers of all sizes, the biomechanical constraints on muzzle length decrease with an allometric increase in the size of the cranium and associated muscle forces; in short, larger bones, teeth and muscles require less effort to break the same materials. This can explain the previously observed allometric correlation in cranial length across several macropodines [2], in which obligate browsers with short faces (Setonix brachyurus and Dendrolagus sp.) progress in positive craniofacial allometry to mixed feeders (W. bicolor, Petrogale xanthropus and smaller Macropus spp.), to medium-large grazers (larger Macropus spp.) with relatively long faces. However, positive allometry in muzzle length is most unlikely among browsers, which progressively take greater quantities of bulkier, lower quality plant tissues as BM increases [49], requiring relatively greater bite forces and shorter muzzles to do so. This culminated in the extreme muzzle reduction of the extinct short-faced kangaroos (Sthenurinae), all of which are generally considered to have been browsers [50]. The largest kangaroo to ever exist, Procoptodon goliah, was a specialist browser that represented an extreme of muzzle reduction [51], further challenging the proposal that a relationship exists between facial elongation and body size for kangaroos and their relatives.
5. Conclusion
Our results indicate that although there are conditional trends in positive craniofacial allometry among some Macropodiformes, these are largely products of various biomechanical demands associated with herbivory and do not represent the taxon as a whole. Instead, muzzle length across the Macropodiformes is associated with a combination of the physical properties of desired vegetation and the cropping behaviours used to access these tissues for further processing. These findings may be useful for extrapolating the potential diets of other species within this taxon, which will assist with conservation and management strategies of endangered species. Additionally, they may be used to infer palaeoecology.
Marsupial herbivores represent an ideal case for examining the direct effects of feeding behaviours on cranial morphology in herbivores, as in all species, the anterior dentition plays a direct role in obtaining food items. These species are, therefore, able to clearly showcase the influences of biting behaviours on cranial form. Our findings across the Macropodiformes may be less apparent for some placental herbivore taxa that use a range of alternative features to acquire vegetation, such as giraffes, tapirs and elephants that have prehensile tongues and trunks to obtain plant matter, respectively. However, similar results may be found, provided that the same feeding apparatus is used within the taxon in question; and that this apparatus is not used for any more strenuous activities, such as gnawing or digging. The taxonomic range to which these correlations may apply requires further investigation.
Supplementary Material
Acknowledgements
The authors would like to thank Stuart Green of the University of New England and staff of the UNE Natural History Museum, Sandy Ingleby of the Australian Museum and Heather Janetzki of the Queensland Museum for allowing access to their respective collections; Karl Vernes, for freely sharing information about potoroid food properties; Karl Little of Armidale Radiology and Tzong-Tyng Hung of the BRIL and National imaging facility at UNSW for CT scanning specimens; and Sam Evans of the University of Newcastle for his assistance with extracting strain values from finite-element models.
Ethics
Dissection of a roadkill specimen was approved the University of New England Animal Ethics Committee, AEC Approval Number: AEC16-042.
Data accessibility
3D shape data, strain magnitudes, MEQ calculations, and FEMs for each species have been submitted to Dryad (http://dx.doi.org/10.5061/dryad.0hr3gk2) [52].
Authors' contribution
D.R.M. carried out all analyses and writing, E.S. assisted with the GMM analyses, J.L. assisted with the FEA methodology and SW assisted with FEA methodology and interpretations. All authors read and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
Work was supported by ARC Discovery grant nos. DP140102659 and DP140102656 to S.W.
References
- 1.Cardini A, Polly PD. 2013. Larger mammals have longer faces because of size-related constraints on skull form. Nat. Commun. 4, 2458 ( 10.1038/ncomms3458) [DOI] [PubMed] [Google Scholar]
- 2.Cardini A, Polly D, Dawson R, Milne N. 2015. Why the long face? Kangaroos and wallabies follow the same ‘rule’ of cranial evolutionary allometry (CREA) as placentals. Evol. Biol. 42, 169–176. ( 10.1007/s1169) [DOI] [Google Scholar]
- 3.Turnbull WD. 1970. Mammalian masticatory apparatus. Fieldiana 18, 147–356. [Google Scholar]
- 4.Schwenk K. 2000. Feeding: form, function and evolution in tetrapod vertebrates. San Diego, CA: Academic Press. [Google Scholar]
- 5.Smith AL, et al. 2015. The feeding biomechanics and dietary ecology of Paranthropus boisei. Anat. Rec. 298, 145–167. ( 10.1002/ar.23073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attard M, Chamoli U, Ferrara TL, Rogers TL, Wroe S. 2011. Skull mechanics and implications for feeding behaviour in a large marsupial carnivore guild: the thylacine, Tasmanian devil and spotted-tailed quoll. J. Zool. 285, 292–300. ( 10.1111/j.1469-7998.2011.00844.x) [DOI] [Google Scholar]
- 7.Goswami A, Milne N, Wroe S. 2011. Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proc. R. Soc. B 278, 1831–1839. ( 10.1098/rspb.2010.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingenberg C.P. 1996. Multivariate allometry. In Advances in morphometrics (eds Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE), pp. 23–49. Berlin, Germany: Springer. [Google Scholar]
- 9.Demment MW, Van Soest PJ. 1985. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Nat. 125, 641–672. ( 10.1086/284369) [DOI] [Google Scholar]
- 10.Gordon IJ, Illius AW. 1988. Incisor arcade structure and diet selection in ruminants. Funct. Ecol. 2, 15–22. ( 10.2307/2389455) [DOI] [Google Scholar]
- 11.Hume ID. 1989. Optimal digestive strategies in mammalian herbivores. Physiol. Zool. 62, 1145–1163. ( 10.1086/physzool.62.6.30156206) [DOI] [Google Scholar]
- 12.Gordon IJ, Illius AW. 1994. The functional significance of the browser-grazer dichotomy in African ruminants. Oecol. 98, 167–175. ( 10.1007/BF00341469) [DOI] [PubMed] [Google Scholar]
- 13.Bodmer RE. 1990. Ungulate frugivores and the browser-grazer continuum. Oikos 57, 319–325. ( 10.2307/3565960) [DOI] [Google Scholar]
- 14.Shipley LA. 1999. Grazers and browsers: how digestive morphology affects diet selection. In Grazing behavior of livestock and wildlife (eds Launchbaugh KL, Sanders KD, Mosley JC), pp. 20–27, Moscow, Russia: University of Idaho. [Google Scholar]
- 15.Sanson GD. 2006. The biomechanics of browsing and grazing. Am. J. Bot. 93, 1531–1545. ( 10.3732/ajb.93.10.1531) [DOI] [PubMed] [Google Scholar]
- 16.Sanson GD. 1989. Morphological adaptations of teeth to diets and feeding in the Macropodoidea. In: Kangaroos, wallabies and Rat-kangaroos, vol. 1 (eds Grigg G, Jarman P, Hume I), pp. 151–168. Chipping Norton, NSW: Surrey Beatty. [Google Scholar]
- 17.Janis C. 2008. An evolutionary history of browsing and grazing ungulates. In The ecology of browsing and grazing. Ecological studies, (eds Gordon IJ, Prins HHT), pp. 21–45. Berlin, Germany: Springer. [Google Scholar]
- 18.Janis CM. 1990. Correlation of cranial and dental variables with dietary preferences in mammals: a comparison of macropodoids and ungulates. Mem. Queensl. Mus. 28, 349–366. [Google Scholar]
- 19.Fletcher TM, Janis CM, Rayfield EJ. 2010. Finite element analysis of ungulate jaws: can mode of digestive physiology be determined. Palaeontol. Electron. 13, 1–15. (http://palaeo-electronica.org/2010_3/234/index.html) [Google Scholar]
- 20.Sharp AC. 2015. Comparative finite element analysis of the cranial performance of four herbivorous marsupials. J. Morphol. 276, 1230–1243. ( 10.1002/jmor.20414) [DOI] [PubMed] [Google Scholar]
- 21.Richardson K. 2012. Australia's amazing kangaroos: their conservation, unique biology and coexistence with humans. Collingwood, Victoria: CSIRO Publishing. [Google Scholar]
- 22.Milne N, O'Higgins P. 2002. Inter-specific variation in Macropus crania: form, function and phylogeny. J. Zool. 256, 523–535. ( 10.1017/S0952836902000572) [DOI] [Google Scholar]
- 23.Sanson G. 1980. The morphology and occlusion of the molariform cheek teeth in some Macropodinae (Marsupialia: Macropodidae). Aust. J. Zool. 28, 341–365. ( 10.1071/ZO9800341) [DOI] [Google Scholar]
- 24.Arman SD, Prideaux GJ. 2015. Dietary classification of extant kangaroos and their relatives (Marsupialia: Macropodoidea). Austral. Ecol. 40, 909–922. ( 10.1111/aec.12273) [DOI] [Google Scholar]
- 25.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: the R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 26.Adams DC, Collyer ML, Kaliontzopoulou A, Sherratt E.2017. Geomorph: software for geometric morphometric analyses. R package version 3.0.5. See https://cran.r-project.org/package=geomorph .
- 27.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2018. nlme: linear and nonlinear mixed effects models. R package version 3.1–137, See https://CRAN.R-project.org/package=nlme.
- 28.Rohlf FJ, Slice D. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39, 40–59. ( 10.2307/2992207) [DOI] [Google Scholar]
- 29.Klingenberg CP, Barluenga M, Meyer A. 2002. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56, 1909–1920. ( 10.1554/0014-3820(2002)056%5B1909:SAOSSQ%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 30.Mitchell KJ, et al. 2014. Molecular phylogeny, biogeography, and habitat preference evolution of marsupials. Mol. Biol. Evol. 31, 2322–2330. ( 10.1093/molbev/msu176) [DOI] [PubMed] [Google Scholar]
- 31.Phillips MJ, Haouchar D, Pratt RC, Gibb GC, Bunce M. 2013. Inferring kangaroo phylogeny from incongruent nuclear and mitochondrial genes. PLoS ONE 8, e57745 ( 10.1371/journal.pone.0057745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strait DS, et al. 2010. The structural rigidity of the cranium of Australopithecus africanus: implications for diet, dietary adaptations, and the allometry of feeding biomechanics. Anat. Rec. 293, 583–593. ( 10.1002/ar.21122) [DOI] [PubMed] [Google Scholar]
- 33.Strait DS, Wang Q, Dechow PC, Ross CF, Richmond BG, Spencer MA, Patel BA. 2005. Modeling elastic properties in finite-element analysis: how much precision is needed to produce an accurate model? Anat. Rec. 283A, 275–287. ( 10.1002/ar.a.20172) [DOI] [PubMed] [Google Scholar]
- 34.Rayfield EJ. 2007. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planet. Sci. 35, 541–576. ( 10.1146/annurev.earth.35.031306.140104) [DOI] [Google Scholar]
- 35.Warburton NM. 2009. Comparative jaw muscle anatomy in kangaroos, wallabies, and rat-kangaroos (Marsupialia: Macropodoidea). Anat. Rec. 292, 875–884. ( 10.1002/ar.20905) [DOI] [PubMed] [Google Scholar]
- 36.Grosse IR, Dumont ER, Coletta C, Tolleson A. 2007. Techniques for modeling muscle-induced forces in finite element models of skeletal structures. Anat. Rec. 290, 1069–1088. ( 10.1002/ar.20568) [DOI] [PubMed] [Google Scholar]
- 37.Ledogar JA, Luk THY, Perry JMG, Neaux D, Wroe S. 2018. Biting mechanics and niche separation in a specialized clade of primate seed predators. PLoS ONE 13, e0190689 ( 10.1371/journal.pone.0190689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers TJ. 2001. Prediction of marsupial body mass. Aust. J. Zool. 49, 99–118. ( 10.1071/ZO01009) [DOI] [Google Scholar]
- 39.McHenry CR, Wroe S, Clausen PD, Moreno K, Cunningham E. 2007. Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation. Proc. Natl Acad. Sci. USA 104, 16010–16015. ( 10.1073/pnas.0706086104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Therrien F. 2005. Feeding behaviour and bite force of sabretoothed predators. Zool. J. Linn. Soc. 145, 393–426. ( 10.1111/j.1096-3642.2005.00194.x) [DOI] [Google Scholar]
- 41.Herring SW, Herring SE. 1974. The superficial masseter and gape in mammals. Am. Nat. 108, 561–576. ( 10.1086/282934) [DOI] [Google Scholar]
- 42.Vincent JF. 1982. The mechanical design of grass. J. Mater. Sci. 17, 856–860. ( 10.1007/BF00540384) [DOI] [Google Scholar]
- 43.Janis CM, Ehrhardt D. 1988. Correlation of relative muzzle width and relative incisor width with dietary preference in ungulates. Zool. J. Linn. Soc. 92, 267–284. ( 10.1111/j.1096-3642.1988.tb01513.x) [DOI] [Google Scholar]
- 44.Martin R. 2005. Tree-kangaroos of Australia and New Guinea . Clayton, Australia: CSIRO publishing. [Google Scholar]
- 45.Jarman PJ. 1984. The dietary ecology of macropod marsupials. Proc. Nutr. Soc. Aust. 9, 82–87. [Google Scholar]
- 46.McDowell MC, Haouchar D, Aplin KP, Bunce M, Baynes A, Prideaux GJ. 2015. Morphological and molecular evidence supports specific recognition of the recently extinct Bettongia anhydra (Marsupialia: Macropodidae). J. Mammal. 96, 287–296. ( 10.1093/jmammal/gyv006) [DOI] [Google Scholar]
- 47.Telfer WR, Bowman DM. 2006. Diet of four rock-dwelling macropods in the Australian monsoon tropics. Austral Ecol. 31, 817–827. ( 10.1111/j.1442-9993.2006.01644.x) [DOI] [Google Scholar]
- 48.Rodrigues HG, Hautier L, Evans AR. 2017. Convergent traits in mammals associated with divergent behaviors: the case of the continuous dental replacement in rock-wallabies and African mole-rats. J. Mamm. Evol. 24, 261–274. ( 10.1007/s10914-016-9348-7) [DOI] [Google Scholar]
- 49.Fritz H, Duncan P, Gordon IJ, Illius AW. 2002. Megaherbivores influence trophic guilds structure in African ungulate communities. Oecol. 131, 620–625. ( 10.1007/s00442-002-0919-3) [DOI] [PubMed] [Google Scholar]
- 50.Helgen KM, Wells RT, Kear BP, Gerdtz WR, Flannery TF. 2006. Ecological and evolutionary significance of sizes of giant extinct kangaroos. Aust. J. Zool. 54, 293–303. ( 10.1071/ZO05077) [DOI] [Google Scholar]
- 51.Prideaux GJ, Ayliffe LK, DeSantis LR, Schubert BW, Murray PF, Gagan MK, Cerling TE. 2009. Extinction implications of a chenopod browse diet for a giant Pleistocene kangaroo. Proc. Natl Acad. Sci. USA 106, 11 646–11 650. ( 10.1073/pnas.0900956106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell DR, Sherratt E, Ledogar JA, Wroe S. 2018. Data from: The biomechanics of foraging determines face length among kangaroos and their relatives Dryad Digital Repository. ( 10.5061/dryad.0hr3gk2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mitchell DR, Sherratt E, Ledogar JA, Wroe S. 2018. Data from: The biomechanics of foraging determines face length among kangaroos and their relatives Dryad Digital Repository. ( 10.5061/dryad.0hr3gk2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
3D shape data, strain magnitudes, MEQ calculations, and FEMs for each species have been submitted to Dryad (http://dx.doi.org/10.5061/dryad.0hr3gk2) [52].