Abstract
The use of fat to support the energy needs of reproduction (i.e. capital breeding) has been studied in a diversity of taxa. However, despite reproductive output (i.e. young or eggs) being approximately 70% water, little is known about the availability of internal resources to accommodate the hydric demands of reproduction. Recent research suggests that dehydration increases the catabolism of muscle as a means of maintaining water balance. Accordingly, we investigated the interactive effects of reproductive investment and water deprivation on catabolism and reproductive output in female Children's pythons (Antaresia childreni). Both reproductive and non-reproductive females were either provided water ad libitum or were water-deprived for three weeks at the time when reproductive females were gravid. We found that water-deprived reproductive females had, in general, greater body mass loss, epaxial muscle loss, plasma osmolality and plasma uric acid concentrations relative to the other groups. Furthermore, water-deprived females had similar clutch sizes compared with females with access to water, but produced lighter eggs and lower total clutch masses. Our results provide the first evidence that selective protein catabolism can be used to support water demands during reproduction, and, as a result, these findings extend the capital breeding concept to non-energetic resources.
Keywords: reproductive costs, capital breeder, water constraints, protein catabolism, bound water
1. Introduction
Optimizing reproductive phenology is essential to fitness, with offspring typically being born when trophic resources and abiotic conditions maximize offspring survival [1,2]. However, producing offspring at such a time may require reproductive activities (i.e. migration, mate acquisition and energy allocation) to occur when resources are limited [3]. Multiple strategies exist to cope with this possible temporal conflict. For example, females can unlink resource acquisition from allocation by accumulating resources when available and then relying on internal stores to invest in subsequent reproduction (i.e. capital breeding [4]). To date, such breeding strategies have solely been approached from an energetic viewpoint, where fat stores are primarily used to fuel reproductive efforts [5–7]. However, offspring production requires other nutrients that cannot be supplied by the catabolism of fat alone. For example, if not adequately obtained from feeding, amino acids needed for offspring production must come from the catabolism of muscle mass [8,9].
Water is another high-demand resource required for reproduction and notably embryonic development [10]. While in many environments water is readily available when energy resources are not, this is not the case in arid ecosystems, where access to drinking water may be extremely limited for extended periods. In these ecological contexts, the appearance of offspring coincides with the rainy season to take advantage of this period of high productivity. Such temporal optimization may require females to start investing in reproduction during times of concomitantly limited water and food resources. Capital breeding provides a robust way to support the energy demands of reproduction, especially in seasonal environments [11,12]. Despite the crucial importance of water for reproduction [13], to date it is unknown whether such a capital breeding tactic also pertains to water. We posit that mobilizing stored water (i.e. ‘capital') should provide benefits when environmental water resources (i.e. ‘income') are limiting at the time of reproductive effort.
A water-based capital breeding strategy is challenged by the facts that water imbalance can have debilitating effects on physical, cognitive and physiological functions [14], and most species do not possess distinct water stores. However, bound water is a significant component of all body tissues, typically representing approximately 70% of the wet mass of vertebrates. Thus, internal tissues could provide a water source for reallocation to reproductive needs. While fat is an exceptional source of energy, providing twice the energy per gram as does protein, it is a less effective source for the reallocation of bound water. Bound water represents only approximately 10% of the wet mass of fat, while it represents approximately 76% of skeletal muscle [15]. Overall, wet protein mobilization yields more than five times the total (metabolic and bound) water (0.155 g water kJ−1) than does wet lipids (0.029 g water kJ−1) [16]. Therefore, muscle protein may serve as an important water depot when environmental water resources are not available. In fact, mammals and birds enhance the catabolism of muscle relative to that of fat to maintain water balance [17,18]. However, no previous efforts have examined whether selective protein catabolism (beyond that required for the reallocation of amino acids) can be used to meet the water demands of reproduction.
Children's pythons (Antaresia childreni) reside in the wet–dry forest of northern Australia, and their eggs hatch shortly after the onset of the rainy season. Consequently, reproduction occurs during the latter parts of the dry season when both food and water are extremely limited. In fact, plasma osmolality of Children's pythons increases as the dry season progresses [19]. Children's pythons are typical capital breeders, using energy stores to support reproduction and notably yolk deposition during vitellogenesis. The ensuing period of gravidity is characterized by a shift in maternal thermoregulation, development of the eggshell and the investment of a significant amount of water into developing eggs [20,21]. While reproductive female Children's pythons provided with ad libitum water but no food catabolize muscle to provide energy and amino acids for egg production [9], it is unknown whether additional muscle catabolism can support the investment of water into the eggs when this resource is limited.
We examined the interactive effects of reproduction and water deprivation on plasma osmolality, muscle catabolism and reproductive output in female Children's pythons. We hypothesized that structural proteins serve as a valuable source of water for developing eggs when environmental water availability is insufficient. We tested the following predictions:
(1) Females deprived of water dehydrate (i.e. increase plasma osmolality) more than females with continuous access to water, and the extent of this constraint is greater in gravid relative to non-reproductive females due to the water demand associated with gravidity.
(2) To support embryonic water demands, water-deprived females catabolize more protein than females with ad libitum access to water, as evidenced by a greater loss of muscle and an increased plasma concentration of uric acid, a by-product of protein catabolism.
(3) Protein catabolism is insufficient in covering all water demands associated with gravidity, so water-deprived females will have lower reproductive output than do females that have access to water.
2. Methods
(a). Experimental design
To stimulate the reproductive cycle, snakes were overwintered (6 L : 18 D cycle and a daily temperature cycle of 20°C : 15°C) from 6 December 2016 to 7 February 2017. Snakes were deprived of food from the onset of overwintering to the end of the experiment (approx. five months). Upon emergence, the room temperature was increased to a constant 31.5°C, which is the preferred temperature of gravid Children's pythons [20]. Females were held at a constant temperature without opportunity to thermoregulate to prevent a temperature-based difference in water loss as a result of differential thermoregulation between reproductive and non-reproductive females.
Females were randomly assigned to either the reproductive or non-reproductive groups, with reproductive females, but not non-reproductive ones, being exposed to a different male every 2–3 days, resulting in each reproductive female being sequentially housed with six to eight males during this 18-day breeding period. Each non-reproductive female was yoked to a reproductive female so that the timing of its treatments, measurements and sampling coincided with that of the reproductive female to which it was yoked. At ovulation (based on a peri-ovulation shed [20]), half of the reproductive females and their yoked partners, were deprived of water until the reproductive females laid her eggs (21–23 days). Treatment groups were: gravid with water provided ad libitum (n = 9), non-reproductive with water provided ad libitum (n = 8), gravid with no water provided (n = 10) and non-reproductive with no water provided (n = 8).
Assessments focused on changes that occurred predominantly over the vast majority of the gravidity period. Body mass, snout–vent length (SVL) and epaxial muscle width (at both 50% and 75% of each snake's SVL) were measured at ovulation and again during late gravidity (21 days post-ovulation). Epaxial muscle widths were measured following previously described methods [9,22]. In brief, there is a palpable demarcation where the longissimus dorsi muscle rests against the iliocostalis muscle, so the distance between the lateral edges of the two longissimus dorsi muscles can be measured with digital calipers [22]. Four measurements were taken each at 50% and 75% of the distance from the head to the vent, and an overall average was used for statistical analyses. Blood samples were collected from females once the reproductive female of the yoked partners reached late vitellogenesis and again when it reached late gravidity. From the collected blood samples, plasma osmolality values (vapour pressure osmometer, #5600, Wescor Inc.) and circulating concentrations of nutrients were measured (glucose [blood glucose meter, #EG220546, Medline Industries], triglycerides [assay kit #TR0100, Sigma-Aldrich], total proteins [assay kit #23236, Thermo Scientific]) and their catabolic by-products (ketones [blood ketone meter, # OS020102A, Nova Biomedical] and uric acid [assay kit #A22181, Life Technologies], for fatty acid and protein catabolism, respectively). At oviposition, female body mass, clutch size and clutch mass were measured.
(b). Statistical analyses
One-factor analyses of variance (ANOVAs) were performed to compare mass and epaxial muscle changes over the course of the experimental treatment (21 days) among the four treatment groups. Two-factor ANOVAs were also performed to examine the influences of water treatment (water or no water), reproductive status (reproductive or non-reproductive), and their interaction on epaxial muscle and mass changes.
Repeated-measures analysis of variance (rmANOVA) was used to examine the effect of status (reproductive or non-reproductive) and treatment (water or no water) on biochemical assessments over the two time periods (late vitellogenesis and late gravidity). Three-way interactions using treatment, status and time period as fixed effects and individual identity as a random effect were also tested.
The influence of treatment on clutch size (i.e. number of eggs per clutch) was tested using a linear model with treatment (water or no water) as a fixed effect and the SVL as a covariate. As clutch size and body size are closely related in snakes, size-adjusted fecundity was calculated by extracting residuals of the linear relationship between clutch size and the SVL. The effect of treatment on clutch mass was then tested using a linear model with treatment as a fixed effect and the SVL and size-adjusted fecundity as covariates. The data were checked to ensure they met the assumptions for parametric testing, and transformations were used where necessary. All statistical analyses were performed in R v. 3.3.2 [23] with the packages ‘nlme' and ‘multcomp' [24,25] for rmANOVAs and ‘agricolae' [26] for post hoc tests. Significance was set at α = 0.05.
3. Results
(a). Morphological changes during treatment
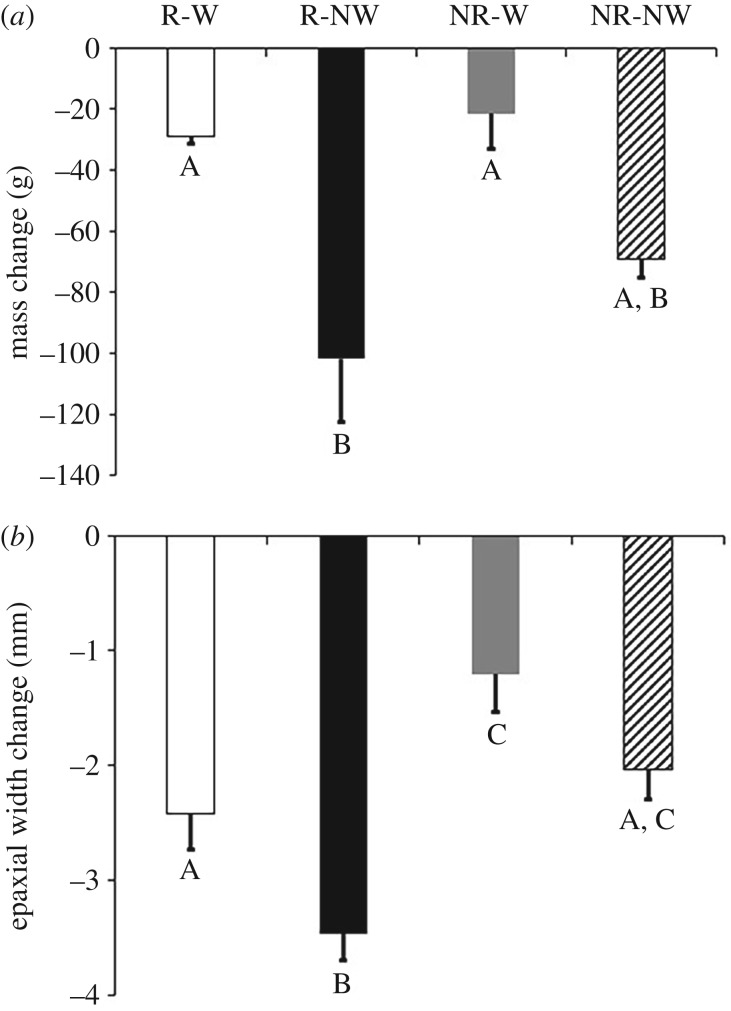
Females at the onset of the experiment had similar SVL (F1,34 = 1.32, p = 0.28) and body mass (F1,34 = 1.17, p = 0.33). We found significant differences in mass loss during the three-week experimental period among the four groups (F3,34 = 8.05, p = 0.004) with higher values in water-deprived reproductive females when compared with water-provided reproductive females (figure 1a). Using a two-factor ANOVA, we found that mass loss was influenced by treatment, being significantly higher in water-deprived females than water-provided females (respectively, −85.46 ± 9.45 g versus −25.19 ± 9.68 g, F1,34 = 19.81, p < 0.001). We found no effect of reproductive status (F1,34 = 2.18, p = 0.14) and no interaction between treatment and status (F1,34 = 0.87, p = 0.35).
Figure 1.
(a) Average mass change (grams) and (b) average epaxial muscle width change (millimetres) measured in non-reproductive (NR) and reproductive (R) female Antaresia childreni with (W) or without (NW) access to water from ovulation to oviposition or its equivalent duration for yoked, non-reproductive females. Error bars represent ±1 s.e.m. Different letters indicate significant differences among groups (HSD post hoc test).
The mean epaxial muscle width was not related to body size (F1,34 = 2.35, p = 0.13) and there was no initial difference in muscle width among experimental groups (F1,34 = 1.35, p = 0.27). Comparing the four experimental groups revealed that epaxial muscle loss was greatest in water-deprived reproductive females compared to the other groups (F3,34 = 11.38, p = 0.0001; figure 1b). A two-factor ANOVA revealed that muscle loss was influenced by treatment with higher values in water-deprived females relative to water-provided females (−2.75 ± 0.18 versus −1.81 ± 0.2 mm, F1,34 = 10.96, p = 0.002). We also found a significant influence of status with higher values in reproductive females relative to non-reproductive females (−2.94 ± 0.19 and −1.62 ± 0.2 mm, F1,34 = 21.8, p < 0.0001). No interaction was found between status and treatment (F1,34 = 0.14, p = 0.71). Epaxial muscle loss and mass loss were correlated (F1,34 = 11.44, p = 0.002), emphasizing that muscle catabolism was associated with mass decreases.
(b). Influence of treatment on blood parameters
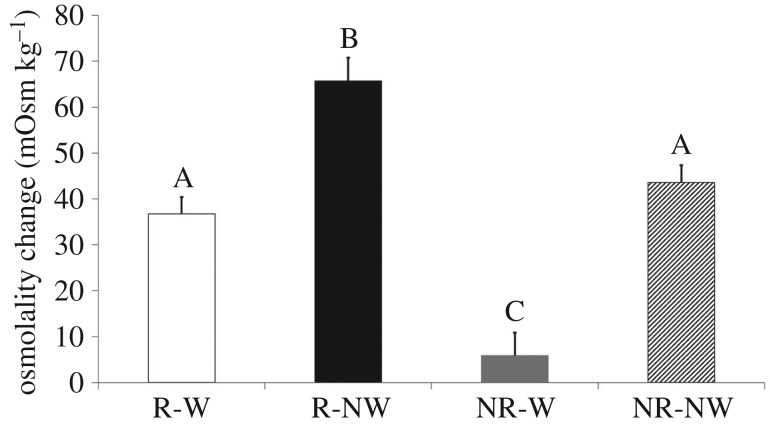
Plasma osmolality prior to the onset of water treatment (late vitellogenesis) was similar among groups (F1,31 = 0.49, p = 0.42). By contrast, at the end of treatment (late gravidity), osmolality was higher in water-deprived females when compared with water-provided ones. We found significant time by water treatment (F1,31 = 56.90, p < 0.001) and time by reproductive status (F1,31 = 34.72, p < 0.001) interactions, but no three-way interaction (F1,31 = 1.00, p = 0.34). When considering osmolality change (i.e. final osmolality minus initial osmolality), we found a significant influence of both treatment (F1,31 = 55.39, p < 0.0001) and reproductive status (F1,31 = 34.97, p < 0.0001), but there was no interaction (F1,31 = 2.13, p = 0.16). An HSD post hoc test revealed that osmolality increases were highest in water-deprived reproductive females (65.70 ± 4.17 mOsm kg−1, all p < 0.05). Osmolality increase was not different between water-deprived non-reproductive females (43.62 ± 4.66 mOsm kg−1) and water-provided reproductive females (36.77 ± 4.39 mOsm kg−1). Finally, the lowest osmolality changes were found in water-provided non-reproductive females (5.87 ± 4.66 mOsm kg−1, HSD post hoc tests, all p < 0.05; figure 2).
Figure 2.
Average plasma osmolality change (mOsm kg−1) measured in non-reproductive (NR) and reproductive (R) female Antaresia childreni with (W) or without (NW) access to water from ovulation to oviposition or its equivalent duration for yoked, non-reproductive females. Error bars represent ±1 s.e.m. Different letters indicate significant differences among groups (HSD post hoc test).
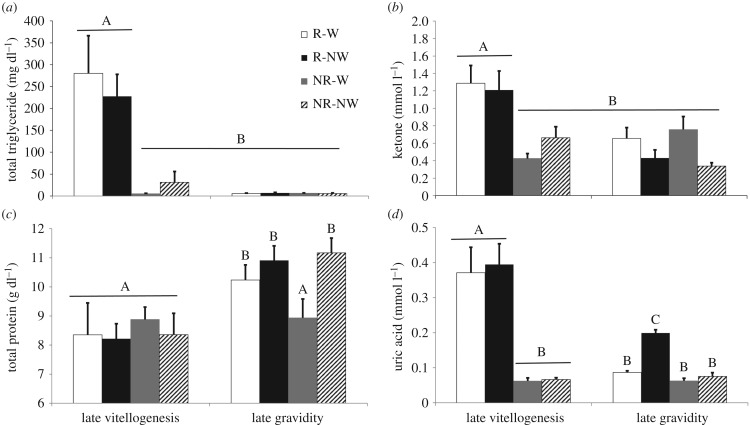
Regarding the biochemical assays, we found a significant time by reproductive status interaction in plasma concentrations of total triglycerides (F1,31 = 18.06, p < 0.001), ketones (F1,31 = 10.72, p = 0.003) and uric acid (F1,31 = 23.51, p < 0.001; figure 3a,b,d) where late vitellogenic females, regardless of water treatment, had higher levels than did non-reproductive females. During late gravidity, water-restricted gravid females had higher uric acid levels compared to the other three groups (all p < 0.05). For total protein, we did not detect any significant interactions (all p > 0.05); however, there was a significant main effect of time where total proteins increased from late vitellogenesis to late gravidity (F1,31 = 16.80, p < 0.005; figure 3c). We did not detect any significant interactions or main effects in plasma glucose concentration (all p > 0.05).
Figure 3.
Mean plasma concentrations of (a) total triglycerides, (b) ketones, (c) total protein and (d) uric acid measured during late vitellogenesis and late gravidity in non-reproductive (NR) and reproductive (R) female Antaresia childreni with (W) or without (NW) access to water from ovulation to oviposition, or its equivalent duration for yoked, non-reproductive females. Error bars represent ±1 s.e.m. Different letters indicate significant differences among groups (HSD post hoc test).
(c). Influence of experimental treatment on reproductive output
Clutch size was influenced by SVL (F1,18 = 6.77, p = 0.02), but not by treatment (12.5 ± 0.7 versus 12.2 ± 0.7 eggs for water-deprived and water-provided females, respectively; F1,18 = 0.09, p = 0.76) or the interaction term (F1,18 = 0.55, p = 0.46).
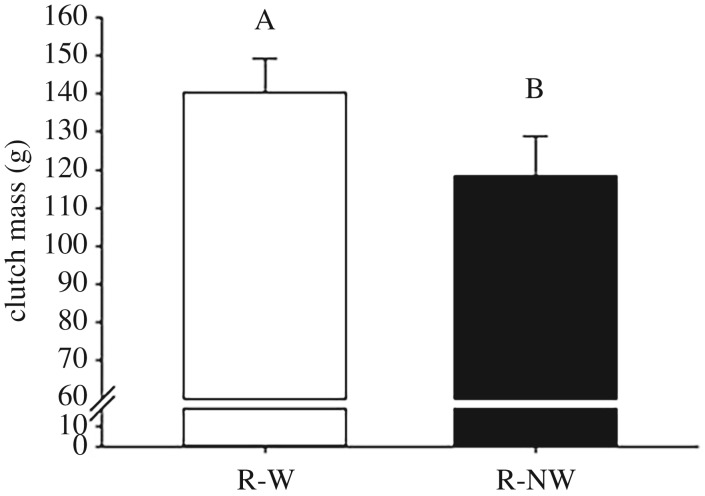
Clutch mass was positively influenced by SVL (F1,15 = 22.86, p < 0.001). We found an influence of size-adjusted fecundity, with relatively more fecund females having heavier clutches (F1,15 = 17.40, p = 0.001). After accounting for these two independent covariates, we detected a significant effect of treatment with water-deprived females producing slightly lighter clutch masses than water-provided females (figure 4; F1,15 = 7.62, p = 0.014). Therefore, water-deprived females produced 16% lighter eggs than did water-provided females (mean egg mass = 9.48 ± 0.36 versus 11.38 ± 0.62 g, respectively, F1,17 = 6.74, p = 0.02). Finally, we found that average epaxial muscle loss was negatively related to clutch mass (F1,17 = 5.65, p = 0.03) and average egg mass (F1,17 = 3.04, p = 0.007). When considering each group separately, the negative relation between epaxial muscle loss and clutch mass was marginal in water-provided ones (F1,8 = 1.31, p = 0.28).
Figure 4.
Influence of treatment on clutch mass (grams) measured in reproductive (R) female Antaresia childreni with (W) or without (NW) access to water from ovulation to oviposition. Plotted values are LS means adjusted for maternal size (SVL) and relative clutch size (see text for statistics). Error bars represent ±1 s.e.m. Different letters indicate significant differences among groups (HSD post hoc test).
4. Discussion
Resource-based trade-offs have shaped many of our current concepts in evolutionary ecology, and energy is usually the currency being balanced. For example, most of our understanding of the gradient between income and capital breeding strategies has focused solely on energetic requirements and cost/benefit balances associated with storage [6,7,27]. Our results provide the first evidence that protein catabolism can be used to support water demands during reproduction, and these findings extend the capital breeding concept to non-energetic resources.
Vitellogenic females had higher plasma concentrations of total triglycerides, ketones and uric acid than did non-reproductive females (figure 3a,b,d), reflecting the energy mobilization required for yolk production into developing follicles of lecitothropic species [28–30]. All plasma osmolalities measured in this study were within the osmolality range of free-ranging A. childreni (279–354 mOsm kg−1) [19], suggesting ecological relevance despite being a laboratory study. Initially, osmolality was similar among all four female groups but, after the water treatment, it was strongly influenced by both treatment and reproductive status. Water-deprived, non-reproductive females had a higher plasma osmolality than did those provided with water. Additionally, females during late gravidity had a higher plasma osmolality than did non-reproductive females, even when both groups had ad libitum access to water (figure 2). These results suggest distinct water imbalances associated with water deprivation and reproduction. Plasma osmolality in females facing both of these challenges simultaneously (i.e. gravid females deprived of water) was significantly different from non-reproductive females deprived of water. Furthermore, the change in osmolality was greater in reproductive, water-deprived females when compared with the three other groups, which underlines the cumulative constraints imposed by reproduction and concurrent water restrictions.
Water deprivation strongly impacted body mass loss and increased protein catabolism in reproductive females (as evidenced by greater loss of epaxial musculature and higher plasma uric acid concentrations; figures 1b, 3d). Because proteins are a much lower energetic source than are lipids (5.3 kJ g−1 and 37.6 kJ g−1, respectively), this suggests that the oxidation of proteins was mostly driven by water needs, as protein supplies five times more total water than do lipids [16]. Muscle loss during reproduction has been documented in insects [31,32], fish [33], reptiles [34], birds [35,36] and mammals [37,38]. Protein mobilization is typically associated with extended fasting and is thought to cover energy and amino acid requirements when lipid reserves and food intake are insufficient [9,39]. Alternatively, protein mobilization can serve as a water resource during times of water restriction or increased water demands [40]. Our study is the first to link muscle atrophy during reproduction to water allocation requirements of developing offspring. Muscle loss associated with fasting and reproduction is known to alter locomotor performance [34,41,42] and induce predation costs [43]. Therefore, using muscle as a water depot under water-limited circumstances leads to an important water-based trade-off between reproduction and performance that is similar to that commonly demonstrated with energy as the currency.
Water deprivation had no impact on clutch size, probably because treatment started at ovulation, after clutch size determination and energy investment is mostly completed [44]. Importantly, water-deprived females produced lighter clutch masses when compared to water-provided females. This impact on reproductive output suggests that maternal catabolism of muscle, while greater in water-deprived females, was not to the extent needed to completely satisfy the water requirements of the developing embryos during gravidity. This implies that there is a trade-off that creates a parent–offspring conflict [45–47]. Evidence for a water-based transgenerational conflict has been previously demonstrated in two viviparous squamates [47,48]. Our study clearly supports the existence of a trade-off between female condition (e.g. muscle mass and osmolality) and reproductive output (e.g. egg size). For the female, loss of epaxial muscle width has been previously correlated with reductions in whole-body performances [49,50] and increases in plasma osmolality alter physiological performances [19,51,52]. From the perspective of the offspring, egg mass in squamates is typically positively correlated with offspring size [53,54]. Additionally, the egg mass difference we report probably reflects reduced water allocation, and lower egg water-content reduces embryonic yolk absorption, resulting in smaller size and reduced offspring performance [55,56].
Our experimental water restriction was limited to the time when females were gravid, which lasted for a rather brief period (three weeks). An even more significant conflict may take place if water deprivation occurs over a longer period and encompasses additional reproductive stages (e.g. vitellogenesis). Furthermore, examining only simple quantitative metrics of reproductive output (e.g. clutch size and egg mass) may miss more cryptic effects, so future research on water-based trade-offs should examine compositional components of eggs, offspring phenotype and offspring performance.
Uricotelic species (i.e. reptiles and birds) produce a highly concentrated, and frequently solid, form of nitrogenous waste. Therefore, the elimination of the nitrogen freed during muscle catabolism requires little water, enabling water acquired from muscle catabolism to be reallocated to internal needs. By contrast, it has been proposed that ureotelic animals do not rely on protein catabolism for water [57], mostly as a result of the nitrogenous wastes requiring a considerable increase in urine production. However, there are examples of ureotelic animals facing times without any external water sources [58–62]. Given the consistent demands for water during reproduction and results presented herein, muscle catabolism to fulfil both energy and water requirements may be a widespread phenomenon deserving of further attention across taxa. This is especially relevant given that current changes in rainfall patterns are challenging reproductive strategies [1,63,64] and can have negative impacts on entire ecosystems [65]. Further alteration in water availability is predicted widely across the globe, and increased muscle mobilization during reproduction may result in higher survival or performance costs of reproduction.
Acknowledgements
This work would not have been possible without assistance from members of the DeNardo lab who helped with breeding colony management and blood collection as well as Department of Animal Care and Technologies staff who assisted with animal care. We thank Dr M. Angilletta for his contributions and O.L. wishes to thank Damien Roussel for fruitful insight on muscle physiology and catabolism.
Ethics
All work was conducted under the oversight of the Arizona State University Institutional Animal Care and Use Committee (protocol no. 17-1532R).
Data accessibility
The datasets supporting this article can be accessed at https://doi.org/10.6084/m9.figshare.6075614.
Authors' contributions
G.A.B., O.L. and D.F.D. designed the study and wrote the manuscript. G.A.B., B.K. and D.F.D. collected the samples. G.A.B. conducted all assays. G.A.B. and O.L. performed the statistical analyses.
Competing interests
We have no competing interests.
Funding
This study is based upon work supported by the National Science Foundation Graduate Research Fellowship for G.A.B. (grant no. 1311230), Arizona State University's Graduate and Professional Students Association JumpStart Grant and Arizona State University's College of Liberal Arts & Sciences Graduate Excellence Fellowship for First Generation Students.
References
- 1.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. ( 10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forrest J, Miller-Rushing AJ. 2010. Toward a synthetic understanding of the role of phenology in ecology and evolution. Phil. Trans. R. Soc. B 365, 3101–3112. ( 10.1098/rstb.2010.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houston AI, Stephens PA, Boyd IL, Harding KC, McNamara JM. 2007. Capital or income breeding? A theoretical model of female reproductive strategies. Behav. Ecol. 18, 241–250. ( 10.1093/beheco/arl080) [DOI] [Google Scholar]
- 4.Jönsson KI, Jonsson KI. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66. ( 10.2307/3545800) [DOI] [Google Scholar]
- 5.Pelisson P-F, Bel-Venner M-C, Rey B, Burgevin L, Martineau F, Fourel F, Lecuyer C, Menu F, Venner S. 2012. Contrasted breeding strategies in four sympatric sibling insect species: when a proovigenic and capital breeder copes with a stochastic environment. Funct. Ecol. 26, 198–206. ( 10.1111/j.1365-2435.2011.01925.x) [DOI] [Google Scholar]
- 6.Stephens PA, Houston AI, Harding KC, Boyd IL, McNamara JM. 2014. Capital and income breeding: the role of food supply. Ecology 95, 882–896. ( 10.1890/13-1434.1) [DOI] [PubMed] [Google Scholar]
- 7.Williams CT, Klaassen M, Barnes BM, Buck CL, Arnold W, Giroud S, Vetter SG, Ruf T. 2017. Seasonal reproductive tactics: annual timing and the capital-to-income breeder continuum. Phil. Trans. R. Soc. B 372, 20160250 ( 10.1098/rstb.2016.0250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe RR. 2002. Regulation of muscle protein by amino acids. J. Nutr. 132, 3219S–3224S. ( 10.1093/jn/131.10.3219S) [DOI] [PubMed] [Google Scholar]
- 9.Lourdais O, Lorioux S, DeNardo DF. 2013. Structural and performance costs of reproduction in a pure capital breeder, the children's python Antaresia childreni. Physiol. Biochem. Zool. 86, 176–183. ( 10.1086/669127) [DOI] [PubMed] [Google Scholar]
- 10.Ross MG, Desai M. 2005. Gestational programming: population survival effects of drought and famine during pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 288, R25–R33. ( 10.1152/ajpregu.00418.2004) [DOI] [PubMed] [Google Scholar]
- 11.Varpe O, Jorgensen C, Tarling GA, Fiksen O. 2009. The adaptive value of energy storage and capital breeding in seasonal environments. OIKOS 118, 363–370. ( 10.1111/j.1600-0706.2008.17036.x) [DOI] [Google Scholar]
- 12.Sainmont J, Andersen KH, Varpe Ø, Visser AW. 2014. Capital versus income breeding in a seasonal environment. Am. Nat. 184, 466–476. ( 10.1086/677926) [DOI] [PubMed] [Google Scholar]
- 13.Kleiner SM. 1999. Water. J. Am. Diet. Assoc. 99, 200–206. ( 10.1016/S0002-8223(99)00048-6) [DOI] [PubMed] [Google Scholar]
- 14.Popkin BM, D'Anci KE, Rosenberg IH. 2010. Water, hydration, and health. Nutr. Rev. 68, 439–458. ( 10.1111/j.1753-4887.2010.00304.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candlish J. 1981. Metabolic water and the camel's hump a text book survey. Biochem. Educ. 9, 96–97. ( 10.1016/0307-4412(81)90212-0) [DOI] [Google Scholar]
- 16.Jenni L, Jenni-Eiermann S. 1998. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol. 29, 521 ( 10.2307/3677171) [DOI] [Google Scholar]
- 17.Bintz GL, Mackin WW. 1980. The effect of water availability on tissue catabolism during starvation in Richardson's ground squirrels. Comp. Biochem. Physiol. A Physiol. 65, 181–186. ( 10.1016/0300-9629(80)90220-0) [DOI] [Google Scholar]
- 18.Gerson AR, Guglielmo CG.. 2011. House sparrows (Passer domesticus) increase protein catabolism in response to water restriction. Am. J. Physiol. Integr. Comp. Physiol. 300, R925–R930. ( 10.1152/ajpregu.00701.2010) [DOI] [PubMed] [Google Scholar]
- 19.Brusch GA, Billy G, Blattman JN, DeNardo DF. 2017. Reproduction alters hydration state but does not impact the positive effects of dehydration on innate immune function in children's pythons (Antaresia childreni). Physiol. Biochem. Zool. 90, 646–654. ( 10.1086/694834) [DOI] [PubMed] [Google Scholar]
- 20.Lourdais O, Heulin B, DeNardo DF. 2008. Thermoregulation during gravidity in the children's python (Antaresia childreni): a test of the preadaptation hypothesis for maternal thermophily in snakes. Biol. J. Linn. Soc. 93, 499–508. ( 10.1111/j.1095-8312.2007.00925.x) [DOI] [Google Scholar]
- 21.Stahlschmidt Z, Brashears J, DeNardo D. 2011. The use of ultrasonography to assess reproductive investment and output in pythons. Biol. J. Linn. Soc. 103, 772–778. ( 10.1111/j.1095-8312.2011.01671.x) [DOI] [Google Scholar]
- 22.Lourdais O, Brischoux F, Barantin L. 2005. How to assess musculature and performance in a constricting snake? A case study in the Colombian rainbow boa (Epicrates cenchria maurus). J. Zool. 265, 43–51. ( 10.1017/S095283690400603X) [DOI] [Google Scholar]
- 23.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 24.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in genneral parametric models. Biometrical J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro J, Bates D, DebRoy S, Sarkar D.. 2016. nlme: linear and nonlinear mixed effects models. R Packag. version. R package.
- 26.De Mendiburu F. 2016. agricolae: statistical procedures for agricultural research. R package.
- 27.Warne RW, Gilman CA, Garcia DA, Wolf BO.. 2012. Capital breeding and allocation to life-history demands are highly plastic in lizards. Am. Nat. 180, 130–141. ( 10.1086/665995) [DOI] [PubMed] [Google Scholar]
- 28.Key C, Ross C. 1999. Sex differences in energy expenditure in non-human primates. Proc. R. Soc. B 266, 2479–2485. ( 10.1098/rspb.1999.0949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sibly RM, Witt CC, Wright NA, Venditti C, Jetz W, Brown JH. 2012. Energetics, lifestyle, and reproduction in birds. Proc. Natl Acad. Sci. USA 109, 10 937–10 941. ( 10.1073/pnas.1206512109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBride RS, Somarakis S, Fitzhugh GR, Albert A, Yaragina NA, Wuenschel MJ, Alonso-Fernández A, Basilone G. 2015. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish. 16, 23–57. ( 10.1111/faf.12043) [DOI] [Google Scholar]
- 31.Cheng Y, Luo L, Sappington TW, Jiang X, Zhang L, Frolov AN. 2016. Onset of oviposition triggers abrupt reduction in migratory flight behavior and flight muscle in the female beet webworm, Loxostege sticticalis. PLoS ONE 11, e0166859 ( 10.1371/journal.pone.0166859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khuhro NH, Biondi A, Desneux N, Zhang L, Zhang Y, Chen H. 2014. Trade-off between flight activity and life-history components in Chrysoperla sinica. BioControl 59, 219–227. ( 10.1007/s10526-014-9560-4) [DOI] [Google Scholar]
- 33.Lambert Y, Dutil J-D. 2000. Energetic consequences of reproduction in Atlantic cod (Gadus morhua) in relation to spawning level of somatic energy reserves. Can. J. Fish. Aquat. Sci. 57, 815–825. ( 10.1139/f00-022) [DOI] [Google Scholar]
- 34.Lourdais O, Brischoux F, DeNardo D, Shine R. 2004. Protein catabolism in pregnant snakes (Epicrates cenchria maurus Boidae) compromises musculature and performance after reproduction. J. Comp. Physiol. B 174, 383–391. ( 10.1007/s00360-004-0424-6) [DOI] [PubMed] [Google Scholar]
- 35.Cherel Y, Robin J-P, Le Maho Y. 1988. Physiology and biochemistry of long-term fasting in birds. Can. J. Zool. 66, 159–166. ( 10.1139/z88-022) [DOI] [Google Scholar]
- 36.Veasey JS, Houston DC, Metcalfe NB. 2000. Flight muscle atrophy and predation risk in breeding birds. Funct. Ecol. 14, 115–121. ( 10.1046/j.1365-2435.2000.00391.x) [DOI] [Google Scholar]
- 37.Crocker DE, Webb PM, Costa DP, Le Boeuf BJ. 1998. Protein catabolism and renal function in lactating northern elephant seals. Physiol. Zool. 71, 485–491. ( 10.1086/515971) [DOI] [PubMed] [Google Scholar]
- 38.Harlow HJ, Lohuis T, Grogan RG, Beck TDI. 2002. Body mass and lipid changes by hibernating reproductive and nonreproductive black bears (Ursus americanus). J. Mammal. 83, 1020–1025. ( 10.1644/1545-1542(2002)083%3C1020:BMALCB%3E2.0.CO;2) [DOI] [Google Scholar]
- 39.Cherel Y, Robin JP, Heitz A, Calgari C, le Maho Y. 1992. Relationships between lipid availability and protein utilization during prolonged fasting. J. Comp. Physiol. B 162, 305–313. ( 10.1007/BF00260757) [DOI] [PubMed] [Google Scholar]
- 40.Gerson AR, Guglielmo CG. 2011. Flight at low ambient humidity increases protein catabolism in migratory birds. Science 333, 1434–1436. ( 10.1126/science.1210449) [DOI] [PubMed] [Google Scholar]
- 41.Speakman JR. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398. ( 10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zera AJ, Zhao Z. 2006. Intermediary metabolism and life-history trade-offs: differential metabolism of amino acids underlies the dispersal-reproduction trade-off in a wing-polymorphic cricket. Am. Nat. 167, 889–900. ( 10.1086/503578) [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Witter MS, Cuthill IC, Goldsmith AR. 1996. Reduction in escape performance as a cost of reproduction in gravid starlings, Sturnus vulgaris. Proc. R. Soc. Lond. B 263, 619–623. ( 10.1098/rspb.1996.0093) [DOI] [Google Scholar]
- 44.Lourdais O, Bonnet X, Shine R, Taylor EN. 2003. When does a reproducing female viper (Vipera aspis) ‘decide'on her litter size? J. Zool. 259, 123–129. ( 10.1017/S0952836902003059) [DOI] [Google Scholar]
- 45.Crespi B, Semeniuk C. 2004. Parent–offspring conflict in the evolution of vertebrate reproductive mode. Am. Nat. 163, 635–653. ( 10.1086/382734) [DOI] [PubMed] [Google Scholar]
- 46.Pollux BJA, Meredith RW, Springer MS, Garland T, Reznick DN. 2014. The evolution of the placenta drives a shift in sexual selection in livebearing fish. Nature 513, 233–236. ( 10.1038/nature13451) [DOI] [PubMed] [Google Scholar]
- 47.Dupoué A, Brischoux F, Angelier F, DeNardo DF, Wright CD, Lourdais O. 2015. Intergenerational trade-off for water may induce a mother-offspring conflict in favour of embryos in a viviparous snake. Funct. Ecol. 29, 414–422. ( 10.1111/1365-2435.12349) [DOI] [Google Scholar]
- 48.Dupoué A, Le Galliard J-F, Josserand R, DeNardo DF, Decencière B, Agostini S, Haussy C, Meylan S. 2018. Water restriction causes an intergenerational trade-off and delayed mother-offspring conflict in a viviparous lizard. Funct. Ecol. 32, 676–686. ( 10.1111/1365-2435.13009) [DOI] [Google Scholar]
- 49.Stahlschmidt ZR, Lourdais O, Lorioux S, Butler MW, Davis JR, Salin K, Voituron Y, DeNardo DF. 2013. Morphological and physiological changes during reproduction and their relationships to reproductive performance in a capital breeder. Physiol. Biochem. Zool. 86, 398–409. ( 10.1086/670918) [DOI] [PubMed] [Google Scholar]
- 50.Dupoué A, Lourdais O. 2014. Relative reproductive effort drives metabolic changes and maternal emaciation during pregnancy in a viviparous snake. J. Zool. 293, 49–56. ( 10.1111/jzo.12116) [DOI] [Google Scholar]
- 51.Brusch GA, DeNardo DF. 2017. When less means more: dehydration improves innate immunity in rattlesnakes. J. Exp. Biol. 220, 2287–2295. ( 10.1242/jeb.155028) [DOI] [PubMed] [Google Scholar]
- 52.Moeller KT, Butler MW, DeNardo DF. 2013. The effect of hydration state and energy balance on innate immunity of a desert reptile. Front. Zool. 10, 23 ( 10.1186/1742-9994-10-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olsson M, Shine R. 1997. The limits to reproductive output: offspring size versus number in the sand lizard (Lacerta agilis). Am. Nat. 149, 179–188. ( 10.1086/285985) [DOI] [Google Scholar]
- 54.Aubret F, Shine R, Bonnet X. 2004. Adaptive developmental plasticity in snakes. Nature 431, 261–262. ( 10.1038/431261a) [DOI] [PubMed] [Google Scholar]
- 55.Shine R, Brown GP. 2002. Effects of seasonally varying hydric conditions on hatchling phenotypes of keelback snakes (Tropidonophis mairii, Colubridae) from the Australian wet–dry tropics. Biol. J. Linn. Soc. 76, 339–347. ( 10.1046/j.1095-8312.2002.00068.x) [DOI] [Google Scholar]
- 56.Aubret F, Bonnet X, Shine R, Maumelat S. 2005. Why do female ball pythons (Python regius) coil so tightly around their eggs? Evol. Ecol. Res. 7, 743–758. [Google Scholar]
- 57.McCue MD, Sandoval J, Beltran J, Gerson AR. 2017. Dehydration causes increased reliance on protein oxidation in mice: a test of the protein-for-water hypothesis in a mammal. Physiol. Biochem. Zool. 90, 359–369. ( 10.1086/690912) [DOI] [PubMed] [Google Scholar]
- 58.Maltz E, Shkolnik A. 1980. Milk production in the desert: lactation and water economy in the black bedouin goat. Physiol. Zool. 53, 12–18. ( 10.1086/physzool.53.1.30155770) [DOI] [Google Scholar]
- 59.Oftedal OT. 1993. The adaptation of milk secretion to the constraints of fasting in bears, seals, and baleen whales. J. Dairy Sci. 76, 3234–3246. ( 10.3168/jds.S0022-0302(93)77660-2) [DOI] [PubMed] [Google Scholar]
- 60.Wynne-Edwards KE. 1998. Evolution of parental care in Phodopus: conflict between adaptations for survival and adaptations for rapid reproduction. Am. Zool. 38, 238–250. ( 10.1093/icb/38.1.238) [DOI] [Google Scholar]
- 61.Benoit JB, Hansen IA, Attardo GM, Michalková V, Mireji PO, Bargul JL, Drake LL, Masiga DK, Aksoy S. 2014. Aquaporins are critical for provision of water during lactation and intrauterine progeny hydration to maintain tsetse fly reproductive success. PLoS Negl. Trop. Dis. 8, e2517 ( 10.1371/journal.pntd.0002517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarli J, Lutermann H, Alagaili AN, Mohammed OB, Bennett NC. 2016. Seasonal reproduction in the Arabian spiny mouse, Acomys dimidiatus (Rodentia: Muridae) from Saudi Arabia: the role of rainfall and temperature. J. Arid Environ. 124, 352–359. ( 10.1016/j.jaridenv.2015.09.008) [DOI] [Google Scholar]
- 63.Thomas DW. 2001. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600. ( 10.1126/science.1057487) [DOI] [PubMed] [Google Scholar]
- 64.Visser ME, Both C, Lambrechts MM. 2004. Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 35, 89–110. ( 10.1016/S0065-2504(04)35005-1) [DOI] [Google Scholar]
- 65.McCluney KE, et al. 2012. Shifting species interactions in terrestrial dryland ecosystems under altered water availability and climate change. Biol. Rev. 87, 563–582. ( 10.1111/j.1469-185X.2011.00209.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article can be accessed at https://doi.org/10.6084/m9.figshare.6075614.