ABSTRACT
Gene expression has been considered a highly accurate process, and deviation from such fidelity has been shown previously to be detrimental for the cell. More recently, increasing evidence has supported the notion that the accuracy of gene expression is indeed flexibly variable. The levels of errors during gene expression differ from condition to condition and even from cell to cell within genetically identical populations grown under the same conditions. The different levels of errors resulting from inaccurate gene expression are now known to play key roles in regulating microbial stress responses and host interactions. This minireview summarizes the recent development in understanding the level, regulation, and physiological impact of errors during gene expression.
KEYWORDS: mistranslation, phenotypic heterogeneity, protein synthesis, stress response
IMPORTANCE
Gene expression has been considered a highly accurate process, and deviation from such fidelity has been shown previously to be detrimental for the cell. More recently, increasing evidence has supported the notion that the accuracy of gene expression is indeed flexibly variable.
INTRODUCTION
Gene expression is a fundamental process in all living cells and controls the accurate flow of genetic information from DNA to RNA to protein. To ensure the accuracy of gene expression, extensive substrate selection and proofreading mechanisms are utilized at each step during DNA replication, transcription, and translation (1–3). For instance, aminoacyl-tRNA synthetases selectively pair each amino acid with the correct tRNAs and utilize editing functions to hydrolyze mismatched aminoacyl-tRNAs (4, 5). Despite such conserved quality control mechanisms, the fidelity of gene expression is not fixed under all conditions (Fig. 1). Genetic and environmental changes can substantially increase the levels of errors during gene expression. In this minireview, we summarize recent developments in our understanding of the prevalence, regulation, and physiological impact of gene expression errors, with a focus on microbial organisms.
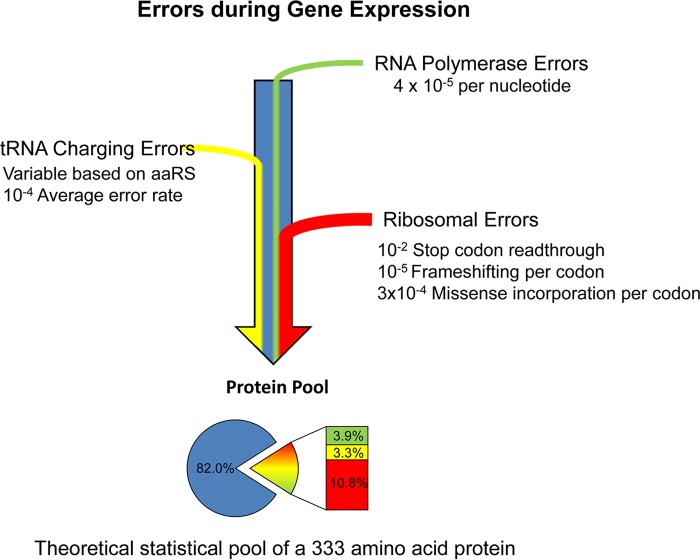
FIG 1 .
Errors during gene expression. Nonheritable errors during gene expression can come from transcription and translation. For a coding gene with around 300 to 400 codons, approximately 10% to 20% of the proteins made contain at least one error, such as missense incorporation, frameshifting, or stop codon readthrough. This fraction of erroneous proteins significantly increases when the error rates per codon are increased by genetic and environmental factors, leading to a statistical proteome containing very diverse protein variants encoded by the same gene. aaRS, aminoacyl-tRNA synthetase.
FIDELITY OF GENE EXPRESSION
Transcriptional fidelity.
Accurate transcription of DNA into mRNA is essential for the transfer of genetic information to the protein synthesis machinery. Despite the clear role that transcriptional accuracy must play in gene expression, transcriptional error rates have been estimated to reach 10−5 errors per nucleotide (2). Traditionally, transcriptional error rates are determined using in vitro transcription by RNA polymerases or in vivo reporters (6). Several recent studies have used high-throughput RNA sequencing with improved fidelity during cDNA synthesis and sequencing to determine global transcriptional error rates in multiple bacteria (7). The transcriptional error rates measured by RNA sequencing match the in vitro error rates on the order of 10−5. Interestingly, transcriptional error rates are similar between extracellular Escherichia coli and endosymbiotic bacteria despite the striking differences in genome sizes and growth conditions (7). In addition, the transcriptional error rate is not affected by growth stages or nutrient sources (7). It appears that transcriptional fidelity is optimized during evolution to resist perturbation by environmental cues. Only a few genetic factors have been identified to control transcriptional fidelity; these include transcription elongation factors GreA and GreB (2, 8) and stringent response regulator DksA (9, 10). Due to their transient nature, the errors generated during transcription are thought to have less of an impact than the DNA replication errors that accumulate over generations. However, increased transcriptional errors have been shown to significantly affect the molecular heterogeneity (noise) of gene expression (discussed below) (10–12).
Translational fidelity.
Compared with transcriptional errors, the overall amino acid misincorporation (missense) rate in the protein is an order higher at approximately 10−4 to 10−3, largely due to imperfections of aminoacyl-tRNA synthesis and ribosomal decoding (4, 13–16). Other types of translational errors, such as stop codon readthrough and frameshifting, could occur more frequently in bacteria and eukaryotes at 10−2 (17–22). It is worth pointing out that under certain circumstances, recoding resulting from translational readthrough or frameshifting depends on the context of mRNA sequences and can lead to production of alternative functional proteins and provide a selective advantage during evolution (20, 23). Such context-dependent recoding events are generally not considered gene expression errors and are beyond the scope of this minireview.
Translational errors have been measured using radiolabeled amino acids (24, 25), enzyme reporters (14, 26–29), mass spectrometry (19, 28, 30), ribosome profiling (31, 32), and fluorescent reporters (18, 21, 33–37). All those assays indeed measure the overall error rates in gene expression, but, given that translation is much more error-prone than DNA replication and transcription, such results serve as a good estimation for the error rate during translation. Despite the technical advancement and growing interest, the picture of the actual rates of different translational errors still remains blurry. It is now increasingly clear that translational fidelity is affected by genetic and environmental factors (3, 38–40), and the same type of translational error may differ from cell to cell (18, 34). To allow accurate quantitation of different translational errors in cells under native growth conditions, further advances in the availability of sensitive reporters and in mass spectrometry technology are much needed.
HETEROGENEITY OF GENE EXPRESSION
Bacterial populations are comprised of millions of clonal cells. Despite the genetic similarity between these cells, individual cells within a population exhibit a wide variety of physiological phenotypes (41). Nearly every aspect of bacteria physiology, including shape, size, growth rate, motility, and stress tolerance, has some level of heterogeneity (noise) within a population. Many of the mechanisms that can lead to population heterogeneity have been reviewed elsewhere (41), and variation in gene expression has been shown to be a critical contributor to the heterogeneity among cells. More-recent work has revealed how different aspects of gene expression, from initiation of transcription to production of a polypeptide, are heterogeneous between single cells in a population.
Transcriptional heterogeneity.
The better-understood aspect of noisy gene expression is transcriptional heterogeneity. Experimental evidence for gene expression noise within a population was first revealed in bacterial cells (42, 43). Ozbudak et al. showed that the expression levels of a fluorescent protein differ from cell to cell within a population of genetically identical Bacillus subtilis cells (43). Using two fluorescence reporters controlled by identical promoters in E. coli, Elowitz et al. found that promoter activity is heterogeneous among cells and is stochastic within the cell, particularly when the transcription level is low (42). Both of those studies used protein fluorescence as the readout for gene expression, and the overall heterogeneity of fluorescence intensity reflected the cumulative noise from transcription, mRNA degradation, translation, protein degradation, and fluorophore maturation. To specifically study transcriptional noise, a breakthrough came from the use of MS2-green fluorescent protein (MS2-GFP) to directly count the number of stable mRNA molecules carrying the MS2 binding sites in E. coli (44). Subsequent studies revealed that transcription initiation does not occur continuously but rather as bursts (45, 46). Variations in promoter activity are large contributors to variations in single-cell gene expression. In 2012, a study characterized the heterogeneity of every known promoter in E. coli and found that different promoters show different levels of heterogeneity in a population (47). Some categories of promoters, such as stress response promoters, are noisier than others (47). Heterogeneity of gene expression was initially thought to be a consequence of the stochastic nature of molecular interactions (42). However, recent analyses of the evolution of synthetic promoters de novo revealed that the heterogeneity of promoter expression is low by default (48). This finding indicates that the high levels of heterogeneity seen in some promoters may have evolved as a beneficial mechanism. Future investigations into the regulation of promoter heterogeneity and evolution of these systems may provide insights into the role and benefits of transcriptional heterogeneity in bacterial populations.
Transcriptional heterogeneity has been directly tied to phenotypic heterogeneity in bacterial populations. The mechanisms by which gene expression heterogeneity can influence bacterial physiology have been previously reviewed (41, 49). Recently, a report showed that the levels of heterogeneity itself are regulated and can influence the fitness of a bacterial population under stress (50). In that work, Carey et al. showed that an E. coli population responds to changes in O2 levels by altering the heterogeneity of a signal transduction system without changing the population mean. This indicates that the mechanisms controlling transcriptional heterogeneity can be regulated independently of the average transcription level and highlights the importance of further studies of single-cell gene expression dynamics.
Despite our improving knowledge of overall transcriptional heterogeneity, the noise of transcriptional errors is poorly understood due to technical challenges. Interestingly, Herman and colleagues have shown that increasing transient transcriptional errors by deleting greAB or dksA genes can alter the stochastic switching frequency of gene expression (10–12). This leads to bistable feedback loops and heritable phenotypic changes. In future studies, it will be intriguing to investigate whether variations of transcriptional errors among individual cells directly correlate with bistable gene expression.
Translational heterogeneity.
Compared to transcriptional noise, the posttranscriptional heterogeneity of gene expression has not been extensively studied. This is primarily due to technical limitations because the noise from transcription is difficult to filter out. However, recently developed reporters can account for changes in single-cell transcription and have provided insights into how variability in posttranscriptional processes may affect the proteome and cell physiology in single cells.
Like changes in transcription, changes in translational rates ultimately have a significant effect on the protein levels in a cell. As such, variations in the overall translational rate in a cell or variations in affinity for ribosome binding sites could contribute to the cell heterogeneity within a population. Despite these similarities between transcription and translation, much less is known about the mechanisms and impact of translation on population heterogeneity. Early work used single fluorescent reporters to determine how translation initiation and codon context affect heterogeneity (43, 51). Those pioneering studies revealed that altering translation initiation and elongation perturbs the overall gene expression noise. However, signals of single fluorescent reporters are heavily influenced by transcriptional levels, making it difficult to fully examine the contribution of translation to the overall gene expression heterogeneity (52).
The recent development of dual-fluorescence reporters to measure the heterogeneity of translational fidelity has been a step forward toward our understanding of posttranscriptional gene expression noise. These reporters use a control fluorescence protein that is translationally fused to a second fluorescence protein in order to normalize differences in transcription and translation initiation in single cells and have enabled the quantification of ribosomal missense errors (34–36), stop codon readthrough (18), and frameshifting events (18, 21, 37). The concept of dual reporters that are translationally fused originated from a dual-luciferase system (14, 29, 53). Compared to luciferase reporters, dual-fluorescence reporters allow quantitation of translational errors within single cells using either fluorescence imaging or flow cytometry. It needs to be noted that in performing quantitation using such reporters, the signal or activity of the first reporter should not be much affected by the fusion. Should this not be the case (i.e., if the first reporter is affected by the fusion), one remedy would be to introduce a linker that allows the reporters to rapidly split following translation (54).
Studies using the dual-fluorescence reporters have revealed that translational errors within single cells are noisy and can lead to phenotypic heterogeneity (18, 34). In mycobacteria, misincorporation of glutamate at glutamine codons occurs at a frequency of approximately 1% and can cause phenotypic resistance to rifampin by producing drug-resistant variants of the target protein RpoB (34, 55). In an elegant study, Javid and colleagues used fluorescence-activated cell sorting to show that misincorporation rates differ from cell to cell and that the subpopulations of cells with high misincorporation rates survive better in the presence of rifampin (34). We have recently developed a dual-fluorescence reporter system to measure stop-codon readthrough and frameshifting and have used fluorescence microscopy to quantitatively demonstrate that such translational errors are heterogeneous among single cells in E. coli (18) and Salmonella enterica serovar Typhimurium (K. Weiss and J. Ling, unpublished results). We further used time-lapse microscopy to show that cells with increased readthrough of the UGA stop codon exhibit an increased rate of recovery from the stationary phase compared to cells with a low readthrough rate (18). Those studies suggest that, in addition to gene expression levels, other factors of gene expression (such as translational fidelity) can play a crucial role in cell physiology at the single-cell level.
GENE EXPRESSION ERRORS AND MICROBIAL STRESSES
Microorganisms constantly experience changing environments and quickly reprogram gene networks to adapt to stress conditions (40, 56, 57). Increasing evidence supports the notion that gene expression errors play a critical role in sensing and responding to various environmental stresses. For example, carbon starvation increases translational frameshifting (58) and stop codon readthrough (22) in E. coli, and oxidative stress increases amino acid misincorporation errors (28, 59, 60). To learn more about the mechanisms and conditions that cause alteration of translational fidelity, readers are referred to two excellent recent reviews (39, 40).
Gene expression errors are regulated by environmental factors but, conversely, also affect adaptation of microbes to environmental conditions, such as stresses (Fig. 2). As discussed above, transcriptional and translational errors are significant sources of molecular noise and lead to a statistical proteome with mixed protein variants encoded by the same gene, providing phenotypic diversity that allows the microbial population to survive and thrive. For example, phenotypic mutations resulting from an error-prone RNA polymerase with a 20-fold increase in transcriptional errors promote evolution of β-lactam resistance (62). Transcriptional errors also lead to heritable phenotypic changes as a consequence of activation of bistable switches that regulate important pathways, including metabolic gene and cellular differentiation pathways (11, 49).
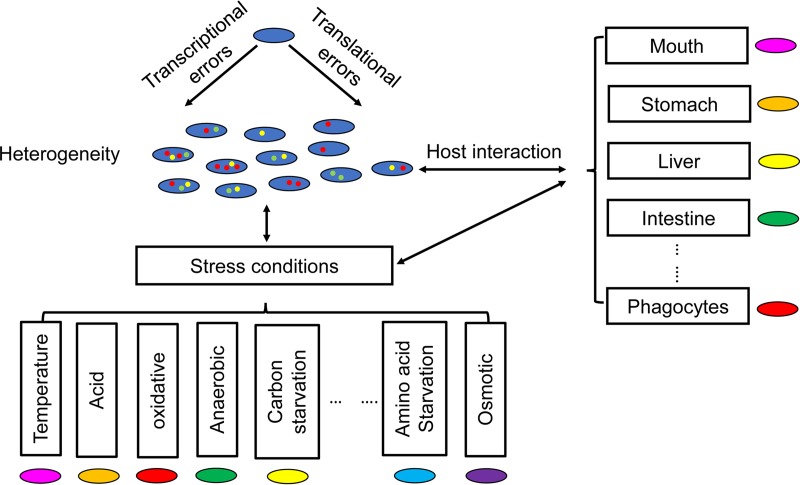
FIG 2 .
Single microbial cells in a population of genetically identical cells display heterogeneity in gene expression errors due to stochasticity of transcription and translation. The mean and heterogeneity of errors can be influenced by environmental factors, such as different stresses. Errors in gene expression in turn make microbial cells better adapted or less well adapted to stress and host environments.
Inaccuracy in the translation machinery appears to have more diverse and profound effects on microbial fitness and stress resistance than transcriptional errors (Table 1). Accumulation of translational errors has been shown to cause proteome destabilization, growth defects, and even cell death (63–66). Reports of recent work from the laboratory of M. Ibba showed that editing deficiencies in phenylalanyl-tRNA synthetase (PheRS), which cause misincorporation of Tyr and meta-Tyr at Phe codons, attenuate amino acid stress response in bacteria and yeast (67, 68). In E. coli, starvation of Phe leads to accumulation of uncharged tRNAPhe, which activates transcription of Phe biosynthesis gene pheA. PheRS editing deficiencies cause mischarging of tRNAPhe and repress transcription of pheA (67). Similarly, uncharged tRNA activates an amino acid starvation response in yeast through the Gcn2/Gcn4 pathway, and PheRS editing deficiency decreases activation of Gcn2p (68). On the other hand, editing defects in aminoacyl-tRNA synthetases benefit bacterial growth when the cognate amino acid is limited in abundance and the mischarged amino acid is abundant (69, 70). Various translational errors have also been reported to improve resistance against antimicrobial, oxidative, and heat stresses (summarized in Table 1). For instance, translational errors lead to resistance against oxidative stress in bacteria and yeasts, but those effects likely occur via distinct mechanisms (71–73).
TABLE 1 .
Effects of translational errors on microbial stress resistance
| Translational error(s) | Stress condition(s) | Organism(s) | Fitness | Reference(s) |
|---|---|---|---|---|
| Ile → norvaline | Amino acid starvation | Escherichia coli | Gain | 69 |
| Ile → Val | Amino acid starvation | Acinetobacter baylyi | Gain | 70 |
| Phe → meta-Tyr | Amino acid starvation | Escherichia coli | Loss | 67 |
| Phe → Tyr | Amino acid starvation | Saccharomyces cerevisiae | Loss | 68 |
| Gln → Glu; Asn → Asp | Antibiotics | Mycobacteria | Gain | 34, 55 |
| Ile → Val | Antibiotics | Escherichia coli | Gain | 82 |
| Met misincorporation | Antibiotics | Escherichia coli | Gain | 83 |
| CUG codon ambiguity | Antifungal drugs | Candida albicans | Gain | 81 |
| Arg → canavanine | Heat stress | Saccharomyces cerevisiae | Gain | 84, 85 |
| CUG codon ambiguity | Oxidative and osmotic stresses | Saccharomyces cerevisiae | Gain | 72 |
| Global mistranslation | Oxidative stress | Escherichia coli | Gain | 27, 71 |
| Stop codon readthrough | Various stresses | Saccharomyces cerevisiae | Gain/loss | 73, 86 |
| Ile → Val | Sporulation | Bacillus subtilis | Loss | 87 |
TRANSLATIONAL ERRORS AND MICROBE-HOST INTERACTIONS
Microbial pathogens must adapt to diverse host environments during infection by triggering specific stress responses and expression of virulence genes (57). In addition to stress resistance, translational errors have also been shown to play a critical role in microbe-host interactions. Modifications of the 16S rRNA gene, such as methylation modifications, are important for maintaining accuracy in translation initiation (74). Deficiencies in 16S rRNA methylation have been reported to decrease virulence in Staphylococcus aureus due to increased sensitivity to oxidative stress (75, 76). Restrictive mutations in the ribosomal protein RpsL enhance translational fidelity (77) and decrease survival of S. enterica serovar Typhimurium in mice (78, 79), suggesting that moderate levels of translational errors in the wild-type bacteria are important for adaption to host environment. In the fungal human pathogen Candida albicans, 97% of CUG codons are translated as Ser and 3% as Leu (80). Bezerra et al. showed that C. albicans strains with increased levels of Leu incorporation at CUG positions cause enhanced host immune response (81), and it has been suggested that CUG ambiguity has evolved to potentially enhance cell surface variability (80). Our overall understanding of how translational fidelity impacts microbe-pathogen interactions is only at the beginning stage, and much work needs to be performed to elucidate the roles of various translational errors in the invasion and survival of different microbial pathogens within hosts.
CLOSING REMARKS AND OUTLOOK
Studies in the past couple of decades have shown that fidelity in gene expression is dynamic and highly regulated. Increased errors during gene expression have various effects on cell fitness and stress responses. However, not all errors are the same, and different types of errors can elicit very different responses even in the same organism. For the most part, the mechanisms by which different gene expression errors lead to physiological changes are not clearly understood and await future investigations. Recent developments in fluorescence reporters have provided a high-throughput platform to determine the error rates during gene expression and now empower us to track various errors under the native growth conditions experienced by microbes (e.g., within biofilms or hosts) at the single-cell and population levels. Translational errors appear to be heterogeneous among single isogenic microbial cells. In future work, it will also be intriguing to understand how such noise in gene expression leads to heterogeneity in diverse microbial phenotypes.
ACKNOWLEDGMENTS
The work in our laboratory was funded by NIGMS R01GM115431 (J.L.).
Footnotes
Citation Evans CR, Fan Y, Weiss K, Ling J. 2018. Errors during gene expression: single-cell heterogeneity, stress resistance, and microbe-host interactions. mBio 9:e01018-18. https://doi.org/10.1128/mBio.01018-18.
REFERENCES
- 1.Francklyn CS. 2008. DNA polymerases and aminoacyl-tRNA synthetases: shared mechanisms for ensuring the fidelity of gene expression. Biochemistry 47:11695–11703. doi: 10.1021/bi801500z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon AJ, Satory D, Halliday JA, Herman C. 2015. Lost in transcription: transient errors in information transfer. Curr Opin Microbiol 24:80–87. doi: 10.1016/j.mib.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaher HS, Green R. 2009. Fidelity at the molecular level: lessons from protein synthesis. Cell 136:746–762. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascarenhas AP, An S, Rosen AE, Martinis SA, Musier-Forsyth K. 2008. Fidelity mechanisms of the aminoacyl-tRNA synthetases, p 153–200. In RajBhandary UL, Köhrer C (ed), Protein engineering. Springer, New York, NY. [Google Scholar]
- 5.Ling J, Reynolds N, Ibba M. 2009. Aminoacyl-tRNA synthesis and translational quality control. Annu Rev Microbiol 63:61–78. doi: 10.1146/annurev.micro.091208.073210. [DOI] [PubMed] [Google Scholar]
- 6.Gamba P, Zenkin N. 2018. Transcription fidelity and its roles in the cell. Curr Opin Microbiol 42:13–18. doi: 10.1016/j.mib.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traverse CC, Ochman H. 2016. Conserved rates and patterns of transcription errors across bacterial growth states and lifestyles. Proc Natl Acad Sci U S A 113:3311–3316. doi: 10.1073/pnas.1525329113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erie DA, Hajiseyedjavadi O, Young MC, von Hippel PH. 1993. Multiple RNA polymerase conformations and GreA: control of the fidelity of transcription. Science 262:867–873. doi: 10.1126/science.8235608. [DOI] [PubMed] [Google Scholar]
- 9.Roghanian M, Zenkin N, Yuzenkova Y. 2015. Bacterial global regulators DksA/ppGpp increase fidelity of transcription. Nucleic Acids Res 43:1529–1536. doi: 10.1093/nar/gkv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satory D, Gordon AJ, Wang M, Halliday JA, Golding I, Herman C. 2015. DksA involvement in transcription fidelity buffers stochastic epigenetic change. Nucleic Acids Res 43:10190–10199. doi: 10.1093/nar/gkv839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon AJ, Halliday JA, Blankschien MD, Burns PA, Yatagai F, Herman C. 2009. Transcriptional infidelity promotes heritable phenotypic change in a bistable gene network. PLoS Biol 7:e44. doi: 10.1371/journal.pbio.1000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon AJ, Satory D, Halliday JA, Herman C. 2013. Heritable change caused by transient transcription errors. PLoS Genet 9:e1003595. doi: 10.1371/journal.pgen.1003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurland CG. 1992. Translational accuracy and the fitness of bacteria. Annu Rev Genet 26:29–50. doi: 10.1146/annurev.ge.26.120192.000333. [DOI] [PubMed] [Google Scholar]
- 14.Kramer EB, Farabaugh PJ. 2007. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA 13:87–96. doi: 10.1261/rna.294907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer EB, Vallabhaneni H, Mayer LM, Farabaugh PJ. 2010. A comprehensive analysis of translational missense errors in the yeast Saccharomyces cerevisiae. RNA 16:1797–1808. doi: 10.1261/rna.2201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaher HS, Green R. 2009. Quality control by the ribosome following peptide bond formation. Nature 457:161–166. doi: 10.1038/nature07582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donoghue P, Prat L, Heinemann IU, Ling J, Odoi K, Liu WR, Söll D. 2012. Near-cognate suppression of amber, opal and quadruplet codons competes with aminoacyl-tRNAPyl for genetic code expansion. FEBS Lett 586:3931–3937. doi: 10.1016/j.febslet.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y, Evans CR, Barber KW, Banerjee K, Weiss KJ, Margolin W, Igoshin OA, Rinehart J, Ling J. 2017. Heterogeneity of stop codon readthrough in single bacterial cells and implications for population fitness. Mol Cell 67:826–836.e5. doi: 10.1016/j.molcel.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohler K, Aerni HR, Gassaway B, Ling J, Ibba M, Rinehart J. 2017. MS-READ: quantitative measurement of amino acid incorporation. Biochim Biophys Acta 1861:3081–3088. doi: 10.1016/j.bbagen.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins JF, Loughran G, Bhatt PR, Firth AE, Baranov PV. 2016. Ribosomal frameshifting and transcriptional slippage: from genetic steganography and cryptography to adventitious use. Nucleic Acids Res 44:7007–7078. doi: 10.1093/nar/gkw530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakauskaite R, Liao PY, Rhodin MH, Lee K, Dinman JD. 2011. A rapid, inexpensive yeast-based dual-fluorescence assay of programmed-1 ribosomal frameshifting for high-throughput screening. Nucleic Acids Res 39:e97. doi: 10.1093/nar/gkr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballesteros M, Fredriksson A, Henriksson J, Nyström T. 2001. Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J 20:5280–5289. doi: 10.1093/emboj/20.18.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling J, O’Donoghue P, Söll D. 2015. Genetic code flexibility in microorganisms: novel mechanisms and impact on physiology. Nat Rev Microbiol 13:707–721. doi: 10.1038/nrmicro3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann RA, Garvin RT, Gorini L. 1971. Alteration of a 30S ribosomal protein accompanying the ram mutation in Escherichia coli. Proc Natl Acad Sci U S A 68:2263–2267. doi: 10.1073/pnas.68.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling J, Yadavalli SS, Ibba M. 2007. Phenylalanyl-tRNA synthetase editing defects result in efficient mistranslation of phenylalanine codons as tyrosine. RNA 13:1881–1886. doi: 10.1261/rna.684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan B, Palioura S, Sabina J, Marvin-Guy L, Kochhar S, Larossa RA, Söll D. 2008. Quality control despite mistranslation caused by an ambiguous genetic code. Proc Natl Acad Sci U S A 105:16502–16507. doi: 10.1073/pnas.0809179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fredriksson A, Ballesteros M, Peterson CN, Persson O, Silhavy TJ, Nyström T. 2007. Decline in ribosomal fidelity contributes to the accumulation and stabilization of the master stress response regulator sigmas upon carbon starvation. Genes Dev 21:862–874. doi: 10.1101/gad.409407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling J, Söll D. 2010. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc Natl Acad Sci U S A 107:4028–4033. doi: 10.1073/pnas.1000315107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devaraj A, Shoji S, Holbrook ED, Fredrick K. 2009. A role for the 30S subunit E site in maintenance of the translational reading frame. RNA 15:255–265. doi: 10.1261/rna.1320109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Boniecki MT, Jaffe JD, Imai BS, Yau PM, Luthey-Schulten ZA, Martinis SA. 2011. Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites. Proc Natl Acad Sci U S A 108:9378–9383. doi: 10.1073/pnas.1016460108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn JG, Foo CK, Belletier NG, Gavis ER, Weissman JS. 2013. Ribosome profiling reveals pervasive and regulated stop codon readthrough in Drosophila melanogaster. Elife 2:e01179. doi: 10.7554/eLife.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baggett NE, Zhang Y, Gross CA. 2017. Global analysis of translation termination in E. coli. PLoS Genet 13:e1006676. doi: 10.1371/journal.pgen.1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyerovich M, Mamou G, Ben-Yehuda S. 2010. Visualizing high error levels during gene expression in living bacterial cells. Proc Natl Acad Sci U S A 107:11543–11548. doi: 10.1073/pnas.0912989107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su HW, Zhu JH, Li H, Cai RJ, Ealand C, Wang X, Chen YX, Kayani MU, Zhu TF, Moradigaravand D, Huang H, Kana BD, Javid B. 2016. The essential mycobacterial amidotransferase GatCAB is a modulator of specific translational fidelity. Nat Microbiol 1:16147. doi: 10.1038/nmicrobiol.2016.147. [DOI] [PubMed] [Google Scholar]
- 35.Gomes AC, Kordala AJ, Strack R, Wang X, Geslain R, Delaney K, Clark WC, Keenan R, Pan T. 2016. A dual fluorescent reporter for the investigation of methionine mistranslation in live cells. RNA 22:467–476. doi: 10.1261/rna.054163.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman KS, Berg MD, Shilton BH, Brandl CJ, O’Donoghue P. 2017. Genetic selection for mistranslation rescues a defective co-chaperone in yeast. Nucleic Acids Res 45:3407–3421. doi: 10.1093/nar/gkw1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardno TS, Poole ES, Mathew SF, Graves R, Tate WP. 2009. A homogeneous cell-based bicistronic fluorescence assay for high-throughput identification of drugs that perturb viral gene recoding and read-through of nonsense stop codons. RNA 15:1614–1621. doi: 10.1261/rna.1586709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribas de Pouplana LR, Santos MA, Zhu JH, Farabaugh PJ, Javid B. 2014. Protein mistranslation: friend or foe? Trends Biochem Sci 39:355–362. doi: 10.1016/j.tibs.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MH, Pan T. 2017. Function and origin of mistranslation in distinct cellular contexts. Crit Rev Biochem Mol Biol 52:205–219. doi: 10.1080/10409238.2016.1274284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohler K, Ibba M. 2017. Translational fidelity and mistranslation in the cellular response to stress. Nat Microbiol 2:17117. doi: 10.1038/nmicrobiol.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackermann M. 2015. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol 13:497–508. doi: 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- 42.Elowitz MB, Levine AJ, Siggia ED, Swain PS. 2002. Stochastic gene expression in a single cell. Science 297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 43.Ozbudak EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. 2002. Regulation of noise in the expression of a single gene. Nat Genet 31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- 44.Golding I, Paulsson J, Zawilski SM, Cox EC. 2005. Real-time kinetics of gene activity in individual bacteria. Cell 123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez A, Golding I. 2013. Genetic determinants and cellular constraints in noisy gene expression. Science 342:1188–1193. doi: 10.1126/science.1242975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine JH, Lin Y, Elowitz MB. 2013. Functional roles of pulsing in genetic circuits. Science 342:1193–1200. doi: 10.1126/science.1239999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silander OK, Nikolic N, Zaslaver A, Bren A, Kikoin I, Alon U, Ackermann M. 2012. A genome-wide analysis of promoter-mediated phenotypic noise in Escherichia coli. PLoS Genet 8:e1002443. doi: 10.1371/journal.pgen.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf L, Silander OK, van Nimwegen E. 2015. Expression noise facilitates the evolution of gene regulation. Elife 4. doi: 10.7554/eLife.05856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norman TM, Lord ND, Paulsson J, Losick R. 2015. Stochastic switching of cell fate in microbes. Annu Rev Microbiol 69:381–403. doi: 10.1146/annurev-micro-091213-112852. [DOI] [PubMed] [Google Scholar]
- 50.Carey JN, Mettert EL, Roggiani M, Myers KS, Kiley PJ, Goulian M. 2018. Regulated stochasticity in a bacterial signaling network permits tolerance to a rapid environmental change. Cell 173:196–207.e14. doi: 10.1016/j.cell.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blake WJ, KÆrn M, Cantor CR, Collins JJ. 2003. Noise in eukaryotic gene expression. Nature 422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- 52.Paulsson J. 2004. Summing up the noise in gene networks. Nature 427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- 53.Grentzmann G, Ingram JA, Kelly PJ, Gesteland RF, Atkins JF. 1998. A dual-luciferase reporter system for studying recoding signals. RNA 4:479–486. [PMC free article] [PubMed] [Google Scholar]
- 54.Loughran G, Howard MT, Firth AE, Atkins JF. 2017. Avoidance of reporter assay distortions from fused dual reporters. RNA 23:1285–1289. doi: 10.1261/rna.061051.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Javid B, Sorrentino F, Toosky M, Zheng W, Pinkham JT, Jain N, Pan M, Deighan P, Rubin EJ. 2014. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc Natl Acad Sci U S A 111:1132–1137. doi: 10.1073/pnas.1317580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang FC, Frawley ER, Tapscott T, Vázquez-Torres A. 2016. Bacterial stress responses during host infection. Cell Host Microbe 20:133–143. doi: 10.1016/j.chom.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fredriksson A, Ballesteros M, Dukan S, Nyström T. 2006. Induction of the heat shock regulon in response to increased mistranslation requires oxidative modification of the malformed proteins. Mol Microbiol 59:350–359. doi: 10.1111/j.1365-2958.2005.04947.x. [DOI] [PubMed] [Google Scholar]
- 59.Netzer N, Goodenbour JM, David A, Dittmar KA, Jones RB, Schneider JR, Boone D, Eves EM, Rosner MR, Gibbs JS, Embry A, Dolan B, Das S, Hickman HD, Berglund P, Bennink JR, Yewdell JW, Pan T. 2009. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462:522–526. doi: 10.1038/nature08576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bullwinkle TJ, Reynolds NM, Raina M, Moghal AB, Matsa E, Rajkovic A, Kayadibi H, Fazlollahi F, Ryan C, Howitz N, Faull KF, Lazazzera BA, Ibba M. 2014. Oxidation of cellular amino acid pools leads to cytotoxic mistranslation of the genetic code. Elife 3. doi: 10.7554/eLife.02501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reference deleted.
- 62.Goldsmith M, Tawfik DS. 2009. Potential role of phenotypic mutations in the evolution of protein expression and stability. Proc Natl Acad Sci U S A 106:6197–6202. doi: 10.1073/pnas.0809506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. 2008. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nangle LA, De Crecy Lagard V, Doring V, Schimmel P. 2002. Genetic code ambiguity. Cell viability related to the severity of editing defects in mutant tRNA synthetases. J Biol Chem 277:45729–45733. doi: 10.1074/jbc.M208093200. [DOI] [PubMed] [Google Scholar]
- 65.Karkhanis VA, Mascarenhas AP, Martinis SA. 2007. Amino acid toxicities of Escherichia coli that are prevented by leucyl-tRNA synthetase amino acid editing. J Bacteriol 189:8765–8768. doi: 10.1128/JB.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ling J, Cho C, Guo LT, Aerni HR, Rinehart J, Söll D. 2012. Protein aggregation caused by aminoglycoside action is prevented by a hydrogen peroxide scavenger. Mol Cell 48:713–722. doi: 10.1016/j.molcel.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bullwinkle TJ, Ibba M. 2016. Translation quality control is critical for bacterial responses to amino acid stress. Proc Natl Acad Sci U S A 113:2252–2257. doi: 10.1073/pnas.1525206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohler K, Mann R, Bullwinkle TJ, Hopkins K, Hwang L, Reynolds NM, Gassaway B, Aerni HR, Rinehart J, Polymenis M, Faull K, Ibba M. 2017. Editing of misaminoacylated tRNA controls the sensitivity of amino acid stress responses in Saccharomyces cerevisiae. Nucleic Acids Res 45:3985–3996. doi: 10.1093/nar/gkx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pezo V, Metzgar D, Hendrickson TL, Waas WF, Hazebrouck S, Döring V, Marlière P, Schimmel P, De Crécy-Lagard V. 2004. Artificially ambiguous genetic code confers growth yield advantage. Proc Natl Acad Sci U S A 101:8593–8597. doi: 10.1073/pnas.0402893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bacher JM, Waas WF, Metzgar D, de Crécy-Lagard V, Schimmel P. 2007. Genetic code ambiguity confers a selective advantage on Acinetobacter baylyi. J Bacteriol 189:6494–6496. doi: 10.1128/JB.00622-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan Y, Wu J, Ung MH, De Lay N, Cheng C, Ling J. 2015. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Res 43:1740–1748. doi: 10.1093/nar/gku1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santos MA, Cheesman C, Costa V, Moradas-Ferreira P, Tuite MF. 1999. Selective advantages created by codon ambiguity allowed for the evolution of an alternative genetic code in Candida spp. Mol Microbiol 31:937–947. doi: 10.1046/j.1365-2958.1999.01233.x. [DOI] [PubMed] [Google Scholar]
- 73.True HL, Lindquist SL. 2000. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- 74.Das G, Thotala DK, Kapoor S, Karunanithi S, Thakur SS, Singh NS, Varshney U. 2008. Role of 16S ribosomal RNA methylations in translation initiation in Escherichia coli. EMBO J 27:840–851. doi: 10.1038/emboj.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kyuma T, Kizaki H, Ryuno H, Sekimizu K, Kaito C. 2015. 16S rRNA methyltransferase KsgA contributes to oxidative stress resistance and virulence in Staphylococcus aureus. Biochimie 119:166–174. doi: 10.1016/j.biochi.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 76.Kyuma T, Kimura S, Hanada Y, Suzuki T, Sekimizu K, Kaito C. 2015. Ribosomal RNA methyltransferases contribute to Staphylococcus aureus virulence. FEBS J 282:2570–2584. doi: 10.1111/febs.13302. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal D, Gregory ST, O’Connor M. 2011. Error-prone and error-restrictive mutations affecting ribosomal protein S12. J Mol Biol 410:1–9. doi: 10.1016/j.jmb.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 78.Björkman J, Samuelsson P, Andersson DI, Hughes D. 1999. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella Typhimurium. Mol Microbiol 31:53–58. doi: 10.1046/j.1365-2958.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- 79.Björkman J, Hughes D, Andersson DI. 1998. Virulence of antibiotic-resistant Salmonella Typhimurium. Proc Natl Acad Sci U S A 95:3949–3953. doi: 10.1073/pnas.95.7.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miranda I, Silva-Dias A, Rocha R, Teixeira-Santos R, Coelho C, Gonçalves T, Santos MA, Pina-Vaz C, Solis NV, Filler SG, Rodrigues AG. 2013. Candida albicans CUG mistranslation is a mechanism to create cell surface variation. MBio 4:e00285-13. doi: 10.1128/mBio.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bezerra AR, Simões J, Lee W, Rung J, Weil T, Gut IG, Gut M, Bayés M, Rizzetto L, Cavalieri D, Giovannini G, Bozza S, Romani L, Kapushesky M, Moura GR, Santos MA. 2013. Reversion of a fungal genetic code alteration links proteome instability with genomic and phenotypic diversification. Proc Natl Acad Sci U S A 110:11079–11084. doi: 10.1073/pnas.1302094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bacher JM, Schimmel P. 2007. An editing-defective aminoacyl-tRNA synthetase is mutagenic in aging bacteria via the SOS response. Proc Natl Acad Sci U S A 104:1907–1912. doi: 10.1073/pnas.0610835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwartz MH, Waldbauer JR, Zhang L, Pan T. 2016. Global tRNA misacylation induced by anaerobiosis and antibiotic exposure broadly increases stress resistance in Escherichia coli. Nucleic Acids Res 44:10292–10303. doi: 10.1093/nar/gkw856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall BG. 1983. Yeast thermotolerance does not require protein synthesis. J Bacteriol 156:1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trotter EW, Kao CM, Berenfeld L, Botstein D, Petsko GA, Gray JV. 2002. Misfolded proteins are competent to mediate a subset of the responses to heat shock in Saccharomyces cerevisiae. J Biol Chem 277:44817–44825. doi: 10.1074/jbc.M204686200. [DOI] [PubMed] [Google Scholar]
- 86.Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. 2012. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature 482:363–368. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kermgard E, Yang Z, Michel AM, Simari R, Wong J, Ibba M, Lazazzera BA. 2017. Quality control by isoleucyl-tRNA synthetase of Bacillus subtilis is required for efficient sporulation. Sci Rep 7:41763. doi: 10.1038/srep41763. [DOI] [PMC free article] [PubMed] [Google Scholar]