Abstract
Like traditional organisms, eusocial insect societies express traits that are the target of natural selection. Variation at the colony level emerges from the combined attributes of thousands of workers and may yield characteristics not predicted from individual phenotypes. By manipulating the ratios of worker types, the basis of complex, colony-level traits can be reduced to the additive and non-additive interactions of their component parts. In this study, we investigated the independent and synergistic effects of body size on nest architecture in a seasonally polymorphic harvester ant, Veromessor pergandei. Using network analysis, we compared wax casts of nests, and found that mixed-size groups built longer nests, excavated more sand and produced greater architectural complexity than single-sized worker groups. The nests built by polymorphic groups were not only larger in absolute terms, but larger than expected based on the combined contributions of both size classes in isolation. In effect, the interactions of different worker types yielded a colony-level trait that was not predicted from the sum of its parts. In nature, V. pergandei colonies with fewer fathers produce smaller workers each summer, and produce more workers annually. Because body size is linked to multiple colony-level traits, our findings demonstrate how selection acting on one characteristic, like mating frequency, could also shape unrelated characteristics, like nest architecture.
This article is part of the theme issue ‘Interdisciplinary approaches for uncovering the impacts of architecture on collective behaviour'.
Keywords: ant nest architecture, network analysis, polymorphism, collective behaviour, emergence
1. Introduction
Social insect nests serve complex physiological functions [1,2], organize labour [3] and act as protective fortresses for the colonies living within. Each nest is an extension of the colony phenotype and represents the response of its many builders to the external environment. Although every species produces a distinctive architecture, individual nests vary considerably around a mean set of characteristics, and some variants may increase colony fitness. Among ground-nesting species, variation in nest architecture has been attributed to season [4], soil type [5], soil moisture gradients [6,7], the presence of food or brood [8] and changes in colony size [9]. Though less well understood, differences in worker attributes, like age, morphology, experience or genetic background also have profound effects on nest structure. For example, old Pogonomyrmex badius workers excavate larger nests than young workers [10], and physical differences in male and female workers control the frequency of tunnel bifurcations in some termite species [11]. Likewise, large Solenopsis invicta workers in isolation excavate less than workers from their colony's natural size distribution [12].
Across colony ontogeny and during each annual cycle, social insect colonies alternately invest in growth and reproduction. Worker age structure [13], body size [14,15] and labour allocation [13,16] have all been demonstrated to vary with these changes in colony investment. Therefore, in each season, the abundance and composition of the work force available to build a new nest are distinct. When distinct worker types are combined, their individual contributions to nest architecture may be additive or non-additive. For example, if small workers tend to build chambers and large workers tend to build shafts, a colony's nest architecture might be predicted from the number of large and small workers present. Alternatively, various ratios of interacting workers may yield new structures or processes not predicted by the performance of any single worker type in isolation. Like other colony-level traits, the additive and non-additive elements of nest architecture can be investigated by manipulating a colony's underlying worker composition while holding environmental factors constant.
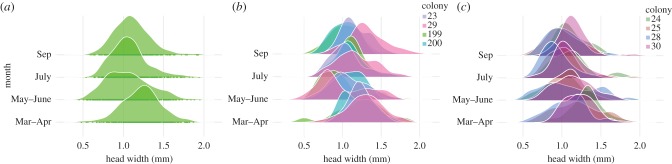
Veromessor pergandei is a polymorphic seed harvesting ant of the Sonoran and Mojave deserts, and an ideal species for studying the interplay between worker variation and variation in nest architecture. Within colonies, large workers are more than two times the size of small workers, and the abundance and frequency of each body size varies considerably across populations [17], across seasons [14,15] (figure 1a) and with respect to colony identity (figure 1b,c) [18]. Body size does not predict the seed size that each individual collects, or the tendency of an individual to participate in nest excavation and foraging behaviour. Rather, changes in body size allow V. pergandei colonies to maintain a stable work force, despite seasonal variation in resource abundance [15,18]. The largest colonies maintain a large foraging force by dramatically reducing new worker body size following sexual reproduction. Colonies with a high degree of seasonal polymorphism also contain fewer patrilines than smaller colonies, with less variable size frequency distributions [18].
Figure 1.
Average worker body size is related to season and colony identity in V. pergandei. (a) Distribution of worker head widths for eight monogyne V. pergandei colonies, sampled in four periods across 1 year (N = 1690 workers, from Kwapich et al. [18]). Colony-level variation in body size is shown across four periods for (b) large colonies and (c) small colonies. Colonies are shown in the same order in each season. Each colour represents a unique colony. Colonies that maintained a larger forager population and produced more workers annually did so by reducing worker size between spring and summer. On average, large colonies also contained fewer patrilines (1–4 versus 4–9). (Online version in colour.)
Though no published descriptions of a complete nest exist to our knowledge, several partial excavations have revealed the incredible scale of V. pergandei nest architecture. Nests of mature colonies are characterized by a wide, central shaft that slants downward at a 35° angle, and reaches an excess of 3 m in depth. Numerous horizontal chambers and ancillary shafts project from the main shaft, and lateral tunnels may connect as many as 10 satellite entrances or secondary nests [9,19]. Foragers initiate the excavation of new nests along foraging routes (CL Kwapich 2015–2017, personal observation), and like other members of the genus, nest relocation takes place year-round, especially following rains [15,19–21]. In effect, V. pergandei nests excavated in different seasons, by colonies that differ in worker number and pedigree may have unique architectures due to the underlying size distributions of their builders.
In this study, we measured the additive and non-additive effects of worker body size on nest architecture in V. pergandei. We compared nests made by combined and isolated size classes to determine (i) if nest structures scale to the average worker body size in a group, (ii) if workers of different sizes are responsible for particular features of the architecture (chambers, branches, etc.) and (iii) if the nests created by each size class in isolation differ from those produced by the interactions of multiple worker size classes in combination.
2. Methods
We compared nests excavated by V. pergandei workers from three, artificial body-size frequency distributions. Ants were foragers, collected from 13 colonies located in Casa Grande, AZ, USA, in September and October of 2017. Although never studied explicitly in this species, we chose foragers for a number of reasons that support their role in the establishment of new nests: across ant species, foragers are associated with the initiation of new nests [22–24]; we observed that foraging activity ceased or was strongly reduced when wild V. pergandei colonies excavated new nests; in a study of forager allocation across the annual cycle, marked foragers represented a large proportion of the excavating force; new nests were initiated along foraging routes and foragers were the only individuals to travel a substantial distance from the nest on these routes [18].
In V. pergandei, total body length ranges from approximately 3.5 to 8.4 mm [17], and all worker size classes contribute to the remodelling of old nests and excavation of new ones [15]. Head width is a good predictor of body size and has been shown to be correlated with both mesosoma length (r = 0.96) and mandible length (r = 0.89) across V. pergandei populations [17]. In our focal population, worker head widths (measured across the full width of the eyes) ranged continuously from 0.50 to 1.90 mm and served as a proxy for body size within colonies. To maximize observable differences in nest excavation, we selected workers from the largest and smallest thirds of the size distribution. In a pilot study, a nest made by all size classes was also cast and has been included in electronic supplementary material, S1.
Foragers from 13 colonies were vacuumed directly from foraging trails using a modified DeWalt®, 20 V Max shop vacuum. Workers and accompanying soil were kept overnight in 30 cm × 15 cm storage bins, offered a mixture of grass seed, nutritional agar and cotton-plugged test tubes containing water. Twenty-four hours after capture, workers were divided into three treatment groups containing 60 individuals each. The first treatment contained 60 workers from the smallest body-size class only (head widths 0.5–1.0 mm), the second treatment contained 60 workers from the largest size class only (head widths 1.4–1.9 mm) and the third treatment contained a mixed group of 30 small and 30 large workers. Workers were sorted by size using soft forceps and a two-dimensional wedge-micrometre printed on clear acetate; similar to that developed by Porter [25].
Each group of 60 workers was introduced into a 19 l bucket containing 23 kg of sand and 1.5 l of deionized water packed into place (37 cm deep and 29.5 cm in diameter). Silica pool filtration sand was selected because of its comparatively uniform particle size (Quikrete®, 0.45–0.85 mm, 20–40 mesh). Sand was compacted so that nest structures could be built without collapsing, and so that chambers and tunnels would maintain their integrity when liquid wax was later introduced as a casting material.
To ensure that digging took place in the centre of each bucket, workers were released into a 10 × 10 cm clear plastic box with a 1.5 cm hole drilled in the centre. The box also contained a ball of wet cotton, a cube of nutritional agar and beetle larvae as a food source. Workers began excavating sand between 6 and 35 min after introduction, and were allowed to dig for 48 h at 26°C before each trial was terminated (n = 39 nests obtained from three different treatments for each of the 13 colonies). Each box was closely monitored for the appearance of dead workers. Dead workers were replaced with individuals from the same colony and size class as soon as they were noticed (fewer than 5 in any replicate). Short film clips were taken of mixed-size class replicates before termination, to demonstrate that both size classes participated in excavation.
After 48 h, boxes were removed. Excavated sand was collected from the floor of each box, then dried and weighed to determine the total amount of sand excavated. Paraffin wax was heated and poured into the entrance of each nest to make a cast of the excavated space. Wax was chosen as a casting material because of its ability to flow into even the smallest of the excavated spaces and create a complete record before cooling [26,27]. After the wax hardened, surrounding sand was removed to reveal the architecture of each nest. Nests were carefully exhumed in pieces, arranged on a flat surface and photographed with a scale. Digital photographs were imported into ImageJ (US National Institutes of Health, Bethesda, MD, USA) and measurements were taken by tracing the length of each shaft and chamber after calibrating a 0.50 cm scale.
(a). Description of nest features
Nests consisted of a single entrance connected to a central shaft. The central shaft often branched into ancillary shafts with varying branching degrees as well as developing chambers. The fate of incipient tunnels could not be determined, but projections from a shaft greater than 1 cm in length (approx. two body lengths) were scored as unique branches. Developing chambers were identified as broadened horizontal projections from a shaft without additional branching of their own, and frequently contained clusters of workers visible through the wax cast. Nest length was measured as the sum of all branch lengths and of all chamber lengths, whereby chamber length was measured as a straight line between the shaft from which it arose and the far wall opposite to the shaft.
Prior to nest casting, a shop vacuum was used to remove loose sand and to create a shallow conical pit around the entrance of each nest. This allowed liquid wax to pool as it flowed into the nest entrance, rather than spreading across the surface of the bucket. The wide, conical feature visible in photographs at the top of each nest cast is, therefore, an artefact of the casting procedure and not a structure built by the ants.
Accurate measurements of shaft diameters and/or chamber volume cannot be taken from the exterior of wax casts because each feature is surrounded by a thick sheath of sand mixed with wax. Detailed measurements can only be taken from the internal diameter of cross sections of these nest structures, where a sand-free ring of wax is clearly visible. We did not measure shaft diameter directly. For the purposes of this study, we focused on the overall number and distribution of nest features, as well as the amount of sand displaced by each nest and the total length excavated.
(b). Nest casts as directed networks
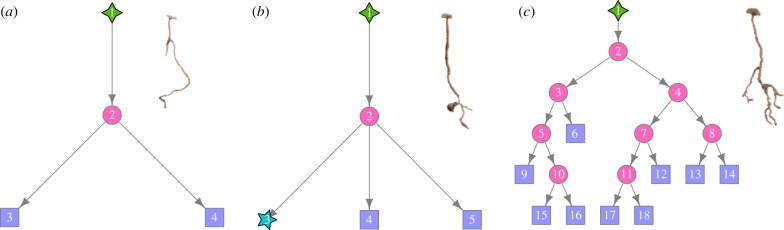
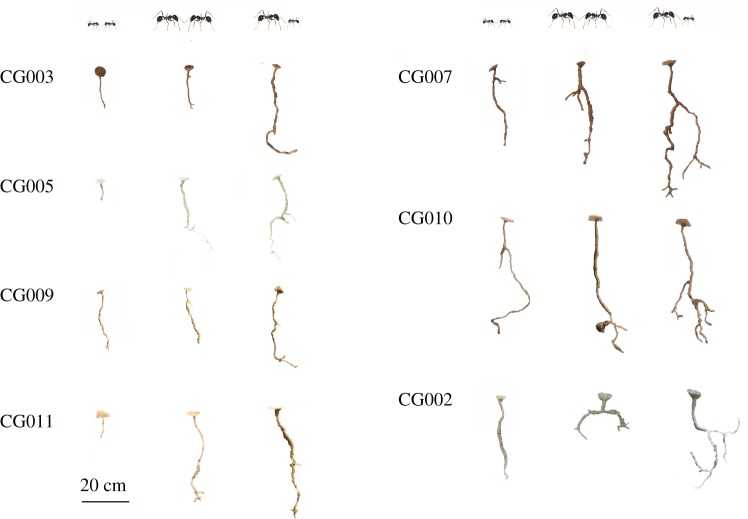
We studied architectural complexity across different experimental treatments by interpreting the structure of each excavated nest as a network [12,28]. We considered a nest as a connected graph, G(V; E), consisting of a set V = {1, …, N} of N vertices (or nodes) and a set E = {〈i1, j1〉, …, 〈ik, jk〉} of k edges connecting a pair of nodes i and j. Nest entrance, branching points, chambers and shaft terminations represent different types of nodes of the network. Shafts represent edges of the network connecting a pair of nodes. A distinctive trait of V. pergandei nests is the absence of multiple shafts connecting the same pair of points (e.g. a branching point with a chamber) that would form a closed loop. Consequently, the nest architecture was well described by means of directed edges pointing downward from the entrance. The resulting network is, therefore, a directed acyclic tree. Additionally, edges were described by an attribute giving the length of the corresponding shaft. We numbered nodes following a breadth-first search strategy to traverse the resulting tree whereby the root of the tree (i.e. the nest entrance) was always node 1 and the last leaf of the tree (i.e. the right-most node) was node N. Figure 2 shows examples of the networks resulting from three different group compositions of workers and their corresponding nest casts.
Figure 2.
Example of networks and corresponding nest casts produced by 60 small (a), 60 large (b), and a mixed group of 30 large and 30 small workers (c) from a single colony, CG010. Diamonds represent nest entrances, circles represent branching points, stars represent chambers, and squares represent terminations. Network edges are not scaled to shaft length. (Online version in colour.)
(c). Body size and excavation
During excavation, V. pergandei workers transport boluses of sand between their mandibles and a psammaphore (basket of hairs) on the ventral side of the head. We measured the amount of sand carried per excavation bout across a full range of worker sizes. To do so, foragers were collected from three field colonies in November of 2017. After 24 h, mixed-size groups of workers were placed in clear plastic boxes with a 1.5 cm hole in the centre. Each box was centred on top of a bucket containing 0.45–0.85 mm sand as detailed above. As workers departed with sand loads, they were collected using a mouth aspirator (n = 32). Each captured ant and the sand she carried were photographed on a gridded Petri dish. The number of collected sand grains was related to head width through linear regression, and an average number of grains collected per trip was calculated for those workers that belonged to the designated ‘large’ and ‘small’ size classes.
(d). Analysis of nest architecture
To determine if worker size influenced nest size, we compared nest length and sand weight excavated by three different worker size distributions. Owing to the normality of data, we used Linear Mixed Models (LMMs, R v. 3.4.0, package lme4). Nest length and sand weight represented our response variables, treatment was the fixed effect and colony identity was the random effect. Post hoc, pairwise comparisons between treatments were made with Tukey's HSD tests (R package lsmeans). Additionally, differences between expected nest size (length or sand weight) and the observed nest sizes of mixed groups of 30 small and 30 large workers were determined using similarly defined LMMs. For each colony, the expected nest size was estimated as the average nest length or weight of sand excavated by both single-sized worker groups from the same colony—in other words, 50% of the nest produced by the small-size group added to 50% of that produced by the large-size worker group for each colony (n = 13 colonies).
The complexity of nest architecture can be described through network analysis [12,28]. The number of nodes and the number of edges provide a direct way to assess the size of a network. Although nodes and edges represent very different structures of a nest, their total numbers are correlated because in a directed acyclic tree the number of nodes equals the number of edges plus one. Therefore, we considered only the number of edges. Additionally, an ensemble of directed networks can also be characterized in terms of the in- and out-degree distributions, which give the probability of finding a node in the network with a certain number x ≥ 0 of ingoing and outgoing edges. For the purposes of our study, we focused on the out-degree distribution only and ignored nodes without outgoing edges (i.e. x > 0) to characterize the number and type of branches in different nests. The effects of treatment on each of the components of complexity were assessed using a Generalized Linear Mixed Model (GLMM) with a logarithmic link function. Number of edges and branching factor represented our response variables with a Poisson distribution; treatment was the fixed effect, and colony identity the random effect. Post hoc, pairwise comparisons between treatments were made with Tukey's HSD tests. Additionally, we analysed the distribution of node types in the network by looking at the proportion of each individual node type in each network. The relative difference in proportions of node types across treatments was analysed using a two-sample t-test.
To eliminate the effects of colony-level variation on nest size and structure, dimensionless differences in nest length, excavated sand and nest complexity (as a number of edges) were expressed as ratios between treatment groups, within colonies. These relative differences in nest size and complexity are reported as means with standard deviations. Data were normally distributed and a single one-sample t-test was used to determine if dimensionless values were equivalent between treatments (reference mean μ = 1, R package stats).
All data were plotted using R packages ggplot2 and ggjoy. Network drawings were generated using the R package igraph. Nest images were prepared for figures using Microsoft Paint 3D v.1703. Accompanying data files (electronic supplementary material, S2), source code (electronic supplementary material, S3) and network drawings (electronic supplementary material, S4) are included in the electronic supplementary material of this paper.
3. Results
(a). Nest length and sand weight
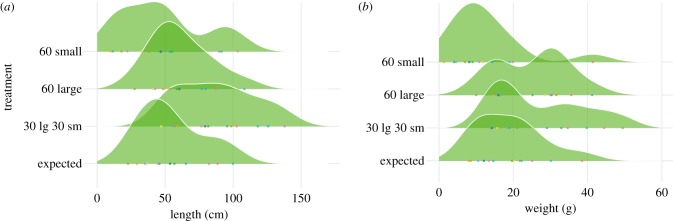
Over 48 h, groups of 60 workers produced nests ranging from 10 to 137 cm in total length (figure 3a). Nest length differed significantly between treatments (LMM, ANOVA, p < 0.0000, table 1). The nests produced by a mix of large and small workers averaged 85 cm (s.d. 30) in length and were significantly longer than those produced by both small workers alone (48 cm, s.d. 31) and large workers alone (61 cm, s.d. 22; both Tukey HSD, p < 0.004; electronic supplementary material, S5). In effect, nests excavated by a mix of worker sizes were 2.64 (s.d. 2.11) times longer than those excavated by small workers, and 1.43 (s.d. 0.38) times longer than those excavated by large workers from the same colony. The nests built by polymorphic groups were not only larger in absolute terms, but 1.67 (s.d. 0.44) times longer than expected based on the summed contributions of each colony's large and small workers in isolation (LMM, ANOVA, p < 0.0000, table 1).
Figure 3.
Distributions of (a) nest length and (b) excavated sand weight by treatment. Colonies are denoted by points and their identity by colour. Nests excavated by a mixed group of 30 large and 30 small individuals were significantly longer than those built by 60 large or 60 small workers alone. Nests built by mixed groups were also significantly longer than expected based on estimates from each single-sized worker group (expected). Mixed groups excavated significantly more sand than expected based on the number of large and small workers present. (Online version in colour.)
Table 1.
The influence of worker body size on nest length, excavated sand weight, observed and expected length, and weight were assessed with LMMs with colony identity as a random effect. The influence of worker body size on edge number was assessed with a Poisson-distributed GLMM, with a log-link function and colony identity as a random effect. Type II Wald χ2-tests were used to determine the overall significance of treatment in each model. Significance levels less than 0.05 are shown in italics.
| fixed effect | estimate | s.e. | t/z | p-value |
|---|---|---|---|---|
| nest length | χ2 = 47.50, p < 0.0000 | |||
| (intercept) | 47.67 | 7.32 | 6.51 | — |
| 60 lg | 13.44 | 5.85 | 2.29 | 0.033 |
| 30 lg + 30 sm | 39.65 | 5.85 | 6.78 | <0.0000 |
| obs. versus exp. nest length | χ2 = 63.74, p < 0.0000 | |||
| (intercept) | 87.32 | 7.02 | 12.45 | — |
| expected | −32.93 | 4.13 | −7.98 | <0.0000 |
| sand weight | χ2 = 40.69, p < 0.0000 | |||
| (intercept) | 12.55 | 2.86 | 4.39 | — |
| 60 lg | 12.53 | 2.47 | 5.07 | <0.0000 |
| 30 lg + 30 sm | 14.67 | 2.50 | 5.87 | <0.0000 |
| obs. versus exp. sand weight | χ2 = 11.91, p = 0.0006 | |||
| (intercept) | 26.99 | 3.03 | 8.91 | — |
| expected | −8.00 | 2.32 | −3.45 | 0.0022 |
| edge number | χ2 = 20.20, p < 0.0000 | |||
| (intercept) | 1.26 | 0.19 | 6.68 | — |
| 60 lg | 0.26 | 0.19 | 1.41 | 0.16 |
| 30 lg + 30 sm | 0.72 | 0.17 | 4.23 | <0.0000 |
The weight of the sand excavated by workers ranged between 1 and 49 g (figure 3b), and differed significantly among treatments (LMM, ANOVA, p < 0.0000, table 1). Small workers excavated an average of 13 g (s.d. 10), while large workers excavated 26 g (s.d. 9) over 48 h. Workers from the mixed-size treatment excavated an average of 27 g (s.d. 12), or 3.33 times (s.d. 3.0) more sand than small workers within the same colony (Tukey HSD, p < 0.001). While they did not excavate significantly more sand than large workers alone, mixed groups dug 1.51 times (s.d. 0.49) more sand than expected based on the mean contribution of both single-sized worker groups in isolation (LMM, ANOVA, p < 0.0005, table 1).
(b). Nest structure and complexity
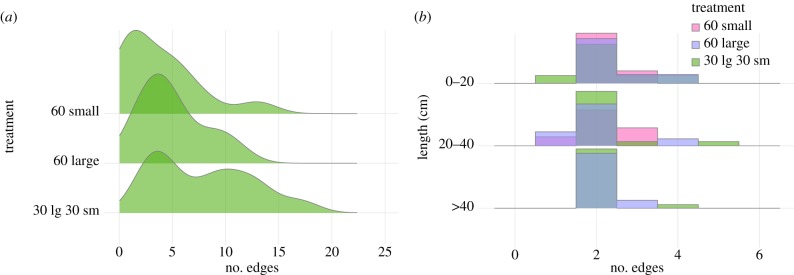
The networks representing each nest contained between 1 and 17 edges (i.e. between 2 and 18 nodes). Figure 4a shows the distributions of the number of edges in a network organized by treatment. The effect of treatment was statistically significant (Poisson GLMM, ANOVA, p < 0.0000). Networks produced by 60 small workers were not significantly different in size from those produced by 60 large workers (Tukey HSD, p = 0.34). However, when the two worker sizes collaborated in the mixed-size treatment, the resulting networks were significantly larger than both those of only small workers alone and those of only large workers alone (both Tukey HSD, p < 0.0095, figure 5). Similar results hold when taking into account the possibility of colony-level variation. Nests built by mixed groups had, on average, 3.6 times (s.d. 3.0) more edges than those produced by small workers alone (t12 = 3.14, p = 0.0086) and 1.89 times (s.d. 1.7) more edges than those produced by large workers in isolation (t12 = 2.75, p = 0.018).
Figure 4.
Distributions of (a) total number of edges in a network by treatment and (b) out-degree distributions by treatment as a function of the distance from the nest entrance.
Figure 5.
A selection of casts showing relative nest size and complexity by treatment (size classes are indicated above) and colony identity (displayed to the left of each group of casts). (Online version in colour.)
The presence and abundance of nodes representing chambers and terminations were not significantly different between treatments, indicating that no single body size is responsible for building these particular features of the nest architecture. However, mixed-worker size groups produced a significantly higher proportion of nodes representing branches, when compared to both groups of only small workers (t16.23 =−3.26, p = 0.0047) and groups of only large workers (t18.49 =−2.3, p = 0.033) with, respectively, 1.98 times and 1.40 times more branching nodes. These results provide further evidence that the interaction between differently sized workers yields greater architectural complexity.
Across treatments and depths, branching tended to take the form of simple bifurcations. This result can be observed in figure 4b, where most of the probability mass of the out-degree distribution of nodes is represented by nodes with degree 2. Though not statistically significant, nodes that produced more than two branches tended to be closer to the nest entrance and were only rarely observed at greater distances.
(c). Body size and excavation
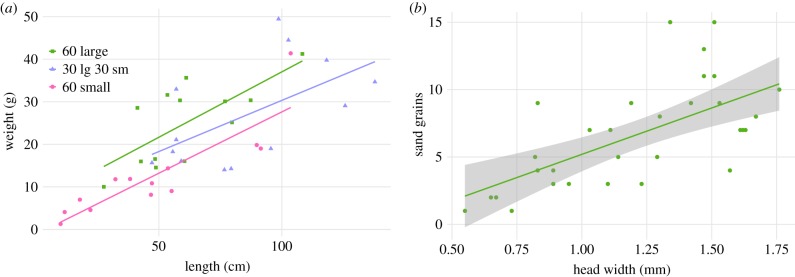
Across treatments, nest length was a significant predictor of the weight of sand excavated per cm (linear regression, F1,37 = 45, R2 = 0.54, p < 0.0000, n = 39 nests). The amount of sand excavated per centimetre was consistent with the average body size of the workers present, so that large workers excavated more sand per centimetre than small workers, while mixed groups excavated an intermediate amount (figure 6a). These results suggest that workers might use their body as a template to determine shaft width, rather than excavating shafts sufficiently wide for the entire range of body sizes within the colony. Although the overall amount of sand excavated by workers in the mixed-size treatment was intermediate between that of large and small workers alone, the amount of sand excavated as a function of nest length increased more slowly in the mixed treatment. The slope of the line describing sand weight excavated per cm is similar between the large and small worker treatments (small workers: y = −1.17 + 0.29x; large workers: y = 6.31 + 0.31x), but is significantly lower in the mixed group (y = 6.29 + 0.24x). This decrease may be explained by the number of extra, concurrently developing shafts, which were not yet fully formed in the mixed-worker treatment.
Figure 6.
(a) Large workers excavated more sand per centimetre than small workers (large y = 6.31 + 0.31x, F1,11 = 11.03, R2 = 0.46, p = 0.0068; small y = −1.17 + 0.29x, F1,11 = 34.21, R2 = 0.73, p = 0.0001) while mixed groups excavated an intermediate amount of sand per centimetre (mixed y = 6.29 + 0.24x, F1,11 = 5.71, R2 = 0.28, p = 0.036). (b) Worker head width was a significant predictor of the amount of sand carried per excavation bout (y = −1.69 + 6.88x, F1,30 = 20.93, R2 = 0.41, p < 0.0001).
In a separate experiment, we observed workers carrying between 1 grain and 15 grains of sand per excavation bout. Worker head width was a significant predictor of the amount of sand carried (figure 6b, linear regression: F1,30 = 20.93, R2 = 0.41, p < 0.0001, n = 32 ants). On average, large workers carried 9 (s.d. 3) grains of sand per trip while small workers carried only 3 (s.d. 2) grains per trip (sample of 10 small and 12 large workers). While large workers frequently carried fewer grains than the maximum amount recorded for their size class, small workers never carried large boluses and occasionally exited the nest facing backwards and dragging their loads. Body size is, therefore, likely to account for the significant difference in total weight of sand excavated by large and small worker groups in isolation (table 1). As in wild colonies [15], both small and large workers were observed depositing sand grains on the surface within seconds of one another in the mixed-size treatment (electronic supplementary material, video S1).
4. Discussion
Across social insect societies, worker heterogeneity has been demonstrated to influence colony performance, from the selection of better nest sites to increases in foraging duration [29–31]. In this study, polymorphic worker groups created longer nests, excavated more sand and produced greater architectural complexity than single-sized worker groups. The nests built by mixed-size groups were both larger in absolute terms and larger than expected based on the mean contribution of both size classes in isolation. The amount of sand excavated per centimetre was related to body size, but specific features, like chambers and nodes with numerous branches, were not produced by a particular worker size class or a combination of sizes. Instead, all workers generated the same basic nest components, and tended to excavate nests that branched more at shallow depths. In polymorphic groups, non-additive increases in nest length were most frequently associated with an increase in the number of simple bifurcations and the resulting increase in the number of edges across the network describing the nest.
In nature, body size range and frequency in V. pergandei vary considerably across seasons [14,15], populations [17] and with respect to colony identity [18]. Large colonies that invest more resources in reproduction experience the greatest seasonal reduction in worker body size, while producing the most workers annually. These colonies also tend to contain significantly fewer patrilines (4 or fewer, range = 1 to 9 fathers) [18]. Owing to the relationship between nest architecture and polymorphism, nests built in different seasons by colonies with different pedigrees may differ markedly in form and may also differ in function.
Because body size is related to multiple colony-level traits in V. pergandei, selection acting on features tied to worker body size, such as mating frequency and colony size, could also indirectly influence unrelated colony characteristics, like nest architecture. In honeybees, artificial selection on a colony-level trait, pollen hoarding, had downstream effects on numerous other aspects of the colony phenotype as well as underlying worker characteristics [32]. Likewise, nest variants that result from differences in intrinsic worker characteristics and increase colony fitness, could affect other levels of colony organization across generations in V. pergandei.
(a). Sources of variation
Further study is needed to determine why polymorphism influences nest architecture in V. pergandei. One possibility is that the physical interactions of differently sized workers allow for more workers to access the nest at one time. For instance, if traffic in the developing nest is limited by body size, variation in size might increase the number of workers that can occupy a developing shaft. Increased packing could also change the flow of traffic, allowing workers of different sizes to pass one another without stalling. Alternatively, extra digging faces and branching may appear if large workers push small workers aside during excavation, or if queuing time at an active digging face increases for one worker size class when the other is present [33].
In our polymorphic treatment groups, both size classes accessed the nest and deposited soil within seconds of one another (electronic supplementary material, S6) [15]. For this reason, the possibility of ‘shift work’ or a temporal division of digging by size is unlikely. We found that average body size predicted the amount of sand excavated per centimtre. Just as Lasius niger workers use their own body length as a template when placing roofs over columns inside developing nests [34], our results suggest that V. pergandei workers use their own body size to determine shaft width, instead of excavating shafts wide enough for the range of body sizes in their source colony. When both size classes worked together, they excavated an intermediate amount of sand per centimetre, but the rate of excavation per centimetre was lower than that of either monomorphic group. This may be attributable the additional shafts produced by the mixed treatment, which had not yet reached their final diameter. Therefore, we expect that completed shafts accommodate the largest size class present, rather than being intermediate in size.
Social insect colonies are known to have daily behavioural rhythms, and it is common in the laboratory to see pauses in digging activity while large groups of workers engaged in allogrooming or feeding. We did not measure overall and individual activity levels across different treatment groups in our study, but detailed video and tracking of individuals in different contexts may reveal any individual or group-level changes in motivation or overall activity across treatments. In other ant species, a significant amount of excavated material is cached below ground and transported upward in different stages when space is needed, often by multiple, age-correlated worker groups [13,27,35]. Our study design did not allow for observation of below-ground deposition, but it is possible that polymorphic groups were more motivated to remove cached sand or that one size class tended to remove cached sand when the other size class was present.
Although nest size and complexity differed significantly across treatments, similarities in relative complexity were also apparent within colonies (figure 5). For instance, when single-sized worker groups produced large or more complex nests in isolation, the nest produced by both worker types in combination often appeared larger or more complex than others in the same treatment. Since treatments were run concurrently for each colony, it is possible that equivalent nutritional and experiential status influenced the activity levels of workers across all colony members. Alternatively, intrinsic genetic factors may have contributed to the overall behavioural algorithms of workers across size classes. In V. pergandei, adult body size is related to juvenile nutrition rather than patrilineage within colonies [18]. Therefore, it is likely that within colonies, genetic structure was equivalent across treatment groups and could underlie some of the similarities observed across size classes.
(b). Natural nest architecture
The intention of the present study was not to describe the natural nest architecture of V. pergandei colonies but to highlight the individual and synergistic contributions of worker morphology to nest excavation while holding all environmental factors constant. Having established the non-additive effects of worker body size on nest size and architecture, future work can explore the relative influences of body size under additional organizational and environmental conditions. Like other ant species, V. pergandei workers experience carbon dioxide [36], moisture, temperature and soil hardness gradients across the vertical strata of the nest, which may span several metres [9,19]. Across their range, V. pergandei appears in diverse soil types, from pure sand dunes in the Anza-Borrego Desert to mixed gravel and fine particulate alluvial soils of central Arizona, which may have profound effects on the structures they build. Moisture is also a key feature affecting nest development in ants [6]. Although V. pergandei colonies have been observed excavating new nests in all seasons, surface soils only contain substantial moisture during the brief monsoon season, and the pronounced slanting of this species' natural architecture may be a response to the risk of collapse in dry soil.
Although the nests examined in the current study were incomplete and dug in laboratory buckets, they did share characteristics of the partial nest cast by Tschinkel [9] in the Anza-Borrego Desert, including wide central shafts with numerous ancillary shafts branching outward as well as flat horizontal chambers. Unlike the cast of a mature colony, nests that developed over 48 h lacked numerous tunnels with high connectivity below the surface [9]. In other harvester ant species, the top of the nest is occupied by recruitable foragers [28,37] and serves as a depot for incoming seeds, outbound sand and waste [38], as well as sites for warming developing brood. It is likely that these complex, near-surface structures develop in occupied nests over time or in response to particular stimuli. In Acromyrmex lundi, for example, the presence of fungus and brood determines the architecture of developing chambers [8]. Likewise, in the seed harvesting ant P. badius, both chamber density and complexity are associated with increasing worker number across colony ontogeny [10].
(c). Possible benefits of polymorphism
In V. pergandei, worker body size does not influence the size of seeds an individual collects, or the tendency of an individual to participate in nest excavation and foraging behaviour [15]. One clear outcome of polymorphism in our study was a relative increase in the rate of new nest growth. In xeric habitats, reducing exposure to surface temperatures by excavating a larger nest more rapidly could increase individual longevity, which averages just 18 days following the onset of foraging [18]. Many other colony-level characteristics of V. pergandei depend on avoidance of desiccation [39]. For instance, the risks of heat and desiccation drive colonies to shift their foraging schedule to pre-dawn hours during the extreme heat of summer [40]. Unlike other members of the genus, and most other Sonoran desert ants, V. pergandei mating flights occur during the comparatively mild temperature window between February and March, rather than at peak temperatures during the late-summer monsoon season [41]. Even under these conditions, cuticular abrasions suffered while digging can lead to desiccation and death of new queens [43].
Our experiment loosely simulates the initiation of a new nest pioneered by a small group of foragers and the results suggest that polymorphism expedites nest deepening, which may represent another adaptation to desert living in mature colonies. Polymorphism may also benefit colonies earlier in their ontogeny. After founding a new nest, queens produce an initial cohort of tiny, monomorphic workers. Average worker size increases for up to a year with each successive cohort [15,41]. During this time, colonies that develop polymorphic workers early on may be able to relocate nests more quickly in response to competitors or environmental factors.
Although the experimental design differed, a similar study of polymorphism in S. invicta compared tunnel area between worker size classes in a quasi-two-dimensional arena filled with wetted glass particles [11]. Control groups, composed of a random sample of the natural size frequency in each source colony, only excavated significantly more tunnel area than large workers in isolation. In contrast to Gravish et al. [12], we found that mixed-worker groups excavated longer nests than both small and large workers in isolation, but that small workers alone excavated significantly less sand than other treatments. It is unclear whether the excavation abilities of large S. invicta workers were limited by the two-dimensional digging space or represent the natural tendency of workers with different body sizes to perform specialized tasks [42]. Our study took place in three-dimensional space that allowed workers to build structures in any plane, move and interact without physical constraints. We also equalized the ratio of large to small workers in each experimental group, while in the study of S. invicta, small workers were the most common size class present in control samples, which may account for the similarity between nests built by small-only and control groups. In either case, determining the ratio of large to small workers necessary for a polymorphic group to outperform a monomorphic group would provide additional insight into the benefits of excavation in specific seasons for V. pergandei colonies.
5. Conclusion
Social insects modify their environment by building nests. These nests serve numerous important functions for the colonies living within. Each colony's nest architecture is both the result of collective behaviour and a device that can shape collective behaviour [44]. In this study, we used a cross-disciplinary approach to analyse how seasonal and colony-specific variation in worker polymorphism influence variation in nest architecture. Worker groups containing more than one body size produced larger and more complex nests, demonstrating that worker interactions can have non-additive outcomes distinct from those of component worker types in isolation. By increasing nest complexity, polymorphic worker groups excavated larger nests, more rapidly. The interplay between colony genetic architecture (matriline and patriline numbers), seasonality, worker body size and nest architecture has not been considered previously among ants. Our findings suggest that selection on multiple colony-level traits in V. pergandei could influence body size frequency distributions, which in turn, characterize both annual worker production and nest architecture.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Andrew Burchill, Tyler Murdock and two anonymous reviewers for their thoughtful comments on this project. We are grateful to the Arizona Bureau of Land Management for allowing us to conduct studies on public land in Casa Grande, AZ, USA.
Data accessibility
Datasets, source code and additional images supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
C.L.K. designed the study, collected field and laboratory data, analysed the data, participated in figure design and drafted the manuscript; G.V. managed the data and accompanying code, conducted the formal analysis, designed the figures and drafted the manuscript; B.H. participated in the design of the study, edited the manuscript and provided support for this project. All authors gave final approval for publication.
Competing interests
The authors of this study declare no competing interests.
Funding
This work was supported by research funds from Arizona State University given to B.H. G.V. acknowledges support from the National Science Foundation (grant no. 1505048).
References
- 1.Kleineidam C, Ernst R, Roces F. 2001. Wind-induced ventilation of the giant nests of the leaf-cutting ant Atta vollenweideri. Naturwissenschaften 88, 301 ( 10.1007/s001140100235) [DOI] [PubMed] [Google Scholar]
- 2.Turner JS. 2001. On the mound of Macrotermes michaelseni as an organ of respiratory gas exchange. Physiol. Biochem. Zool. Ecol. Evol. Approach. 74, 798–822. ( 10.1086/323990) [DOI] [PubMed] [Google Scholar]
- 3.Tschinkel WR, Hanley N. 2017. Vertical organization of the division of labor within nests of the Florida harvester ant, Pogonomyrmex badius. PLoS ONE 12, e0188630 ( 10.1371/journal.pone.0188630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart LM, Tschinkel WR. 2012. A seasonal natural history of the ant, Odontomachus brunneus. Insectes Soc. 50, 45 ( 10.1007/s00040-011-0186-6) [DOI] [Google Scholar]
- 5.Espinoza DN, Santamarina JC. 2010. Ant tunneling—a granular media perspective. Granul. Matter 12, 607–616. ( 10.1007/s10035-010-0202-y) [DOI] [Google Scholar]
- 6.Pielström S, Roces F. 2014. Soil moisture and excavation behaviour in the chaco leaf-cutting ant (Atta vollenweideri): digging performance and prevention of water inflow into the nest. PLoS ONE 9, e95658 ( 10.1371/journal.pone.0095658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollazzi M, Roces F. 2007. To build or not to build: circulating dry air organizes collective building for climate control in the leaf-cutting ant Acromyrmex ambiguus. Anim. Behav. 74, 1349–1355. ( 10.1016/j.anbehav.2007.02.021) [DOI] [Google Scholar]
- 8.Römer D, Roces F. 2014. Nest enlargement in leaf-cutting ants: relocated brood and fungus trigger the excavation of new chambers. PLoS ONE 9, e97872 ( 10.1371/journal.pone.0097872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschinkel WR. 2015. The architecture of subterranean ant nests: beauty and mystery underfoot. J. Bioeco. 17, 271–291. ( 10.1007/s10818-015-9203-6) [DOI] [Google Scholar]
- 10.Tschinkel WR. 2004. The nest architecture of the Florida harvester ant, Pogonomyrmex badius. J. Insect Sci. 4, 21 ( 10.1093/jis/4.1.21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haifig I, Jost C, Janei V, Costa-Leonardo AM. 2011. The size of excavators within a polymorphic termite species governs tunnel topology. Anim. Behav. 82, 1409–1414. ( 10.1016/j.anbehav.2011.09.025) [DOI] [Google Scholar]
- 12.Gravish N, Garcia M, Mazouchova N, Levy L, Umbanhowar PB, Goodisman MAD, Goldman DI. 2012. Effects of worker size on the dynamics of fire ant tunnel construction. J. R. Soc. Interface 9, 3312–3322. ( 10.1098/rsif.2012.0423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rink WJ, Dunbar JS, Tschinkel WR, Kwapich C, Repp A, Stanton W, Thulman DK. et al. 2013. Subterranean transport and deposition of quartz by ants in sandy sites relevant to age overestimation in optical luminescence dating. J. Archaeol. Sci. 40, 2217–2226. ( 10.1016/j.jas.2012.11.006) [DOI] [Google Scholar]
- 14.Gordon SH. 1978. Food and foraging ecology of a desert harvester ant, Veromessor pergandei (Mayr). Dissertation, University of California, Berkeley, CA. [Google Scholar]
- 15.Rissing SW. 1987. Annual cycles in worker size of the seed-harvester ant Veromessor pergandei (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 20, 117–124. ( 10.1007/BF00572633) [DOI] [Google Scholar]
- 16.Tschinkel WR. 2011. The organization of foraging in the fire ant, Solenopsis invicta. J. Insect Sci. 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson DW. 1978. Size variability in the worker caste of a social insect (Veromessor pergandei Mayr) as a function of the competitive environment. Am. Nat. 112, 523–532. ( 10.1086/283294) [DOI] [Google Scholar]
- 18.Kwapich CL, Gadau J, Hölldobler B. 2017. The ecological and genetic basis of annual worker production in the desert seed harvesting ant, Veromessor pergandei. Behav. Ecol. Sociobiol. 71, 110 ( 10.1007/s00265-017-2333-1) [DOI] [Google Scholar]
- 19.Tevis L. 1958. Interrelations between the harvester ant Veromessor Pergandei (Mayr) and some desert ephemerals. Ecology 39, 695–704. ( 10.2307/1931610) [DOI] [Google Scholar]
- 20.Brown MJF. 1999. Nest relocation and encounters between colonies of the seed-harvesting ant Messor andrei. Insectes Soc. 46, 66–70. ( 10.1007/s000400050114) [DOI] [Google Scholar]
- 21.Pinter-Wollman N, Brown MJF. 2015. Variation in nest relocation of harvester ants is affected by population density and food abundance. Behav. Ecol. 26, 1569–1576. ( 10.1093/beheco/arv108) [DOI] [Google Scholar]
- 22.Pratt SC. 2005. Behavioral mechanisms of collective nest-choice by the ant Temnothorax curvisponosus. Insectes Soc. 52, 383–392. ( 10.1007/s00040-005-0823-z) [DOI] [Google Scholar]
- 23.Tschinkel WR. 2014. Nest relocation and excavation in the florida harvester ant, Pogonomyrmex badius. PLoS ONE 9, e112981 ( 10.1371/journal.pone.0112981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avargues-Weber A, Monnin T. 2009. Dynamics of colony emigration in the ant Aphaenogaster senilis. Insectes Soc. 56, 177–183. ( 10.1007/s00040-009-0009-1) [DOI] [Google Scholar]
- 25.Porter S. 1983. Fast, accurate method of measuring ant head widths. Ann. Entomol. Soc. Am. 76, 866–867. ( 10.1093/aesa/76.5.866) [DOI] [Google Scholar]
- 26.Tschinkel WR. 2010. Methods for casting subterranean ant nests. J. Insect Sci. 10, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murdock TC, Tschinkel WR. 2015. The life history and seasonal cycle of the ant, Pheidole morrisi Forel, as revealed by wax casting. Insectes Soc. 62, 265–280. ( 10.1007/s00040-015-0403-9) [DOI] [Google Scholar]
- 28.Pinter-Wollman N. 2015. Nest architecture shapes the collective behaviour of harvester ants. Biol. Lett. 11, 20150695 ( 10.1098/rsbl.2015.0695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki T, Granovskiy B, Mann RP, Sumpter DJT, Pratt SC. 2013. Ant colonies outperform individuals when a sensory discrimination task is difficult but not when it is easy. Proc. Natl Acad. Sci. USA 110, 13 769–13 773. ( 10.1073/pnas.1304917110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley TD, Visscher PK. 2004. Quorum sensing during nest-site selection by honeybee swarms. Behav. Ecol. Sociobiol. 56, 594–601. ( 10.1007/s00265-004-0814-5) [DOI] [Google Scholar]
- 31.Wiernasz DC, Hines J, Parker DG, Cole BJ. 2008. Mating for variety increases foraging activity in the harvester ant, Pogonomyrmex occidentalis. Mol. Ecol. 17, 1137–1144. ( 10.1111/j.1365-294X.2007.03646.x) [DOI] [PubMed] [Google Scholar]
- 32.Page RE, Fondrk MK. 1995. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: colony-level components of pollen hoarding. Behav. Ecol. Sociobiol. 36, 135–144. ( 10.1007/BF00170718) [DOI] [Google Scholar]
- 33.Bardunias PM, Su NY. 2010. Queue size determines the width of tunnels in the formosan subterranean termite (Isoptera: Rhinotermitidae). J. Insect Behav. 23, 189–204. ( 10.1007/s10905-010-9206-z) [DOI] [Google Scholar]
- 34.Khuong A, Gautrais J, Perna A, Sbaï C, Combe M, Kuntz P, Jost C, Theraulaz G. 2016. Stigmergic construction and topochemical information shape ant nest architecture. Proc. Natl Acad. Sci. USA 113, 1303–1308. ( 10.1073/pnas.1509829113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Römer D, Roces F. 2015. Available space, symbiotic fungus and colony brood influence excavation and lead to the adjustment of nest enlargement in leaf-cutting ants. Insectes Soc. 62, 401–413. ( 10.1007/s00040-015-0419-1) [DOI] [Google Scholar]
- 36.Tschinkel WR. 2013. Florida harvester ant nest architecture, nest relocation and soil carbon dioxide gradients. PLoS ONE 8, e59911 ( 10.1371/journal.pone.0059911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwapich CL, Tschinkel WR. 2013. Demography, demand, death, and the seasonal allocation of labor in the Florida harvester ant (Pogonomyrmex badius). Behav. Ecol. Sociobiol. 67, 2011–2027. ( 10.1007/s00265-013-1611-9) [DOI] [Google Scholar]
- 38.Tschinkel WR, Rink WJ, Kwapich CL. 2015. Sequential subterranean transport of excavated sand and foraged seeds in nests of the harvester ant, Pogonomyrmex badius. PLoS ONE 10, e0139922 ( 10.1371/journal.pone.0139922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson RA. 2000. Water loss in desert ants: caste variation and the effect of cuticle abrasion. Physiol. Entomol. 25, 48–53. ( 10.1046/j.1365-3032.2000.00170.x) [DOI] [Google Scholar]
- 40.Bernstein RA. 1974. Seasonal food abundance and foraging activity in some desert ants. Am. Nat. 108, 490–498. ( 10.1086/282928) [DOI] [Google Scholar]
- 41.Pollock GB, Rissing SW. 1985. Mating season and colony foundation of the seed-harvester ant, Veromessor Pergandei. Psyche 92, 125–134. ( 10.1155/1985/87410) [DOI] [Google Scholar]
- 42.Wilson EO. 1978. Division of labor in fire ants based on physical castes (Hymenoptera: Formicidae: Solenopsis). J. Kansas Entomol. Soc. 51, 615–636. [Google Scholar]
- 43.Johnson RA, Kaiser A, Quinlan M, Sharp W. 2011. Effect of cuticular abrasion and recovery on water loss rates in queens of the desert harvester ant Messor pergandei. J. Exp. Biol. 214, 3495–3506. ( 10.1242/jeb.054304) [DOI] [PubMed] [Google Scholar]
- 44.Pinter-Wollman N, Fiore SM, Theraulaz G. 2017. The impact of architecture on collective behaviour. Nat. Ecol. Evol. 1, 0111 ( 10.1038/s41559-017-0111) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets, source code and additional images supporting this article have been uploaded as part of the electronic supplementary material.