Abstract
Evidence for transgenerational effects of senescence, whereby offspring from older parents have a reduced lifetime reproductive success, is increasing. Such effects could arise from compromised germline maintenance in old parents, potentially reflected in reduced telomere length in their offspring. We test the relationship between parental age and offspring early-life telomere length in a natural population of common terns and find a significant negative correlation between paternal age and offspring telomere length. Offspring telomere length is reduced by 35 base pairs for each additional year of paternal age. We find no correlation with maternal age. These results fit with the idea of compromised germline maintenance in males, whose germline stem cells require continued division.
Keywords: ageing, common tern, Lansing effect, parental effect, Sterna hirundo, terminal restriction fragment analysis
1. Introduction
Organismal senescence occurs throughout the tree of life [1] and is defined by age-specific declines in the two main fitness components: survival probability and offspring production [2]. In addition, there is increasing evidence for transgenerational effects, with offspring from older parents achieving a reduced lifetime reproductive success [3–5]. A suggested explanation for such transgenerational effects is compromised germline maintenance in old individuals [6], leading to increased levels of de novo mutations [5] and/or shorter telomeres [7] in their offspring.
Terminal telomeres are highly conserved repetitive DNA sequences found at the end of eukaryote chromosomes. They maintain chromosome integrity, but shorten with cell division. Their length predicts survival across taxa and thus indicates long-term somatic state [8,9]. Evidence for parental age to affect offspring telomere length mainly comes from humans, in which, interestingly, older fathers pass longer, rather than shorter, telomeres to their offspring [10]. This sex-specific positive correlation between parental age and offspring telomere length, however, does not seem to be general across species (table 1).
Table 1.
Overview of studies on non-human vertebrates testing for effects of paternal age at conception on offspring telomere length (n.s. = non-significant; direction reported for significant effects only).
| class | species | population | paternal age effect | N offspring | reference |
|---|---|---|---|---|---|
| Osteichthyes | Atlantic salmon (Salmo salar) | captive | n.s. | 300 | [11] |
| Reptilia | sand lizard (Lacerta agilis) | wild | negative | 12 | [7] |
| Aves | great reed warbler (Acrocephalus arundinaceus) | wild | n.s. | 139 | [12] |
| European shag (Phalacrocorax aristotelis) | wild | n.s. | 204 | [13] | |
| alpine swift (Apus melba) | wild | negative | 95 | [14] | |
| zebra finch (Taeniopygia guttata) | captive | negative | 139 | [15] | |
| jackdaw (Corvus monedula) | wild | negative | 715 | [16] | |
| common tern (Sterna hirundo) | wild | negative | 142 | this study | |
| Mammalia | house mouse (Mus musculus) | captive | negative | 12 | [17] |
| chimpanzee (Pan troglodytes) | captive | positive | 40 | [18] | |
| Soay sheep (Ovis aries) | wild | n.s. | 164 | [19] |
Here, making use of nearly the entire parental age range (3–24 years), offspring blood samples collected 3 days post-hatching and the gold standard of terminal telomere length measurement (in-gel terminal restriction fragment analysis (TRF)), we investigate the correlation between parental age and offspring telomere length in a natural population of common terns (Sterna hirundo). In this bird species, sex-specific negative correlations between parental age and offspring fitness have been established: daughters of older mothers fledge at a lower weight, remain lighter throughout life and suffer from reduced offspring production, while sons of older fathers live less long [3]. Because common tern erythrocyte telomeres shorten with age and their age-specific length predicts remaining lifespan [20], we hypothesize the paternal age–son lifespan correlation to result from, or be reflected in, telomere patterns.
2. Material and methods
(a). Study species and data collection
The common tern is a sexually and socially monogamous seabird [21]. Our data [22] come from a long-term study of a colony in the Banter See at Wilhelmshaven, Germany. In 1992, 101 adult birds of known age were caught and marked with transponders, and since 1992 all offspring were marked with a transponder shortly prior to fledging. During incubation, which is shared between partners, antennae are placed around each nest to identify breeding individuals. Combined with nest-checks to record reproductive parameters and to mark offspring, these methods enable the documentation of individual age-specific reproductive performance at the colony.
In the breeding seasons of 2013 and 2014, we blood sampled 3-day-old chicks from 116 nests (see [23] for details). From these blood samples, erythrocyte telomere length was measured by in-gel TRF [23]. Variation in chick telomere length was not explained by year, brood size or by offspring sex, hatching order and body mass [23].
(b). Statistical analyses
We analysed parental age effects on offspring telomere length in mixed models with normally distributed errors. These models included nested random effects of parental and brood identity to account for the non-independence of offspring from the same parent (within and between years) and brood, and a linear covariate for parental age. Because transgenerational senescence effects were found to be sex-specific [3], we additionally fitted a two-level class variable for offspring sex (with males as the reference category) and the interaction between offspring sex and parental age.
Parental age was known for 183 chicks hatched in 96 nests from 52 mothers (age: average ± s.d. = 10.1 ± 5.7, range 3–24 years) and 58 fathers (age: average ± s.d. = 9.6 ± 4.7, range 3–21 years). Because the age of both parents was known for a subset of only 88 chicks from 37 unique pairs, and positively correlated (r2 = 0.34, n = 37), models were run for maternal and paternal age separately to maximize sample size for each analysis. Models were run in MLwiN v. 2.26 [24]. Significance (p < 0.05, two-tailed) was assessed using the Wald statistic.
3. Results
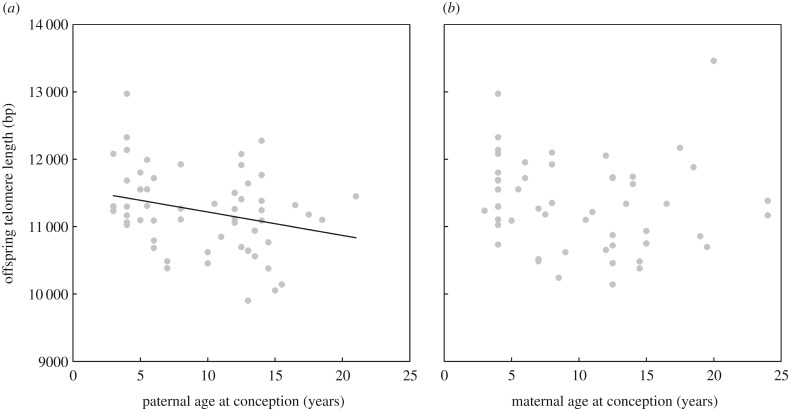
Offspring telomere length was negatively correlated with paternal age. With each additional year of paternal age, offspring telomeres were 35 (±15 s.e., 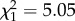 , p = 0.02) base pairs shorter (figure 1a; electronic supplementary material, table S1).
, p = 0.02) base pairs shorter (figure 1a; electronic supplementary material, table S1).
Figure 1.
Common tern offspring telomere length decreased with paternal (a), but not maternal (b), age at conception. Presented are average offspring telomere lengths per parent, but analyses were carried out on the full dataset.
By contrast, there was no significant correlation between maternal age and offspring telomere length. Following [16], assuming both parents to contribute equally to telomere length of offspring, the non-significant negative slope of −15 (±16 s.e., 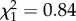 , p = 0.36; figure 1b; electronic supplementary material, table S1) was close to that expected from the paternal age estimate and the correlation between paternal and maternal age (r = 0.58): 0.58 × −35 = −20 base pairs.
, p = 0.36; figure 1b; electronic supplementary material, table S1) was close to that expected from the paternal age estimate and the correlation between paternal and maternal age (r = 0.58): 0.58 × −35 = −20 base pairs.
There was no evidence for a sex difference in offspring telomere length (−3 ± 74 s.e., 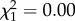 , p = 0.98), nor for an interaction between offspring sex and paternal (−4 ± 18 s.e.,
, p = 0.98), nor for an interaction between offspring sex and paternal (−4 ± 18 s.e., 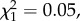 p = 0.82) or maternal (13 ± 17 s.e.,
p = 0.82) or maternal (13 ± 17 s.e., 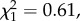 p = 0.43) age.
p = 0.43) age.
4. Discussion
Common tern paternal age was negatively correlated with offspring erythrocyte telomere length measured shortly after hatching, while the weaker and non-significant correlation with maternal age could be attributed to the intra-pair correlation between paternal and maternal age. Because we sampled offspring close after hatching, before parental care affects telomere dynamics (e.g. [13]), and because traits such as clutch and egg size, as well as fledging success [25] and parental feeding rate [26] increase, rather than decrease, with parental age, we expect our result of shorter telomeres in offspring of older fathers to be explained by variation in paternal telomere length at conception. We, however, cannot exclude maternal adjustment of egg physiology in response to paternal age as an alternative explanation. A telomere length variation at conception explanation would fit with the idea of compromised germline maintenance [6] if that leads to short telomeres in male gametes, the production of which, in contrast to that of female gametes, requires continued division of germline stem cells [27].
We initially predicted the effect of paternal age on offspring telomere length to be sex-specific, as we previously found paternal age to negatively affect the lifespan, and thereby lifetime reproductive success, of sons, but not daughters [3]. This prediction was not confirmed. Among adult common terns, however, erythrocyte telomere length is known to be more strongly related to survival in males than females [20], such that our finding may actually underlie or reflect the previously observed negative fitness effect of paternal age on sons [3] even when the negative paternal age effect on offspring telomere length is carried through to adulthood similarly in both sexes.
Interestingly, the negative correlation between paternal age and offspring telomere length we found in common terns corresponds with that found in six out of 11 other non-human study species (table 1), but contrasts with the positive correlation found in humans and chimpanzees (reviewed in [18]). This interspecific difference has been suggested to reflect variation in sperm production rate: species with a larger sperm production rate should have increased telomere-elongating telomerase activity in their spermatogonial stem cells, or increased selective loss of sperm progenitor cells with shorter telomere length [19]. While rates of sperm production have not been assessed, the available data seem to support this idea: species with year-round sperm production, such as humans and chimpanzees, show a positive correlation [10], whereas species with seasonal sperm production but high sperm competition, such as great reed warblers, European shags and Soay sheep, show no correlation, and mostly monogamous species with seasonal sperm production, such as alpine swifts, jackdaws and common terns, show a negative correlation (table 1). We suggest future studies to longitudinally assess the age-specific paternal telomere length in sperm in relation to sperm production rate to test this hypothesis.
Supplementary Material
Acknowledgements
We thank all field workers for collecting and compiling the long-term dataset and are grateful to Peter H. Becker and Ellis Mulder for facilitating the data collection.
Ethics
The study was performed under legal authorization of the city of Wilhelmshaven and the Lower Saxony State Office for Consumer Protection and Food Safety, Germany. Ethical licence numbers for bleeding chicks were 33.9-42502-05-13A329 and 33.9-42502-05-14A438 for 2013 and 2014, respectively.
Data accessibility
The data are available on Dryad (http://dx.doi.org/10.5061/dryad.mc763v6) [22].
Authors' contributions
S.B., O.V. and S.V. designed the study; S.B. and O.V. collected blood samples and analysed the data; C.B. measured telomere lengths in the laboratory of S.V.; S.B. wrote the paper with contributions from O.V., S.V. and C.B. All authors approved publication of this paper and agree to be held accountable for its content.
Competing interests
We have no competing interests.
Funding
O.V. was supported by The Netherlands Organisation for Scientific Research (NWO; grant no. 863.14.010).
References
- 1.Shefferson RP, Jones OR, Salguero-Gómez R. 2017. The evolution of senescence in the tree of life. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Bouwhuis S, Choquet R, Sheldon BC, Verhulst S. 2012. The forms and fitness cost of senescence: age-specific recapture, survival, reproduction, and reproductive value in a wild bird population. Am. Nat. 179, E15–E27. ( 10.1086/663194) [DOI] [PubMed] [Google Scholar]
- 3.Bouwhuis S, Vedder O, Becker PH. 2015. Sex-specific pathways of parental age effects on offspring lifetime reproductive success in a long-lived seabird. Evolution 69, 1760–1771. ( 10.1111/evo.12692) [DOI] [PubMed] [Google Scholar]
- 4.Schroeder J, Nakagawa S, Rees M, Mannarelli M, Burke T. 2015. Reduced fitness in progeny from old parents in a natural population. Proc. Natl Acad. Sci. USA 112, 4021–4025. ( 10.1073/pnas.1422715112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arslan RC, et al. 2017. Older fathers' children have lower evolutionary fitness across four centuries and in four populations. Proc. R. Soc. B 284, 20171562 ( 10.1098/rspb.2017.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maklakov AA, Immler S. 2016. The expensive germline and the evolution of ageing. Curr. Biol. 26, R577–R586. ( 10.1016/j.cub.2016.04.012) [DOI] [PubMed] [Google Scholar]
- 7.Olsson M, Pauliny A, Wapstra E, Uller T, Schwartz T, Blomqvist D. 2011. Sex differences in sand lizard telomere inheritance: paternal epigenetic effects increases telomere heritability and offspring survival. PLoS ONE 6, e17473 ( 10.1371/journal.pone.0017473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonekamp JJ, Simons MJP, Hemerik L, Verhulst S. 2013. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12, 330–332. ( 10.1111/acel.12050) [DOI] [PubMed] [Google Scholar]
- 9.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 20160447 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg DTA, Kuzawa CW. 2018. The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Phil. Trans. R. Soc. B 373, 20160442 ( 10.1098/rstb.2016.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLennan D, Armstrong JD, Stewart DC, McKelvey S, Boner W, Monaghan P, Metcalfe NB. 2018. Links between parental life histories of wild salmon and the telomere lengths of their offspring. Mol. Ecol. 27, 804–814. ( 10.1111/mec.14467) [DOI] [PubMed] [Google Scholar]
- 12.Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D. 2015. Maternal and genetic factors determine early life telomere length. Proc. R. Soc. B 282, 20142263 ( 10.1098/rspb.2014.2263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidinger BJ, Herborn KA, Granroth-Wilding HMV, Boner W, Burthe S, Newell M, Wanless S, Daunt F, Monaghan P. 2016. Parental age influences offspring telomere loss. Funct. Ecol. 30, 1531–1538. ( 10.1111/1365-2435.12630) [DOI] [Google Scholar]
- 14.Criscuolo F, Zahn S, Bize P. 2017. Offspring telomere length in the long lived Alpine swift is negatively related to the age of their biological father and foster mother. Biol. Lett. 13, 20170188 ( 10.1098/rsbl.2017.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguera JC, Metcalfe NB, Monaghan P. 2018. Experimental demonstration that offspring fathered by old males have shorter telomeres and reduced lifespans. Proc. R. Soc. B 285, 20180268 ( 10.1098/rspb.2018.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauch C, Boonekamp JJ, Korsten P, Mulder E, Verhulst S. 2018. Epigenetic inheritance of telomere length in wild birds. bioRxiv 284208. ( 10.1101/284208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Frutos C, Lopez-Cardona AP, Balvis NF, Laguna-Barraza R, Rizos D, Gutierrez-Adan A, Bermejo-Alvarez P. 2016. Spermatozoa telomeres determine telomere length in early embryos and offspring. Reproduction 151, 1–7. ( 10.1530/REP-15-0375) [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg DTA, Tackney J, Cawthon RM, Cloutier CT, Hawkes K. 2017. Paternal and grandpaternal ages at conception and descendant telomere lengths in chimpanzees and humans. Am. J. Phys. Anthropol. 162, 201–207. ( 10.1002/ajpa.23109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froy H, et al. 2017. No evidence for parental age effects on offspring leukocyte telomere length in free-living Soay sheep. Sci. Rep 7, 9991 ( 10.1038/s41598-017-09861-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauch C, Becker PH, Verhulst S. 2014. Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol. Ecol 23, 300–310. ( 10.1111/mec.12602) [DOI] [PubMed] [Google Scholar]
- 21.Becker PH, Ludwigs JD. 2004. Sterna hirundo common tern. In Birds of the western palearctic (ed. Parkin D.), pp. 91–127. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Bouwhuis S, Verhulst S, Bauch C, Vedder O. 2018. Data from: Reduced telomere length in offspring of old fathers in a long-lived seabird Dryad Digital Repository. ( 10.5061/dryad.mc763v6) [DOI] [PMC free article] [PubMed]
- 23.Vedder O, Verhulst S, Bauch C, Bouwhuis S. 2017. Telomere attrition and growth: a life-history framework and case study in common terns. J. Evol. Biol. 30, 1409–1419. ( 10.1111/jeb.13119) [DOI] [PubMed] [Google Scholar]
- 24.Rasbash J, Steele F, Browne WJ, Prosser B. 2005. A user's guide to MLwiN – version 2.0. London, UK: Centre for Multilevel Modelling, University of Bristol. [Google Scholar]
- 25.Zhang H, Vedder O, Becker PH, Bouwhuis S. 2015. Age-dependent trait variation: the relative contribution of within-individual change, selective appearance and disappearance in a long-lived seabird. J. Anim. Ecol. 84, 797–807. ( 10.1111/1365-2656.12321) [DOI] [PubMed] [Google Scholar]
- 26.Limmer B, Becker PH. 2009. Improvement in chick provisioning with parental experience in a seabird. Anim. Behav. 77, 1095–1101. ( 10.1016/j.anbehav.2009.01.015) [DOI] [Google Scholar]
- 27.Gilbert SF. 2013. Developmental biology. Sunderland, MA: Sinauer Associates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bouwhuis S, Verhulst S, Bauch C, Vedder O. 2018. Data from: Reduced telomere length in offspring of old fathers in a long-lived seabird Dryad Digital Repository. ( 10.5061/dryad.mc763v6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data are available on Dryad (http://dx.doi.org/10.5061/dryad.mc763v6) [22].