Abstract
Differences in temporal patterns of activity can modulate the ambient conditions to which organisms are exposed, providing an important mechanism for responding to environmental change. Such differences may be particularly relevant to ecological generalists, which are expected to encounter a wider range of environmental conditions. Here, we compare temporal patterns of activity for partially sympatric populations of a generalist (the lodgepole chipmunk, Tamias speciosus) and a more specialized congener (the alpine chipmunk, Tamias alpinus) that have displayed divergent responses to the past century of environmental change. Although mean activity budgets were similar between species, analyses of individual-level variation in locomotion revealed that T. alpinus exhibited a narrower range of activity patterns than T. speciosus. Further analyses revealed that T. alpinus was more active earlier in the day, when temperatures were cooler, and that activity patterns for both species changed with increased interspecific co-occurrence. These results are consistent with the greater responsiveness of T. alpinus to changes in environmental conditions. In addition to highlighting the utility of accelerometers for collecting behavioural data, our findings add to a growing body of evidence, suggesting that the greater phenotypic variability displayed by ecological generalists may be critical to in situ responses to environmental change.
Keywords: accelerometers, behaviour, climate change, locomotion, plasticity
1. Background
Ecological generalists often display different responses to environmental conditions from ecological specialists, with the latter tending to be more sensitive to external changes [1,2]. This distinction has most often been examined with regard to morphological and physiological attributes, although specialization of behavioural traits [3,4] may also be important in the context of environmental change. For example, temporal differences in activity can alter the conditions to which organisms are exposed [5,6] and species with greater intraspecific variability in such traits are expected to be better able to accommodate environmental changes [7]. Such variability may also facilitate behavioural partitioning of resources when confronted with novel competitors due to climate-induced range shifts and associated changes in community dynamics [8,9]. While the behavioural data needed to evaluate temporal differences in activity have historically been difficult to obtain, the growing use of accelerometers allows remote monitoring of activity in free-living animals [10].
Here, we focus on two co-occurring congeners characterized by distinct responses to the past century of environmental change. The alpine chipmunk (Tamias alpinus, Ta), an ecological specialist endemic to alpine habitats in the Sierra Nevada mountains, has undergone a significant upward range contraction paired with changes in morphology, genetics and diet. By contrast, the more generalist lodgepole chipmunk (T. speciosus, Ts) has not experienced any consistent patterns of change [11–14]. Ecological modelling suggests that Ta's range is constrained by abiotic, climatic factors; by contrast, Ts may be more limited by interspecific competition [14,15].
To determine whether activity patterns contribute to the differential responses of Ta and Ts to environmental conditions, we used accelerometers to characterize patterns of locomotion, examining interspecific differences in activity and the extent to which such differences are associated with external and intrinsic parameters. We also quantified glucocorticoids to assess the impacts of accelerometers on study subjects. Our analyses of both species- and individual-level variability in activity generate intriguing new insights into how activity patterns may contribute to interspecific differences in responses to environmental change.
2. Material and methods
(a). Study species and sites
Ta is a 30–50 g alpine specialist chipmunk; Ts weighs 50–80 g and occurs at and below the treeline. Chipmunks at three study sites (electronic supplementary material, table S1) were captured using grids of Sherman traps that encompassed areas occupied by one or both species.
(b). Accelerometers
Acceleration loggers (Corvus Scientific) consisting of a tri-axial accelerometer, a data logger, and a battery and weighing 1.5–2.5 g (less than 5% body mass; detailed specifications in [16]) were deployed in approximately July–September 2015. Units were affixed to 8–15 individuals per species per site with eyelash extension glue after shaving a dorsal patch of fur. Units activated every 15 min to record 10 s of 20 Hz acceleration readings. A previously validated machine learning system assigned these data to one of three behavioural categories (‘still’, ‘in-place movement’, ‘locomotion’) with 82–90% accuracy [16].
(c). Glucocorticoid analyses
Faeces were collected from traps when animals were captured to deploy and recover accelerometers, and faecal glucocorticoid metabolites (FGMs) were measured as described in [17] (see electronic supplementary material for details).
(d). Climatic data
iButton loggers (DS1921G) were deployed near approximately 75% of trapping stations (within approx. 1 m of the ground) to collect hourly temperature readings. Data collected during accelerometer deployment were used to calculate mean, maximum, minimum and variance in daily temperatures as well as mean daytime and afternoon temperature at each individual's trapping grid. Principal components analysis (PCA) was applied to these data to reduce dimensionality (electronic supplementary material, table S2) and the first PC axis was used as a predictor in models (§2e).
(e). Statistics
(i). Faecal glucocorticoid metabolites
Wilcoxon signed-rank tests were used to compare pre- and post-accelerometer FGMs.
(ii). Activity budgets
Wilcoxon rank-sum tests were used to assess interspecific differences in the proportion of the day spent on each behavioural category (§2b).
(iii). Activity patterns
For each individual, a generalized additive model (GAM; gam package in R) was fitted for behaviour as a function of time of day. The response variable was the number of seconds in each 10 s sampling period (§2b) scored as ‘locomotion’. Each model was plotted and PCA (electronic supplementary material, table S3) was applied to the correlation matrix of locomotion features (table 1; electronic supplementary material, figure S1) extracted from each individual's plot. PCA loadings were un-rotated. We repeated analyses for overall activity (locomotion and in-place movement), which showed reduced interspecific differentiation; thus, we focused only on locomotion for subsequent analyses.
Table 1.
Activity features extracted from individual GAM plots of locomotion as a function of smoothed time.
| feature | description |
|---|---|
| maximum | magnitude of maximum locomotion |
| time of maximum | time of maximum locomotion |
| time of minimum | time of minimum locomotion |
| afternoon (∼10:45–15:15) locomotion | area under the curve (AUC) of afternoon hours divided by AUC of daylight hours |
| morning (∼06:30–10:45) locomotion | AUC of morning hours divided by AUC of daylight hours |
| evening (∼15:15–19:30) locomotion | AUC of evening hours divided by AUC of daylight hours |
| no. of peaks | modality of locomotion curve (e.g. bimodal = 2) |
To group individuals by differences in patterns of daily locomotion, K-means cluster analyses were applied to the first two PCs (electronic supplementary material, table S3) of locomotion features [18]. Sum-of-squared error scree plots were used to determine the optimal number of clusters; each animal was assigned to the cluster with the centroid nearest to its PC position. For each species, χ2-tests were used to determine if the number of individuals per cluster differed from expectation (equal distribution across clusters).
Generalized linear mixed models (GLMMs) were constructed to test whether patterns of locomotion differed between species, whether individuals altered their activity in areas of sympatry versus allopatry and whether patterns of locomotion were associated with selected climatic or phenotypic factors (electronic supplementary material, table S4). A set of models containing all possible subsets of variables was constructed (‘dredge’ function, MuMIn package). All models for which comparisons with the lowest-AIC (Akaike information criterion) model exhibited a ΔAICc of less than 4 were included in model averaging (‘model.avg’) to generate the final optimum model.
3. Results
(a). Effects of accelerometers on faecal glucocorticoid metabolites
No significant differences were detected between pre- and post-accelerometer FGMs (Ta: V = 72, p = 0.37; Ts: V = 261, p = 0.19; electronic supplementary material, figure S2).
(b). Activity budgets
Accelerometers were recovered from 19 Ta (15F, 4M) and 28 Ts (18F, 10M). These units collected data over a mean ± s.d. of 57.4 ± 14 h per individual for Ta and 56.9 ± 18.1 h per individual for Ts. Overall activity budgets were similar across species (figure 1); both species were diurnal, with no significant differences in the proportion of the day spent on any behavioural category (all p > 0.35).
Figure 1.
Daily activity budgets for T. alpinus (a) and T. speciosus (b). Mean proportion of each hour spent still (light shading), moving in place (medium shading) or in locomotion (dark shading) is shown; no significant differences in activity were found between species. Species distributions are shown on the left. (Online version in colour.)
(c). Clustering of activity patterns
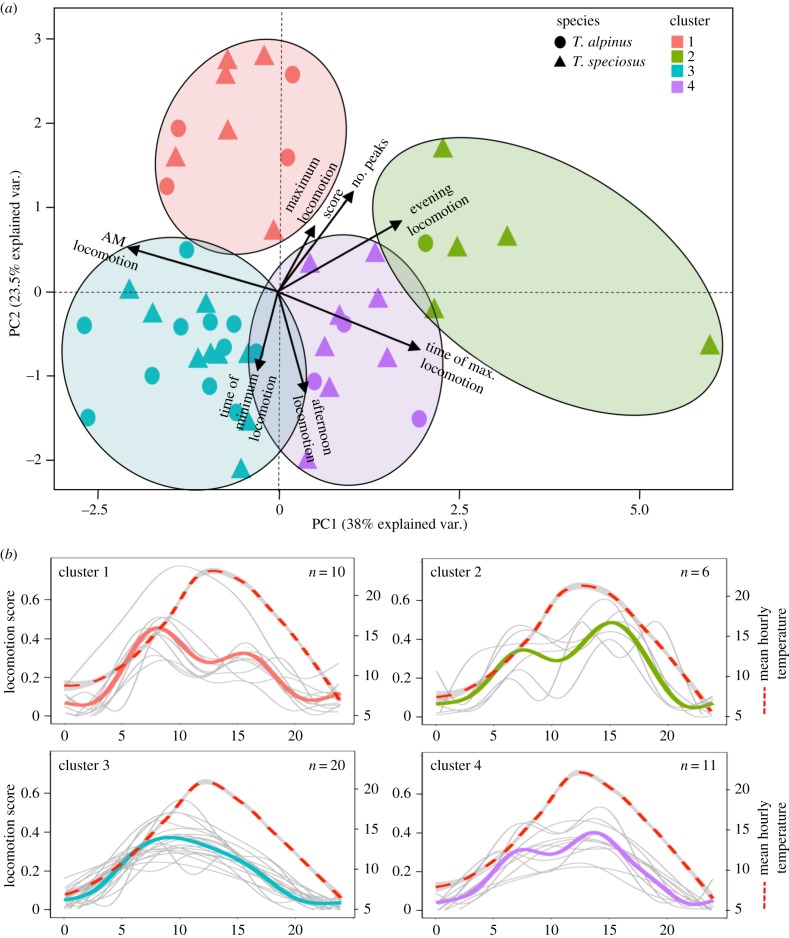
Scree plots (electronic supplementary material, figure S3) indicated four clusters of locomotor activity (figure 2a). While the distribution of individual Ts across clusters was not uneven (χ2 = 1.43, p = 0.70), Ta's distribution differed from expectation (χ2 = 11.9, p = 0.008). Ta was disproportionately abundant in cluster 3 (11/19 individuals); averaged activity patterns (GAM output for all individuals in a cluster; figure 2b) revealed that animals in this cluster were more active in the morning, such that peak locomotion did not coincide with peak daily temperatures (figure 2b). By contrast, Ta was underrepresented in clusters 2 (1/19) and 4 (3/19), in which animals exhibited locomotion peaks later in the day, when temperatures were higher, and was found in expected numbers in cluster 1 (4/19), where individuals were also more active in the morning.
Figure 2.
Clustering of activity patterns. (a) A biplot showing clustering of activity data along the first two PCs of locomotion-based features (table 1). Arrows represent vectors indicating the direction and magnitude of each variable's PC score along the two axes. Each point represents an individual chipmunk (shape denotes species and colour denotes cluster). (b) GAM locomotion curves for each cluster; averaged values are depicted in bold, coloured by cluster, with curves for all individuals in each cluster in grey. Dotted red lines show smoothed hourly temperatures from data collection periods and sampling localities averaged across all individuals in each cluster.
(d). Locomotion and environmental parameters
Species, deployment date and species co-occurrence score were retained in GLMMs predicting the first PCs of locomotion data; sex and the interaction between species and species co-occurrence were also retained, although they had limited predictive power (table 2). Model results were consistent with cluster analyses, suggesting that activity patterns of the focal species were significantly different. More specifically, Ta exhibited higher proportions of activity in the mornings and earlier activity peaks in comparison with Ts (table 2; electronic supplementary material, table S3). As co-occurrence with heterospecifics increased, activity patterns for both species changed, with individuals becoming more active in the evening and less active in the morning (electronic supplementary material, table S3). As the season progressed (i.e. later deployment dates), peak activity for both species shifted to later in the day (table 2).
Table 2.
Final model-averaged GLMM results predicting the first principal component of locomotion curve data (electronic supplementary material, table S2). Significant terms are in bold type.
| estimate | adjusted s.e. | z-value | p-value | relative importance | |
|---|---|---|---|---|---|
| (intercept) | −0.01 | 0.25 | 0.06 | 0.95 | |
| species co-occurrence score | 1.12 | 0.51 | 2.21 | 0.03 | 0.81 |
| species (Ts) | 0.51 | 0.21 | 2.45 | 0.01 | 0.79 |
| start date | −1.889 | 0.52 | 3.65 | 0.0003 | 1.00 |
| sex (M) | −0.19 | 0.21 | 0.89 | 0.37 | 0.09 |
| species×co-occurrence score | 0.30 | 0.41 | 0.73 | 0.47 | 0.15 |
| random effect | variance | (s.d.) | |||
|---|---|---|---|---|---|
| site | 0.06 | 0.25 |
4. Discussion
Our results indicate that accelerometers provide valuable information on activity patterns of small mammals. With validation [16], accelerometer data can be used to monitor specific behavioural categories, including locomotion, which has clear implications for patterns of habitat use. Our FGM analyses revealed no post-deployment changes, suggesting that accelerometers were not stressful to study animals.
While overall activity budgets for the study species were similar, individual patterns of locomotion revealed important interspecific differences that were likely masked by marked intraspecific variation during analyses of mean activity budgets. Specifically, our findings indicated that Ta was more likely to exhibit higher proportions of and peak values of locomotor activity during the morning, when temperatures were cooler. This finding is consistent with the suggested greater sensitivity of this species to thermal conditions [14,19]. Given the correlational nature of our study and the limited sample size and spatio-temporal scope of our study, we cannot conclude that these results are due solely to temperature, with no input from other environmental parameters (e.g. predation risk, vapour pressure, forage quality). Our findings do, however, underscore the importance of exploring individual variation in activity patterns rather than simply assessing differences in average activity [18].
Members of both species displayed altered locomotor activity in areas of sympatry, with a shift towards greater locomotion later in the day. Areas of sympatry tended to have lower ambient daytime temperatures than sites inhabited by exclusively T. alpinus, which were above the treeline and, therefore, experienced greater exposure and warmer daytime temperatures. Thus, at least for T. alpinus, increased activity later in the day was not due to warmer temperatures in areas of sympatry. Interspecific competition provides a logical hypothesis to explain differences in locomotion in areas of sympatry, although other, unmeasured habitat variables may also contribute to these differences.
Among individuals, Ta exhibited less diverse temporal patterns of locomotion in comparison with Ts, suggesting that the former species has reduced variability in activity patterns. In addition to undergoing more pronounced spatial, genetic, dietary and morphological responses to the past century of environmental change [11–14], Ta is more ecologically specialized than Ts, and Ta's reduced variability in activity patterns is consistent with the prediction that taxa with reduced behavioural diversity or flexibility may be less able to cope in situ with rapidly changing environments [7,20]. Ultimately, improved understanding of interactions between ecological specialization and phenotypic variability should enhance understanding of biotic responses to environmental change.
Supplementary Material
Acknowledgements
We thank E. Klobetz-Rassam for endocrine analyses; C. Hendrickson, T. Maxwell, A. Petrosky and R. Cruz for data collection; and three anonymous reviewers for comments on the manuscript.
Ethics
All procedures were approved by UC Berkeley's Animal Care and Use Committee (IACUC protocol number: 2015-01-7084).
Data accessibility
Data are available via Dryad: https://doi.org/10.5061/dryad.ch3k2s1 [21].
Authors' contributions
T.T.H. and E.A.L. designed this project. R.P. conducted endocrine work. T.T.H. conducted data collection and analyses and wrote the manuscript with input from both the other authors. All authors have approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants to T.T.H. from NSF, American Society for Mammalogists and Museum of Vertebrate Zoology and Department of Integrative Biology, UC Berkeley.
References
- 1.Clavel J, Julliard R, Devictor V. 2011. Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ. 9, 222–228. ( 10.1890/080216) [DOI] [Google Scholar]
- 2.Devictor V, Julliard R, Jiguet F. 2008. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117, 507–514. ( 10.1111/j.0030-1299.2008.16215.x) [DOI] [Google Scholar]
- 3.Gallagher AJ, Hammerschlag N, Cooke SJ, Costa DP, Irschick DJ. 2015. Evolutionary theory as a tool for predicting extinction risk. Tree 30, 61–65. ( 10.1016/j.tree.2014.12.001) [DOI] [PubMed] [Google Scholar]
- 4.Wong B, Candolin U. 2015. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. ( 10.1093/beheco/aru183) [DOI] [Google Scholar]
- 5.Salinas-Melgoza A, Salinas-Melgoza V, Wright TF. 2013. Behavioral plasticity of a threatened parrot in human-modified landscapes. Biol. Conserv. 159, 303–312. ( 10.1016/j.biocon.2012.12.013) [DOI] [Google Scholar]
- 6.Smith AT. 1974. The distribution and dispersal of pikas: influences of behavior and climate. Ecology 55, 1368–1376. ( 10.2307/1935464) [DOI] [Google Scholar]
- 7.Beever EA, Hall LE, Varner J, Loosen AE, Dunham JB, Gahl MK, Smith FA, Lawler JJ. 2017. Behavioral flexibility as a mechanism for coping with climate change. Front. Ecol. Environ. 15, 299–308. ( 10.1002/fee.1502) [DOI] [Google Scholar]
- 8.Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. 2013. Climate change and the past, present, and future of biotic interactions. Science 341, 499–504. ( 10.1126/science.1237184) [DOI] [PubMed] [Google Scholar]
- 9.Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482. ( 10.1890/070037) [DOI] [Google Scholar]
- 10.Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. 2013. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelem. 1, 20 ( 10.1186/2050-3385-1-20) [DOI] [Google Scholar]
- 11.Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264. ( 10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 12.Rubidge EM, Patton JL, Lim M, Burton AC, Brashares JS, Moritz C. 2012. Climate-induced range contraction drives genetic erosion in an alpine mammal. Nat. Clim. Change 2, 285–288. ( 10.1038/nclimate1415) [DOI] [Google Scholar]
- 13.Walsh RE, Assis A, Paula A, Patton JL, Marroig G, Dawson TE, Lacey EA. 2016. Morphological and dietary responses of chipmunks to a century of climate change. Glob. Change Biol. 22, 3233–3252. ( 10.1111/gcb.13216) [DOI] [PubMed] [Google Scholar]
- 14.Rubidge EM, Monahan WB, Parra JL, Cameron SE, Brashares JS. 2011. The role of climate, habitat, and species co-occurrence as drivers of change in small mammal distributions over the past century. Glob. Change Biol. 17, 696–708. ( 10.1111/j.1365-2486.2010.02297.x) [DOI] [Google Scholar]
- 15.Heller HC. 1971. Altitudinal zonation of chipmunks (Eutamias): interspecific aggression. Ecology 52, 312–319. ( 10.2307/1934590) [DOI] [Google Scholar]
- 16.Hammond TT, Springthorpe D, Walsh RE, Berg-Kirkpatrick T. 2016. Using accelerometers to remotely and automatically characterize behavior in small animals. J. Exp. Biol. 219, 1618–1624. ( 10.1242/jeb.136135) [DOI] [PubMed] [Google Scholar]
- 17.Hammond TT, Palme R, Lacey EA. 2015. Contrasting stress responses of two co-occurring chipmunk species (Tamias alpinus and T. speciosus). Gen. Comp. Endocrinol. 211, 114–122. ( 10.1016/j.ygcen.2014.11.013) [DOI] [PubMed] [Google Scholar]
- 18.Hertel AG, Swenson JE, Bischof R. 2017. A case for considering individual variation in diel activity patterns. Behav. Ecol. 28, 1524–1531. ( 10.1093/beheco/arx122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller HC, Poulson T. 1972. Altitudinal zonation of chipmunks (Eutamias): adaptations to aridity and high temperature. Am. Midl. Nat. 87, 296–313. ( 10.2307/2423563) [DOI] [Google Scholar]
- 20.Muñoz AR, Márquez AL, Real R. 2015. An approach to consider behavioral plasticity as a source of uncertainty when forecasting species' response to climate change. Ecol. Evol. 5, 2359–2373. ( 10.1002/ece3.1519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond TT, Palme R, Lacey EA. 2018. Data from: Ecological specialization, variability in activity patterns, and response to environmental change Dryad Digital Repository. ( 10.5061/dryad.ch3k2s1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hammond TT, Palme R, Lacey EA. 2018. Data from: Ecological specialization, variability in activity patterns, and response to environmental change Dryad Digital Repository. ( 10.5061/dryad.ch3k2s1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available via Dryad: https://doi.org/10.5061/dryad.ch3k2s1 [21].