Abstract
Within jawed vertebrates, pelvic appendages have been modified or lost repeatedly, including in the most phylogenetically basal, extinct, antiarch placoderms. One Early Devonian basal antiarch, Parayunnanolepis, possessed pelvic girdles, suggesting the presence of pelvic appendages at the origin of jawed vertebrates; their absence in more derived antiarchs implies a secondary loss. Recently, paired female genital plates were identified in the Late Devonian antiarch, Bothriolepis canadensis, in the position of pelvic girdles in other placoderms. We studied these putative genital plates along an ontogenetic series of B. canadensis; ontogenetic changes in their morphology, histology and elemental composition suggest they represent endoskeletal pelvic girdles composed of perichondral and endochondral bone. We suggest that pelvic fins of derived antiarchs were lost, while pelvic girdles were retained, but reduced, relative to Parayunnanolepis. This indicates developmental plasticity and evolutionary lability in pelvic appendages, shortly after these elements evolved at the origin of jawed vertebrates.
Keywords: placoderm, fossilized ontogeny, atavism, allometric growth, perichondral bone, endochondral bone
1. Introduction
Jawed vertebrates (Gnathostomata) share short-based pectoral and pelvic appendages supported by endoskeletal elements [1]. Pectoral girdle-supported fins first evolved in fossil jawless osteostracans [2], whereas pelvic fins originated in the most basal gnathostomes, the antiarch placoderms [3,4], based on dermal and endoskeletal pelvic girdles in a specimen of the Early Devonian Parayunnanolepis xitunensis [3]. Beyond this, pelvic fins were reported in the Late Devonian (ca 380 million years ago) derived antiarch Bothriolepis canadensis [5,6], but this has been questioned, and pelvic appendage absence in derived antiarchs is interpreted as a secondary loss [3,7,8]. Furthermore, other skeletal elements, dorsal to the posterior ventrolateral (PVL) plates (pelvic girdle position in other placoderms [9]) of the Bothriolepis thoracic shield, were interpreted as an anal plate [5,10] or female dermal genital plates [11]. Here, bony pelvic plates in 32 Bothriolepis specimens ranging from 28 to 186 mm total dorsal armour length (TALd) are described. Based on their morphology, histology and ontogenetic changes, we suggest that these represent the endoskeletal pelvic girdles of Bothriolepis, as do the aforementioned anal and genital plates.
The Bothriolepis pelvic girdle is relatively smaller than in Parayunnanolepis, and fin radials are lost (but with occasional atavistic reappearance), suggesting reduction of the pelvic girdle in Bothriolepis as part of the loss of pelvic appendages in antiarchs. We also document the presence of perichondral bone, previously problematic in antiarchs [12], and endochondral bone in Bothriolepis (electronic supplementary material, table S1).
2. Material and methods
A size series of 340 specimens (4–205 mm TALd) of B. canadensis (Upper Devonian Escuminac Formation, Canada) was examined (Leica MZ9.5). Specimens preserving a pelvic girdle (32) were photographed (Nikon D80/Leica IC80) and drawn (camera lucida). Specimens are housed in the Musée d'Histoire Naturelle de Miguasha (MHNM) and the Natural History Museum (UK; NHMUK).
Pelvic girdle growth was studied, measuring length (Lpelg) and width (Wpelg) of left and right (L, R) girdles. Analyses were performed using R (v. 3.3.2; libraries PSYCH, lmodel2, car) to estimate: (i) girdle bilateral symmetry by comparing the mean of ‘L minus R’ with a mean of zero (t-test), (ii) Spearman rank correlation of the number of concentric lines on the girdle surface relative to Lpelg, Wpelg and TALd, and (iii) allometric growth with major axis and two ordinary least square regressions on log10 transformed data (electronic supplementary material).
Girdles were removed from three specimens and embedded in EpoFix Resin (Struers). Thin sections were prepared (Secotom-50), ground, polished (LaboPol-35, Struers) and sealed (Araldite 2020 epoxy, coverslips). Slides were examined (Leica DMLB microscope) and photographed (AmScope: MUIOOO camera, Toupview, v. 3.7 2013).
Elemental composition analyses were performed with a scanning electron microscope (SEM; JEOL 6460LV) equipped with an energy dispersive spectrometer (Cu calibrated; 100 s; EDS, INCA X-sight, Oxford Instruments).
3. Results
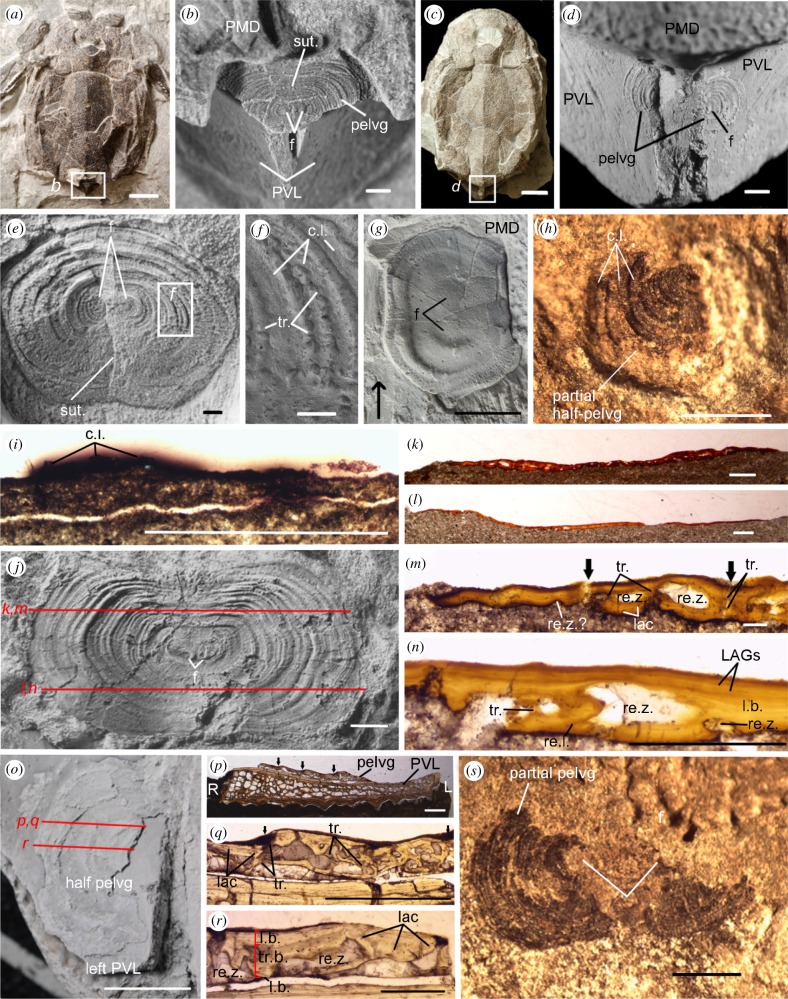
Pelvic girdles were observed within the trunkshield above the posterior margin of the PVL plates (figure 1a–d) in 32 specimens larger than 28 mm TALd (electronic supplementary material, table S2). In 73 smaller specimens (4–28 mm TALd), girdles were not observed (unmineralized/not yet developed); thus TALd at the onset of girdle development is unknown. The pelvic girdle includes two symmetrical semicircular bony plates (half-girdles; electronic supplementary material, table S3). Half-girdles join at a median suture in 25 specimens, including the smallest (figure 2a–c), being separated in seven specimens. Left and right half-girdles show contiguous ossification centres (foci) surrounded by hemi-concentric lines (figure 1b,d,e,g,j,s), interpreted as growth lines, increasing significantly in number with TALd and girdle width (electronic supplementary material, table S4). In two specimens (greater than 100 mm), the median suture is imperceptible, but foci and a few concentric lines appear as bulges on the surface (figure 1g; electronic supplementary material, figure S1), making the girdle appear unpaired.
Figure 1.
Bothriolepis canadensis pelvic girdles. Immature specimens (a,b) MHNM 02-3333, (c,d) MHNM 02-3397. (e,f) Girdle with concentric lines (ventral view, SEM), medium-sized specimen (MHNM 02-3668). (g) Large specimen (MHNM 02-3461; arrow towards head). (h,i) Partial pelvic girdle, immature specimen (MHNM 02-1421). (j–n) Medium-sized specimen (MHNM 02-988). (o–r) Medium-sized specimen (MHNM 02-2119) with partial left girdle. (s) Immature specimen (MHNM 02-3170). c.l., concentric line; f, focus; L, left; lac, lacunae; LAGs, lines of arrested growth; l.b., lamellar bone; pelvg, pelvic girdle; PMD, posterior median dorsal plate; PVL, posterior ventrolateral plates; R, right; re.l., reversal (cement) line; re.z., resorption zone; sut., suture; tr., trabeculae; tr.b., trabecular bone. Black arrows, limits between concentric lines. (b,d,e,g,j,o) Specimens dusted with ammonium chloride. Scale bars: (a,c,g) 10 mm, (b,d,e,h–j,p,q,s) 1 mm, (f,k,l) 0.5 mm, (m) 0.01 mm, (n,r) 0.005 mm and (o) 5 mm. (Online version in colour.)
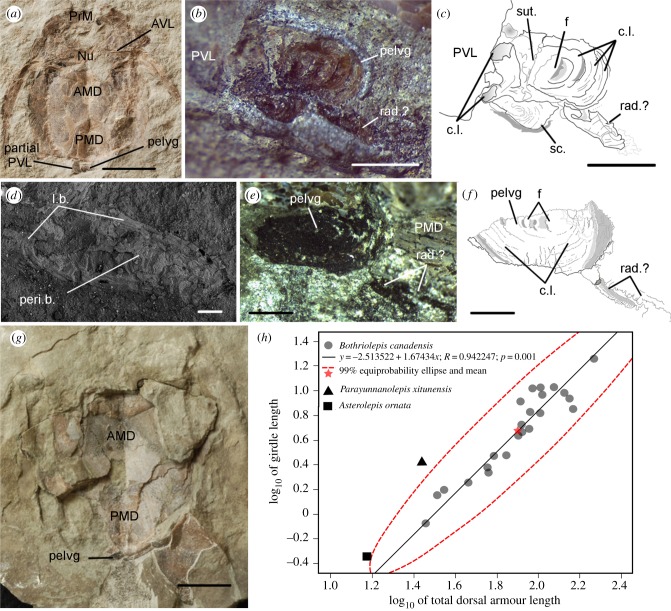
Figure 2.
Bothriolepis canadensis pelvic girdles. (a–d) Immature specimen (MHNM 02-1454), pelvic girdle and radial. (e–g) Immature specimen (MHNM 02-2426), pelvic girdle and radials. (h) Scatterplot of girdle length relative to TALd with MA regression plus 99% equiprobability ellipse. AMD, anterior median dorsal plate; AVL, anterior ventrolateral plate; c.l., concentric lines; f, focus; l.b., lamellar bone; Nu, nuchal plate; pelvg, pelvic girdle; Peri.b., perichondral bone; PMD, posterior median dorsal plate; PrM, premedian plate; PVL, posterior ventrolateral plate; rad., radial; sc., scale; sut., suture. Scale bars, (a,g) 10 mm; (b,c,e,f) 1 mm and (d) 0.1 mm. (Online version in colour.)
The Bothriolepis pelvic girdle lacks articular surfaces for fin radials. Ossified elements, showing similar relative positions and orientations to the girdle, are present in two small specimens (figure 2a–g; electronic supplementary material, table S2). These elements, whether flat with peripheral lamellar bone or tubular, are interpreted as fin radials.
In most specimens, including the smallest, the girdle elemental composition shows a low carbon content (4–20%) and a calcium : phosphorus ratio (2.06–2.71) similar to Bothriolepis dermal bones (1.66–2.31; electronic supplementary material, table S5). High carbon content is measured in one specimen (60%, MHNM 02-2426, 48.69 mm), suggesting the presence of cartilage, while calcium (6.90%) and phosphorus (0.61%) suggest mineralization was initiated, but incomplete. Despite these chemical differences, morphology is similar between carbon-rich (figure 2e–g) and ossified girdles (figure 1s). Mineralization in the pelvic girdle suggests the presence of bone; histologically, dorsal and ventral lamellar sheaths (perichondral) cover intermediate trabecular (endochondral) bone (figure 1j–r), and bone resorption and remodelling is observable in this region (re.z, re.l, figure 1m,n,r; electronic supplementary material). The perichondral sheaths show lines of arrested growth, suggesting, as for the surface concentric lines, cyclical bone deposition around the girdle (figure 1n).
The girdle appears wider than long in small and medium-sized (80–100-mm) specimens, while nearly as long as wide in large specimens (figure 1j versus e). Pelvic girdle length and width show positive allometric growth relative to TALd; this tendency is stronger for girdle length (electronic supplementary material, table S6). The pelvic girdle of Parayunnanolepis is proportionally larger than in juvenile Bothriolepis (figure 2h; electronic supplementary material). One small juvenile of the antiarch Asterolepis ornata (14–16 mm TALd; figure 4.35 in [13]) shows a 0.45 mm pelvic element with similar position and symmetrical appearance to Bothriolepis. This element shows slight overlap with the 99% equiprobability ellipse of Bothriolepis girdle length relative to TALd (figure 2h), but is smaller than in the smallest Bothriolepis specimen. No dermal pelvic girdle, intromittent organs or genital plates were observed in B. canadensis.
4. Discussion
(a). Bothriolepis pelvic girdle and fins among placoderms
The so-called ‘median anal plate’ of Bothriolepis, with its circular shape and concentric ridges [5] resembles the pelvic girdle described above, but this resemblance is less obvious in Stensiö's ‘anal plate’ (fig. 54 in [10]). Additionally, the anal plate in other placoderms is more posterior relative to the pelvic girdles [14], the latter located above the posterior margin of the PVL plates in all placoderms and in the same relative position when PVL plates are absent [9]. The putative Bothriolepis ‘genital plates’ (fig. 2h–j in [11]) closely resemble separated half-girdles in terms of position, presence of medial focal points and small size relative to the trunkshield length, with separation resulting from fracture at the weaker medial suture postmortem (e.g. figure 1o). Considering the inferred mating behaviour in antiarchs, with male claspers gripping the internal ornament of the female genital plates [11], it is unlikely that the Bothriolepis pelvic girdle had any reproductive function.
The ‘pelvic bone’ figured by Stensiö (figs 59 and 60 in [6]) differs from the pelvic girdle described earlier, and ‘fins’ [6] are too anterolaterally positioned and have not been observed in any of the 340 specimens examined here. Bothriolepis pelvic half-girdles are small and ovoid, as in rhenanid and ptyctodontid placoderms [9,15,16], but are fused, rather than independent. The presence of a suture between half-girdles, as well as proximity of their foci, indicates early ontogenetic fusion.
(b). Paedomorphosis and the phylogenetic reduction of the pelvic girdle
Paired appendage losses are frequent among gnathostomes, notably in teleosts, where pelvic fins are often modified, reduced or lost [1,17]. The sequence of gnathostome appendage development is proximo-distal, while loss of elements is distal to proximal, i.e. addition or truncation are more frequent distally [17,18].
The presence of ossified fin radials in only a few immature Bothriolepis individuals of our series suggests the absence of pelvic fins in this derived antiarch, with the occasional reappearance of radials, i.e. an atavism [19]. The positive allometry of the pelvic girdle relative to TALd in Bothriolepis suggests the pelvic girdle had a great potential for variation (i.e. developmental plasticity, evolutionary lability) [20]. Heterochronic patterns such as paedomorphosis are recognized to have played a role in antiarch evolution [20]. The Bothriolepis pelvic girdle seems significantly reduced in size (paedomorphic) relative to the basal Parayunnanolepis (figure 2h), but without a comparative ontogenetic trajectory, this inference remains tentative (electronic supplementary material). However, if the pelvic element in the 14–16 mm Asterolepis specimen [13] is a girdle, the absence of preserved pelvic girdle in Bothriolepis smaller than 28 mm suggests paedomorphosis as well, with either belated onset of development (girdle absent) or belated mineralization relative to Asterolepis. Consequently, a paedomorphic evolutionary trend in antiarch pelvic appendages, proceeding from the loss of the distal fin to the size reduction of the proximal girdle, with adult morphologies resembling early ontogenetic stages (i.e. no radials/fin absence, small girdles) is suggested. The lack of the dermal pelvic girdle, present in other placoderms [9], and rarity of scales ([21]; figure 2b,c; electronic supplementary material, figure S1) constitute a reduction of the post-armour dermal skeleton, which also suggests paedomorphosis in Bothriolepis. However, the absence of pelvic fins in Bothriolepis species showing a heavily scale-covered tail [22] excludes a direct link between the reduction of the pelvic appendages and the reduction of the post-armour dermal skeleton in B. canadensis.
The ontogenetic series of B. canadensis presented here is of great importance in the interpretation of the pelvic skeletal elements, previously documented as an anal plate [5,10] or female genital plates [11], but here identified as the pelvic girdle. Moreover, taking into account the ontogenetic stages of the specimens of Parayunnanolepis and Asterolepis, within the Bothriolepis pelvic girdle ontogenetic trajectory, allows us to infer that paedomorphosis may underlie the secondary loss of pelvic appendages in antiarchs. Integration of data from fossil early vertebrate ontogenies, relatively rare although increasingly recovered from nursery assemblages like those at Miguasha, adds crucial information to long-standing questions involving not only the origin of the gnathostome appendages, but also their evolutionary development (e.g. sequence of appendage loss).
Supplementary Material
Acknowledgements
We thank C. Belzile (ISMER-UQAR; SEM-EDS), A. Caron (UQAR; statistical advice), M. Chevrinais (UQAR; thin sections), I. Upeniece (University of Latvia; A. ornata data), J. Willett (MHNM; preparation) and three anonymous referees for their constructive comments.
Data accessibility
Data are available as electronic supplementary material.
Authors' contributions
R.C. conceived the project, all authors examined fossil specimens; F.C. performed data analyses, constructed figures and wrote the electronic supplementary material with input from R.C. and Z.J. F.C. wrote the first version; all authors contributed to the final version. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
F.C. was supported by Parc national de Miguasha, R.C. by NSERC (238612, 2017-06200) and the Research Chair in Paleontology and Evolutionary Biology – UQAR.
References
- 1.Larouche O, Zelditch ML, Cloutier R. 2017. Fin modules: an evolutionary perspective on appendage disparity in basal vertebrates. BMC Biol. 15, 32 ( 10.1186/s12915-017-0370-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janvier P. 2007. Homologies and evolutionary transitions in early vertebrate history. In Major transitions in vertebrate evolution (eds Anderson JS, Sues H-D), pp. 57–121. Bloomington, IN: Indiana University Press. [Google Scholar]
- 3.Zhu M, Yu X, Choo B, Wang J, Jia L. 2012. An antiarch placoderm shows that pelvic girdles arose at the root of jawed vertebrates. Biol. Lett. 8, 453–456. ( 10.1098/rsbl.2011.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King B, Qiao T, Lee MSY, Zhu M, Long JA. 2016. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Syst. Biol. 66, 499–516. ( 10.1093/sysbio/syw107) [DOI] [PubMed] [Google Scholar]
- 5.Patten W. 1904. New facts concerning Bothriolepis. Biol. Bull. 7, 113–124. ( 10.2307/1535537) [DOI] [Google Scholar]
- 6.Stensiö EA. 1948. On the Placodermi of the Upper Devonian of East Greenland. Palaeozool. Groenl. 2, 1–622. [Google Scholar]
- 7.Arsenault M, Desbiens S, Janvier P, Kerr J. 2004. New data on the soft tissues and external morphology of the antiarch Bothriolepis canadensis (Whiteaves, 1880), from the Upper Devonian of Miguasha, Quebec. In Recent advances in the origin and early radiation of vertebrates (eds Arratia G, Wilson MVH, Cloutier R), pp. 439–454. München, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 8.Béchard I, Arsenault F, Cloutier R, Kerr J. 2014. The Devonian placoderm fish Bothriolepis canadensis revisited with three-dimensional digital imagery. Palaeontol. Electron. 17, 19 (palaeo-electronica.org/content/2014/647-3d-bothriolepis) [Google Scholar]
- 9.Trinajstic K, Boisvert C, Long J, Maksimenko A, Johanson Z. 2015. Pelvic and reproductive structures in placoderms (stem gnathostomes). Biol. Rev. 90, 467–501. ( 10.1111/brv.12118) [DOI] [PubMed] [Google Scholar]
- 10.Stensiö EA. 1931. Upper Devonian vertebrates from East Greenland collected by the Danish Greenland expeditions in 1929 and 1930. Medd. Grønl. 86, 1–213. [Google Scholar]
- 11.Long JA, et al. 2015. Copulation in antiarch placoderms and the origin of gnathostome internal fertilization. Nature 517, 196–199. ( 10.1038/nature13825) [DOI] [PubMed] [Google Scholar]
- 12.Downs JP, Donoghue PCJ. 2009. Skeletal histology of Bothriolepis canadensis (Placodermi, Antiarchi) and evolution of the skeleton at the origin of jawed vertebrates. J. Morphol. 270, 1364–1380. ( 10.1002/jmor.10765). [DOI] [PubMed] [Google Scholar]
- 13.Upeniece I. 2011. Palaecology and juvenile individuals of the Devonian placoderm and acanthodian fishes from Lode site, Latvia. PhD Thesis, University of Latvia, Riga. [Google Scholar]
- 14.Miles RS, Westoll TS. 1968. The placoderm fish Coccosteus cuspidatus Miller ex Agassiz from the Middle Old Red Sandstone of Scotland. Part I. Descriptive morphology. Trans. R. Soc. Edinburgh 67, 373–476. ( 10.1017/S0080456800024078) [DOI] [Google Scholar]
- 15.Ørvig T. 1960. New finds of acanthodians, arthrodires, crossopterygians, ganoids and dipnoans in the Upper Middle Devonian Calcareous Flags (Oberer Plattenkalk) of the Bergisch Gladbach-Paffrath Trough. Paläontol. Z. 34, 295–335. ( 10.1007/BF02986872) [DOI] [Google Scholar]
- 16.Miles RS. 1967. Observations on the ptyctodont fish, Rhamphodopsis Watson. J. Linn. Soc. Lond. Zool. 47, 99–120. ( 10.1111/j.1096-3642.1967.tb01398.x) [DOI] [Google Scholar]
- 17.Don EK, Currie PD, Cole NK. 2013. The evolutionary history of the development of the pelvic fin/hindlimb. J. Anat. 222, 114–133. ( 10.1111/j.1469-7580.2012.01557.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberch P, Blanco MJ. 1996. Evolutionary patterns in ontogenetic transformation: from laws to regularities. Int. J. Dev. Biol. 40, 845–858. [PubMed] [Google Scholar]
- 19.Hall BK. 1984. Developmental mechanisms underlying the formation of atavisms. Biol. Rev. 59, 89–124. ( 10.1111/j.1469-185X.1984.tb00402.x) [DOI] [PubMed] [Google Scholar]
- 20.Werdelin L, Long JA. 1986. Allometry in the placoderm Bothriolepis canadensis and its significance to antiarch evolution. Lethaia 19, 161–169. ( 10.1111/j.1502-3931.1986.tb00727.x) [DOI] [Google Scholar]
- 21.Burrow CJ, Turner S. 1999. A review of placoderm scales, and their significance in placoderm phylogeny. J. Vertebr. Paleontol. 19, 204–219. ( 10.1080/02724634.1999.10011135) [DOI] [Google Scholar]
- 22.Long JA, Werdelin L. 1986. A new Late Devonian bothriolepid (Placodermi, Antiarchi) from Victoria, with descriptions of other species from the state. Alcheringa 10, 355–399. ( 10.1080/03115518608619146) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as electronic supplementary material.