Abstract
Cell migration is an essential part of many (patho)physiological processes, including keratinocyte re-epithelialization of healing wounds. Physical forces and mechanical cues from the wound bed (in addition to biochemical signals) may also play an important role in the healing process. Previously, we explored this possibility and found that polyacrylamide (PA) gel stiffness affected human keratinocyte behaviour and that mechanical deformations in soft (approx. 1.2 kPa) PA gels produced by neighbouring cells appeared to influence the process of de novo epithelial sheet formation. To clearly demonstrate that keratinocytes do respond to such deformations, we conducted a series of experiments where we observed the response of single keratinocytes to a prescribed local substrate deformation that mimicked a neighbouring cell or evolving multicellular aggregate via a servo-controlled microneedle. We also examined the effect of adding either Y27632 or blebbistatin on cell response. Our results indicate that keratinocytes do sense and respond to mechanical signals comparable to those that originate from substrate deformations imposed by neighbouring cells, a finding that could have important implications for the process of keratinocyte re-epithelialization that takes place during wound healing. Furthermore, the Rho/ROCK pathway and the engagement of NM II are both essential to substrate deformation-directed keratinocyte migration.
Keywords: mechanobiology, mechanosensing, actomyosin, polyacrylamide gel, non-muscle myosin II
1. Introduction
Cell migration is an essential part of many physiological and pathological processes in the body, including tissue morphogenesis [1], inflammation [2] and wound healing [3]. Re-epithelialization during cutaneous wound healing is a result of migration, proliferation and differentiation of the peripheral keratinocytes along the edge of the wound [4–6]. Aberrant or delayed re-epithelialization increases the risk of infection and the severity of scar tissue formation, and it contributes to the development and persistence of chronic wounds [4,7,8]. The regulation of re-epithelialization and keratinocyte migration is understood largely in terms of biochemical signals [6]. Recent interest in the mechanobiology of tissues, however, suggests that physical forces and mechanical cues from the wound bed could also play an important role in the healing process [4,9–11].
The biophysical mechanisms by which cells sense and respond to mechanical signals, however, remain poorly understood. In addition to the multi-protein complexes that form at the anchoring junctions between the cell and the substrate [12,13], actin–myosin interactions with the cytoskeleton remain a focal point of study for elucidating the mechanisms involved in mechanosensing, particularly those involving non-muscle myosin II (NM II) [14–16]. NM II engages with actin to generate cytoskeletal traction forces that are transmitted through an integrated complex of focal adhesion associated proteins to the extracellular matrix (ECM) via integrins, the main transmembrane receptors for ECM proteins [17,18]. An increase in ECM/substrate stiffness recruits more integrin-associated structural and signalling proteins to the site of adhesion, including focal adhesion kinase (FAK). Increased FAK activity at these adhesive contacts also stimulates the Rho/ROCK (Rho kinase) pathway. Rho is a family of small GTPases that activate ROCK, which in turn regulates myosin light chain phosphorylation, NM II engagement and contraction with actin, which leads to increases in both cortical tension and transmembrane force generation that promotes cell migration [19,20] or substrate displacement [21,22]. Collectively, cellular components that contribute to myosin contraction, such as Rho/ROCK, appear to be vital to mechanosensing for a range of cell phenotypes [20,22], possibly through a mechanism involving substrate deformation-dependent mechanical feedback. Indeed, several studies have indicated that Rho/ROCK and NM II are important to keratinocyte behaviour, including regulation of keratinocyte differentiation [23–28].
It is important to understand not only the basic mechanisms underlying cellular mechanosensation, but also how this process controls individual and coordinated physiological and pathophysiological cellular behaviours, such as those involved in tissue self-assembly [29–32], ECM remodelling [33,34], wound healing [4,9–11], fibrosis [35,36] and cancer metastasis [37–41]. For example, the influence of ECM remodelling and stiffness on tumour progression has been the subject of some recent cancer studies [39,41]. With respect to wound healing, Zarkoob et al. [42] explored previously how substrate stiffness affected primary human keratinocyte behaviour and found that mechanical cues influenced the process of de novo epithelial sheet formation. Specifically, the process by which human keratinocyte formed multicellular aggregates was significantly different on soft (approx. 1.2 kPa) versus stiff (approx. 24 kPa) polyacrylamide (PA) gels [42]. Keratinocytes on soft PA gels exhibited smaller spread contact areas, increased migration velocities, increased rates of aggregate formation and more cells per aggregate, respectively [42]. In addition, the keratinocytes on soft PA gels appeared to migrate directly towards an evolving multicellular aggregate in a cooperative manner, ostensibly in response to mechanical cues that propagated through the deforming substrate from the aggregate. Such directed migration was not apparent on the stiff PA gels.
These observations were near completely analogous to those published by Reinhart-King et al. [43] with respect to bovine aortic endothelial cells plated at low density on 2.5–5.5 kPa PA gels. Both Reinhart-King et al. and Zarkoob et al. [42] hypothesized that such enhanced cooperativity is due to the ability of a cell to sense and respond to substrate deformations induced by the neighbouring cells. In our previous study of multicellular aggregate formation, because each cell likely modulates the tractions it exerts on the substrate in response to those of adjacent contracting cells, the mechanical environment is spatially heterogeneous and temporally dynamic, which precluded any analysis aimed at unambiguously correlating cell migratory behaviours to mechanical signals communicated through substrate deformations imposed by neighbouring cells.
The purpose of this study was to demonstrate that keratinocytes alter their migratory pathways by sensing of the substrate deformation field. Using a servo-controlled microneedle embedded in a PA gel substrate, we produce spatio-temporally controlled deformations at a defined distance in proximity to an otherwise randomly migrating keratinocyte. The magnitude and rate of change of substrate deformations induced by the embedded microneedle are selected to roughly mimic those induced by an evolving multicellular aggregate during the process of de novo epithelial sheet formation, as observed in our previous study [42]. We also examined the effect of adding either Y27632, a Rho kinase inhibitor, or blebbistatin, a NM II inhibitor, on the response of the cells to PA gel deformations. Both of these chemicals are commonly used to investigate the role that internal force generation plays in the mechanosensing process [44–47]. The results of this study indicate that keratinocytes do sense and respond to mechanical signals comparable to those that originate from substrate deformations imposed by neighbouring cells, a finding that could have important implications for the process of keratinocyte re-epithelialization that takes place during normal and pathologic wound healing.
2. Material and methods
2.1. Polyacrylamide gel preparation
Thin PA gels approximately 100 µm in thickness were polymerized on the surfaces of glass bottom Petri dishes (MatTec Corp., Ashland, MA, USA) as described previously [42,48]. Briefly, a ratio of 2.0%/0.25% acrylamide/bis-acrylamide was used to fabricate soft PA gels with a nominal stiffness of 1.2 kPa based on gel formulations published elsewhere [49,50]. We use the term, soft, to denote that this PA gel formulation is identical to the formulation of the soft gels used in our previous work [42]. Consistent with this reported modulus, we estimated a zero-strain PA gel modulus of 1.3 ± 0.5 kPa by using a manual glass bead indentation technique with a Hertzian contact model [51]. FluoroSpheres® Carboxylate-Modified Microspheres (no. F8812, Life Technologies, Carlsbad, CA, USA) measuring 0.5 µm in diameter were embedded inside the gels to facilitate deformation tracking. Pepsin-digested collagen was attached covalently to the surface of the gel with Sulfo-SANPAH. The samples were sterilized under UV light for 15 min and stored at 4°C for later use.
2.2. Cell culture
Neonatal human epidermal keratinocytes (HEKn) (Fisher Scientific, Waltham, MA, USA) were cultured in keratinocyte serum-free medium (Invitrogen) supplemented with 1% penicillin–streptomycin and 0.1% amphotericin B in a humidified incubator maintained at 37°C and 5%/95% CO2/air. Passage three cells were plated at a density of approximately 200 cells cm−2 onto the surface of the PA gels, a density that was effectively 20 times less than used in our previous experiments [42]. The calcium of the medium was then elevated from a baseline concentration of approximately 0.09 to 1.2 mM by adding CaCl2 to the medium in order to trigger cell–cell anchoring junction assembly and de novo epithelial sheet formation via the calcium switch [42,52,53]. HEKn were allowed to attach and equilibrate for 3 h in the incubator before imaging with the microscope.
2.3. Time-lapse live cell imaging
Once the samples had equilibrated, they were transferred to a temperature-controlled microscope enclosure (CO2 Microscope Cage Incubation System, Okolab, Pozzuoli, Italy) with recirculating air that integrates with a micro-environmental gas chamber (H201-K-Frame, Okolab, Pozzuoli, Italy) (figure 1a). This set-up provides 37°C, humidified air with 5% CO2 via a manual gas mixer and pump to the samples so that extended time-lapse imaging is possible. Time-lapse images were acquired with a Nikon Ti-E inverted microscope equipped with wide-field epifluorescence and differential interference contrast (DIC) microscopy capabilities and a DS-Qi1 Nikon camera. DIC and fluorescent images pairs were acquired with a CFI plan Apo 10× DIC objective combined with a 1.5× magnifier.
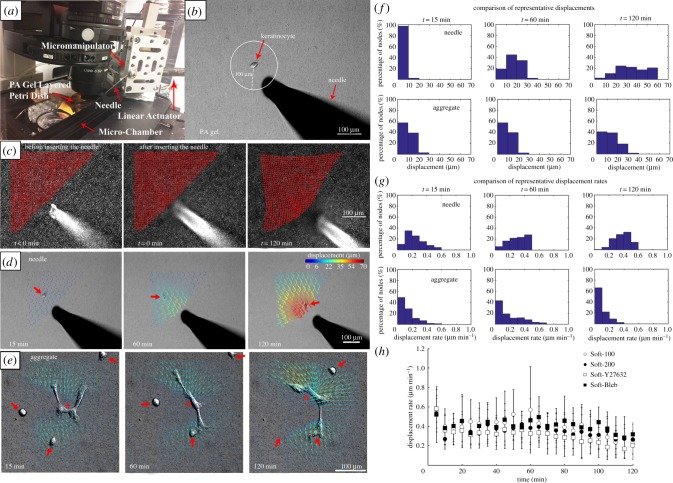
Figure 1.
Experimental set-up to apply controlled mechanical deformations to PA gels. (a) The PA gel is placed within a microscope-mounted micro-chamber contained with an environmentally controlled enclosure. A servo-controlled liner actuator drives needle movement via a high-precision micromanipulator. (b) A single keratinocyte is identified and the needle is inserted a prescribed distance away from the centroid of the cell, in this instance approximately 100 µm. (c) Substrate deformation in response to needle movement are calculated by tracking the movement of embedded fluorescent microspheres at each node (red points) of a user-defined grid superimposed on the image. (d) A displacement field can be calculated from the nodal displacements. Here, the series of images depicts the evolution of substrate displacement vectors, where the arrows indicate direction and the displacement magnitudes are colour-coded. Large red arrows denote the position of the migrating cell. Note that although the needle moves at the prescribed rate of 1 µm min−1, the rate of gel displacement decreases with distance from the needle. (e) Displacement fields for a prototypical evolving multicellular aggregate (*) at equivalent time points. The three single cells denoted with large red arrows eventually join the aggregate (adapted from [42]). (f) Histograms showing the corresponding substrate displacements displayed in (d,e). (g) Histograms showing the corresponding substrate displacement rates corresponding to (d,e). (h) Comparison of the substrate displacement rates in the PA gel initially located approximately 100 µm from the microneedle tip. (Online version in colour.)
2.4. Microneedle-induced polyacrylamide gel deformations
Controlled local mechanical deformations in the PA gel substrate were produced using a sterile 0.25 × 75 mm acupuncture needle (Tai-Chi Brand, Suzhou Shenlong Medical Apparatus Co., Ltd, Suzhou, China) secured to a high-resolution linear actuator (M-230.25, PI (Physik Instrumente) LP, Auburn, MA, USA) and inserted into the gel. The actuator was controlled with MTESTQuattro software (ADMET, Norwood, MA, USA), and mounted to a high-precision, manual linear stage (462 XYZ-M ULTRAlign, Newport Corp., Irvine, CA, USA).
Single keratinocytes that were at least 350 µm away from any neighbouring cells were located and imaged. Circles measuring either 100 or 200 µm in radius and centred on the cell nucleus were superimposed on the live image using Nikon Elements software. The needle tip was then inserted to a depth of approximately 40 µm below the surface of the gel at a location on the circle that was intentionally irrespective of the direction of cell migration (figure 1b). The needle (figure 1c,d) was then displaced at a constant rate of 1 µm min−1 in order to roughly mimic both the average instantaneous velocity of single keratinocytes and the rate of change of keratinocyte-induced substrate deformations we previously observed on soft PA gels [42]. To ensure that the microneedle-induced displacements were comparable to those observed in our previous work, tracked substrate displacements and displacements rates produced by the microneedle (see §2.7 PA gel substrate displacement tracking) were compared with a region of equivalent area centred on an evolving multicellular aggregate over a 2-h time window (figure 1e). Histograms at select time points (figure 1f,g) indicate that despite some expected differences in the distribution of displacements and displacement rates, particularly towards t = 2 h, there is reasonable overlap between the substrate deformations generated in a representative microneedle experiment and those generated by a prototypical multicellular aggregate as observed in our previous work [42]. In order to assess for experimental reproducibility, substrate displacement rates in the PA gel initially located approximately 100 µm from the microneedle tip were tracked and compared across all of our experimental conditions (figure 1h). The results confirm that no significant differences exist with time or by experimental condition as assessed by a two-way ANOVA.
Following needle insertion, time-lapse images were acquired at 5-min intervals for 2 h, roughly the time at which time the needle began to exit the image field of view. As many as three sequential experiments were conducted on each gel, such that all observations were conducted within 10 h of the start of the experiment. The 2 h imaging period for any given cell was determined to be of acceptable duration because it was substantially larger than the average persistence time characteristic of a keratinocyte migrating on a soft PA gel (14.4 ± 17.0 min). To calculate this persistence time, a parallel set of control experiments were conducted where the needle was absent and images were acquired every 5 min over 24 h. The mean squared displacements calculated from these time-lapse images were fit to the persistent random walk model of cell motility [54–56] using the method of Wu et al. [56] and averaged to extract the persistence time.
2.5. Chemical inhibitors
The Rho kinase inhibitor, Y27632 (ALX-270-333-M001, Enzo Life Sciences, Inc., Farmingdale, NY, USA) [24–27,57], and a NM II inhibitor, blebbistatin (ab120425, Abcam, Cambridge, MA, USA) [58,59], were added to the culture medium of some experiments in order to assess how inhibiting components of the mechanosensing and force generating machinery of the cytoskeleton affected keratinocyte behaviour in response to the needle-induced substrate deformations. One hour after the cells were added to the PA gels (i.e. 2 h before imaging began) either Y27632 or blebbistatin was added to the culture medium to produce a final concentration of 50 µM of inhibitor.
2.6. Experimental conditions
Four different experimental conditions involving needle-induced substrate deformations on soft PA gels were investigated, where the initial distance of the needle away from the cell (Soft-100, Soft-200) and the effects of chemical inhibition (Soft-Y27632, Soft-Bleb) on cell migration were investigated (table 1). An additional control condition (Soft-Control), where the needle was absent, was also examined. Ten cells were analysed for each condition.
Table 1.
Experimental conditions.
| condition | gel (kPa) | needle location (µm) | chemical inhibitor | n |
|---|---|---|---|---|
| Soft-100 | 1.2 | 100 | — | 10 |
| Soft-200 | 1.2 | 200 | — | 10 |
| Soft-Y27632 | 1.2 | 100 | Y27632 50 µM | 10 |
| Soft-Bleb | 1.2 | 100 | blebbistatin 50 µM | 10 |
| Soft-Control | 1.2 | — | — | 10 |
2.7. Polyacrylamide gel substrate displacement tracking
The PA gel substrate displacements were measured, as was done previously [42], by using a custom matlab (Mathworks, Inc., Natick, MA, USA) algorithm based on spatial cross-correlation between subsequent image pairs of the embedded fluorescent microspheres [60]. Specifically, an array of evenly spaced grid points was placed on a subset of the image that contained the cell and extended to the location of microneedle insertion. Subwindows around each grid point were then correlated between image pairs to obtain the corresponding displacements in the substrate. The grid point positions were updated and used to generate the subwindows for correlation between the next image pair. In this manner, the grid point positions track with the microspheres in the deforming substrate. Thus, the two-dimensional position vector of the substrate, 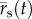 , and the incremental substrate displacement between frames,
, and the incremental substrate displacement between frames, 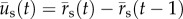 , at time, t, are straightforward to describe.
, at time, t, are straightforward to describe.
2.8. Cell motility
The two-dimensional position of the cell, 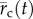 , was determined by hand tracking the location of the nucleus in each image using the Manual Tracking plugin in ImageJ (National Institutes of Health, Bethesda, MD, USA). In the experiments involving the needle, it was necessary to distinguish between cell movement from cell motility versus cell movement stemming from needle-induced deformations in the substrate. Thus, the total incremental displacement of the cell,
, was determined by hand tracking the location of the nucleus in each image using the Manual Tracking plugin in ImageJ (National Institutes of Health, Bethesda, MD, USA). In the experiments involving the needle, it was necessary to distinguish between cell movement from cell motility versus cell movement stemming from needle-induced deformations in the substrate. Thus, the total incremental displacement of the cell, 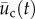 , between image pairs is given by:
, between image pairs is given by:
| 2.1 |
where 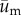 is the incremental displacement vector of the cell due to cell motility and
is the incremental displacement vector of the cell due to cell motility and 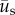 is the incremental displacement vector of the underlying substrate. Here,
is the incremental displacement vector of the underlying substrate. Here, 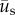 was made coincident with the position of the cell via linear interpolation from the displacements measured in the surrounding set of tracked grid points. For the limiting case of no substrate displacement (i.e. when there is no needle and
was made coincident with the position of the cell via linear interpolation from the displacements measured in the surrounding set of tracked grid points. For the limiting case of no substrate displacement (i.e. when there is no needle and 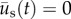 ), cell movement is from cell motility alone. The position vector of the cell due to cell migration alone,
), cell movement is from cell motility alone. The position vector of the cell due to cell migration alone, 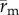 , was then calculated by substituting the relationship
, was then calculated by substituting the relationship 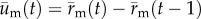 into equation (2.1) and rearranging to give:
into equation (2.1) and rearranging to give:
| 2.2 |
Both 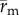 and
and 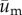 , which we refer to as the adjusted position vector and adjusted cell displacement vector, respectively, were used in the data analysis described below.
, which we refer to as the adjusted position vector and adjusted cell displacement vector, respectively, were used in the data analysis described below.
2.9. Directional analysis and circular statistics
We posit that if a cell is preferentially migrating in a direction coincident with the direction of substrate displacement—as opposed to changing direction randomly—then the distribution of the direction vectors should deviate significantly from a uniform distribution (i.e. random cell migration). Furthermore, the distribution should have a mean direction that coincides with the mean direction of substrate displacement. To determine if such a relationship was present, an angle distribution of the direction of cell migration was obtained by calculating the angle of the adjusted cell displacement vector, 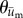 , which is given by
, which is given by 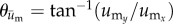 , where
, where 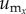 , and
, and 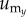 are the components of
are the components of 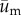 . In a similar manner, the angle distribution of the direction of substrate displacement,
. In a similar manner, the angle distribution of the direction of substrate displacement, 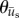 , was calculated from
, was calculated from 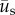 . Both
. Both 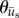 and
and 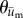 were calculated for each frame, binned together for all 10 experiments associated with a given condition, and represented as polar histograms. The CircStat tool box for matlab [61] was then used to compare the two distributions via a modified Rayleigh test for uniformity, or V test [62], to determine if the distribution for
were calculated for each frame, binned together for all 10 experiments associated with a given condition, and represented as polar histograms. The CircStat tool box for matlab [61] was then used to compare the two distributions via a modified Rayleigh test for uniformity, or V test [62], to determine if the distribution for 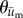 was random (i.e. distributed uniformly around a circle), or if the distribution for
was random (i.e. distributed uniformly around a circle), or if the distribution for 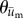 was not random and its mean direction coincided with the average direction of
was not random and its mean direction coincided with the average direction of 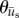 .
.
3. Results
Control experiments without the insertion of a needle into the gel were performed in order to obtain baseline information on single keratinocyte migration behaviours. These cells dynamically interrogated and deformed the substrate as they moved randomly all over the gel surface (electronic supplementary material, movie S1). The average cell speed, net distance travelled, and maximum distance travelled over the 24 h period were 1.3 ± 0.3 µm min−1, 178.1 ± 123.7 µm, and 238.2 ± 115.1 µm, respectively (table 2). The persistence time of these cells was calculated as 14.4 ± 17.0 min. Consequently, a duration of 2 h for each microneedle experiment was deemed more than sufficient for determining whether directed migration towards the needle was occurring for each condition examined.
Table 2.
Baseline keratinocyte migration metrics on a non-deformed substrate results.
| condition | speed (µm min−1) | net distance travelled (µm) | max distance travelled (µm) |
|---|---|---|---|
| Soft-100 | 2.1 ± 0.7 | 106.2 ± 46.8 | 110.0 ± 42.3 |
| Soft-200 | 1.6 ± 0.6 | 82.5 ± 54.3 | 104.4 ± 64.5 |
| Soft-Y27632 | 2.2 ± 1.0 | 142.7 ± 95.6 | 152.3 ± 86.3 |
| Soft-Bleb | 1.5 ± 0.6 | 48.0 ± 35.8 | 64.8 ± 42.7 |
| Soft-Controla | 1.3 ± 0.3 | 178.1 ± 123.7 | 238.2 ± 115.1 |
aOver 24 h.
Next, the servo-controlled needle was inserted into the gel a nominal distance of 100 µm away from the cell nucleus and displaced at a constant rate of 1 µm min−1 to produce a constant and repeatable deformation in the gel over a 2 h period (electronic supplementary material, movie S2). These cells, which prior to the insertion of the needle were migrating in random directions, typically altered course and moved in the direction of needle displacement (figure 2). This behaviour was also observed, though to a lesser extent, in experiments where the needle was nominally inserted 200 µm from the cell centre (electronic supplementary material, movie S3).
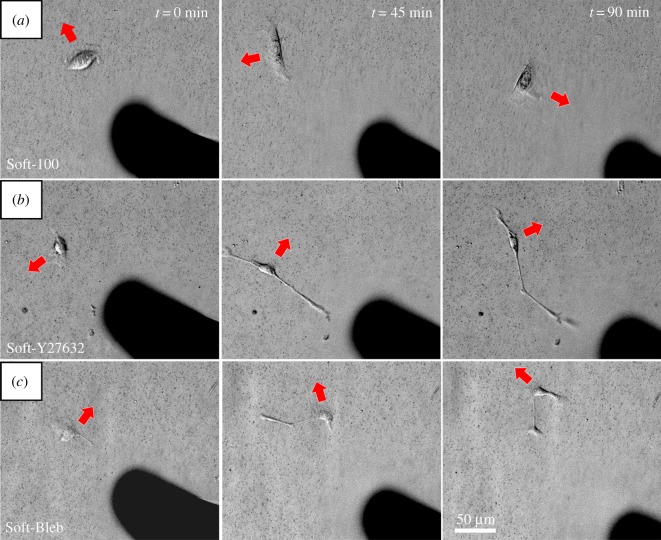
Figure 2.
Direction of keratinocyte migration in response to needle-induced substrate deformations at t = 0, t = 45, and t = 90 min. Red arrows indicate the current direction of cell movement. (a) For Soft-100, the cell, which is initially migrating away from the needle gradually reorients and moves in the direction in which the needle is pulling the substrate. (b) For Soft-Y27632 and (c) Soft-Bleb, the cells move in a manner that is unresponsive to the substrate deformations produced by needle movement. Also, note that exposure to both drugs perceptibly altered cell morphology. (Online version in colour.)
The addition of Y27632 (electronic supplementary material, movie S4) and blebbistatin (electronic supplementary material, movie S5) altered keratinocyte morphology and migration behaviour (figure 2). Both drugs induced the formation of highly extended cells with quasi-filopodial-like and quasi-lamellipodial-like cellular protrusions that seemed to preferentially align with the substrate deformations produced by the movement of the embedded microneedle (electronic supplementary material, movies S4 and S5). Keratinocytes that were not exposed to these drugs did not exhibit this prominent morphological change. Most importantly, keratinocytes exposed to both blebbistatin and Y27632 were completely unresponsive to substrate deformations produced by the needle; their migration path was unaffected by the superimposed substrate deformation field.
Post-experiment analysis found no significant differences in the magnitude or rate of substrate displacements induced by the needle amongst the four experimental conditions. However, the true needle tip to cell nuclei distance for circles measuring 100 and 200 µm was less than the nominal distance at 84.0 ± 5.7 µm, 85.9 ± 4.4 µm, 86.4 ± 6.0 µm, and 182.3 ± 5.4 µm for Soft-100, Soft-Y27632, Soft-Bleb, and Soft-200, respectively.
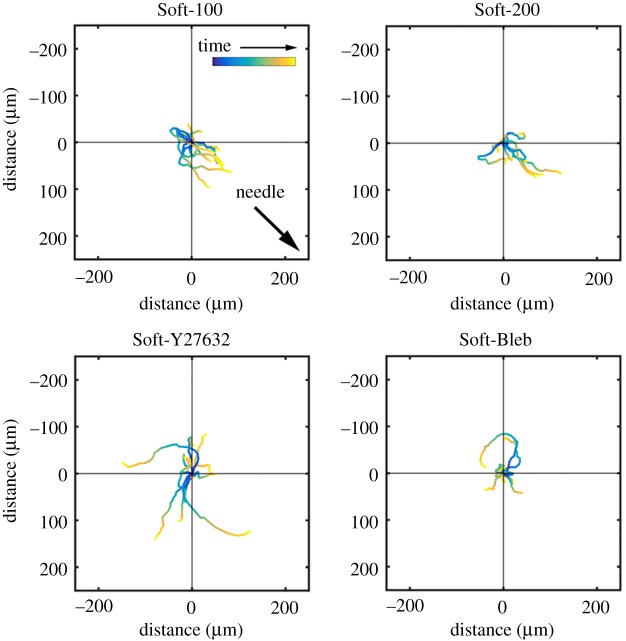
In order to confirm that the keratinocytes were migrating towards the needle and not simply carried by the underlying deforming substrate, we calculated the adjusted cell position and plotted the adjusted cell paths for all experiments (figure 3). These paths clearly show that the cells primarily migrated in the direction of the needle and that exposure to either Y27632 or blebbistatin blocked this effect. A comparison of the distribution of 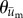 to the direction of needle displacement (figure 4) also quantitatively and statistically supported this notion. V-tests confirmed that for both Soft-100 and Soft-200 the direction of cell movement coincided with the direction of needle movement (p < 0.001). V-tests also indicated that the direction of cell movement for Soft-Y27632 and Soft-Bleb samples was not significantly different from a uniform distribution and did not coincide with the direction of needle-induced substrate displacements.
to the direction of needle displacement (figure 4) also quantitatively and statistically supported this notion. V-tests confirmed that for both Soft-100 and Soft-200 the direction of cell movement coincided with the direction of needle movement (p < 0.001). V-tests also indicated that the direction of cell movement for Soft-Y27632 and Soft-Bleb samples was not significantly different from a uniform distribution and did not coincide with the direction of needle-induced substrate displacements.
Figure 3.
Adjusted cell motility on PA gels. Each colour line shows the trace of 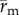 , the position vector of the cell due to cell migration effects alone (see equation (2.2)). Each cell path is colour-coded to show the relative time along the path from its beginning at the origin (dark blue) to the final tracked position (yellow). The majority of cells moved toward the displacing needle for needles nominally positioned either 100 or 200 µm from the cell centroid at the start of the experiment (i.e. Soft-100 and Soft-200, respectively). Exposure to the Rho kinase inhibitor Y27632 or the actin–myosin inhibitor blebbistatin interrupted this directed migration. (Online version in colour.)
, the position vector of the cell due to cell migration effects alone (see equation (2.2)). Each cell path is colour-coded to show the relative time along the path from its beginning at the origin (dark blue) to the final tracked position (yellow). The majority of cells moved toward the displacing needle for needles nominally positioned either 100 or 200 µm from the cell centroid at the start of the experiment (i.e. Soft-100 and Soft-200, respectively). Exposure to the Rho kinase inhibitor Y27632 or the actin–myosin inhibitor blebbistatin interrupted this directed migration. (Online version in colour.)
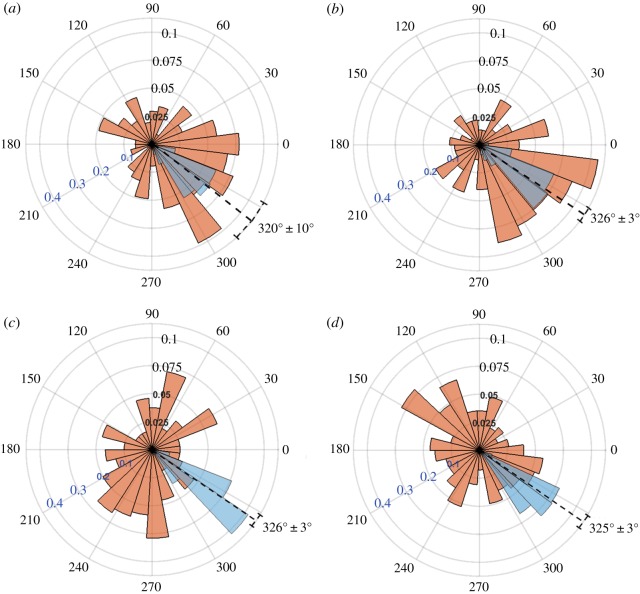
Figure 4.
Polar histograms showing the combined angle distributions in degrees of adjusted cell displacement 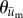 (red) and substrate displacement
(red) and substrate displacement 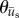 (blue) for all 10 replicates of the (a) Soft-100, (b) Soft-200, (c) Soft-Y27632 and (d) Soft-Bleb experiments. Each distribution is normalized by the total number of observations with the black radial text associated with
(blue) for all 10 replicates of the (a) Soft-100, (b) Soft-200, (c) Soft-Y27632 and (d) Soft-Bleb experiments. Each distribution is normalized by the total number of observations with the black radial text associated with 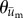 and the blue radial text associated with
and the blue radial text associated with 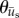 . The dashed line shows the mean and standard deviation of the angle of needle movement. For all four conditions, the displacements of the substrate aligned with the direction of needle movement. In addition, for Soft-100 and Soft-200, V-tests indicate that the direction of cell movement is coincident with that of the needle-induced substrate deformations, but not for soft-Y27632 or Soft-Bleb (p < 0.001). (Online version in colour.)
. The dashed line shows the mean and standard deviation of the angle of needle movement. For all four conditions, the displacements of the substrate aligned with the direction of needle movement. In addition, for Soft-100 and Soft-200, V-tests indicate that the direction of cell movement is coincident with that of the needle-induced substrate deformations, but not for soft-Y27632 or Soft-Bleb (p < 0.001). (Online version in colour.)
4. Discussion
During wound healing, the composition and structure of the wound bed transitions dramatically from that of the initial provisional fibrin clot to a dense, vascularized, cellular bed of granulation tissue, to a final state of remodelled replacement ECM [63]. Concurrently, the mechanical properties of the wound site change continuously, with atomic force microscope measurements on wounded rat skin showing increases in stiffness from 18.5 kPa at day 7 to 29.4 kPa by day 9 [64], compared to median measurements in human papillary and reticular dermis of 0.82 and 1.12 kPa, respectively [65]. Given that a variety of mechanical cues and physical contexts haven been shown to regulate physiological and pathophysiological cellular behaviours [48,66–70], it is reasonable to ask whether such cues also have the potential to guide temporal and spatial aspects of keratinocyte re-epithelialization of the wound site.
Here, we set out to test the hypothesis that keratinocytes adherent to soft substrates can sense and respond to substrate deformations, and that this might be the explanation for the enhanced cooperativity in de novo epithelial sheet formation that we observed in our previous work [42]. Towards this end, we designed an experiment in which we experimentally imposed substrate deformations similar in magnitude and rate of deformation to those that can be generated by an evolving multicellular aggregate of keratinocytes. By imposing these experimental deformations within a defined length scale relative to an otherwise randomly migrating isolated keratinocyte, we were able to consistently induce substrate deformation-directed keratinocyte migration. Furthermore, using a Rho kinase inhibitor (Y27632) and a NM II inhibitor (blebbistatin), we have demonstrated that the Rho/ROCK pathway and the engagement of NM II are both essential to the observed process of substrate deformation-directed keratinocyte migration.
Our observations are consistent with the pioneering work of Lo et al. [70], who, in addition to showing that cells preferentially migrate up gradients of rigidity (i.e. durotaxis), also used a blunted microneedle to demonstrate that localized mechanical deformations in the substrate could direct 3T3 fibroblast migration either towards or away from the needle, depending on whether the substrate was pulled or pushed, respectively. More recently, Plotnikov et al. [45] obtained similar results following the same methodology, where a needle was inserted 10 µm away from an isolated cell, and then instantaneously stretched to the elastic limit and held in place for 45 min. By contrast, we applied a controlled, constant rate of substrate deformation with the needle inserted 100 and 200 µm from the cell centre and quantitatively tracked both the imposed substrate deformations and the cell migration path over a 2 h period. Although the cellular response was essentially the same, the nature of the mechanical cues involved was temporally and spatially different from these earlier studies. Because the microneedle imposed substrate deformations at a constant rate, and cells were migrating in random directions with respect to the subsequently imposed substrate deformation, the potential mechanical cues a given cell experienced were varied. The diversity of spatial and temporal mechanical inputs likely contributed to the variability in the cell paths taken (figure 3), where not all cells followed the needle-induced deformations for the duration of the experiment.
Multicellular aggregating behaviour on soft but not stiff PA gel substrates is a phenomenon that has been observed by others [43,44]. In particular, Reinhart-King et al. [43] found that endothelial cell pairs on soft PA gels were able to interact with each other by sensing mechanical forces exerted between them through the substrate. The dispersion, or tendency of these cell pairs to migrate away from their initial location, was reduced significantly compared to that of single cells. Guo et al. [44] posited that this type of behaviour could be explained in terms of comparisons between mechanical signals from the cell and the substrate and mechanical signals between the cell and neighbouring cells. Cells migrate away from each other if the mechanical signals are greater in the substrate than from neighbours, and towards each other when the signals are reversed. The tendency for keratinocytes to migrate in the direction of needle displacement observed here is consistent with this hypothesis.
A common explanation for what guides directed cell migration is that deformation of the substrate induced either by a needle or an adjacent cell or aggregate of cells produces a durotactic gradient in substrate rigidity via nonlinear strain stiffening of the substrate in response to cell traction forces. For small deformations, PA gels are generally considered linear elastic materials, which implies that local substrate stiffness does not change with increasing strain. However, nonlinear elastic behaviour has been reported for PA gels used in traction force microscopy experiments subject to imposed surface displacements of as little as 2–6 µm [71] (note that substrate displacements as large as 60–70 µm were generated in our microneedle experiments). Regardless of the elastic or nonlinear elastic constitutive behaviour of the underlying substrate, however, geometric nonlinearities exist within a deforming substrate such that a migrating cell will perceive an increase in apparent substrate stiffness when migrating over a region of substrate that is strained as opposed to an area that is unstrained.
As a purely speculative explanation of the durotactic phenomenon, consider the following conceptual model of a migrating keratinocyte, initially present within an area of unstrained substrate (zero-strain condition). Within this keratinocyte, both ‘stable’ and ‘tugging’ focal adhesions are present, the latter of which are thought to be asynchronously sampling the extracellular mechanical environment [45]. When the cell encounters an area of strained substrate, it is possible that ‘tugging’ focal adhesions are more efficiently converted to ‘stable’ focal adhesions within the advancing lamellipodia. Consequently, ‘stable’ focal adhesions present on areas of strained substrate will accumulate, eventually outnumbering those present on areas of unstrained substrate. Ultimately, this relative imbalance could bias the migration of the cell to follow a path of increasing strain (or apparent stiffness) gradient. In essence, this model is consistent with the results of Plotnikov et al. [45] where softer substrates were observed to promote increased numbers of ‘tugging’ focal adhesions and rigid substrates supported a relative increase in the numbers of ‘stable’ focal adhesion contacts.
As an alternative to the durotactic hypothesis, it is also possible that substrate deformations generated by our microneedle created a gradient in matrix protein (collagen) density and that the keratinocyte was simply migrating along a path of increasing sites of adhesive contact availability in a mechanism that is often referred to as haptotaxis. Alternatively, our data are also potentially consistent with the more recently described phenomenon of topotaxis [72], where the keratinocyte was following changes in PA gel topography in a way that favoured directed cell migration towards the microneedle. Unfortunately, based on the present experimental dataset, we are unable to further characterize whether or not the substrate deformation-directed migration of keratinocytes is best categorized as durotaxis, haptotaxis, topotaxis or a combination of these biophysical mechanisms. Without question, future investigations are needed to better define the mechanism(s) underlying the keratinocyte's migratory response to imposed substrate deformations.
Regardless of which mechanical or biochemical cue(s) are responsible, the addition of either Y27632 or blebbistatin interrupted directed cell migration towards the needle-induced substrate deformations. Plotnikov et al. [45] observed that ROCK inhibition mediated via exposure to Y27632 did not specifically alter the number of focal adhesion contacts in migrating mouse embryonic fibroblasts, but it did promote a switching of focal adhesions from ‘stable’ to ‘tugging’ states. Consistent with our conceptual model of durotaxis, this implies that if Y27632-treated keratinocytes possess increased numbers of focal adhesions relegated to a ‘tugging’-only state, they should statistically be less likely to exhibit substrate deformation-directed migration patterns due to their inability to preferentially form ‘stable’ focal adhesions on areas of strained substrate.
In addition to direct ROCK inhibition, downregulation of NM II activity is a well-known downstream effect of Y27632. Based on this fact, it is reasonable to expect that blebbistatin-exposed keratinocytes might exhibit a similar loss of substrate deformation-directed migration to that observed for Y27632-treated cells. Indeed, this was the case. Multiple studies have shown that intracellular tension via actin–myosin engagement is necessary for the integration of key mechanosensing proteins (e.g. vinculin) into focal adhesions [13,16,73], and that interrupting NM II contractility via exposure to blebbistatin or Y27632 interferes with this process [16,45].
Lastly, note that lamellipodia-mediated cellular migration is an exquisitely complex cellular process, requiring spatio-temporal coordination in the assembly of lamellipodia at the leading edge of the cell, together with the formation/dissolution of focal adhesion contacts and the modulation of actomyosin contractility within the cell cortex [74]. Based on their molecular targets alone, we cannot say for certain whether or not the loss of substrate deformation-directed migration caused by Y27632 and blebbistatin exposure was due to an acquired mechanosensory defect versus a global loss of coordination within the molecular machinery of the migratory apparatus. The observation that drug-treated keratinocytes often exhibited cellular protrusions that seemed to preferentially align with substrate deformation gradients seems to suggest that within some localized areas of focal adhesion contact formation, the cell was still able to sense deformation gradients in the substrate. Presumably, localized mechanosensing failed to exert a global effect on keratinocyte migration due to the overall loss of molecular coordination amongst the various components of the migratory apparatus.
With regards to the generalizability of our results, it is important to note that major differences in cell migration have been observed in two-dimensional versus three-dimensional culture environments. As such, there is no reason to expect that the results we observed for epidermal keratinocytes are universally applicable to all eukaryotic cell types. Cells in three-dimensional constructs, e.g. fibroblasts migrating within a fibrillar collagen gel, likely employ different migration machinery (actin-based protrusion and contraction driver blebs) and adhesion kinetics [22,59,75–77] compared to cells migrating in two dimensions. Although it is unclear whether or not the biophysical mechanism(s) of cell mechanosensation differs in two-dimensional versus three-dimensional environments, durotactic migration has been reported in three-dimensional culture platforms [76,78]. However, from both a mathematical and physiological standpoint, the epidermis constitutes a two-dimensional manifold, a surface that separates an organism from its external environment. Arguably, to this first-order approximation, epidermal keratinocytes are constrained in vivo to two-dimensional migration. Based on this assumption, our experiments are comparable—in terms of their physiologic relevance—to the classic two-dimensional scratch assay that is widely used as a model of wound re-epithelialization in vitro [79].
To date, the implications for single-cell keratinocyte mechanosensation of substrate deformations during wound healing are uncertain, as epidermal re-epithelialization is dogmatically viewed as a form of collective cellular migration initiated from the leading edge of the wounded epithelium. However, in our previous work with keratinocytes [42], we frequently observed that peripheral cells of a multicellular aggregate forming on a soft PA gel extended cellular processes in a direction of increasing substrate deformation generated by nearby cells and cell aggregates. This response suggests that mechanical sensation of substrate deformations may also apply to multicellular aggregates, and by extension, to the leading edge of a wounded epidermis during the process of re-epithelialization. Whether or not single-cell or multicellular aggregate sensation of substrate deformations plays a significant role during the course of natural wound healing, its continued study is warranted to support the development of novel therapeutic strategies targeting the treatment of chronic skin wounds. As a case in point, individual keratinocyte migration and multicellular aggregate formation may represent the primary mechanism by which liquid spray keratinocyte cell-based therapies contributed to the enhanced re-epithelialization of human venous stasis ulcers observed in a recent clinical trial [80].
5. Conclusion
In this work, we investigated the keratinocyte's migratory response to controlled substrate deformations originating approximately 100 and 200 µm away from an otherwise randomly migrating cell. Most keratinocytes altered their migration pathway, preferring a course that vectorially aligned with the direction of substrate deformation. Addition of either Y27632 or blebbistatin impaired the cellular migratory response to these mechanical deformations. Collectively, these results provide compelling evidence supporting the notion that keratinocytes are mechanosensitive cells, further implying that the mechanobiology of keratinocytes could be an important factor that contributes to the process of re-epithelialization in states of both health and disease.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
Data supporting this paper are contained within the paper and as the electronic supplementary information. Additional information will be provided upon request.
Authors' contributions
H.Z. and S.C. performed the experiments, H.Z., J.C.S. and E.A.S. analysed the data, E.A.S. provided the reagents, and H.Z., J.C.S. and E.A.S. wrote the manuscript. All authors gave final approval of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Support for this work was provided by the National Science Foundation (CAREER 1452728) to E.A.S. In addition, J.C.S. acknowledges the Dermatology Foundation for their support of this work through a career development award.
References
- 1.Juliano RL, Haskill S. 1993. Signal transduction from the extracellular matrix. J. Cell Biol. 120, 577–585. ( 10.1083/jcb.120.3.577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandquist JC, Swenson KI, DeMali KA, Burridge K, Means AR.. 2006. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J. Biol. Chem. 281, 35 873–35 883. ( 10.1074/jbc.M605343200) [DOI] [PubMed] [Google Scholar]
- 3.Martin P. 1997. Wound healing—aiming for perfect skin regeneration. Science 276, 75–81. ( 10.1126/science.276.5309.75) [DOI] [PubMed] [Google Scholar]
- 4.Evans ND, Oreffo ROC, Healy E, Thurner PJ, Man YH.. 2013. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 28, 397–409. ( 10.1016/j.jmbbm.2013.04.023) [DOI] [PubMed] [Google Scholar]
- 5.Wong VW, Akaishi S, Longaker MT, Gurtner GC.. 2011. Pushing back: wound mechanotransduction in repair and regeneration. J. Invest. Dermatol. 131, 2186–2196. ( 10.1038/jid.2011.212) [DOI] [PubMed] [Google Scholar]
- 6.Pastar I, et al. 2014. Epithelialization in wound healing: a comprehensive review. Adv. Wound Care 3, 445–464. ( 10.1089/wound.2013.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Veer WM, Bloemen MCT, Ulrich MMW, Molema G, van Zuijlen PP, Middelkoop E, Niessen FB.. 2009. Potential cellular and molecular causes of hypertrophic scar formation. Burns 35, 15–29. ( 10.1016/j.burns.2008.06.020) [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Zhang Y, Zhang N, Wang C, Herrler T, Li Q.. 2015. An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell. Mol. Life Sci. 72, 2091–2106. ( 10.1007/s00018-015-1853-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa R, Orgill DP.. 2009. Mechanobiology of cutaneous wound healing and scarring. In (ed. A Gefen) Bioengineering research of chronic wounds. Studies in mechanobiology, tissue engineering and biomaterials, vol. 1. Berlin, Germany: Springer. [Google Scholar]
- 10.Kenny FN, Connelly JT. 2015. Integrin-mediated adhesion and mechano-sensing in cutaneous wound healing. Cell Tissue Res. 360, 571–582. ( 10.1007/s00441-014-2064-9) [DOI] [PubMed] [Google Scholar]
- 11.Rosinczuk J., Taradaj J, Dymarek R, Sopel M.. 2016. Mechanoregulation of wound healing and skin homeostasis . BioMed Res. Int. 2016, 3943481 ( 10.1155/2016/3943481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton ER, Astudillo P, Humphries MJ, Humphries JD.. 2016. Mechanosensitivity of integrin adhesion complexes: role of the consensus adhesome. Exp. Cell Res. 343, 7–13. ( 10.1016/j.yexcr.2015.10.025) [DOI] [PubMed] [Google Scholar]
- 13.Atherton P, Stutchbury B, Jethwa D, Ballestrem C.. 2016. Mechanosensitive components of integrin adhesions: role of vinculin. Exp. Cell Res. 343, 21–27. ( 10.1016/j.yexcr.2015.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti MA, Adelstein RS. 2008. Nonmuscle myosin II moves in new directions. J. Cell Sci. 121, 11–18. ( 10.1242/jcs.007112) [DOI] [PubMed] [Google Scholar]
- 15.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR.. 2009. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790. ( 10.1038/nrm2786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfenson H, Bershadsky A, Henis YI, Geiger B.. 2011. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J. Cell Sci. 124, 1425–1432. ( 10.1242/jcs.077388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janoštiak R, Pataki AC, Brábek J, Rösel D.. 2014. Mechanosensors in integrin signaling: the emerging role of p130Cas. Eur. J. Cell Biol. 93, 445–454. ( 10.1016/j.ejcb.2014.07.002) [DOI] [PubMed] [Google Scholar]
- 18.Schwartz MA. 2010. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2, a005066 ( 10.1101/cshperspect.a005066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Ingber DE. 2005. Cell tension, matrix mechanics, and cancer development. Cancer Cell 8, 175–176. ( 10.1016/j.ccr.2005.08.009) [DOI] [PubMed] [Google Scholar]
- 20.Huveneers S, Danen EH.. 2009. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069. ( 10.1242/jcs.039446) [DOI] [PubMed] [Google Scholar]
- 21.Wang JH, Lin JS. 2007. Cell traction force and measurement methods. Biomech. Model. Mechanobiol. 6, 361–371. ( 10.1007/s10237-006-0068-4) [DOI] [PubMed] [Google Scholar]
- 22.Hoon JL, Tan MH, Koh CG. 2016. The regulation of cellular responses to mechanical cues by Rho GTPases. Cells 5, E17 ( 10.3390/cells5020017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lock FE, Hotchin NA. 2009. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS ONE 4, e8190 ( 10.1371/journal.pone.0008190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMullan R, et al. 2003. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr. Biol. 13, 2185–2189. ( 10.1016/j.cub.2003.11.050) [DOI] [PubMed] [Google Scholar]
- 25.Terunuma A, Limgala RP, Park CJ, Choudhary I, Vogel JC.. 2010. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng. Part A 16, 1363–1368. ( 10.1089/ten.tea.2009.0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandham VD, Maddala RL, Rao V, Jin JY, Epstein DL, Hall RP, Zhang JY.. 2013. Effects of Y27632 on keratinocyte procurement and wound healing. Clin. Exp. Dermatol. 38, 782–786. ( 10.1111/ced.12067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman S, Liu X, Meyers C, Schlegel R, McBride AA.. 2010. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J. Clin. Invest. 120, 2619–2626. ( 10.1172/JCI42297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida FV, Walko G, McMillan JR, McGrath JA, Wiche G, Barber AH, Connelly JT.. 2015. The cytolinker plectin regulates nuclear mechanotransduction in keratinocytes. J. Cell Sci. 128, 4475–4486. ( 10.1242/jcs.173435) [DOI] [PubMed] [Google Scholar]
- 29.Boerckel JD, Uhrig BA, Willett NJ, Huebsch N, Guldberg RE.. 2011. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc. Natl Acad. Sci. USA 108, E674–E680. ( 10.1073/pnas.1107019108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly DJ, Prendergast PJ. 2005. Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J. Biomech. 38, 1413–1422. ( 10.1016/j.jbiomech.2004.06.026) [DOI] [PubMed] [Google Scholar]
- 31.Isaksson H, Comas O, van Donkelaar CC, Mediavilla J, Wilson W, Huiskes R, Ito K.. 2007. Bone regeneration during distraction osteogenesis: mechano-regulation by shear strain and fluid velocity. J. Biomech. 40, 2002–2011. ( 10.1016/j.jbiomech.2006.09.028) [DOI] [PubMed] [Google Scholar]
- 32.Tatsumi R. 2010. Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim. Sci. J. 81, 11–20. ( 10.1111/j.1740-0929.2009.00712.x) [DOI] [PubMed] [Google Scholar]
- 33.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ.. 2009. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28, 4326–4343. ( 10.1038/onc.2009.299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray A, Slama ZM, Morford RK, Madden SA, Provenzano PP.. 2017. Enhanced directional migration of cancer stem cells in 3D aligned collagen matrices. Biophys. J. 112, 1023–1036. ( 10.1016/j.bpj.2017.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinz B. 2006. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur. J. Cell Biol. 85, 175–181. ( 10.1016/j.ejcb.2005.09.004) [DOI] [PubMed] [Google Scholar]
- 36.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA.. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363. ( 10.1038/nrm809) [DOI] [PubMed] [Google Scholar]
- 37.Jaalouk DE, Lammerding J. 2009. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63–73. ( 10.1038/nrm2597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gargalionis AN, Basdra EK, Papavassiliou AG. 2018. Mechanosignalling in tumour progression. J. Cell. Mol. Med. 22, 704–705. ( 10.1111/jcmm.13452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schedin P, Keely PJ. 2011. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 3, a003228 ( 10.1101/cshperspect.a003228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mierke CT, Rosel D, Fabry B, Brabek J.. 2008. Contractile forces in tumor cell migration. Eur. J. Cell Biol. 87, 669–676. ( 10.1016/j.ejcb.2008.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mierke CT. 2014. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep. Prog. Phys. 77, 076602 ( 10.1088/0034-4885/77/7/076602) [DOI] [PubMed] [Google Scholar]
- 42.Zarkoob H, Bodduluri S, Ponnaluri SV, Selby JC, Sander EA.. 2015. Substrate stiffness affects human keratinocyte colony formation. Cell. Mol. Bioeng. 8, 32–50. ( 10.1007/s12195-015-0377-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhart-King CA, Dembo M, Hammer DA. 2008. Cell–cell mechanical communication through compliant substrates. Biophys. J. 95, 6044–6051. ( 10.1529/biophysj.107.127662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo WH, Frey MT, Burnham NA, Wang Y-L.. 2006. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 90, 2213–2220. ( 10.1529/biophysj.105.070144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM.. 2012. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527. ( 10.1016/j.cell.2012.11.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dupont S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183. ( 10.1038/nature10137) [DOI] [PubMed] [Google Scholar]
- 47.Foolen J, Janssen-van den Broek MW, Baaijens FP. 2014. Synergy between Rho signaling and matrix density in cyclic stretch-induced stress fiber organization. Acta Biomater. 10, 1876–1885. ( 10.1016/j.actbio.2013.12.001) [DOI] [PubMed] [Google Scholar]
- 48.Pelham RJ Jr, Wang Y.. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility . Proc. Natl Acad. Sci. USA 94, 13 661–13 665. ( 10.1073/pnas.94.25.13661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aratyn-Schaus Y, Aratyn-Schaus Y, Oakes PW, Stricker J, Winter SP, Gardel ML.. 2010. Preparation of complaint matrices for quantifying cellular contraction. J Vis Exp. (46), e2173 ( 10.3791/2173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ.. 2002. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 282, C595–C605. ( 10.1152/ajpcell.00270.2001) [DOI] [PubMed] [Google Scholar]
- 51.Kraning-Rush CM, Carey SP, Califano JP, Reinhart-King CA.. 2012. Quantifying traction stresses in adherent cells. Methods Cell Biol. 110, 139–178. ( 10.1016/B978-0-12-388403-9.00006-0) [DOI] [PubMed] [Google Scholar]
- 52.Watt FM. 1987. Influence of cell shape and adhesiveness on stratification and terminal differentiation of human keratinocytes in culture. J. Cell Sci. Suppl. 8, 313–326. ( 10.1242/jcs.1987.Supplement_8.17) [DOI] [PubMed] [Google Scholar]
- 53.Zamansky GB, Nguyen U, Chou IN. 1991. An immunofluorescence study of the calcium-induced coordinated reorganization of microfilaments, keratin intermediate filaments, and microtubules in cultured human epidermal keratinocytes. J. Invest. Dermatol. 97, 985–994. ( 10.1111/1523-1747.ep12491899) [DOI] [PubMed] [Google Scholar]
- 54.Dunn GA. 1983. Characterising a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 12, 14–33. ( 10.1007/978-3-0348-9352-7_1) [DOI] [PubMed] [Google Scholar]
- 55.Othmer HG, Dunbar SR, Alt W. 1988. Models of dispersal in biological systems. J. Math. Biol. 26, 263–298. ( 10.1007/BF00277392) [DOI] [PubMed] [Google Scholar]
- 56.Wu PH, Giri A, Wirtz D. 2015. Statistical analysis of cell migration in 3D using the anisotropic persistent random walk model. Nat. Protoc. 10, 517–527. ( 10.1038/nprot.2015.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strudwick XL, Lang DL, Smith LE, Cowin AJ, Brandner JM.. 2015. Combination of low calcium with Y-27632 rock inhibitor increases the proliferative capacity, expansion potential and lifespan of primary human keratinocytes while retaining their capacity to differentiate into stratified epidermis in a 3D skin model. PLoS ONE 10, e0123651 ( 10.1371/journal.pone.0123651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar S, Egelhoff T, Baskaran H. 2009. Insights into the roles of non-muscle myosin IIA in human keratinocyte migration. Cell. Mol. Bioeng. 2, 486–494. ( 10.1007/s12195-009-0094-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM.. 2007. Myosin IIA regulates cell motility and actomyosin–microtubule crosstalk. Nat. Cell Biol. 9, 299–309. ( 10.1038/ncb1540) [DOI] [PubMed] [Google Scholar]
- 60.Raghupathy R, Witzenburg C, Lake SP, Sander EA, Barocas VH.. 2011. Identification of regional mechanical anisotropy in soft tissue analogs. J. Biomech. Eng. 133, 091011 ( 10.1115/1.4005170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berens P. 2009. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1–21. ( 10.18637/jss.v031.i10) [DOI] [Google Scholar]
- 62.Zar J. 2010. Biostatistical analysis, 5th edn. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 63.Witte MB, Barbul A. 1997. General principles of wound healing. Surg. Clin. North Am. 77, 509–528. ( 10.1016/S0039-6109(05)70566-1) [DOI] [PubMed] [Google Scholar]
- 64.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister J-J, Hinz B.. 2006. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 172, 259–268. ( 10.1083/jcb.200506179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Achterberg VF, Buscemi L, Diekmann H, Smith-Clerc J, Schwengler H, Meister J-J, Wenck H, Gallinat S, Hinz B.. 2014. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 134, 1862–1872. ( 10.1038/jid.2014.90) [DOI] [PubMed] [Google Scholar]
- 66.Trappmann B, et al. 2012. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11, 642–649. ( 10.1038/nmat3339) [DOI] [PubMed] [Google Scholar]
- 67.Connelly JT, Gautrot JE, Trappmann B, Tan DW-M, Donati G, Huck WTS, Watt FM.. 2010. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 12, 711–718. ( 10.1038/ncb2074) [DOI] [PubMed] [Google Scholar]
- 68.Engler AJ, Sen S, Sweeney HL, Discher DE.. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. ( 10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 69.Discher DE, Janmey P, Wang YL. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143. ( 10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]
- 70.Lo CM, Wang H-B, Dembo M, Wang Y-L.. 2000. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152. ( 10.1016/S0006-3495(00)76279-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boudou T, Ohayon J, Picart C, Pettigrew RI, Tracqui P.. 2009. Nonlinear elastic properties of polyacrylamide gels: implications for quantification of cellular forces. Biorheology 46, 191–205. ( 10.3233/BIR-2009-0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park J, Kim D-H, Levchenko A. 2018. Topotaxis: a new mechanism of directed cell migration in topographic ECM gradients. Biophys. J. 114, 1257–1263. ( 10.1016/j.bpj.2017.11.3813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carisey A, et al. 2013. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 23, 271–281. ( 10.1016/j.cub.2013.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ridley AJ. 2015. Rho GTPase signalling in cell migration. Curr. Opin Cell Biol. 36, 103–112. ( 10.1016/j.ceb.2015.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM.. 2015. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 6, 8720 ( 10.1038/ncomms9720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doyle AD, Yamada KM. 2016. Mechanosensing via cell–matrix adhesions in 3D microenvironments. Exp. Cell Res. 343, 60–66. ( 10.1016/j.yexcr.2015.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vu LT, Jain G, Veres BD, Rajagopalan P.. 2015. Cell migration on planar and three-dimensional matrices: a hydrogel-based perspective. Tissue Eng. Part B Rev. 21, 67–74. ( 10.1089/ten.TEB.2013.0782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hadjipanayi E, Mudera V, Brown RA. 2009. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil. Cytoskeleton 66, 121–128. ( 10.1002/cm.20331) [DOI] [PubMed] [Google Scholar]
- 79.Cory G. 2011. Scratch-wound assay. In (eds C Wells, M Parsons) Cell migration. Methods in molecular biology (methods and protocols), vol. 769. New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- 80.Kirsner RS, Marston WA, Snyder RJ, Lee TD, Cargill DI, Slade HB.. 2012. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 380, 977–985. ( 10.1016/S0140-6736(12)60644-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this paper are contained within the paper and as the electronic supplementary information. Additional information will be provided upon request.