Abstract
Carcinogenesis is an evolutionary process whereby cells accumulate multiple mutations. Besides the ‘driver mutations’ that cause the disease, cells also accumulate a number of other mutations with seemingly no direct role in this evolutionary process. They are called passenger mutations. While it has been argued that passenger mutations render tumours more fragile due to reduced fitness, the role of passenger mutations remains understudied. Using evolutionary computational models, we demonstrate that in the context of tumour suppressor gene inactivation (and hence fitness valley crossing), the presence of passenger mutations can accelerate the rate of evolution by reducing overall population fitness and increasing the relative fitness of intermediate mutants in the fitness valley crossing pathway. Hence, the baseline rate of tumour suppressor gene inactivation might be faster than previously thought. Conceptually, parallels are found in the field of turbulence and pattern formation, where instabilities can be driven by perturbations that are damped (disadvantageous), but provide a richer set of pathways such that a system can achieve some desired goal more readily. This highlights, through a number of novel parallels, the relevance of physical sciences in oncology.
Keywords: evolutionary theory, mathematical models, fitness valley, tumour evolution
1. Introduction
The development and progression of cancer is an evolutionary process whereby cells accumulate multiple mutations, which enables them to break out of homeostasis and to proliferate out of control. The mutations that enable this process typically confer a selective advantage to cells and have been called driver mutations [1–5]. Tumours, however, are highly heterogeneous and cells also contain a variety of other mutations, called passenger mutations [1–5]. They arise from random mutations in sequences that do not contribute directly to disease, facilitated by exposure to mutagenic processes and lack of repair [6]. While passenger mutations have been thought to have minimal biological consequences on the disease process, the properties and role of passenger mutations remain poorly understood. Recent data indicate that passenger mutations carry a certain fitness cost [7–9], and that they might therefore render tumours more fragile, which could be exploited therapeutically [4,7,10]. One particular evolutionary process that is central to carcinogenesis and cancer progression is the inactivation of tumour suppressor genes (TSGs) [9,10]. This typically requires two mutational hits because both copies of the gene need to be inactivated to achieve full loss of function [11]. Different TSGs display different characteristics, and, in principle, the inactivation of only one copy of the gene either results in no change in the fitness of the cell, or it could entail a certain selective disadvantage. To inform model assumptions, we will specifically consider the TSG APC, which becomes inactivated early in the development of colorectal cancer [12]. In this case, data indicate that heterozygous APC+/− cells can experience reduced fitness, which means that a fitness valley has to be crossed for the inactivation of APC to occur. Experiments with colorectal cancer cell lines revealed that a truncating mutation in APC has a dominant effect resulting in a spindle checkpoint defect, aneuploidy, and a reduced proliferation rate of cells [13,14]. Similar effects have been found in vivo in APCMin/+ mice [15], which have an APC+/− germ line mutation. In general, if a copy of a TSG is lost as a consequence of aneuploidy, the cell is likely to suffer a fitness reduction (e.g. references [16,17]). Motivated by these studies, our paper investigates the effect of passenger mutations on the evolutionary dynamics of TSG inactivation, assuming that a fitness valley needs to be crossed.
2. Results
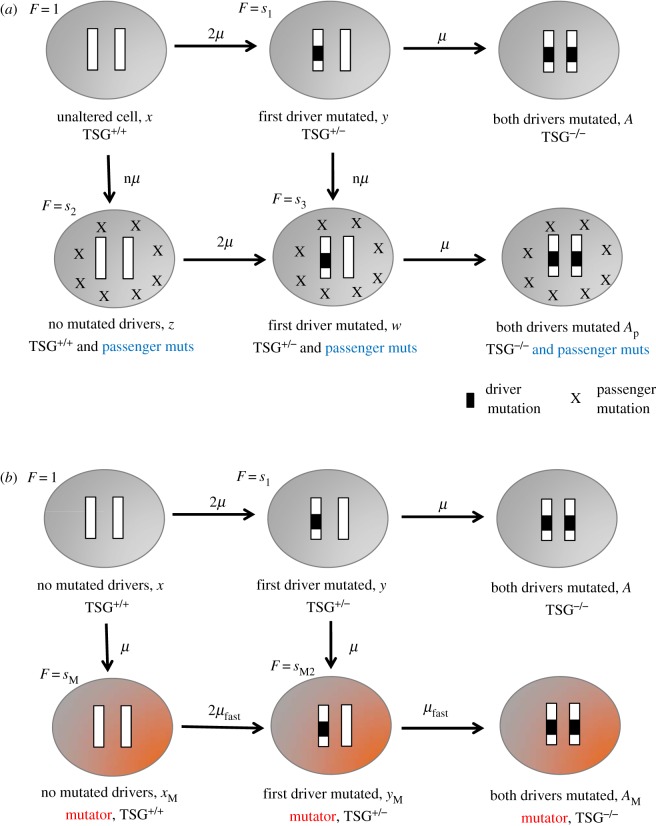
We consider a computational model for the inactivation TSGs [18,19], where cells only acquire an advantage once they have accumulated two separate mutations, but are neutral or disadvantageous in the presence of only one of the mutations. For convenience, cells with both copies of the TSG present are referred to as TSG+/+, and cells with one or both copies of the TSG inactivated are referred to as TSG+/− and TSG−/− respectively. Much evolutionary work has been performed that studied how fast such fitness valleys/plateaus can be crossed, depending on the scenario under consideration [20–31]. To study the role of passenger mutations, we employ a stochastic agent-based model that is also referred to as a contact process. This model assumes the existence of N spots, which can either be empty or contain a cell. Each time step, the system is sampled M times, where M is the number of cells present. If the chosen spot contains a cell, it can divide and die with defined probabilities. When a cell is chosen to divide, a target spot is chosen randomly from the whole system, and division only proceeds if this target spot is empty. Upon division, mutations can occur that give rise to different cell genotypes (figure 1a). TSG+/+ cells without passenger mutations are denoted by x and attempt division with a probability Lx per cell per update. TSG+/− cells without passenger mutations are denoted by y and have a fitness cost of s1 (s1 ≤ 1), such that their division probability is s1 Lx. TSG+/+ cells that also contain passenger mutations are denoted by z and have a fitness cost s2 (s2 < 1). The assumption of a possible fitness cost of passenger mutations is in agreement with previous experimental studies [7–9]. At the same time we note, however, that this blurs the definition of a passenger mutation, which is elaborated on further in the discussion section. TSG+/− cells that also contain passenger mutations are denoted by w and have a fitness cost s3 (s3 < 1). Both TSG+/− populations, y and w, can give rise to the advantageous TSG−/− double mutant. All the mutation processes are defined in figure 1a. For simplicity, each cell type is assumed to die with the same rate D. The model was simulated repeatedly, and the fraction of realizations when an advantageous TSG−/− mutant had been generated by a defined time threshold was determined. We compared simulations without passenger mutations (n = 0) with those that did allow the generation of passenger mutations (n > 0). Two different regimes have been observed [25]: In one regime, the double-hit mutant arises without the intermediate TSG+/− mutant reaching fixation, a process called stochastic tunnelling. The second regime can be called sequential fixation, were the intermediate TSG+/− mutant fixates before the double mutant is created.
Figure 1.
Schematic representation of computational models. (a) Model of TSG inactivation in the context of passenger mutations. The cell types and associated fitness values (F) are explained in the main text. The arrows show the mutational steps that generate the different cell types. The top row of cell types depicts standard evolutionary processes where the two copies of the TSG are sequentially inactivated. In addition, the model assumes that with a rate nμ, cells can accumulate passenger mutations. Cells with passenger mutants can also inactivate the TSG, as shown. (b) Model of TSG (APC) inactivation in the context of Lynch syndrome and mutator phenotypes in colorectal cancer. This model does not contain passenger mutations. The basic evolutionary processes (along the top row of cells) are the same as above. The difference is that unmutated TSG+/+ cells can inactivate mismatch repair mechanisms with a rate μ, giving rise to mutator phenotypes that are characterized by an elevated mutation rate μfast. (Online version in colour.)
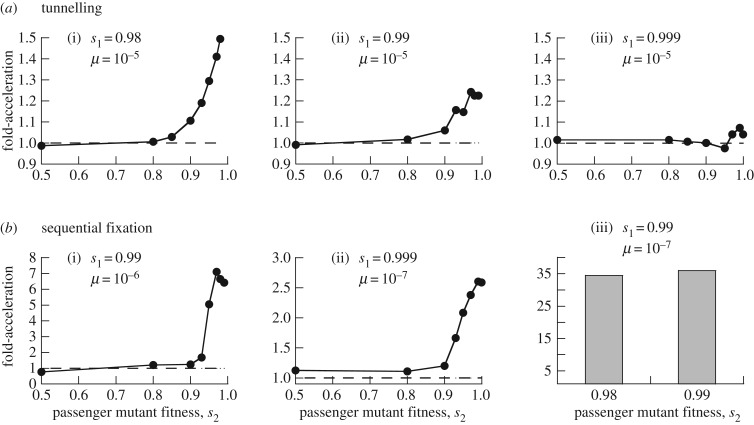
For the tunnelling regime, we find that the presence of passenger mutations can accelerate the generation of the advantageous double mutant (figure 2a(i)). The magnitude of this effect increases with higher fitness of the passenger mutants, s2 (figure 2a(i)). This requires that (i) the number of passenger mutations that can be accumulated, n, is sufficiently large relative to the inverse of the mutation rate and (ii) the intermediate TSG+/− mutation reduces the fitness to a lesser degree in cells with passenger mutations than in cells without passenger mutations, i.e. there are epistatic interactions between drivers and passengers [33,34]. The exact condition is s3 > s1s2, see the electronic supplementary material for computational details. This is a necessary condition for the passenger mutations to accelerate evolution (both in the tunnelling regime and in the sequential fixation regime, see below). In the Discussion section, we describe a specific example where the fitness of TSG+/− mutants is context dependent, indicating that an assumed occurrence of epistasis in such cells is biologically relevant.
Figure 2.
Accelerated crossing of fitness valleys (i.e. inactivation of TSGs) in the presence of passenger mutations. The computer simulations were run repeatedly, and the fraction of realizations in which the double mutant was created before a time threshold Tthr was determined. The number of simulations/sample sizes required by rare events is large and were chosen using reference [32]. For a fixed margin of error, the more rare the events, the larger the sample size. The margins of errors of our study are acceptable, as even in the worst situation (N = 543 449, recorded fraction = 0.000077), the margin of error is less than one third of the estimated proportion. Each graph plots the ‘fold acceleration’, which is the fraction of runs where the double mutant was generated in the presence of passenger mutations with fitness s2, divided by the same measure in the absence of passenger mutations. The Z-score for population proportions was used to determine whether the difference in outcome between simulations with and without passenger mutations was statistically significant (the distribution under the null hypothesis, when the two true proportions are the same, is asymptotically normal). (a) Parameter regime where the double mutant evolved through a tunnelling pathway. Panels (i–iii) show that a reduced cost of the intermediate TSG+/− mutant (higher value of s1) leads to a reduced effect of passenger mutations on the rate of evolution. Differences between outcomes with and without passenger mutations were statistically significant for (i) s2 ≥ 0.93, (ii) s2 ≥ 0.93, and (iii) s2 = 0.99. (b) Parameter regime where the double mutant evolves by sequential fixation, in which passenger mutants have a stronger accelerating effect on the rate of evolution. Differences between outcomes with and without passenger mutations were statistically significant (p < 0.05) for (i) s2 ≥ 0.95, (ii) s2 ≥ 0.93, and (iii) s2 = 0.98 & s2 = 0.99. In panel (iii), the fold acceleration was only determined for the highest values of s2 (where the effect is strongest), due to the extensive computational cost associated with this parameter set. Remaining parameters were chosen as follows. Lx = 0.15, D = 0.01, s3 = s2. n = 5 × 10−2/μ, N = 2500. The results do not depend on the assumption s3 = s2. How s3 needs to depend on s2 and s1 for the results to hold is defined in the electronic supplementary material. The time thresholds are given as follows for the individual graphs. (a) (i) Tthr = 8000; (ii) Tthr = 8000, (iii) Tthr = 5000; (b) (i) Tthr = 120 000; (ii) Tthr = 1 200 000; (iii) Tthr = 1 500 000.
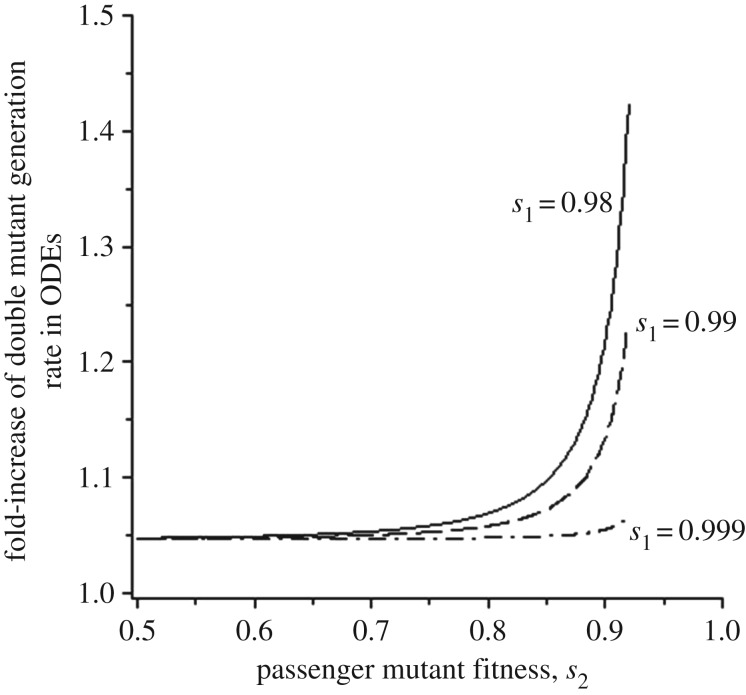
The reason for the accelerated evolution in the presence of passenger mutations is that these mutations increase the relative fitness of the intermediate TSG+/− cells through a set of complex interactions. If the wild-type population consists mostly of cells without passenger mutations, the evolutionary dynamics are largely driven by the x, y system, which is relatively slow due to the more pronounced disadvantage of y. The larger the proportion of cells with passenger mutations, however, the more the evolutionary dynamics are driven by the z, w system, where the intermediate mutant suffers an overall lower fitness cost. This allows the total population of intermediate TSG+/− cells to persist at a higher selection–mutation balance, making it more likely to generate the double mutant. The average rate of double mutant generation can be calculated from ordinary differential equations (ODEs, see electronic supplementary material), which is a reasonable model that quantifies population dynamics in the tunnelling regime. The rate of double mutant generation is increased by the presence of passenger mutants, with more pronounced effects for larger values of s2 (figure 3), thus explaining our observations. If the fitness of passenger mutants crosses a threshold (which depends on the total rate of passenger mutant generation), the cells with passenger mutations, z, outcompete those without passenger mutations, x (because z is generated by x). In this regime, the advantageous double mutants are created fastest because the intermediate TSG+/− mutants (w) have the highest relative fitness out of all scenarios. This might be a biologically relevant parameter region given the ubiquitous occurrence of passenger mutations in cancer cells, and even in aged non-cancerous tissue [35,36]. We note that the accelerating effect exhibited by passenger mutants is not due to their presence providing additional targets for further mutations to occur. The total number of cells in the model is the same in the presence and absence of passenger mutants. The accelerating effect of passengers stems from the overall reduction in population fitness and the consequent elevation of the relative fitness of intermediate TSG+/− mutants.
Figure 3.
The average behaviour of the contact process can be described by ordinary differential equations given in the electronic supplementary material. From these ODEs, equilibrium populations sizes, and hence the average rate of double mutant generation at equilibrium can be calculated. In the presence of passenger mutations, this is given by (s1y* + s3w*)[1 − (x* + y* + z* + w*)/k], where * denotes equilibrium population sizes. This is divided by the rate of double mutant generation in the absence of passenger mutations, given by s1y*[1 − (x* + y*)/k]. This yields the fold increase of the double mutant generation rate that is mediated by passenger mutations, and is plotted in the graph for different values of s1 (fitness cost of TSG+/− cells without passenger mutations, y). Parameter values were chosen as follows: r = 0.1, d = 0.01, μ = 10−5, n1 = 2, n2 = 5000, k = 2500.
The extent to which passenger mutants accelerate fitness valley crossing further depends on the relative fitness of intermediate TSG+/− mutants without passenger mutations (s1, the fitness cost of population y). The closer the fitness of the intermediate mutant y-population is to the fitness of the wild-type x-population (s1 → 1), the less pronounced the accelerating effect (compare figure 2a(i–iii)). This is also seen in the ODE predictions, which show that for larger values of s1, the average rate of double mutant generation is accelerated by passenger mutations to a lesser extent (figure 3). The reason is that for higher s1, the intermediate TSG+/− mutants (y) have less of a disadvantage compared to population x, which leaves less room for improvement by the z, w interactions. Thus, if the intermediate TSG+/− mutant is almost neutral with respect to the wild-type, passenger mutants are not likely to accelerate evolution in the tunnelling regime.
Next, consider the parameter regime where the intermediate mutant fixates prior to the generation of the double mutant (sequential fixation). This tends to occur for parameters where the generation of the double mutant takes a longer period of time due to lower mutation rates or smaller population sizes. In this scenario, the accelerating effect of passenger mutations can be significantly more pronounced than in the tunnelling regime (figure 2b). For a physiologically realistic rate of gene inactivation (10−7 per gene per division), even if the TSG+/− mutants without passenger mutations only have a 0.1% fitness cost (s1 = 0.999), and if the passenger mutations lead to a 1% fitness cost (s2 = 0.99), the presence of passenger mutations can accelerate the emergence of the double mutant almost threefold (figure 2b(ii)). If the fitness cost of the intermediate TSG+/− mutant is 1%, the acceleration can be up to 35-fold (figure 2b(iii)). The reason is that the fixation probability of the TSG+/− mutants is markedly higher when the dynamics are governed more by the z,w system compared to the x,y system.
These results remain robust if instead of assuming that all passenger mutants have the same fitness cost, those fitness cost values are taken from a power function distribution between zero and one, with averages given by s2 and s3. This potentially allows for some significantly deleterious passenger mutants even though many of them can be close to neutral (electronic supplementary material). Results are further shown to remain robust in a spatially explicit model, where dividing cells place their offspring in a randomly chosen spot nearby (electronic supplementary material). Finally, the same patterns are observed in a constant population Moran process, which represents tissues where normal cells are maintained at carrying capacity and their homeostatic turnover is driven by cell death (electronic supplementary material).
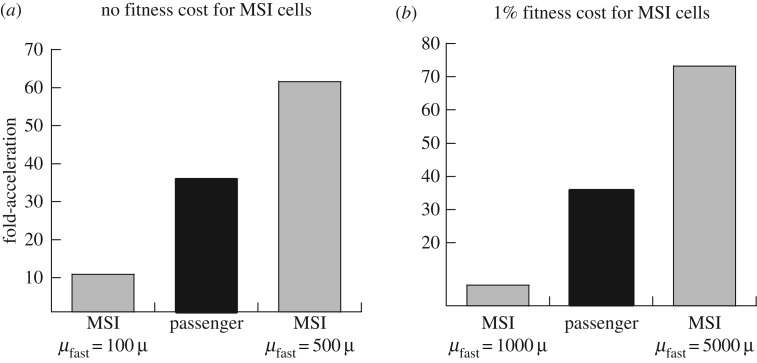
While our models have shown that the presence of passenger mutations can accelerate the rate of TSG inactivation, the question arises how significant this acceleration can be. To gauge that, we compare the degree of acceleration that can be observed in our passenger mutation model to the acceleration observed in the context of a different and unrelated process that occurs in colorectal carcinogenesis, and that is known to lead to clinically significant accelerations in evolutionary processes: tumour initiation in Lynch syndrome patients [37]. It is known that Lynch syndrome patients develop colorectal tumours at a significantly faster rate than the general population. While the average age of onset in the general population is around 64 years, it is less than 45 years for Lynch syndrome patients [38,39]. This is because Lynch syndrome patients are characterized by a germ line mutation in one copy of a mismatch repair (MMR) gene. Hence a single point mutation can frequently generate MMR-deficient cells that promote mutant accumulation and hence the inactivation of APC. Therefore, we describe tumour formation in the context of Lynch syndrome (without passenger mutations) in the same kind of computational framework studied so far (figure 1b), and determine the extent to which evolution is accelerated in that model. If the degree of acceleration observed in the Lynch syndrome model is of a similar magnitude as the acceleration observed in the passenger model, then there is indication that passenger-induced acceleration can be clinically highly relevant. If the acceleration in the passenger model is much less than that in the Lynch syndrome model, then the passenger-induced acceleration is less relevant. In particular, we investigated by how much the mutation rate in the MMR-deficient cells has to be increased to obtain a degree of evolution acceleration that is comparable to that observed with passenger mutations. To do so, we assumed that genes are inactivated with a rate of 10−7 per division, and that an intermediate TSG+/− cell carries a 1% fitness cost, consistent with data that documented reduced growth of APC+/− cells [13]. As before, passenger mutants were also assumed to carry a 1% fitness cost. If we assume that MMR-deficient cells do not carry a fitness cost, we obtain that MMR-deficient cells need to have a 100–500 fold increase in their mutation rate to accelerate evolution to a similar degree as seen in corresponding passenger mutant simulations (figure 4a). If MMR-deficient cells have a 1% fitness cost, then the fold increase in the mutation rate has to be 1000–5000 fold to match the acceleration afforded by the presence of passenger mutations (figure 4b). Because this increase in mutation rate is thought to be typical for MMR deficient colorectal cells [40], this suggests that passenger mutations can have an accelerating effect that is similar in magnitude to acceleration in Lynch syndrome, pointing to potentially strong biological relevance. If APC+/− cells are characterized by a significantly lower fitness cost, or if the assumed epistatic interactions between drivers and passengers are significantly weaker, this effect would be reduced.
Figure 4.
Rate of TSG inactivation in two types of simulations: assuming Lynch syndrome, which involves the acquisition of microsatellite instability or MSI, i.e. cells with a faster mutation rate (figure 1b); and assuming the passenger mutant pathway (figure 1a). The computer simulations were run repeatedly, and the fraction of realizations in which the double mutant was created before a time threshold Tthr was determined. For the Lynch syndrome model, simulations with different accelerated mutation rates, μfast, were run. The fraction of runs that resulted in double mutant generation were divided by the fraction obtained without the existence of mutator phenotypes (MSI cells), which is the fold-acceleration depicted by the grey bars. The black bar shows the fold-acceleration derived from the passenger mutation model (without mutator cells), but with otherwise identical parameters. This was done in two settings (a) assuming that mutator cells do not suffer from a fitness cost; (b) assuming that mutator cells are characterized by a 1% fitness cost, brought about by the frequent generation of deleterious mutations. The parameters were chosen as follows. Lx = 0.15, D = 0.01, s1 = 0.99, s2 = 0.99, s3 = 0.99, μ = 10−7. For (a) sM = 1, sM2 = 1. For (b) sM = 0.99, sM2 = 0.99, n = 5 × 10−2/μ, Tthr = 1 500 000, N = 2500.
3. Discussion and conclusion
Previous work reported that the presence of passenger mutations can make tumours more fragile in certain circumstances due to a reduction in overall fitness [4,7,10]. Here we have shown that in the context of fitness valley crossing, costly passenger mutations can actually accelerate evolution because they reduce overall population fitness and thereby provide an environment in which intermediate TSG+/− mutants enjoy a higher relative fitness. Although passenger mutations are selected against, their accumulation (even at low numbers) provides access to additional pathways to cancer where the fitness valley is shallower and easier to cross. It has been previously suggested that the process of carcinogenesis could be promoted through a reduction of overall population fitness due to ageing and other insults, thus providing a more favourable fitness landscape for the evolution of malignant cells [41]. Our passenger mutations model fits well into this concept.
This brings up the question whether the slightly disadvantageous mutants considered here should be considered ‘passenger mutations’. Passenger mutations can be defined as mutations that do not directly drive cancer initiation and progression, as opposed to driver mutations, such as mutations in oncogenes, TSGs or repair genes. In this sense, the mutations considered in our model should be classified as passenger mutations. As our model shows, such mutants can indirectly accelerate tumour evolution through altering the fitness landscape, but this would not make them driver mutations. While in the literature, passenger mutations are sometimes referred to as ‘neutral’, this is not based on specific measurements. The only studies attempting to quantify the fitness of passenger mutations that we are aware of indicate that they might have a certain disadvantage [7–9], although further studies will be required to gain more detailed information. It is important to point out that our model does not require all ‘passenger’ mutants to have a selective disadvantage. While in the main model, we assumed that passenger mutants had a slightly reduced fitness, in §2.1 of the electronic supplementary material, we considered a model where the fitness of passenger mutants was taken from a random distribution. In other words, some of the passenger mutants could be neutral, while others could have various degrees of disadvantage, with the average fitness over all passenger mutants being slightly lower than that of wild-type cells. Given, however, that our model suggests that passenger mutations can actually accelerate certain evolutionary processes, our study is further useful in the sense that it provides a basis for discussing the definition of what constitutes passenger mutations.
Another interesting aspect of the work presented here is the indication that passenger mutations can in principle accelerate tumour evolution to an extent that might comparable to that observed in patients with a predisposition to genetic instability (Lynch syndrome). This suggests that the ‘baseline’ rate of TSG inactivation in the absence of genetic predisposition and genetic instability can be significantly faster than previously thought, which might be conceptually important for understanding the ability of cells to accumulate a number of carcinogenic mutations in a relatively short period of time [42]. This applies not only to tumour progression, but also to cancer initiation in healthy tissue, which has been shown to contain a significant number of passenger (and driver) mutations [36], especially at advanced age [35]. In other words, our result leads to the hypothesis that in the absence of passenger mutations, the onset of a cancer that is initiated by TSG inactivation (e.g. colorectal cancer) would be delayed significantly (by perhaps 20 years if the magnitude of the acceleration from passenger mutations is indeed comparable to that seen in Lynch syndrome patients [38,39]).
Our analysis identified possible epistatic interactions between driver and passenger mutations [33,34] to be important for the reported dynamics, and the literature supports this notion. Indeed, it has been pointed out that the classification of mutations into passengers and drivers might be an over-simplification, because the fitness of a given cancer phenotype can be context-dependent [43]. More specifically, we turn again to the TSG APC in colorectal carcinogenesis. There are mouse strains that are heterozygous for the APCMin (multiple intestinal neoplasia) mutation, called APCMin/+ mice. They frequently develop intestinal tumours [44,45]. Significant variation in tumour incidence occurs among APCMin/+ mice with identical APC mutations and which are kept under identical laboratory conditions [44,45]. This variation is caused by differences in the genetic background of the APC mutation, which in turn depends on variation in ‘modifier genes’ in different mouse strains [44–46]. These are not involved directly in the process of carcinogenesis, but modify the phenotypic properties of APC+/− cells. This indicates that passengers can modulate the fitness of TSG+/− cells, as required by our model to observe accelerated evolution.
Our work adds to the growing literature that investigates the dynamics of fitness valley crossing under various conditions [20–22,24,26–29,47–49]. Beyond this immediate discipline, however, it is also interesting to consider our results in a wider scientific sense. In the presence of passenger mutations, cellular evolvability is predicted to increase through the introduction of disadvantageous cells. The presence of these disadvantageous cells lowers overall population fitness, allowing intermediate TSG+/− mutants to have an overall higher relative fitness, which promotes faster generation of the TSG−/− double mutant. Studies of the onset of turbulence as well as pattern forming systems have revealed mechanisms of instability that act in a very similar manner. For example, in turbulence it has been shown that three-dimensional perturbations on parallel shear flows are damped more strongly than two-dimensional ones; but because of the slow decay, they provide a new base flow on which a new and richer class of fluctuations can grow more rapidly (details in electronic supplementary material). This provides a fundamental connection between principles in the physical sciences and the particular oncology question under consideration. This is a good example of how seemingly different scientific disciplines can inform each other and drive progress.
Supplementary Material
Data accessibility
Computer code for the basic contact process and moran process is available at: https://github.com/dwodarz/Passenger-Mutation-code.
Authors' contributions
D.W. conceived the project, performed numerical simulations and wrote the paper. N.L.K. performed mathematical calculations and wrote the paper. A.C.N. contributed the parallels to the field of turbulence and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This study was funded in part by NIH grant U01CA187956.
References
- 1.Bozic I, et al. 2010. Accumulation of driver and passenger mutations during tumor progression. Proc. Natl Acad. Sci. USA 107, 18 545–18 550. ( 10.1073/pnas.1010978107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenman C, et al. 2007. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158. ( 10.1038/nature05610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaves M, Maley CC. 2012. Clonal evolution in cancer. Nature 481, 306–313. ( 10.1038/nature10762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatenby RA, Cunningham JJ, Brown JS. 2014. Evolutionary triage governs fitness in driver and passenger mutations and suggests targeting never mutations. Nat. Commun. 5, 5499 ( 10.1038/ncomms6499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haber DA, Settleman J. 2007. Cancer: drivers and passengers. Nature 446, 145–146. ( 10.1038/446145a) [DOI] [PubMed] [Google Scholar]
- 6.Futreal PA. 2007. Backseat drivers take the wheel. Cancer Cell 12, 493–494. ( 10.1016/j.ccr.2007.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland CD, Korolev KS, Kryukov GV, Sunyaev SR, Mirny LA. 2013. Impact of deleterious passenger mutations on cancer progression. Proc. Natl Acad. Sci. USA 110, 2910–2915. ( 10.1073/pnas.1213968110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland CD, Yaglom JA, Wojtkowiak JW, Scott JG, Morse DL, Sherman MY, Mirny LA. 2015. Passenger DNA alterations reduce cancer fitness in cell culture and mouse models. bioRxiv 026302. [Google Scholar]
- 9.McFarland CD, Yaglom JA, Wojtkowiak JW, Scott JG, Morse DL, Sherman MY, Mirny LA. 2017. The Damaging effect of passenger mutations on cancer progression. Cancer Res. 77, 4763–4772. ( 10.1158/1538-7445.AM2017-4763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland CD, Mirny LA, Korolev KS. 2014. Tug-of-war between driver and passenger mutations in cancer and other adaptive processes. Proc. Natl Acad. Sci. USA 111, 15 138–15 143. ( 10.1073/pnas.1404341111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudson AG. 2001. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer 1, 157–162. ( 10.1038/35101031) [DOI] [PubMed] [Google Scholar]
- 12.Polakis P. 1997. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta 1332, F127–F147. [DOI] [PubMed] [Google Scholar]
- 13.Tighe A, Johnson VL, Taylor SS. 2004. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J. Cell Sci. 117, 6339–6353. ( 10.1242/jcs.01556) [DOI] [PubMed] [Google Scholar]
- 14.Green RA, Kaplan KB. 2003. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 163, 949–961. ( 10.1083/jcb.200307070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell CM, Green RA, Kaplan KB. 2007. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J. Cell Biol. 178, 1109–1120. ( 10.1083/jcb.200703186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. 2007. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36. ( 10.1016/j.ccr.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 17.Gordon DJ, Resio B, Pellman D. 2012. Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet. 13, 189–203. ( 10.1038/nrg3123) [DOI] [PubMed] [Google Scholar]
- 18.Vogelstein B, Kinzler KW. 2004. Cancer genes and the pathways they control. Nat. Med. 10, 789–799. ( 10.1038/nm1087) [DOI] [PubMed] [Google Scholar]
- 19.Vogelstein B, Lane D, Levine AJ. 2000. Surfing the p53 network. Nature 408, 307–310. ( 10.1038/35042675) [DOI] [PubMed] [Google Scholar]
- 20.Weinreich DM, Chao L. 2005. Rapid evolutionary escape by large populations from local fitness peaks is likely in nature. Evolution 59, 1175–1182. ( 10.1111/j.0014-3820.2005.tb01769.x) [DOI] [PubMed] [Google Scholar]
- 21.Serra MC, Haccou P. 2007. Dynamics of escape mutants. Theor. Popul. Biol. 72, 167–178. ( 10.1016/j.tpb.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 22.Weissman DB, Desai MM, Fisher DS, Feldman MW. 2009. The rate at which asexual populations cross fitness valleys. Theor. Popul. Biol. 75, 286–300. ( 10.1016/j.tpb.2009.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissman DB, Feldman MW, Fisher DS. 2010. The rate of fitness-valley crossing in sexual populations. Genetics 186, 1389–1410. ( 10.1534/genetics.110.123240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasa Y, Michor F, Nowak MA. 2004. Stochastic tunnels in evolutionary dynamics. Genetics 166, 1571–1579. ( 10.1534/genetics.166.3.1571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komarova NL, Sengupta A, Nowak MA. 2003. Mutation-selection networks of cancer initiation: tumor suppressor genes and chromosomal instability. J. Theor. Biol. 223, 433–450. ( 10.1016/S0022-5193(03)00120-6) [DOI] [PubMed] [Google Scholar]
- 26.Barton NH, Rouhani S. 1987. The frequency of shifts between alternative equilibria. J. Theor. Biol. 125, 397–418. ( 10.1016/S0022-5193(87)80210-2) [DOI] [PubMed] [Google Scholar]
- 27.Carter AJR, Wagner GP. 2002. Evolution of functionally conserved enhancers can be accelerated in large populations: a population-genetic model. Proc. R. Soc Lond. B 269, 953–960. ( 10.1098/rspb.2002.1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura M. 1985. The role of compensatory neutral mutations in molecular evolution. J. Genet. 64, 7–19. ( 10.1007/BF02923549) [DOI] [Google Scholar]
- 29.Durrett R, Schmidtt D. 2008. Waiting for two mutations: with applications to regulatory sequence evolution and the limits of Darwinian evolution. Genetics 180, 1501–1509. ( 10.1534/genetics.107.082610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durrett R, Foo J, Leder K. 2016. Spatial Moran models, II: cancer initiation in spatially structured tissue. J. Math. Biol. 72, 1369–1400. ( 10.1007/s00285-015-0912-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durrett R, Moseley S. 2015. Spatial Moran models I. Stochastic tunneling in the neutral case. Ann Appl Probab 25, 104–115. ( 10.1214/13-AAP989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajian-Tilaki K. 2011. Sample size estimation in epidemiologic studies. Caspian J. Intern. Med. 2, 289–298. [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer B, Siebert R, Traulsen A. 2014. Cancer initiation with epistatic interactions between driver and passenger mutations. J. Theor. Biol. 358, 52–60. ( 10.1016/j.jtbi.2014.05.018) [DOI] [PubMed] [Google Scholar]
- 34.Illingworth CJ, Mustonen V. 2011. Distinguishing driver and passenger mutations in an evolutionary history categorized by interference. Genetics 189, 989–1000. ( 10.1534/genetics.111.133975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holstege H, et al. 2014. Somatic mutations found in the healthy blood compartment of a 115-yr-old woman demonstrate oligoclonal hematopoiesis. Genome Res. 24, 733–742. ( 10.1101/gr.162131.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martincorena I, et al. 2015. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886. ( 10.1126/science.aaa6806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. 2009. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin. Genet. 76, 1–18. ( 10.1111/j.1399-0004.2009.01230.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umar A, et al. 2004. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 96, 261–268. ( 10.1093/jnci/djh034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch HT, Lanspa SJ. 2010. Colorectal cancer survival advantage in MUTYH-associated polyposis and Lynch syndrome families. J. Natl. Cancer Inst. 102, 1687–1689. ( 10.1093/jnci/djq439) [DOI] [PubMed] [Google Scholar]
- 40.Komarova NL, Lengauer C, Vogelstein B, Nowak MA. 2002. Dynamics of genetic instability in sporadic and familial colorectal cancer. Cancer Biol. Ther. 1, 685–692. ( 10.4161/cbt.321) [DOI] [PubMed] [Google Scholar]
- 41.DeGregori J. 2011. Evolved tumor suppression: why are we so good at not getting cancer? Cancer Res. 71, 3739–3744. ( 10.1158/0008-5472.CAN-11-0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomlinson I, Bodmer W. 1999. Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nat. Med. 5, 11–12. ( 10.1038/4687) [DOI] [PubMed] [Google Scholar]
- 43.Marusyk A, Almendro V, Polyak K.. 2012. Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334. ( 10.1038/nrc3261) [DOI] [PubMed] [Google Scholar]
- 44.Montagutelli X. 2000. Effect of the genetic background on the phenotype of mouse mutations. J. Am. Soc. Nephrol. 11 Suppl 16, S101–S105. [PubMed] [Google Scholar]
- 45.McCart AE, Vickaryous NK, Silver A. 2008. Apc mice: models, modifiers and mutants. Pathol. Res. Pract. 204, 479–490. ( 10.1016/j.prp.2008.03.004) [DOI] [PubMed] [Google Scholar]
- 46.Nadeau JH. 2001. Modifier genes in mice and humans. Nat. Rev. Genet. 2, 165–174. ( 10.1038/35056009) [DOI] [PubMed] [Google Scholar]
- 47.Iwasa Y, Michor F, Nowak MA. 2004. Evolutionary dynamics of invasion and escape. J. Theor. Biol. 226, 205–214. ( 10.1016/j.jtbi.2003.08.014) [DOI] [PubMed] [Google Scholar]
- 48.Komarova NL, Urwin E, Wodarz D. 2012. Accelerated crossing of fitness valleys through division of labor and cheating in asexual populations. Sci. Rep. 2, 917 ( 10.1038/srep00917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komarova NL, Shahriyari L, Wodarz D. 2014. Complex role of space in the crossing of fitness valleys by asexual populations. J. R Soc. Interface 11, 20140014 ( 10.1098/rsif.2014.0014) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Computer code for the basic contact process and moran process is available at: https://github.com/dwodarz/Passenger-Mutation-code.