Abstract
Despite recent clinical guidelines, the optimal therapeutic strategy for the management of refractory chronic cough is still a challenge. The present systematic review was designed to assess the evidence for efficacy and safety of gabapentin in the treatment of chronic cough. A systematic search of PubMed, Embase, Cochrane Library databases, and publications cited in bibliographies was performed. Articles were searched by two reviewers with a priori criteria for study selection. Seven relevant articles were identified, including two randomized controlled trials, one prospective case-series designed with consecutive patients, one retrospective case series of consecutive patients, one retrospective case series with unknown consecutive status, and two case reports comprising six and two patients, respectively. Improvements were detected in cough-specific quality of life (Leicester Cough Questionnaire score) and cough severity (visual analogue scale score) following gabapentin treatment in randomized controlled trials. The results of prospective case-series showed that the rate of overall improvement of cough and sensory neuropathy with gabapentin was 68%. Gabapentin treatment of patients with chronic cough showed superior efficacy and a good safety record compared with placebo or standard medications. Additional randomized and controlled trials are needed.
Keywords: Gabapentin, Cough, Treatment, Review Literature as Topic, Safety
Introduction
Chronic cough is the most common disease in respiratory specialty and community clinics that impairs quality of life (QoL) and increases the economic burden1,2. The common underlying causes associated with chronic cough included asthma syndromes, gastroesophageal reflux disease, upper airway disorders, and various combinations of these diseases3. Although most patients are treated effectively, cough can persist even after extensive examination or treatment trials in some outpatients. These patients are diagnosed as unexplained chronic cough, idiopathic cough, or refractory chronic cough when there is no identifiable cause4,5. This is always a considerable challenge in clinical practice. Considering the significant medical burden of cough, it is important to establish more effective treatment of patients, especially with chronic cough.
A major hindrance for the development of chronic cough treatment is inadequate understanding of the pathophysiological mechanism of cough. Although it is induced by a variety of stimuli, cough reflex is mediated by the vagal primary afferent nerve that is distributed in the bronchial tree6 and in the extra visceral areas (main bronchus, trachea, and larynx)7. It is widely accepted that the sensitivity of the cough reflex is increased in chronic cough8,9. Meanwhile, more and more evidences showed that the sensory disorder of the laryngeal branches of vagus nerve is an important pathogenetic mechanism10,11,12,13,14,15,16,17,18. As pain and cough share the remarkably similar pathways, gabapentin, traditionally used in treatment of neuropathic pain, was recently used as a non-specifc antitussives for chronic idiopathic cough15,17,19.
Gabapentin has a similar lipophilic structure to the neurotransmitter gamma aminobutyric acid which notoriously performs central action20. Accordingly, it may also cause central nervous system side effects in the patients taking it. Systematically evaluating the evidence regarding the use of gabapentin medications in the treatment of refractory chronic cough cases may provide views about their efficacy and a better understanding of the studies related to this new therapeutic strategy. Current study is limited by a relatively small sample size, and there was no systematic review of studies regarding gabapentin antitussive therapies. Therefore, our systematic review was conducted to examine the articles concerning the use of gabapentin in the management of chronic cough. We performed the present systematic review to objectively assess the safety and efficacy of gabapentin in chronic cough so as to provide valuable references for clinical medication.
Materials and methods
1. Data sources and literature search strategy
Data searching were performed using PubMed, Embase, CBM, and Cochrane Library database by two authors independently, from their dates of inception to 1 July 2017. No language and geographical restriction was imposed. We translated non-English publications into English when they were satisfied for inclusion criteria. Search keywords used included: (cough or bronchitis or respiratory tract infections or irritable larynx or pharyngeal diseases or laryngeal diseases or postviral vagal neuropathy or central sensitisation) and (neurontin or gabapentin or gralise or neuromodulator). Unpublished data or data derived only from abstracts were not used. We also searched reference lists of all primary studies and review articles.
2. Inclusion and exclusion criteria
We used following inclusion criteria to select studies for the systematic review: (1) adults with chronic cough of unknown etiology at least 8 weeks' duration, (2) purpose of the use of gabapentin for the treatment of cough, and (3) outcome measures standard should be inclued. Exclusion criteria are as follows: (1) patients younger than 18 years; (2) nonhuman studies; (3) cough due to clear etiology like reflux disease, sinonasal pathology, allergy, pulmonary diseases, angiotensin-converting enzyme inhibitors (ACEIs) and so on; (4) studies with unclear or incomplete information.
3. Outcome measures
One or more of the following outcomes should be included: cough severity score, Leicester Cough Questionnaire (LCQ), visual analogue scale (VAS), QoL, cough severity, cough frequency, clinician assessment, urge-to-cough score, and laryngeal dysfunction score.
4. Data extraction and quality assessment
Data were selected and extracted from all of the included studies independently by two authors. Each study was noted by the major information including the reference, country, year of publication, study type, number of participants, patient age, medication regimen, follow-up time, inclusion and exclusion criteria of study, definition of effective treatment, and medication adverse effects. Two reviewers independently evaluated each relevant article and reached agreement for study inclusion. The quality of each trial was assessed by the following trial design features: (1) consecutive status of patients for non randomized controlled trails, (2) type of study outcome, (3) whether study outcome was chosen a priori, and (4) exclusion/inclusion criteria for patient enrollment. Owing to the limited numbers of studies, variability in the quality of researches, and inconsistent reporting of outcomes, no metaanalysis was attempted.
Results
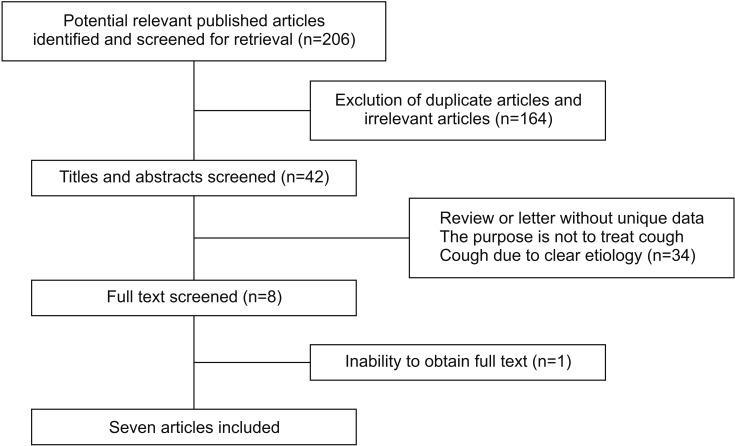
Initial data search identified a total of 206 potentially relevant articles (Figure 1). We removed 30 duplicated articles and excluded 134 after screening the title and abstracts. After reviewing the full-text articles, 34 studies were excluded, mainly because they were reviews and letters without new data or were not satisfactory for the study selection; one study was not included because of the inability to obtain full text21; we included one article which was a case report about evidence for chronic cough as a sensory vagal neuropathy, owing to it was also a cause of refractory chronic cough18; a study published in China was also included as it was a relatively complete randomized controlled trials (RCTs)22; eventually, all together seven papers were selected for further analysis, and a detailed description of these studies is provided (Table 1).
Figure 1. Flowchart for study selection.
Table 1. Study type, intervention, sample size, and patient age range of included studies.
| Study | Country (year) | Study type | No. of patients | Patient age (yr) | Intervention |
|---|---|---|---|---|---|
| Van de Kerkhove et al.23 | Belgium (2012) | Retrospective cohort, consecutive patients | 10 male, 41 female | Mean±SD 47±14 | Gabapentin 75 mg qd to 1,200 mg daily over 4 wk |
| Ryan et al.6 | Australia (2014) | Case report | 1 male, 1 female | A 61-year-old female | 1,800 mg/day for 1 mo |
| A 69-year-old male | 1,800 mg/day for 3 mo | ||||
| Bastian and Bastian24 | Retrospective case series | 12 | Range 23–80 | Median final dose 1,350 mg | |
| Ting and Na22 | China (2016) | Randomized, placebo-controlled patient blinded trial | Gabapentin: 11 male, 19 female; placebo: 8 male, 18 female | Range 18–65 | Gabapentin 300–1,800 mg/day vs. placebo for 12 wk; included a 6-day dose escalation, 8-wk treatment, a 6-day dose reduction |
| Gabapentin: mean 50.5 Placebo: mean 52.2 | |||||
| Lee and Woo14 | USA (2005) | Prospective case series, consecutive | 9 male, 17 female | Mean±SD 51.2±17.0; range 14–80; median 50.5 | Gabapentin 100–900 mg daily >4 wk, nonresponders stop at 4 wk, responders continue dose for 3 mo and then reduction |
| Ryan et al.17 | Australia (2012) | Randomized, double-blinded, placebo-controlled trial; note that patients and research staff were blinded; investigators assessing outcomes were not blinded; block randomization, sex stratified | Gabapentin: 12 male, 20 female; placebo: 10 male, 20 female | Gabapentin: mean±SD 62.7±14.0; placebo: mean±SD 60.9±12.9 | Gabapentin 300–1,800 mg/day vs. placebo for 84 days; included a 6-day dose escalation, 8-wk treatment, a 6-day dose reduction |
| Mintz and Lee15 | Canada (2006) | Case series, consecutive status unknown | 6 female | Mean 59; range 34–77 | Gabapentin 100 mg bid to 1,600 mg daily dose |
qd: once daily; bid: twice a day.
Among these two studies were placebo controlled RCTs17,22. The RCTs were placebo controlled, one study participants and researchers were blinded (investigators evaluating the outcome were not blinded)17, and the other did not reported blind method22. One study was a prospective case series design of consecutive patients14; two studies were retrospective trails23,24, one was a retrospective cohort of consecutive patients, consecutive status in the other retrospective case series was not reported. The rest of the two studies were a case report of two patients consist of one male and one female and a case series of six participants whose consecutive status were not clear6,15. Two studies from same authors were funded by the National Health and Medical Research Council of Australia6,17. The rest of the studies did not describe the financial support. All of the studies included patients with cough for at least 8 weeks (Table 2).
Table 2. Diseases excluded, follow-up, and cough duration of included studies.
| Study | Inclusion/exclusion criteria | Follow-up | Duration of cough |
|---|---|---|---|
| Van de Kerkhove et al.23 | All patients failed empirical treatment trials with proton pump inhibitors (≥6 wk of omeprazole 40 mg twice daily), nasal decongestants, (≥6-wk fluticasone 100 μg twice daily or equivalent) and inhaled steroids (≥6-wk fluticasone 250 μg twice daily or equivalent) | Mean follow-up, unknown | Median duration 48 mo |
| Ryan et al.6 | Normal spirometry and a negative response to previously trialled proton pump inhibitor, inhaled corticosteroid treatment, oral corticosteroids or nasal steroid treatment. | 1 mo | 30 mo |
| 3 mo | 96 mo | ||
| Bastian and Bastian24 | Gastroesophageal reflux disease, asthma, and allergy with no reduction of cough were included | ≥6 mo | Median 60 mo |
| Ting and Na22 | Normal spirometry and a negative response to previously trialled proton pump inhibitor, corticosteroid treatment, antitussive, SABA | NR | Gabapentin: median 32 mo; range 13–96 mo |
| Structural disease were exclued | Placebo: median 39 mo; range 11–90 mo | ||
| Lee and Woo14 | Prior workup included (not systematic or uniform across all patients): | Mean follow-up, unknown | Median 7.5 mo; range 1.5–240 mo |
| Modified barium swallow | |||
| CT | |||
| MRI | |||
| pH testing | |||
| Ryan et al.17 | Smoking | Treatment visits after 4 wk and 8 wk of treatment; additional assessment 4 wk after drug cessation | Gabapentin: median 36 mo; range 18–150 mo |
| Pulmonary disease or infection (including asthma, productive cough) | Placebo: median 48 mo; range 18–156 mo | ||
| Reflux | |||
| Postnasal drip | |||
| ACEI | |||
| Pregnant/breastfeeding | |||
| Impaired liver function | |||
| Mintz and Lee15 | Not systematic or uniform across patients: gastroesophageal reflux disease, asthma, postnasal drip exclusion mentioned in the abstract; article mentions privious workup, including bronchoscopy, upper gastrointestinal series, bronchoalveolar lavage, methacholine challenge test, serology (ANA, IgG, pertussis IgA, α-1-antitrypsin, etc.) | 12 mo | Median 7.5 mo; range 1.5–240 mo; mean±SD 31.4±57.4 |
SABA: short-acting β2 agonist; NR: not reported in study; CT: computed tomography; MRI: magnetic resonance imaging; ACEI: angiotensin-converting enzyme inhibitors; ANA: anti-nuclear antibody.
The inclusion/exclusion criteria adopted by the investigators included a negative response to empirical treatment trials with proton pump inhibitors, inhaled corticosteroid, oral corticosteroid or nasal corticosteroid treatment6,21,22; normal spirometry6,21; smoking17; modified barium swallow, computed tomography (CT), magnetic resonance imaging (MRI), pH testing14; gastroesophageal reflux disease and asthma15,17,23.
In the research of Lee and Woo14, most of the patients had received previous treatment and examination, including the treatment of gastroesophageal reflux disease, modified barium swallow, CT, MRI, and pH monitoring, but it was unclear whether these had been systematically carried out in all patients. In the study by Mintz and Lee15, all patients were treated initially with corticosteroids therapy, not every patient had been evaluated for postnasal drip but did have prior empirical reflux treatment trials and surveys for pulmonary disease in some way. Only an RCT designed by Ryan et al.17 mentioned smoking and ACEI as inclusion criteria.
Different outcome criteria were used to evaluate the clinical therapeutic effect of gabapentin (Table 3). The two RCTs used LCQ instruments, VAS and an objective cough monitor to determine cough frequency17,22. Two studies categorized treatment response based on self-reported percent improvement. A study by Lee and Woo14 simply referred to the improvement of clinical symptoms (yes or no), and if the system response was reported by patient's self-reported or physician assessment was not clear15. A cough severity score, which was correlated with the average of the four LCQ scores, was performed by Van de Kerkhove et al.23. Mintz and Lee15 evaluated the symptom response using clinician reports15. One RCT by Ryan et al.17 also used capsaicin cough challenge as a measurement to evaluate objective outcome, in which participants' cough threshold was assessed in response to the chemical tussive capsaicin.
Table 3. Outcome measure, results, and side effects of included studies.
| Study | Outcome | Result | Side effect |
|---|---|---|---|
| Van de Kerkhove et al.23 | Cough Severity score | Eight subjects discontinued during treatment due to adverse effects; eight subject did not start the treatment because of a fear of side-effects; 35 patients a mean reduction in cough severity score of 2.8 was seen | Fatigue (n=5) dizziness (n=3) 19%, nausea 9% |
| Ryan et al.6 | Leicester Cough Questionnaire (LCQ) | Improved | NR |
| Quality of life | |||
| Cough severity | |||
| Bastian and Bastian24 | Symptom response (patient report) | 10 Responded | NR |
| 69% Reduction of symptoms | |||
| Ting and Na22 | LCQ | 54 Patients improved LCQ and VAS scores, decreased cough times | Adverse events reported in 40% of gabapentin group: two patients withdrewed because of side effect; adverse events reported in 23.1% of placebo group |
| VAS | |||
| Cough frequency | |||
| Lee and Woo14 | Symptom response (unclear if clinician or patient report), yes or no | 69% Improved; variable responses depending on presence of neuropathy upon laryngeal electromyography | Dizziness or somnolence in 18% of all enrolled patients |
| Ryan et al.17 | Primary end point: cough specific quality of life | Improved cough-specific QoL scores, cough severity, cough frequency compared with placebo; no effect on capsaicin cough reflex sensitivity | Gabapentin group: 31% such as fatigue, confusion, dizziness, dry mouth, and/or nausea; headache, blurred vision, and memory loss reported in only one patient each |
| Secondary end points: cough severity, capsaicin cough reflux sensitivity, cough frequency using objective cough monitor, urge-to-cough score, laryngeal dysfunction score | Effects not sustained after treatment cessation | Placebo group: 10% | |
| Mintz and Lee15 | Clinician assessment | Three of six complete resolution, one 10% to 15% improved, one probably improved, one decreased frequency and intensity of cough | Fatigue in 17%, drowsiness in 17% |
NR: not reported in study; VAS: visual analogue scale; QoL: quality of life.
Gabapentin treatment improved symptoms of cough17. In the two RCTs improvements were seen in cough-specific QoL (LCQ score), cough severity (VAS score), and there was a reduction in cough frequency17,22. The RCT using capsaicin cough challenge showed that capsaicin cough reflex sensitivity did not change significantly with gabapentin treatment17. One study showed the rate of treatment response was 68% using a binary rating of improvement, compared with 80% in the patients with demonstrable findings of laryngeal neuropathy, while patients without evidences of motor neuropathy had only a 37.5% response rate14. In the study using clinician assessment response, half of the patients had complete remission and the remaining patients had improved to varying degree in cough15. One RCT study also evaluated clinical symptom improvement time and found the onset of action of gabapentin was within 4 weeks, and the treatment effect was maintained with maximal dosing during 8 weeks17. Mintz and Lee15 noted the duration of improvement ranged from 6 months to ongoing. The improvement in cough-specific quality of life was not sustained after discontinuing gabapentin and the LCQ score returned to baseline values17. A similar trend was also found in cough severity and cough frequency.
Adverse effects may come from gabapentin treatment of chronic cough. Reported side effects included fatigue, dry mouth, drowsiness, and dizziness14,15,17,22,23. Rates of patient-reported side effects in chronic cough patients ranged from 40% to 18% with varying degrees of severity14,15,17,22,23. In two studies, side effects was not reported6,24. One RCT found side effects in 31% of gabapentin group while 10% of patients taking placebo, including confusion, dizziness, nausea, dry mouth, fatigue, headache, blurred vision, and memory loss17. The other RCT reported adverse events in 40% of gabapentin group vs 23.1% in the placebo group, with similar side effects as Ryan et al.17 reported.
Discussion
This systematic review sought to examine evidence for efficacy and safety of gabapentin in the treatment of chronic cough. We examined the data in the current studies regarding this management strategy. There were considerable distinctions in the quality and type of studies on the treatment of chronic cough, which used gabapentin of interest. Although two RCTs were included, the quality of one study left much to be desired, and owing to the limited numbers of participants, calculation couldn't be evaluated comprehensively17,22.
The other researches included one non-controlled prospective case series of consecutive participants14, one non-controlled retrospective cohort of consecutive cases23, one retrospective case series24, and two case reports whose consecutive status is unknown6,15. Even though all included researches involved adults with chronic cough of unknown etiology at least 8 weeks' duration, there were considerable variations between preliminary investigation and previous therapy trails in these studies. Potential heterogeneity may be derived from the exclusion of some particular diagnoses, such as reflux or asthma.
Although different outcome measures were used, it was observed which benefited from gabapentin. Patients experienced improvements were seen in lower LCQ score, VAS score, and a reduction in cough frequency6,17,22,23. Additionally, over 68% cases obtained some treatment response, and more than 80% had a reduction in varying degrees in symptoms of cough14,15,22,23,24. Nevertheless, there remain some problems, in the case of the optimum dose, treatment duration, time to the best benefit, and symptom reduction rates after therapeutics. Unfortunately, only one RCT reported treatment response, which observed no improvement continuously in cough-specific quality of life after discontinuing gabapentin and the LCQ score returned to baseline values. This may indicate long-term maintenance therapy is suitable for refractory chronic cough, which should be examined by further studies. The neuromodulatory effect of gabapentin on central sensitisation (CS) was noted by one RCT, which reported patients in the treatment group with CS symptoms had noticeable improvements in LCQ score vs those without symptoms of CS17. Lee and Woo14 reported higher treatment response rate in the patients with demonstrable findings of laryngeal neuropathy than patients without evidences of motor neuropathy. A case report noted that both refractory chronic cough patients with Arnold's nerve reflex hypersensitivity were successfully treated with gabapentin6. Laryngeal irritability, such as laryngospasm and throat clearing, was attributable to cases with additional symptoms14,24. In addition, Madanick et al.21 reported cases seen in a tertiary care esophageal clinic for esophageal diseases and swallowing for chronic cough that the symptoms improved in most patients with a low-dose gabapentin, no matter the results of reflux testing. Further researches about these subgroups may provide more useful information about the gabapentin treatment on these type-specific patients.
Side effects such as fatigue/drowsiness/dry mouth/lethargy and dizziness generated early and alleviated voluntarily within a short period in the majority of patients, and only a few patients withdraw because of adverse effects17,22. Gabapentin in overdose often generates mild toxicity with clinical presentations without the need for medical treatment25,26. Further investigation into gabapentin long-period security, optimum dose, treatment duration is necessary.
There are variations on the design of study, quality of studies, medical intervention, dosage, and outcomes in included articles. Owing to these inconsistencies, a formal metaanalysis was not conducted. This systematic review showed superior efficacy and a good safety compared with placebo or standard medications in the use of gabapentin for patients with chronic cough, and further more RCTs are needed.
Footnotes
Authors' Contributions: Conceptualization: Shi G, Zhao H. Methodology: Shen Q, Zhan C, Ma J. Formal analysis: Shi G, Shen Q. Data curation: Shen Q, Zhan C. Writing - original draft preparation: Shi G, Shen Q. Writing - review and editing: Mohammed A. Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Gibson PG, Ryan NM. Cough pharmacotherapy: current and future status. Expert Opin Pharmacother. 2011;12:1745–1755. doi: 10.1517/14656566.2011.576249. [DOI] [PubMed] [Google Scholar]
- 2.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 4.Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708–715. doi: 10.1164/rccm.201007-1017CI. [DOI] [PubMed] [Google Scholar]
- 5.Pratter MR. Unexplained (idiopathic) cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):220S–221S. doi: 10.1378/chest.129.1_suppl.220S. [DOI] [PubMed] [Google Scholar]
- 6.Ryan NM, Gibson PG, Birring SS. Arnold's nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy. J Thorac Dis. 2014;6(Suppl 7):S748–S752. doi: 10.3978/j.issn.2072-1439.2014.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canning BJ, Chang AB, Bolser DC, Smith JA, Mazzone SB, McGarvey L, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest. 2014;146:1633–1648. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127:1710–1713. doi: 10.1378/chest.127.5.1710. [DOI] [PubMed] [Google Scholar]
- 9.Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24:267–271. doi: 10.1016/j.pupt.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17–21. doi: 10.1016/j.otohns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1:414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SM, Misono S. Use of specific neuromodulators in the treatment of chronic, idiopathic cough: a systematic review. Otolaryngol Head Neck Surg. 2013;148:374–382. doi: 10.1177/0194599812471817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cukier-Blaj S, Bewley A, Aviv JE, Murry T. Paradoxical vocal fold motion: a sensory-motor laryngeal disorder. Laryngoscope. 2008;118:367–370. doi: 10.1097/MLG.0b013e31815988b0. [DOI] [PubMed] [Google Scholar]
- 14.Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253–257. doi: 10.1177/000348940511400401. [DOI] [PubMed] [Google Scholar]
- 15.Mintz S, Lee JK. Gabapentin in the treatment of intractable idiopathic chronic cough: case reports. Am J Med. 2006;119:e13–e15. doi: 10.1016/j.amjmed.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Murry T, Branski RC, Yu K, Cukier-Blaj S, Duflo S, Aviv JE. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope. 2010;120:1576–1581. doi: 10.1002/lary.20985. [DOI] [PubMed] [Google Scholar]
- 17.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 18.Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J Voice. 2011;25:596–601. doi: 10.1016/j.jvoice.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Fan H, Yu W, Zhang Q, Cao H, Li J, Wang J, et al. Efficacy and safety of gabapentin 1800 mg treatment for post-herpetic neuralgia: a meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2014;39:334–342. doi: 10.1111/jcpt.12167. [DOI] [PubMed] [Google Scholar]
- 20.Kimos P, Biggs C, Mah J, Heo G, Rashiq S, Thie NM, et al. Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain. 2007;127:151–160. doi: 10.1016/j.pain.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Madanick R, Sigmon L, Ferrell K, Shaheen N, Dellon E. Gabapentin for the treatment of chronic cough: a novel approach to treating a challenging clinical problem. Am J Gastroenterol. 2012;107(Suppl 1):S27–S28. [Google Scholar]
- 22.Ting L, Na C. The efficiency and safety of gabapentin in the treatment of pertinacious chronic cough. Int Med Health Guid News. 2016;22:665–668. [Google Scholar]
- 23.Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, Dupont LJ. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9. doi: 10.1186/1745-9974-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastian ZJ, Bastian RW. The use of neuralgia medications to treat sensory neuropathic cough: our experience in a retrospective cohort of thirty-two patients. PeerJ. 2015;3:e816. doi: 10.7717/peerj.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer JH, Barr AN, Rogers SL, Fischer PA, Trudeau VL. Lack of serious toxicity following gabapentin overdose. Neurology. 1994;44:982–983. doi: 10.1212/wnl.44.5.982. [DOI] [PubMed] [Google Scholar]
- 26.Verma A, St Clair EW, Radtke RA. A case of sustained massive gabapentin overdose without serious side effects. Ther Drug Monit. 1999;21:615–617. doi: 10.1097/00007691-199912000-00006. [DOI] [PubMed] [Google Scholar]