Abstract
Introduction
The purpose of this study is to explore 13 cytokine predictors of chemotherapy-related cognitive impairment (CRCI) in breast cancer survivors (BCS) 6 months to 10 years after chemotherapy completion using a multivariate, non-parametric approach.
Methods
Cross sectional data collection included completion of a survey, cognitive testing, and non-fasting blood from 66 participants. Data were analyzed using random forest regression to identify the most significant predictors for each of the cognitive test scores.
Results
A different cytokine profile predicted each cognitive test. Adjusted R2 for each model ranged from 0.71–0.77 (p’s < 9.50−10). The relationships between all the cytokine predictors and cognitive test scores were non-linear.
Conclusions
Our findings are unique to the field of CRCI and suggest non-linear cytokine specificity to neural networks underlying cognitive functions assessed in this study.
Keywords: chemotherapy-related cognitive impairment, cytokines, machine learning, random forest regression, breast cancer survivors
Graphical Abstract
1.0 Introduction
As many as 78% of breast cancer survivors (BCS) report ongoing cognitive changes after breast cancer treatment is completed (Janelsins et al., 2014, Wefel et al., 2015). For some BCS, these changes can last decades after treatment (Koppelmans et al., 2012, Amidi et al., 2015). Typically, cognitive dysfunction occurs in the domains of memory, attention, processing speed, and executive function (Janelsins et al., 2014, Ono et al., 2015, Wefel et al., 2015). Collectively, dysfunction in these domains will be referred to in this article as chemotherapy-related cognitive impairments (CRCI). CRCI are most often attributed to chemotherapy toxicity, however, several other mechanisms have been proposed, including the indirect neurotoxic effects of cytokine dysregulation initiated by cancer pathologies and perpetuated by cancer treatments (Ahles and Saykin, 2007, Janelsins et al., 2014, Vardy et al., 2008).
Both the pathology of cancer and cancer treatment can elevate peripheral pro-inflammatory cytokines in women with breast cancer (Patel et al., 2015, Seruga et al., 2008, Kesler et al., 2013a, Cheung et al., 2013). Evidence from animal and human research indicate that high levels of circulating inflammatory cytokines can access the brain and cause damage, resulting in behavioral symptoms including depression, sleep problems, fatigue, and cognitive dysfunction (Cheung et al., 2013, Miller et al., 2008, Pomykala et al., 2013, Seruga et al., 2008, Palesh et al., 2012). Prolonged exposure to such cytokines can have neurodegenerative effects on the brain. A link between inflammatory cytokines and CRCI has been made in BCS (Cheung et al., 2015, Pomykala et al., 2013, Kesler et al., 2013a, Janelsins et al., 2012, Patel et al., 2015, Vardy et al., 2017, Ganz et al., 2013, Williams et al., 2018); but the evidence is heterogeneous in regards to specific cytokines and cognitive outcomes being measured, and the magnitude and directions of the relationships between inflammatory and cognitive variables in these studies.
This heterogeneity of study findings could be a result of the limitations in the methods that have been employed to understand the complex relationships between cytokines and behaviors. Cytokines are involved in many aspects of the innate and adaptive immune systems, and their concentrations are influenced by both upstream and downstream factors such as other chemokines, circulating hormones, circadian rhythms, and receptor inhibition (Hunter and Jones, 2015). Furthermore, evidence suggests that relationships between biological factors and cognitive function in cancer survivors are not linear (Kesler et al., 2016), therefore; statistical methods that do not assume linearity are preferable for studying these relationships. The vast majority of research conducted in this area have utilized univariate methods (e.g. linear modeling) to examine cytokine predictors of CRCI. We know that CRCI likely arise from a large number of varying and interacting factors that require multivariate statistical methods (Kesler et al., 2017a). Additionally, if the true relationships between cytokines and cognitive outcomes is in fact complex, multivariate, and non-linear, then an alternative mathematical approach is necessary to identify predictors of CRCI.
Our group was among the first to employ machine learning to the study of neuroimaging biomarkers of CRCI in cancer survivors (D'Agata et al., 2013, Kesler et al., 2013b, Hosseini and Kesler, 2014, Kesler et al., 2017a, Kesler et al., 2017d) and survivors of ALL (Kesler et al., 2016). Multivariate random forest regression (RFR) is a non-linear/non-parametric machine learning approach that inherently models complex interactions among predictors (Breiman, 2001). We have demonstrated that RFR is superior to traditional linear modeling in predicting CRCI from neuroimaging biomarkers in BCS (Kesler et al., 2017b), but to our knowledge, RFR has not been utilized to evaluate cytokine predictors of CRCI in BCS. This study aims to explore cytokine predictors of CRCI in BCS 6 months to 10 years after chemotherapy completion using non-parametric modeling. We hypothesized that cytokines would be significant predictors of cognitive performance and that the relationships between the various cytokines and cognitive outcomes would be non-linear.
2.0 Materials and Methods
2.1 Participants
BCS who completed chemotherapy 6 months to 10 years prior were recruited through community oncology centers, a breast cancer patient and survivor navigation center, the local chapter of the Oncology Nursing Society, and the Army of Women (Dr. Susan Love Research Foundation). All participants were female, had a history of nonmetastatic breast cancer, and had been without cancer recurrence or secondary cancers for six months to 10 years prior to study enrollment. Women between the ages of 21 to 65 years old, able to read and write in English, and of any ethnic or racial group were included. Women over age 65 were excluded to control for age related cognitive decline (Aartsen et al., 2002). Women on systemic steroids, and/or with a history of inflammatory comorbidities (i.e. arthritis, DM II, autoimmune diseases) which can impact cytokine concentrations, were excluded. Women with a pre-cancer history of sleep apnea, severe insomnia, severe cognitive impairments (diagnosed by a physician), a verbal learning disability, or other neurological or psychiatric disorders that can impact cognition or emotions and interfere with completion of surveys and cognitive testing were excluded. Women currently receiving hormonal therapy for their breast cancer were not excluded. This study was approved by the Institutional Review Board at the University of Texas at Austin and in accordance with the Declaration of Helsinki. All participants provided written, informed consent.
2.2 Demographics and Clinical Measures
A self-report questionnaire was used to collect demographic information (age, education, race, ethnicity, marital status, income, employment) and disease and treatment information (type of breast cancer, type of cancer treatments, date of chemotherapy completion, current medications, comorbidities, menopausal status, and genetic testing information). BMI was measured because cytokines such as IL- 6, TNF-α, IL-8, IL-18, and IL-1ra are consistently associated with obesity indices in large samples (O'Connor et al., 2009). Height was measured to the nearest 0.5 cm with participants’ back to the wall, without shoes, looking straight ahead. All participants were weighed with the same digital scale (Tanita Model WB-300 Plus Arlington Heights, Illinois) to the nearest 100 g, wearing no shoes and light clothing. BMI was calculated using weight in kilograms and height in cm.
2.3 Self Report Measures
Psychosocial Factors
Several psychosocial factors that have been linked to CRCI in BCS including depressive symptoms, fatigue, anxiety (Bower and Lamkin, 2013, Cheung et al., 2013, Shilling et al., 2006, Vardy, 2009) perceived stress, sleep quality, and loneliness (Henneghan et al., 2017, Reid-Arndt and Cox, 2012) were evaluated with various self-report measures. Emotional distress was measured using two Patient-Reported Outcomes Measurement Information System (PROMIS) scales, the Emotional Distress – Anxiety – Short Form 8a and Emotional Distress – Depression–Short Form 8a (Cella et al., 2010). Fatigue was measured using the PROMIS Fatigue Short form 8a, an 8 item scale that asks how frequently a person experienced fatigue related feelings in the past seven days. Answer choices for all 3 PROMIS scales range from 1 (“never”) to 5 (“always”) and total scores can range from 8–40 with higher scores indicated more depressive, anxiety, or fatigue related symptoms.
Perceived stress was measured using the Perceived Stress Scale (Cohen et al., 1983), a 10-item scale measuring the degree that life circumstances are appraised as having been stressful in the previous week. Responses for each item range from 0 (“never”) to 4 (“very often”). Loneliness was measured using the UCLA-R Loneliness Scale version 3 Survey (Russell, 1996). This 20-item instrument quantifies how people experience their loneliness. Each item asks how often each feeling is experienced— answer choices are “never”, “rarely”, “sometimes”, and “always” (Russell, 1996). Higher scores indicate more loneliness and perceived isolation. Sleep quality was measured using the Pittsburgh Sleep Quality Index (Buysse et al., 1989), a self-administered 19-item questionnaire that evaluates 7 components of sleep: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction. Scores can range from 0 to 21 with higher scores indicating worse sleep quality. Daytime sleepiness was also measured with the Epworth Sleepiness Scale, an 8-item instrument, with each item response choices are 0 to 3, with higher scores indicating greater daytime sleepiness (Enderlin et al., 2011).
Perceived Cognitive Function
Perceived cognitive function was measured with the Functional Assessment of Cancer Therapy-Cognitive Function Instrument version 3 (FACT-Cog), a 34-item self-administered questionnaire that measures how often specific dysfunctions have been experienced in the last 7 days (Von Ah and Tallman, 2015, Cheung et al., 2014). Total scores can range from 0 to 148; higher scores indicate better cognitive function and quality of life.
2.4 Cognitive Function Assessment
The International Cognition and Cancer Task Force recommends that the following well-established, valid, and reliable cognitive measures be used in CRCI research-- Hopkins Verbal Learning Test Revised (a measure of immediate, [HVLT-I], and delayed verbal memory,[HVLT-D], (Benedict et al., 1998)), Trail Making Test A (Trails A) and Trail Making Test B (Trails B, measures of processing speed, executive function, attention, and cognitive flexibility (Tombaugh, 2004)), and Controlled Oral Word Association Test (COWA, a measure of verbal fluency and word finding (Benton et al., 1983)). Standardized testing protocols were used when administering these measures. Two trained research staff scored tests separately. Raw test scores were used in the analyses.
2.5 Data Collection Procedures
Potential participants were screened for eligibility via telephone and those who were eligible provided verbal consent. Written consent forms were then mailed or emailed to participants along with a link to the online survey and scheduled for in-person data collection. Data collected for this study included survey responses, anthropometric measures, standardized cognitive tests, and blood samples. Twenty-four to 48 hours prior to the data collection appointments, participants were emailed a link to the structured questionnaire including demographic and clinical questions and all self-report instruments. Face-to-face data collection included anthropometric assessment, standardized cognitive testing, and a blood draw. Appointments were scheduled between 1 and 4 hours of participants waking in the morning. Cognitive tests were administered according to standard procedures and blood draws completed at the end of the appointment.
2.6 Blood Collection and Assay Procedures
Non-fasting blood samples (10 ml) were drawn for all participants between 1–4 hours of waking. Blood was collected into serum separator tubes (BD Franklin Lakes, New Jersey) and allowed to clot at room temperature for 30 min – 2 hr, then transported (in a portable cooler that maintained room temperature) to the University of Texas at Austin School of Nursing BioBehavioral lab. In the lab, the samples were centrifuged at 3,330 rpm for 15 min, then serum was aliquoted using filtered pipettes into 1ml polypropylene tubes and stored at −80°C until subsequent batch analyses. The inflammatory cytokines in this study were chosen based on evidence supporting their associations with CRCI in BCS, especially Interleukin 6 (IL-6) and Tumor Necrosis Factor alpha (TNF-α)(Kesler et al., 2013a, Ganz et al., 2013, Pomykala et al., 2013, Lyon et al., 2016).
Human high sensitivity T cell magnetic, premixed, multiplex assays were purchased from EMD Millipore (EMD Millipore; Darmstadt, Germany). The assay kits included 13 analytes, including IL-6 and TNF-α, and others involved in different aspects of the inflammatory process. The cytokines included in the analyses for this manuscript were the only ones evaluated. The HSTCMAG28SPMX13 multiplex assay kits used for this study were purchased with reagents pre-mixed for the 13 cytokines measured. These cytokines are key components representative of multiple aspects of the inflammatory response in that some are pro-inflammatory (IL-6, TNF-α, IL-1β), while others are anti-inflammatory (IL-10, IL-4), or chemokines (IFN-γ, GM-CSF). The availability of multiplex technology allows for multiple analytes to be assayed simultaneously, which saves significant time, money, and sample relative to the more traditional approach using ELISA kits.
Since this was an exploratory study using a distinct data analysis approach, we chose to include all 13 analytes in the analyses— including IL-6, TNF-α, Interferon gamma (IFN-g), Granulocyte-macrophage colony-stimulating factor (GM-CSF), Interleukin 10 (IL-10), Interleukin 12 (IL-12p10), Interleukin 13 (IL-13), Interleukin 1 beta (IL-1β), Interleukin 2 (IL-2), Interleukin 4 (IL-4), Interleukin 5 (IL-5), Interleukin 7 (IL-7), and Interleukin 8 (IL-8). Standards, controls, and samples were added, in duplicate, to 96-well plates and analyzed according to manufacturer’s recommendations. Plates were then read using a Luminex 200 (Luminex Corporation; Austin, TX) to determine mean fluorescent intensity for each well. Five-parameter logistic regression was used to construct a standard curve, which was then used to calculate control and sample unknown concentrations. R2 for the standard curves ranged from 0.998 – 1, all controls were within the range supplied by the manufacturer, and coefficient of variation between sample duplicates averaged 6%.
2.7 Data Analysis Plan
Data were analyzed with SPSS 23.0 (IBM) and R Studio (R Foundation). Continuous variables are presented as mean and standard deviation (SD), and discreet variables as frequencies and percentages. The primary analysis for this study began with examining relationships between the cytokines and cognitive outcomes using simple scatter plots and lines of best fit functions, which revealed non-linear patterns. Therefore, we conducted RFR (Breiman, 2001) to predict cognitive test scores. RFR uses random subsets of features (i.e. independent variables) to create an ensemble of decision trees that predict a continuous outcome (Breiman, 2001, Breiman et al., 1984). The outcome is predicted at each bootstrap iteration using data not included in that bootstrap sample (i.e. “out-of-bag” sample) and therefore, random forest models include an intrinsic cross-validation. The out-of-bag predictions are aggregated to determine an error rate (Liaw and Wiener, 2002) and model accuracies based on out-of-bag cross-validation have been shown to be comparable to those derived from cross-validation methods using separate test samples (Breiman, 1996, Kesler et al., 2017c). RFR is relevantly robust to over-fitting, and an appropriate choice when the sample size is small and/or there are a large number of predictors (Breiman, 2001).
All of the cytokines, self-reported psychosocial variables, BMI, years of education, age, and time since chemotherapy were used as RFR features. The psychosocial variables were combined using Principal Component Analysis (Henderson et al., 1990) consisting of the total scores for PROMIS scales, Perceived Stress Scale, UCLA-R Loneliness Scale, and Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale. The first component score from the within class pooled variance-covariance matrix was chosen to establish the coefficients for the composite scores, and this component accounted for 54% of the total variance of the 7 self-report measures.
Separate models were tested with each of the cognitive outcomes as dependent variables (Table 1). A Bonfereroni adjustment was made to the p values for the RFR model testing (0.05/5= p<.01) to limit Type I error. All models remained significant after the adjustment was made. Feature importance was determined using the increase in mean squared error which reflects the increase in error of the model if the feature was randomized. Therefore, higher values indicate greater importance of that variable to the model. The ability to quantify feature importance is another advantage of random forest models compared to alternate machine learning approaches. Partial dependence plots were created for the most important predictors to illustrate the shape of the relationship between the feature and the outcome. All RFR data analyses were performed in the R Statistical Package (R Foundation) including the “randomForest”, “caret” and "Hmisc" libraries.
Table 1.
Defined Features for each Random Forest Classification Model and Dependent Variables
| Dependent Variables | Defined Variables |
| HVLT_i | Hopkins Verbal Learning Test Immediate Recall |
| HVLT_d | Hopkins Verbal Learning Test Delayed Recall |
| COWAT | Controlled Oral Word Association Test |
| TrailA | Trail Making Test A |
| TrailB | Trail Making Test B |
| Factcog | FACT-Cog Version 3 total score |
| Features | Defined Variable |
| Age | Age in years |
| Ed | Years of completed formal education |
| TimeTx | Time in months since completion of chemotherapy treatment |
| BMI | Body mass index |
| IL_6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor alpha |
| GM_CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN_g | Interferon gamma |
| IL_10 | Interleukin 10 |
| IL_12p70 | Interleukin 12 |
| IL_13 | Interleukin 13 |
| IL_1β | Interleukin 1 beta |
| IL_2 | Interleukin 2 |
| IL_4 | Interleukin 4 |
| IL_5 | Interleukin 5 |
| IL_7 | Interleukin 7 |
| IL_8 | Interleukin 8 |
| Psych | Composite score for all self-report measures (PROMIS Anx, PROMIS Dep, PROMIS Fat, PSS, UCLA-R, PSQI, ESS) |
Abbreviations: PROMIS Anx: PROMIS Emotional Distress – Anxiety – Short Form 8a, PROMIS dep: PROMIS Emotional Distress – Depression–Short Form 8a, PROMIS fat: PROMIS Fatigue Short form 8a, PSS: Perceived Stress Scale, UCLAR: UCLA-R Loneliness Scale, PSQI: Pittsburgh Sleep Quality Index, ESS: Epworth Sleepiness Scale
3.0 Results
3.1 Sociodemographics and treatment characteristics
One hundred and nine women were screened for this study. Of these, 21 did not meet study inclusion criteria due to the following factors (stage IV breast cancer, chemotherapy outside of outlined timeframe, history of inflammatory comorbidities or other comorbidities that interfere with normal cognitive processes, older than 65 years, and systemic steroid treatment) and 13 either lived too far away or were unable to be scheduled for appointments. Seventy-five women were enrolled in the study from April 2016— January 2017, and blood samples were obtained from 66 participants. The final sample used for this study consisted of 66 women. The sample was on average 49 years of age (SD 8.77). The majority were white (93.4%), non-Hispanic (95.5%), college educated (80.3%), partnered (69.7%), and working full or part time (86.4%). The majority had a history of stage II or III (81.8%) invasive ductal carcinoma breast cancer (69.7%) that was hormone receptor positive (84.8%). Almost all were treated with surgery (98.5%); the majority had radiation therapy (60.6%) in addition to anthracycline-based chemotherapy (56.1%) and some type of hormonal therapy (84.6%) and 66.7% were currently on hormonal therapy. Approximately 71.2% were post-menopausal. On average, the women in the sample had completed chemotherapy three years prior (37 months ± 27.67 months).
3.2 Inflammatory and cognitive characteristics
The raw scores for cytokine concentrations (pg/ml) were skewed and unevenly distributed, however, RFR modeling is non-parametric; therefore, raw scores were utilized in the multivariate modeling. Similarly, raw cognitive test scores were used in data analyses. Descriptive statistics for the key study variables are displayed in Table 2.
Table 2.
Descriptive Statistics for all Study Variables (N=66)
| Variable | Mean (SD) | Min, Max |
|---|---|---|
| Age | 49 (8.77) | 27, 65 |
| Years of Education | 16.7 (2.16) | 12, 22 |
| Months since Chemotherapy | 35.7 (27.12) | 6.83, 120.84 |
| BMI | 27.36 (5.46) | 18.62, 42.46 |
| IL-6 (pg/mL) | 2.25 (1,80) | 0.05, 7.62 |
| TNF- α (pg/mL) | 5.91 (1.40) | 2.98, 10.09 |
| GM-CSF (pg/mL) | 129.54 (182.97) | 4.13, 1000.00 |
| IFN-g (pg/mL) | 7.80 (6.21) | 0.49, 28.23 |
| IL-10 (pg/mL) | 10.15 (9.34) | 1.22, 48.40 |
| IL-12p70 (pg/mL) | 2.77 (1.77) | 0.21, 9.00 |
| IL-13 (pg/mL) | 11.40 (29.72) | 0.11, 214.63 |
| IL-1β (pg/mL) | 1.02 (0.60) | 0.24, 4.52 |
| IL-2 (pg/mL) | 1.21 (0.87) | 0.21, 4.52 |
| IL-4 (pg/mL) | 21.15 (13.93) | 7.32, 81.51 |
| IL-5 (pg/mL) | 2.93 (7.40) | 0.10, 55.36 |
| IL-7 (pg/mL) | 7.69 (2.71) | 2.85, 14.39 |
| IL-8 (pg/mL) | 5.75 (6.51) | 0.20, 48.70 |
| HVLT Immediate Recall | 29.80 (3.60) | 21, 36 |
| HVLT Delayed Recall | 10.61 (1.47) | 6, 12 |
| COWAT | 40.18 (11.29) | 16, 71 |
| Trail Making Test A | 26.36 (9.17) | 11.5, 54 |
| Trail Making Test B | 56.72 (23.03) | 26.5, 179.5 |
| FACT- Cog Total^ | 94.99 (34.87) | 19, 147 |
= Fact Cog Total: lower scores indicate lower overall functioning
Abbreviations: IL-6: Interleukin 6, TNF-α: Tumor Necrosis Factor alpha, GM-CSF: Granulocytemacrophage colony-stimulating factor, IFN-g: Interferon gamma, IL-10: Interleukin 10, IL-12p10: Interleukin 12 p 10, IL-13: Interleukin 13, IL-1β: Interleukin 1 beta, IL-2 Interleukin 2, IL-4: Interleukin 4, IL-5: Interleukin 5, IL-7: Interleukin 7, IL-8: Interleukin 8; FACT-Cog Total: Functional Assessment of Cancer Treatment- Cognition Version 3, HVLT: Hopkins Verbal Learning Test, COWA: Controlled Oral Word Association
3.3 Cytokine Predictors of Cognitive Function
Bivariate Analyses
Initially we approached this analysis by looking at linear relationships between the individual cytokines and cognitive outcomes. Some small correlations were seen between Trails A, HVLT-I and FACT-Cog and some of the cytokines; however, these relationships were not significant after a Bonferroni adjustment was made to the p values to control for Type 1 error. Bivariate correlations are displayed in Supplementary Table 1. Next, scatter plots and lines of best fit were used to visualize the nature of the relationships between each of the cytokines and the cognitive outcomes. These plots illustrated that the relationships between the cytokines and cognitive outcomes were non-linear. Examples of 2 of these plots are displayed in Supplementary Figure 1.
Random Forest Regression Models
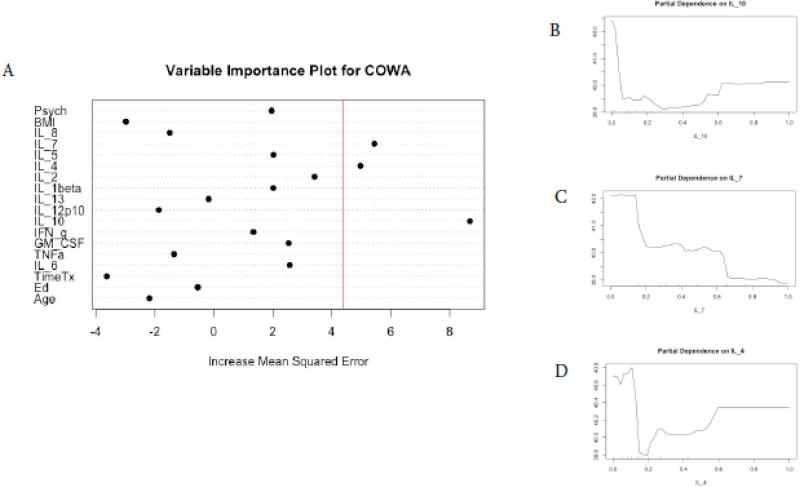
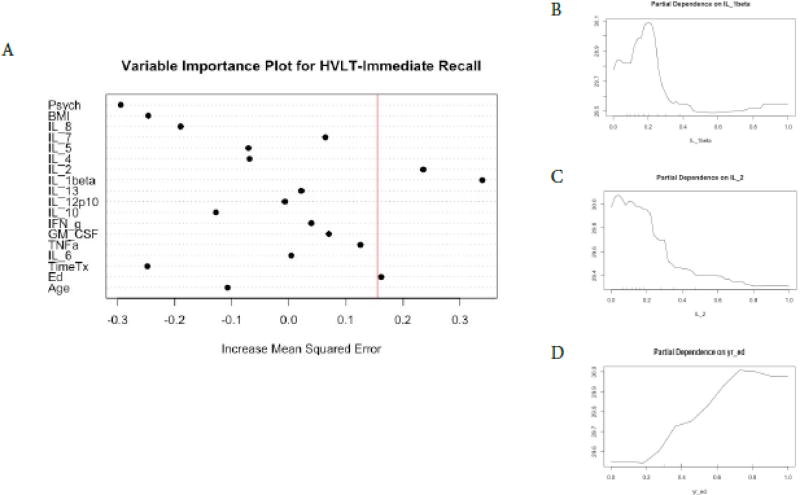
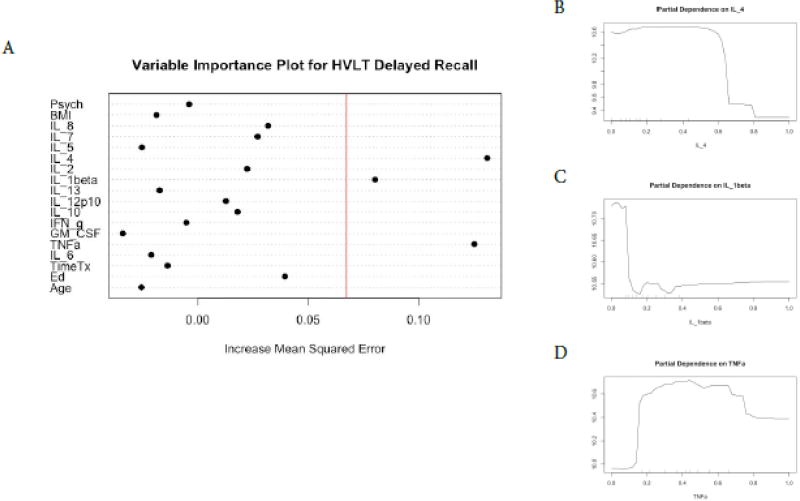
We found a different profile of cytokine predictors for each of the cognitive tests and no cytokine predictors for FACT-Cog scores (Figures 1–6). In terms of COWA performance, the RFR adjusted R squared = 0.72, F = 10.07, and p = 1.17−10. The most important variables in descending order were IL-10, IL-7, and IL-4 (Figure 1, panel A). Favorable performance on COWA (greater number of words) were associated with levels of IL-10 <0.1 pg/ml, IL-7 levels less than 0.18 pg/ml, and IL-4 levels < 0.14 and > 0.6 pg/ml (Figure.1, panels B–D). RFR for HVLT-I performance, the adjusted R squared=0.71, F= 10.02, and p=1.27−10. The most important variables in descending order were IL-1β, IL-2, and years of education (Figure 2, panel A). Favorable performance on HVLT-I (more recalled words) was associated with IL-1β levels between 0.1– 0.25 pg/ml and IL-2 < 0.2 pg/ml, and there was a linear relationship between years of education and HVLT-I (Figure 2, panel B–D). For HVLT-D performance, the RFR adjusted R squared=0.75, F=11.80, and p=7.34−12. The most important variables in descending order were IL-4, IL-1β, TNF-α (Figure 3, panel A) Favorable performance on HVLT-D (more recalled words) was associated with IL-4 < 0.6 pg/ml, IL-1β < 0.1 pg/ml, and TNF-α levels between 0.2 – 0.7 pg/ml (Figure 3, panels B–D).
Figure 1. Random Forest Regression with COWA as Dependent Variable.
The Variable importance plot (A) for COWA scores illustrates that IL-10, IL-7, and IL-4 have the highest Increased Mean Squared Error in the model and therefore are the most important features for the model predicting COWA scores. The red line represents the a prior threshold (1 SD above the mean) for determining the most important model features. The partial dependence plots for COWA scores on IL-10 levels (B), on IL-7 (C), and on IL-4 (D) illustrate the marginal effect of the these cytokines on COWA scores after partialling out the influence of all other variables in the model (Y-axis in pane "A").
Figure 2. Random Forest Regression with HVLT-Immediate as Dependent Variable.
The Variable importance plot (A) for HVLT-I scores illustrates that IL-1beta has the highest Increased Mean Squared Error in the model, followed by IL-2, IL-1beta, and years of education. The red line represents the a prior threshold (1 SD above the mean) for determining the most important model features. The partial dependence plot for HVLT-I scores on IL-1beta levels (B) and IL-2 levels (C) illustrate the marginal effect of the these cytokines on HVLT-I scores after partialling out the influence of all other variables in the model. Years of education and HVLT-I are linearly related (d) between 0.2 and 0.8 years of education.
Figure 3. Random Forest Regression with HVLT-Delayed as Dependent Variable.
The Variable importance plot (A) for HVLT-D scores illustrates that IL4 IL-1beta and TNF-alpha have the highest Increased Mean Squared Error in the model and therefore the most important predictors of HVLT-D scores. The red line represents the a prior threshold (1 SD above the mean) for determining the most important model features. The partial dependence plot for HVLT-D scores on IL-4 levels (B), IL-1beta (C), and TNF-a (D) illustrate the marginal effect of the these cytokines on HVLT-D scores after partialling out the influence of all other variables in the model.
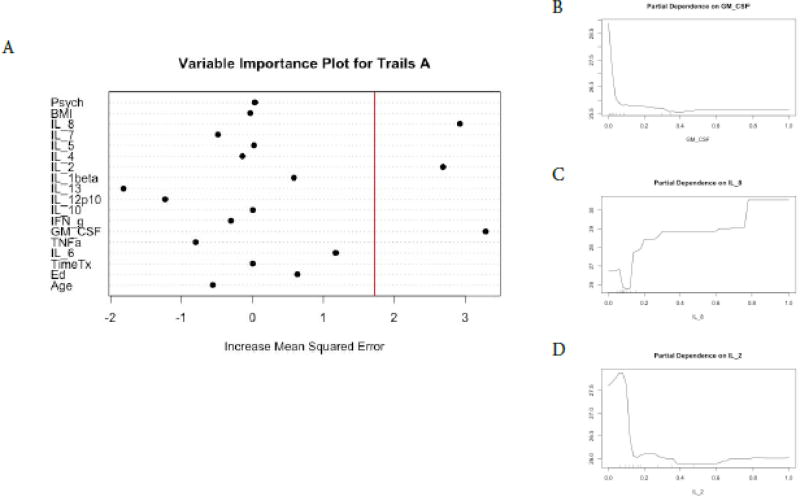
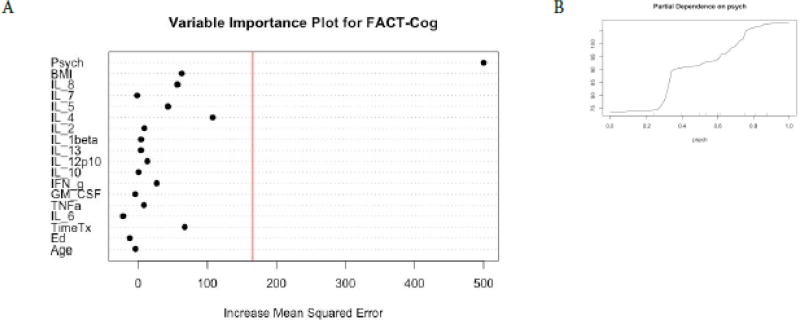
For Trails A performance, the RFR adjusted R squared = 0.72, F = 10.43, p =6.44−11. The most important variables in descending order were GM-CSF, IL-8, and IL-2 (Figure 4, panel A). Favorable performance on Trails A (less time to complete test) was associated with GM-CSF < 0.5 pg/ml, IL-2 < 0.1 pg/ml, and IL-8 > 0.8 pg/ml (Figure 4, panels B–D). For Trails B performance, the RFR adjusted R squared = 0.77, F = 8.89 p = 9.50−10. The most important variables in descending order were IL-1β and IL-2 (Figure 5, panel A). Favorable performance on Trails B (less time to complete test) was associated with IL-1beta < 0.1 pg/ml and IL-2 > 0.1 pg/ml (Figure 5, panels B and C). For FACT-Cog scores, the RFR adjusted R squared = 0.86, F = 23.68, p < 0.0001 and the psychosocial composite score identified as the most important feature to the model (Figure 6,). Individual relationships were examined between the instruments originally used for the PCA analyses to produce the psychosocial composite scores and FACT-Cog scores. These moderate to strong inverse relationships (R’s −0.43–0.67, p’s <0.0001) suggest favorable FACT-Cog scores are associated with lower levels of psychosocial symptoms.
Figure 4. Random Forest Regression with Trails A as Dependent Variable.
The Variable importance plot (A) for Trails A scores illustrates that GM-CSF, IL-8, and IL-2 have the highest Increased Mean Squared Error in the model and therefore the most important predictors of Trails A scores. The red line represents the a prior threshold (1 SD above the mean) for determining the most important model features. The partial dependence plot for Trails A scores on GM-CSF levels (B), IL-8 levels (C), IL-2 levels (D) illustrate the marginal effect of these cytokines on Trails A scores after partialling out the influence of all other variables in the model.
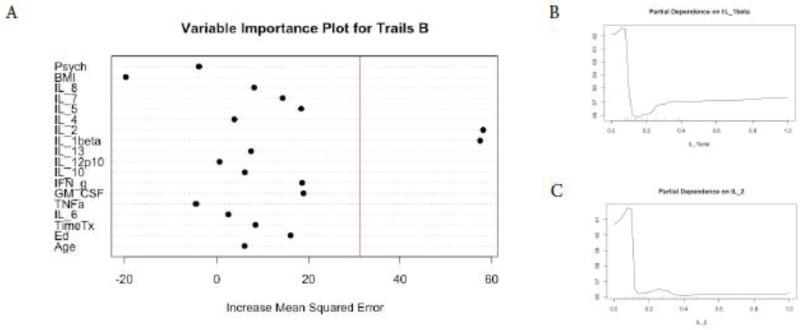
Figure 5. Random Forest Regression Trails B as Dependent Variable.
The Variable importance plot (A) for Trails B scores illustrates that IL-1 beta and IL-2 have the highest Increased Mean Squared Error in the model and therefore the most important predictors of Trails B scores. The red line represents the a prior threshold (1 SD above the mean) for determining the most important model features. The partial dependence plot for Trails B scores on IL-1 beta levels (B) IL-2 levels (C) illustrate the marginal effect of these cytokines on Trails B scores after partialling out the influence of all other variables in the model.
Figure 6. Random Forest Regression with FACT-Cog as Dependent Variable.
The Variable importance plot (A) for Fact-Cog total scores illustrates that none of the cytokine features were important variables in predicting FACT-Cog scores. The red line represents the a prior threshold (1 SD above the mean) for determining the most important model features. The psychosocial composite score was the most important feature in this model. A partial dependence plot for Psych composite scores (B).
4.0 Discussion
We conducted exploratory analyses using preliminary data in an observational study evaluating serum based cytokine predictors of neuropsychological functioning in 66 BCS. Interesting patterns emerged from the data suggesting that unique cytokine algorithms are associated with each of the cognitive domains in this population (measured by cognitive test performance) and that the relationships between the individual cytokines and cognitive test scores were non-linear. Our findings support a growing body of research that links pro-inflammatory cytokines to both cognitive performance (Ganz et al., 2013, Lyon et al., 2016, Patel et al., 2015, Janelsins et al., 2012, Williams et al., 2018) and neuroimaging changes (Kesler et al., 2013a, Pomykala et al., 2013) in BCS with CRCI.
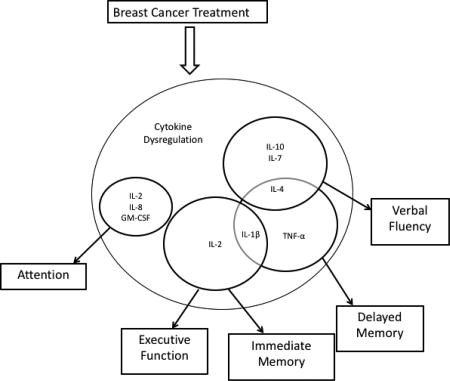
Our findings suggest that specific cytokines are the most important predictors of cognitive performance and vary depending on cognitive domain assessed, even when entering BMI, age, psychological variables, and time since end of chemotherapy into the models. We found associations between IL-2, IL-4, IL-1β, TNF-α and verbal memory, both immediate and delayed recall, expanding on findings reported in other studies linking TNF-α (Cheung, 2014, Kesler et al., 2013a), IL-7 and IL-5 (Lyon et al., 2016) to memory performance in BCS and sTNFRI and sTNFRII to visual memory in breast cancer patients(Williams et al., 2018). Our findings suggest that IL-2, IL-4, IL-1β may also be important to memory performance in BCS and that specific concentrations of these cytokines are optimal.
We also found links between GM-CSF, IL-2, IL-8, IL-1β and executive functioning in this sample of women expanding on other study findings linking IL-6, IL-4, and IL-8 to executive attention and IL-1β to processing speed in BCS (Cheung, 2014, Janelsins et al., 2012). Furthermore, our findings suggested that lower levels of GM-CSF, IL-2, IL-1β, and higher levels of IL-8 are optimal for executive functioning. We also observed unique associations between lower levels of IL-10, IL-7, and IL-4 and better verbal fluency in this sample. Cognitive domains are associated with various brain regions in the brain. It is possible that cytokine concentrations vary across brain regions, or that cytokine receptors are specific to different brain regions.
In animal models, cytokine receptors have been found in discrete brain regions (Arisi, 2014, Scheinert et al., 2015). The hippocampus and temporal lobes are integral regions of memory networks and hippocampal neurons possess IL-1β receptors in adult mice (Arisi, 2014). Additionally, specific cytokines, including IL-1β, IL-10, IL-6, and IL-4, have been shown to circulate in the frontal cortex (part of the executive function networks) in rats (Scheinert et al., 2015). In animal models, IL-4 accumulation in the meninges has been linked to performance on memory tasks, suggesting that IL-4 impacts cognitive function through meningeal cells specifically (Derecki et al., 2010, Gadani et al., 2012).
The mechanisms by which peripheral inflammation interferes with neural processing in humans are not fully established (Thomson et al., 2014), but there is a body of research that suggests ongoing cross talk between the peripheral immune system and the brain (Patterson, 2015). It is possible that the neural impact of cytokines is more local rather than global, and that certain cytokines interfere with specific functional networks in the brain. More neurogenesis occurs in the hippocampus compared to other areas of the brain possibly making this area and the associated functional networks vulnerable to the neurotoxic effects of inflammation. Links have been established between IL-1β and neuronal death in the hippocampus (Ryan et al., 2013). A study conducted on postmortem brains of persons with various dementia types revealed regional variations in cytokines and chemokines in the brains of those who had a history of neurodegeneration compared to controls suggesting region specific concentrations of various cytokines including C-reactive protein, IL-1β, IL-6, IL-7, IL-8, and IL-16 (Chen et al., 2016). Together, these findings suggest that there are cytokine-specific neural pathways in the brain that interfere with cognitive functional networks, but more research is needed.
Contrary to findings by Ganz and colleagues (Ganz et al., 2013) and Janelsins et al. (Janelsins et al., 2012) we did not find any significant cytokine predictors of perceived cognitive functioning in our sample. As an ad hoc analysis, we tested linear models of self-report cognitive function and cytokines and found no significant effects. Our RFR model indicated that psychosocial functioning was the most important variable associated with perceived cognitive functioning, congruent with previous work (Bower and Lamkin, 2013, Cheung et al., 2014, Janelsins et al., 2016, Reid-Arndt and Cox, 2012). These finding have important implications for CRCI research. Cognitive test performance has been the gold standard for measuring CRCI (Nelson and Suls, 2013), but more recently the sensitivity and ecological validity of these standardized tests for detecting CRCI have been called into question (Janelsins et al., 2016). Some argue that investigators should focus on perceived cognitive function as patients’ self-report is a more accurate representation of functioning in everyday lives (Janelsins et al., 2016, Henneghan et al., 2017). However, there is a well-documented and important confounding relationship between psychological distress and self-report (Shilling et al., 2006, Wefel et al., 2015, Vardy, 2009) and our findings further emphasize that psychosocial factors must be accounted for when considering perceived cognitive functioning as an outcome in BCS research. Even though the relationship between psychological distress and perceived cognitive functioning is well-established, researchers do not always covary for or fully consider these factors.
Study Limitations
Although this study has several strengths and our approach to evaluating the relationships between cytokines and CRCI is distinct, it is not without limitations. We included 17 predictors in our models so it is possible that the associations identified in this study are spurious, however RFR is appropriate to model a large number of predictors (Breiman, 2001) and we adjusted p values to control for Type I error. One of the disadvantages to machine learning is that the models can "overfit" the data, especially when small samples and/or a large number of features are involved. We used random forest out-of-bag cross-validation in this study but further validation with a new and larger sample is required. There are also many other machine learning approaches that could be used and future studies should compare them to see if another is better than RFR as this is beyond the scope of this paper. We used a limited number of peripheral inflammatory cytokines as a proxy for neuroinflammation. It is possible that other cytokines, neurotransmitters, and/or cytokine polymorphisms are contributing to neural network functioning, but these were not measured in this study. We did not include chemo naïve or healthy control groups in this cross-sectional study so no conclusions can be made regarding specific treatment or disease effects. Additionally, our neuropsychological test battery was limited, so it is possible that we did not comprehensively capture cognitive performance within this sample.
Clinical and Research Implications
In the current study we identified cytokine correlates of cognitive function in BCS using RFR, a multivariate non-parametric analysis. Since this was an exploratory study, the models were not evaluated for their accuracy at predicting categorical cognitive impairment on the various neurocognitive tests. Future research should not only validate the features identified with the RFR in another larger sample, but also evaluate the accuracy of the algorithm at predicting categorical impairment status. Future studies should also utilize network analyses to determine the overall patterns of cytokines identified in this study. Identifying biological predictors of CRCI has potential broader utility and may lead to identifying those most at risk for the development of CRCI, but first these methods need to be employed in a prospective study with baseline data. The current study was cross sectional, focusing on persistent CRCI in survivors 6 months to 10 years post chemotherapy. It is possible that a combination of serum biomarkers and neuroimaging biomarkers will significantly improve the prediction of impairment and likelihood of future neurodegeneration in BC populations, and our group is working on this possibility.
Conclusion
CRCI is a common and persistent effect of breast cancer treatment. The etiology of this adverse treatment effect is likely multifactorial. Evidence from the current study suggests neurotoxic effects of cytokine dysregulation are contributing to ongoing CRCI in BCS 6 months to 10 years after chemotherapy completion. Our findings also suggest that specific cytokines are associated with individual cognitive test performance and that the relationships between these cytokines and cognitive test performance are non-linear. Although this study is exploratory, the approach is distinct in the field of CRCI research, and the preliminary findings prompt further inquiry.
Supplementary Material
Highlights.
Unique proinflammatory cytokine profiles associated with each cognitive test
Cytokines were more important predictors of CRCI than clinical/demographic variables
Machine learning models of cytokines explained large variances in cognitive performance
Acknowledgments
The authors would like to acknowledge Michelle Harrison, PhD and the Health & Integrative Physiology Lab at the University of Texas at Austin for their assistance with the cytokine assay procedures.
Funding. Research reported in this publication was supported by National Institute of Nursing Research of the National Institutes of Health under award number F31NR015707 and by the National Cancer Institute under award number R01CA172145. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Ashley M. Henneghan was supported by the Doctoral Degree Scholarship in Cancer Nursing, DSCN-15-072 from the American Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Aartsen MJ, Smits CH, Van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P153–62. doi: 10.1093/geronb/57.2.p153. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidi A, Christensen S, Mehlsen M, Jensen AB, Pedersen AD, Zachariae R. Long-term subjective cognitive functioning following adjuvant systemic treatment: 7–9 years follow-up of a nationwide cohort of women treated for primary breast cancer. Br J Cancer. 2015;113:794–801. doi: 10.1038/bjc.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisi GM. Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy Behav. 2014;38:43–7. doi: 10.1016/j.yebeh.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Benton A, Des Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment: A clinical manual. Oxford: Oxford University Press; 1983. [Google Scholar]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. Technical report. Vol. 33. Statistics Department, University of California Berkeley; Berkeley CA 94708: 1996. Out-of-bag estimation; p. 34. 1996b. [Google Scholar]
- Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- Breiman L, Friedman J, Olshen R, Stone C. Classification and regression trees. New York, NY: Chapman & Hall; 1984. [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, Dewalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Oakley AE, Monteiro M, Tuomela K, Allan LM, Mukaetova-Ladinska EB, O'Brien JT, Kalaria RN. Multiplex analyte assays to characterize different dementias: brain inflammatory cytokines in poststroke and other dementias. Neurobiol Aging. 2016;38:56–67. doi: 10.1016/j.neurobiolaging.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, Chan A, Ng T. International Cognition and Cancer Taskforce Meeting. Seattle, Washington: 2014. The association of pro-inflammatory biomarkers and cognitive impairment in asian breast cancer patients: a multi -centered, prospective, cohort study. [Google Scholar]
- Cheung YT, Foo YL, Shwe M, Tan YP, Fan G, Yong WS, Madhukumar P, Ooi WS, Chay WY, Dent RA, Ang SF, Lo SK, Yap YS, Ng R, Chan A. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67:811–20. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Cheung YT, Lim SR, Ho HK, Chan A. Cytokines as mediators of chemotherapy-associated cognitive changes: current evidence, limitations and directions for future research. PLoS One. 2013;8:e81234. doi: 10.1371/journal.pone.0081234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Lee JA, Fan G, Tan YP, Yong WS, Madhukumar P, Loo SK, Ang SF, Wong M, Chay WY, Ooi WS, Dent RA, Yap YS, Ng R, Chan A. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26:1446–51. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- D'Agata F, Costa T, Caroppo P, Baudino B, Cauda F, Manfredi M, Geminiani G, Mortara P, Pinessi L, Castellano G, Bisi G. Multivariate analysis of brain metabolism reveals chemotherapy effects on prefrontal cerebellar system when related to dorsal attention network. EJNMMI Res. 2013;3:22. doi: 10.1186/2191-219X-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–80. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderlin CA, Coleman EA, Cole C, Richards KC, Kennedy RL, Goodwin JA, Hutchins LF, Mack K. Subjective sleep quality, objective sleep characteristics, insomnia symptom severity, and daytime sleepiness in women aged 50 and older with nonmetastatic breast cancer. Oncol Nurs Forum. 2011;38:E314–25. doi: 10.1188/11.ONF.E314-E325. [DOI] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–9. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WG, Fisher SG, Cohen N, Waltzman S, Weber L. Use of principal components analysis to develop a composite score as a primary outcome variable in a clinical trial. The VA Cooperative Study Group on Cochlear Implantation. Control Clin Trials. 1990;11:199–214. doi: 10.1016/0197-2456(90)90014-s. [DOI] [PubMed] [Google Scholar]
- Henneghan A, Stuifbergen A, Becker H, Kesler S, King E. Modifiable correlates of perceived cognitive function in breast cancer survivors up to 10 years after chemotherapy completion. Journal of Cancer Survivorship. 2017 doi: 10.1007/s11764-017-0661-9. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Kesler SR. Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc. 2014;20:391–401. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, Ambrosone CA, Ahles TA, Morrow GR. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.5826. JCO2016685856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–13. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, Heckler CE, Roscoe JA, Katz AW, Williams JP, Morrow GR. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Supportive Care in Cancer. 2012;20:831–9. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013a;30(Suppl):S109–16. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Rao A, Blayney DW, Oakley Girvan I, Karuturi M, Palesh O. Predicting long-term cognitive outcome following breast cancer with pre-treatment resting state fMRI and random forest machine learning. Front Human Neurosci. 2017a;11:555. doi: 10.3389/fnhum.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Adams M, Packer M, Rao V, Henneghan AM, Blayney DW, Palesh O. Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav. 2017b;7:e00643. doi: 10.1002/brb3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Gugel M, Huston-Warren E, Watson C. Atypical Structural Connectome Organization and Cognitive Impairment in Young Survivors of Acute Lymphoblastic Leukemia. Brain Connect. 2016;6:273–82. doi: 10.1089/brain.2015.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Noll K, Cahill DP, Rao G, Wefel JS. The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. J Neurooncol. 2017c;131:565–574. doi: 10.1007/s11060-016-2328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Rao V, Ray WJ, Rao A. Probability of Alzheimer's disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2017d;9:67–75. doi: 10.1016/j.dadm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A. 2013b;110:11600–5. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–6. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Liaw A, Wiener M. Classification and Regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- Lyon DE, Cohen R, Chen H, Kelly DL, Mccain NL, Starkweather A, Ahn H, Sturgill J, Jackson-Cook CK. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-Immune Mechanisms of Behavioral Comorbidities in Patients With Cancer. Journal of Clinical Oncology. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WL, Suls J. New approaches to understand cognitive changes associated with chemotherapy for non-central nervous system tumors. J Pain Symptom Manage. 2013;46:707–21. doi: 10.1016/j.jpainsymman.2012.11.005. [DOI] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Ogilvie JM, Wilson JS, Green HJ, Chambers SK, Ownsworth T, Shum DH. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol. 2015;5:59. doi: 10.3389/fonc.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, Savard J, Rotatori M, Kesler S, Telli M, Mustian K. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Wong AL, Wong FL, Breen EC, Hurria A, Smith M, Kinjo C, Paz IB, Kruper L, Somlo G, Mortimer JE, Palomares MR, Irwin MR, Bhatia S. Inflammatory Biomarkers, Comorbidity, and Neurocognition in Women With Newly Diagnosed Breast Cancer. J Natl Cancer Inst. 2015;107:1–7. doi: 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology. 2015;96:11–8. doi: 10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, Cheng I, Ahn R, Breen EC, Irwin MR, Silverman DH. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7:511–23. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid-Arndt SA, Cox CR. Stress, coping and cognitive deficits in women after surgery for breast cancer. J Clin Psychol Med Settings. 2012;19:127–37. doi: 10.1007/s10880-011-9274-z. [DOI] [PubMed] [Google Scholar]
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- Ryan SM, O'Keeffe GW, O'Connor C, Keeshan K, Nolan YM. Negative regulation of TLX by IL-1beta correlates with an inhibition of adult hippocampal neural precursor cell proliferation. Brain Behav Immun. 2013;33:7–13. doi: 10.1016/j.bbi.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Scheinert RB, Asokan A, Rani A, Kumar A, Foster TC, Ormerod BK. Some hormone, cytokine and chemokine levels that change across lifespan vary by cognitive status in male Fischer 344 rats. Brain Behav Immun. 2015;49:216–32. doi: 10.1016/j.bbi.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog--methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Research and Treatment. 2006;95:125–9. doi: 10.1007/s10549-005-9055-1. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Mccoll A, Cavanagh J, Graham GJ. Peripheral inflammation is associated with remote global gene expression changes in the brain. J Neuroinflammation. 2014;11:73. doi: 10.1186/1742-2094-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T. Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Vardy J. Cognitive function in breast cancer survivors. Cancer Treat Res. 2009;151:387–419. doi: 10.1007/978-0-387-75115-3_24. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–9. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- Vardy JL, Stouten-Kemperman MM, Pond G, Booth CM, Rourke SB, Dhillon HM, Dodd A, Crawley A, Tannock IF. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9728-5. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Tallman EF. Perceived cognitive function in breast cancer survivors: evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy-cognitive function instrument. J Pain Symptom Manage. 2015;49:697–706. doi: 10.1016/j.jpainsymman.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–38. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Shah R, Shayne M, Huston AJ, Krebs M, Murray N, Thompson BD, Doyle K, Korotkin J, Van Wijngaarden E, Hyland S, Moynihan JA, Cory-Slechta DA, Janelsins MC. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. 2018;314:17–23. doi: 10.1016/j.jneuroim.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.