Abstract
Background
The approaches to the diagnosis and treatment of chronic spontaneous urticaria (CSU) differ in various parts of the world. We sought to determine the adherence to international and national urticaria guidelines as well as the motives to deviate from the guidelines among physicians worldwide.
Methods
A web-based questionnaire was created and launched via e-mail by the World Allergy Organization (WAO) to representatives of all WAO Member Societies, the members of the American Academy of Allergy, Asthma & Immunology (AAAAI) and the members of the WAO Junior Members Group (JMG), regardless of the specialty, affiliation, or nationality in March 2017.
Results
We received 1140 completed surveys from participating physicians from 99 countries. Virtually all participants (96%) were aware of at least one urticaria guideline and reported that they follow a guideline. However, one in five physicians who follow a guideline (22%) reported to deviate from it. Reliance on own clinical experience is the most frequent reason for deviation from guidelines or not following them (44%). Young (< 40 years) and less experienced physicians more often follow a guideline and less often deviate than older and experienced ones. Physicians who follow a urticaria guideline showed higher rates of routinely ordering a complete blood count, the erythrocyte sedimentation rate, C-reactive protein, anti-thyroid antibodies, and thyroid-stimulating hormone and of performing the autologous serum skin test as compared to those who do not. Physicians who follow a urticaria guideline showed higher rates of using second generation antihistamines as their first-line treatment of CSU (p = 0.001) and more frequently observed higher efficacy of these drugs (or had more confidence that it would work, p < 0.019) as compared to those who do not follow the guidelines.
Conclusions
Physicians’ characteristics (e.g. age, clinical experience, and specialty) and country specifics and regional features (e.g. availability of drugs for CSU treatment) importantly influence adherence to urticaria guidelines and CSU patient care and should be addressed in more detail in future research.
Electronic supplementary material
The online version of this article (10.1186/s40413-018-0193-4) contains supplementary material, which is available to authorized users.
Keywords: Chronic spontaneous urticaria, Guidelines, Worldwide, Guideline adherence, Urticaria treatment, Urticaria management, Global survey
Background
Chronic spontaneous urticaria (CSU) is a mast cell-driven disease that is defined as the occurrence of wheals, angioedema, or both for more than 6 weeks due to known or unknown causes [1]. CSU affects up to 1% of the general population [2, 3]. It exerts a devastating impact on patients’ quality of life [4, 5].
The approaches to the diagnostic workup and treatment of CSU patients differ in various parts of the world, and there are discrepancies between national consensus papers and guidelines and the international EAACI/GA2LEN/EDF/WAO guideline [1, 6, 7]. The impact of guidelines on the diagnostic workup and treatment strategy selection in every day clinical practice needs further research. How many physicians know urticaria guidelines? How many physicians use them to guide their clinical practice? What are the reasons for not following the available guidelines? What is the impact of following the guidelines on the quality of care for urticaria patients? These questions need to be addressed on a global level. The answers to these questions can be of significant value in updating and revising the current guidelines and improving patient care.
The World Allergy Organization (WAO) Junior Members Group (JMG) Steering Committee developed a questionnaire to survey the opinions on a whole variety of questions regarding CSU management and the use of guidelines. The questionnaire targeted the WAO members, including, but not limited to the representatives of the constituent national societies of WAO, having the authority to vote on their behalf, the WAO JMG and the American Academy of Allergy, Asthma & Immunology (AAAAI) members. We sought to determine, in physicians from around the world, the knowledge of and adherence to international and national urticaria guidelines as well as the motives to deviate from them.
Methods
Study survey
A web-based questionnaire (Additional file 1: Figure S1) was created and circulated among the members of the WAO JMG Steering Committee for revisions (July–September 2016). The protocol was approved by the WAO Executive Committee and Board of Directors (25 October 2016). The questionnaire was created de novo and has not been previously validated. The final version consisted of 24 questions including survey participant demographic information (country of residence, gender, age, specialty, clinical experience and type of practice) and those concerning CSU management (patients’ age, number of CSU patients seen per week, number of CSU patients with angioedema, awareness, adherence and/or deviation of current guidelines, examination of a CSU patient, including general laboratory work-up and targeted search for the cause of the disease, and treatment options used). There were 11 single-choice and 13 multiple-choice questions.
Recruitment and dissemination
The survey was beta tested and approved by the WAO JMG Steering Committee and WAO leadership before dissemination among participants. It was disseminated via email by the WAO office to representatives of WAO Member Societies as well as members of AAAAI and the WAO JMG in March 2017, with no restrictions applied to the specialty, affiliation, or nationality of the participants. The email contained a link (Internet address) to the online questionnaire that was unique to each participating member. A reminder to participate was sent in April 2017. Participants were given 30 days to reply and were guaranteed complete anonymity.
Statistical analysis
SPSS v.22 (Armonk, NY: IBM Corp, USA) was used for all analyses. Analyses of the difference in frequencies across groups were performed with the Pearson Chi-squared test and a p value ≤0.05 was considered significant.
Results
Demographics of study participants
A total of 32,356 individuals from 149 countries were invited to take part in the survey. We received 1140 (3.5%) completed surveys from participating physicians from 99 countries, with most residing in Europe (33.2%) and North America (28.9%) (Table 1). Most of the respondents were allergists/clinical immunologists (88.7%), followed by pediatricians (16.5%), dermatologists (4.5%) and general practitioners (2.2%) (Table 2). One hundred and fifty-seven participants had more than one specialty. The majority of participants were ≥ 40 years old (74.8%) and almost half of respondents had clinical experience of > 19 years (43.9%). Two thirds and half of the participants worked in academic institutions and/or had a private practice. Most participants (88.9%) reported to see primarily outpatients, both adults and children with CSU (53.7%). Only 22% of physicians reported to see ≥10 CSU patients per week.
Table 1.
A geographical distribution of the respondents participating in the survey (n = 1140)
| North America (n = 330, 28.9%) |
Latin America (n = 193, 16.9%) |
Europe (n = 379, 33.2%) |
Africa/Middle-East (n = 64, 5.6%) |
Asia-Pacific (n = 174, 15.3%) |
|---|---|---|---|---|
| Canada | Argentina | Albania | Algeria | Australia |
| United States | Bolivia | Armenia | Cyprus | Bangladesh |
| US Virgin Islands | Brazil | Austria | Egypt | Cambodia |
| Chile | Azerbaijan | Ethiopia | Hong Kong | |
| Colombia | Belarus | Iran | India | |
| Costa Rica | Belgium | Israel | Indonesia | |
| Cuba | Bulgaria | Kenya | Japan | |
| Dominican Republic | Croatia | Lebanon | Jordan | |
| Ecuador | Czech Republic | Oman | Korea | |
| El Salvador | Denmark | Qatar | Kuwait | |
| Guatemala | Estonia | South Africa | Malaysia | |
| Honduras | Finland | Tunisia | Mongolia | |
| Mexico | France | Nepal | ||
| Panama | Georgia | New Zealand | ||
| Paraguay | Germany | Pakistan | ||
| Peru | Greece | Peoples Republic of China | ||
| Uruguay | Guernsey | Philippines | ||
| Venezuela | Hungary | Saudi Arabia | ||
| Iceland | Singapore | |||
| Ireland | Sri Lanka | |||
| Italy | Taiwan | |||
| Kosovo | Thailand | |||
| Latvia | United Arab Emirates | |||
| Lithuania | Uzbekistan | |||
| Macedonia | Viet Nam | |||
| Moldova | ||||
| Montenegro | ||||
| Netherlands | ||||
| Poland | ||||
| Portugal | ||||
| Romania | ||||
| Russia | ||||
| Serbia | ||||
| Slovakia | ||||
| Slovenia | ||||
| Spain | ||||
| Sweden | ||||
| Switzerland | ||||
| Turkey | ||||
| Ukraine | ||||
| United Kingdom |
Table 2.
Characteristics of survey respondents (n = 1140)
| Characteristics of respondents | Geographical regions | Total % (n) |
|||||
|---|---|---|---|---|---|---|---|
| NA % (n/total) |
LA % (n/total) |
EU % (n/total) |
AME % (n/total) |
AP % (n/total) |
|||
| Specialtya (n = 1138) | |||||||
| Allergists/ Clinical Immunologists | 98.2 (324/330) | 97.4 (187/192) | 83.1 (315/379) | 79.7 (51/64) | 76.9 (133/173) | 88.7 (1010) | |
| Dermatologists | 0.3 (1/330) | 0.5 (1/192) | 9.8 (37/379) | 0 | 6.9 (12/173) | 4.5 (51) | |
| Pediatricians | 4.2 (14/330) | 25.5 (49/192) | 17.4 (66/379) | 23.4 (15/64) | 25.4 (44/173) | 16.5 (188) | |
| General Practitioners | 0.6 (2/330) | 3.1 (6/192) | 1.6 (6/379) | 6.2 (4/64) | 4 (7/173) | 2.2 (25) | |
| Gender (n = 1117) | |||||||
| Male | 58.5 (189/323) | 55.1 (102/185) | 43.7 (163/373) | 57.1 (36/63) | 58.4 (101/173) | 53.0 (591) | |
| Female | 41.5 (134/323) | 44.9 (83/185) | 56.3 (210/373) | 42.9 (27/63) | 41.6 (72/173) | 47.0 (526) | |
| Age, years (n = 1132) | |||||||
| < 40 | 22.9 (75/327) | 27.6 (53/192) | 28.2 (106/376) | 17.2 (11/64) | 23.1 (133/173) | 25.2 (285) | |
| ≥40 | 77.1 (252/327) | 72.4 (139/192) | 71.8 (270/376) | 82.8 (53/64) | 76.9 (133/173) | 74.8 (847) | |
| Clinical experience, years (n = 1130) | |||||||
| ≤19 | 50.3 (164/326) | 53.9 (104/193) | 58.2 (217/373) | 60.9 (39/64) | 63.2 (110/174) | 56.1 (634) | |
| Over 19 | 49.7 (162/326) | 46.1 (89/193) | 41.8 (156/373) | 39.1 (25/64) | 36.8 (64/174) | 43.9 (496) | |
| Place of worka (n = 1140) | |||||||
| Private practice | 63.9 (211/330) | 82.4 (159/193) | 34.6 (131/379) | 54.7 (35/64) | 46.0 (80/174) | 54.0 (616) | |
| University clinic | 70.3 (232/330) | 68.4 (132/193) | 57.5 (218/379) | 59.4 (38/64) | 71.8 (125/174) | 65.3 (745) | |
| Hospital | 11.8 (39/330) | 39.4 (76/193) | 43.3 (164/379) | 42.2 (27/64) | 58.6 (102/174) | 35.8 (408) | |
| Specialized urticaria centre | 0.9 (3/330) | 4.7 (9/193) | 2.9 (11/379) | 3.1 (2/64) | 0.3 (3/174) | 2.4 (28) | |
| Department (n = 1140) | |||||||
| Outpatients | 97.3 (321/330) | 89.6 (173/193) | 82.3 (312/379) | 81.3 (52/64) | 89.1 (155/174) | 88.9 (1013) | |
| Inpatients | 0.9 (3/330) | 8.8 (17/193) | 12.4 (47/379) | 10.9 (7/64) | 6.9 (12/174) | 7.5 (86) | |
| Outpatients and inpatients | 1.8 (6/330) | 1.6 (3/193) | 5.3 (20/379) | 7.8 (5/64) | 4.0 (7/174) | 3.6 (41) | |
| Age of patients (n = 1131) | |||||||
| Adults | 16.5 (54/327) | 17.2 (33/192) | 42.0 (158/376) | 10.9 (7/64) | 30.8 (53/172) | 27.0 (305) | |
| Children | 9.5 (31/327) | 16.7 (32/192) | 19.4 (73/376) | 18.8 (12/64) | 27.9 (71/172) | 19.3 (219) | |
| Adults and children | 74.0 (242/327) | 66.1 (127/192) | 38.6 (145/376) | 70.3 (45/64) | 41.3 (48/172) | 53.7 (607) | |
| Number of CSU patients per week (n = 1127) | |||||||
| < 10 | 75.5 (247/327) | 78.8 (152/193) | 80.7 (302/374) | 71.0 (44/62) | 78.4 (134/171) | 78.0 (879) | |
| ≥10 | 24.5 (80/327) | 21.2 (41/193) | 19.3 (72/374) | 29.0 (18/62) | 21.6 (37/171) | 22.0 (248) | |
| Patients with angioedema, % from the total number of CSU patients (n = 1131) | |||||||
| ≤20 | 37.7 (123/326) | 58.5 (113/193) | 51.7 (195/377) | 55.6 (35/63) | 66.3 (114/172) | 51.3 (580) | |
| > 20 | 62.3 (203/326) | 41.5 (80/193) | 48.3 (182/377) | 44.4 (28/63) | 33.7 (58/172) | 48.7 (551) | |
| Adherence to the urticaria guidelinesa (n = 1126) | |||||||
| Follow the guidelines | Any of three below | 88.0 (286/325) | 93.8 (180/192) | 97.3 (365/375) | 85.7 (54/63) | 89.5 (153/171) | 92.2 (1038) |
| EAACI/WAO/GA2LEN/EDF | 16.3 (53/325) | 78.1 (150/192) | 82.4 (309/375) | 68.3 (43/63) | 63.2 (108/171) | 58.9 (663) | |
| US practice parameters | 81.2 (264/325) | 32.3 (62/192) | 9.1 (34/375) | 28.6 (18/63) | 29.8 (51/171) | 38.1 (429) | |
| National | 7.1 (23/325) | 26.0 (50/192) | 32.5 (122/375) | 14.3 (9/63) | 30.4 (52/171) | 22.7 (256) | |
| Follow any but deviate | 40.0 (80/200) | 28.1 (25/89) | 49.4 (79/160) | 58.1 (18/31) | 53.8 (49/91) | 22.3 (251) | |
| Do not follow | 12.0 (39/325) | 6.3 (12/192) | 2.7 (10/375) | 14.3 (9/63) | 10.5 (18/171) | 7.8 (88) | |
AME Africa/Middle-East, AP Asia-Pacific, EU Europe, LA Latin America, NA North America. arespondents could choose more than one option
More than 90% of physicians follow the urticaria guidelines, but almost one-fourth of them deviate
Virtually all participants (1086 of 1126, 96%) were aware of one or more urticaria guidelines, and almost all of them reported to follow a guideline (n = 1038 of 1086, 96%) (Fig. 1). The most widely used guideline was the international EAACI/GA2LEN/EDF/WAO urticaria guideline [1] (58.9%), followed by the American AAAAI/ACAAI Joint Task Force practice parameters for the diagnosis and management of acute and chronic urticaria [7] (38.1%) and national guidelines (22.7%). Expectedly, the US Joint Task Force practice parameters are used more often in North America and the EAACI/GA2LEN/EDF/WAO urticaria guidelines are more known in other countries of the world. One in five physicians who follow a guideline (22%) reported to deviate from it.
Fig. 1.
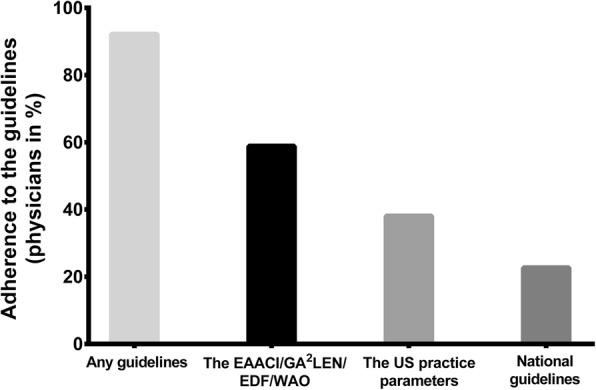
Adherence to the urticaria guidelines. Physicians were asked if they know and follow the current guidelines for management of urticaria. Results are expressed as percentage of participants who chose the corresponding guidelines (one respondent could choose several answers)
Reliance on own clinical experience is the most frequent reason for deviation from the guidelines or not following them
Of the 339 (30%) physicians who do not follow a guideline or follow a guideline but deviate from it, 149 (43.9%) reported that they rely more on their own clinical experience (Table 3). It was the most frequent reason in all regions of the world except for Latin America, where the most common reason, provided by 14.3% of respondents, was that some of the guidelines’ recommendations cannot be implemented in their country of residence. The second most prevalent reason for deviating from guideline recommendations (29.8%) was that the approach to CSU management recommended by the guidelines was seen as overly simplified and not reflecting the complexity of the disease. The least frequent reasons were the discrepancy and/or disagreement between the guidelines (3.8%) and a negative experience with following the guidelines in clinical practice (3.2%). Nine percent of participants did not agree with the guidelines’ recommendations and/or conclusions.
Table 3.
Reasons why physicians don’t follow or deviate from the guidelines
| Reasons | Geographical regions | Total n = 339a % (n) |
||||
|---|---|---|---|---|---|---|
| NA n = 236a % (n) |
LA n = 98a % (n) |
EU n = 167a % (n) |
AME n = 38a % (n) |
AP n = 104a % (n) |
||
| The guidelines do not undergo revision frequently enough | 4.2 (10) | 4.1 (4) | 3.0 (5) | 5.3 (2) | 6.7 (7) | 8.2 (28) |
| I rely more on my own clinical experience | 27.1 (64) | 10.2 (10) | 20.4 (34) | 34.2 (13) | 26.9 (28) | 43.9 (149) |
| I do not agree with the guidelines’ recommendations and/or conclusions | 7.2 (17) | 3.1 (3) | 4.2 (7) | 2.6 (1) | 3.8 (4) | 9.4 (32) |
| Some of the recommendations are unclear to me and require further details | 3.4 (8) | 1.0 (1) | 8.4 (14) | 2.6 (1) | 11.5 (12) | 10.6 (36) |
| Some of the guidelines’ recommendations cannot be implemented in my country of residence | 0.8 (2) | 14.3 (14) | 12.0 (20) | 26.3 (10) | 22.1 (23) | 20.3 (69) |
| I had a negative experience with following the guidelines in my clinical practice | 1.7 (4) | 0 (0) | 3.6 (6) | 2.6 (1) | 0 (0) | 3.2 (11) |
| Overly simplified approach to CSU management recommended by the guidelines that does not reflect the complexity of the disease | 22.9 (54) | 7.1 (7) | 15.6 (26) | 10.5 (4) | 9.6 (10) | 29.8 (101) |
| The discrepancy and/or disagreement between the guidelines | 3.0 (7) | 2.0 (2) | 1.8 (3) | 2.6 (1) | 0 (0) | 3.8 (13) |
AME Africa/Middle-East, AP Asia-Pacific, EU Europe, LA Latin America, NA North America. athe total number of respondents was 339. However, there was overlapping in the data analysis because respondents could choose more than one option
Young and less experienced physicians more often follow a guideline and less often deviate than older and experienced ones
Physicians who are less than 40 years of age more often reported that they adhere to urticaria guidelines and less often deviate as compared to responding physicians of ≥40 years of age (p = 0.001 and p = 0.023, respectively). Responding physicians with clinical experience of > 19 years statistically more often deviate from the guidelines and less frequently follow them as compared to responding physicians with clinical experience of 19 years or less (p = 0.025 and p < 0.001, respectively) (Tables 4 and 5).
Table 4.
Factors linked to adherence to the urticaria guidelines
| Factors | % (n) of physicians, who follow the guidelines | % (n) of physicians, who don’t follow the guidelines | X2 | p |
|---|---|---|---|---|
| Age, years | ||||
| < 40 (n = 283) | 96.9 (274) | 3.1 (9) | 11.152 | 0.001 |
| ≥40 (n = 836) | 90.7 (758) | 9.3 (78) | ||
| Clinical experience, years | ||||
| ≤19 years (n = 630) | 95.9 (604) | 4.1 (26) | 28.017 | < 0.001 |
| Over 19 years (n = 487) | 87.3 (425) | 12.7 (62) | ||
| Departmenta | ||||
| Outpatients (n = 1006) | 92.0 (926) | 8.0 (80/85) | 0.431 | 0.511 |
| Inpatients (n = 84) | 94.0 (79) | 6.0 (5) | ||
| Age of patientsa | ||||
| Adults (n = 300) | 92.3 (277) | 7.7 (23) | 0.029 | 0.865 |
| Children (n = 193) | 92.7 (179) | 7.3 (14) | ||
| CSU patients per week | ||||
| < 10 (n = 870) | 92.2 (802) | 7.8 (68) | 0.076 | 0.783 |
| ≥10 (n = 247) | 92.7 (229) | 7.3 (18) | ||
aOnly in physicians who chose one of the options
Values marked in bold indicate a statistically significant difference (p < 0.05)
Table 5.
Factors linked to the deviation from the urticaria guidelines
| Factors | % (n) of physicians, who deviate from the guidelines | % (n) of physicians, who don’t deviate from the guidelines | X2 | p |
|---|---|---|---|---|
| Age, years | ||||
| < 40 (n = 140) | 35.7 (50) | 64.3 (90) | 5.194 | 0.023 |
| ≥40 (n = 428) | 46.7 (200) | 53.3 (228) | ||
| Clinical experience, years | ||||
| ≤19 years (n = 323) | 39.9 (129) | 60.1 (194) | 5.049 | 0.025 |
| Over 19 years (n = 245) | 49.4 (121) | 50.6 (124) | ||
| Patientsa | ||||
| Outpatients (n = 510) | 44.5 (227) | 55.5 (283) | 0.029 | 0.865 |
| Inpatients (n = 44) | 43.2 (19) | 56.8 (25) | ||
| Age of patientsa | ||||
| Adults (n = 150) | 55.3 (83) | 44.7 (67) | 7.712 | 0.005 |
| Children (n = 92) | 37.0 (34) | 63.0 (58) | ||
| CSU patients per week | ||||
| < 10 (n = 441) | 44.0 (194) | 50.0 (247) | 0.002 | 0.961 |
| ≥10 (n = 128) | 43.7 (56) | 56.3 (72) | ||
aOnly in physicians who chose one of the options
Values marked in bold indicate a statistically significant difference (p < 0.05)
Physicians who follow a urticaria guideline more often perform diagnostic tests
Physicians who follow a urticaria guideline showed higher rates of routinely ordering a complete blood count (CBC), the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anti-thyroid antibodies, and thyroid-stimulating hormone (TSH) and of performing the autologous serum skin test (ASST) as compared to those who do not (Table 6). CSU due to unknown causes was reported to be much more common than CSU due to known causes, in all regions of the world (90 vs 10%). Autoimmunity was the most common identifiable cause of CSU (51.9%) and malignancy was the least common identifiable cause of CSU (4.5%). Food intolerance was a less frequent cause of CSU in North America (8.0%) as compared to other regions of the world (> 16.0%) (Table 7).
Table 6.
Differences in the approach to the management of CSU in respondents who do and do not follow the guidelines
| Test | Compared groups | n | % (n) of physicians, who follow the guidelines | % (n) of physicians, who don’t follow the guidelines | X2 | p |
|---|---|---|---|---|---|---|
| CBC | Order | 854 | 79.2 (802) | 60.5 (52) | 16.011 | < 0.001 |
| No | 245 | 20.8 (211) | 39.5 (34) | |||
| ESR | Order | 602 | 55.9 (566) | 41.9 (36) | 6.284 | 0.012 |
| No | 497 | 44.1 (447) | 58.1 (50) | |||
| CRP | Order | 527 | 49.0 (496) | 36.0 (31) | 5.299 | 0.021 |
| No | 572 | 51.0 (517) | 64.0 (55) | |||
| Anti-TG/TPO | Order | 559 | 51.9 (526) | 38.4 (33) | 5.826 | 0.016 |
| No | 540 | 48.1 (487) | 61.6 (53) | |||
| TSH | Order | 543 | 50.8 (515) | 32.6 (28) | 10.598 | 0.001 |
| No | 556 | 49.2 (498) | 67.4 (58) | |||
| Total IgE | Order | 481 | 56.1 (445) | 58.1 (36) | 0.138 | 0.710 |
| No | 618 | 43.9 (568) | 41.9 (50) | |||
| ECP | Order | 51 | 4.7 (48) | 3.5 (3) | 0.280 | 0.597 |
| No | 1048 | 95.3 (965) | 96.5 (83) | |||
| D-dimer | Order | 54 | 5.2 (53) | 1.2 (1) | 2.809 | 0.094 |
| No | 1045 | 94.8 (960) | 98.8 (85) | |||
| Skin prick tests | Order | 308 | 71.4 (290) | 79.1 (18) | 2.329 | 0.127 |
| No | 791 | 28.6 (723) | 20.9 (68) | |||
| Allergen-specific IgE | Order | 286 | 26.5 (268) | 20.9 (18) | 1.257 | 0.262 |
| No | 813 | 73.5 (745) | 79.1 (68) | |||
| ANA | Order | 407 | 37.7 (382) | 29.1 (25) | 2.538 | 0.111 |
| No | 692 | 62.3 (631) | 70.9 (61) | |||
| Tryptase | Order | 162 | 84.9 (153) | 89.5 (9) | 1.357 | 0.244 |
| No | 937 | 15.1 (860) | 10.5 (77) | |||
| ASST | Order | 186 | 17.7 (179) | 8.1 (7) | 5.121 | 0.024 |
| No | 913 | 82.3 (834) | 91.9 (79) | |||
| Search for chronic infections | Perform | 364 | 66.2 (342) | 74.4 (22) | 2.394 | 0.122 |
| No | 735 | 33.8 (671) | 25.6 (64) | |||
| Do not order any tests | 182 | 15.4 (156) | 30.2 (26) | 12.621 | < 0.001 | |
| Order at least 1 test | 917 | 84.6 (857) | 69.8 (60) | |||
CBC complete blood count, ESR erythrocyte sedimentation rate, CRP C-reactive protein, TG/TPO thyroglobulin/thyroperoxidase, TSH thyroid-stimulating hormone, ECP eosinophil cationic protein, ANA antinuclear antibodies, ASST autologous serum skin test
Values marked in bold indicate a statistically significant difference (p < 0.05)
Table 7.
Number of respondents from different regions of the world who find these causes of CSU as most common (n = 1098)a
| CSU causes | Geographical regions | Total % (n/total) |
||||
|---|---|---|---|---|---|---|
| NA % (n/total) |
LA % (n/total) |
EU % (n/total) |
AME % (n/total) |
AP % (n/total) |
||
| Idiopathic CSU | 97.5 (315/323) | 80.6 (150/186) | 88 (323/367) | 93.3 (56/60) | 89.5 (145/162) | 90.1 (989/1098) |
| Type-I-allergy | 27.5 (77/280) | 27.1 (46/170) | 17.9 (60/336) | 19.6 (11/56) | 39.6 (59/149) | 25.5 (253/991) |
| Autoimmune CSU | 64.4 (201/312) | 44.8 (81/181) | 46.8 (166/355) | 32.8 (19/58) | 54.1 (80/148) | 51.9 (547/1054) |
| Systemic disorders | 26.7 (79/296) | 29.1 (50/172) | 18.2 (62/340) | 12.3 (7/57) | 20.8 (31/149) | 22.6 (229/1014) |
| Malignancy | 2.4 (7/288) | 6.1 (10/165) | 6.6 (22/334) | 0 (0/56) | 3.6 (5/139) | 4.5 (44/982) |
| Chronic infection | 11.7 (34/290) | 32.8 (58/177) | 27.4 (95/347) | 13.6 (8/59) | 22.3 (33/148) | 22.3 (228/1021) |
| Food intolerance | 8.0 (23/289) | 19.7 (35/178) | 16.3 (56/343) | 22.4 (13/58) | 26.6 (41/154) | 16.4 (168/1022) |
AME Africa/Middle-East, AP Asia-Pacific, EU Europe, LA Latin America, NA North America. athe respondents could choose more than one answer
Adherence to urticaria guidelines is associated with more frequent administration and confidence in higher efficacy of second-generation antihistamines
Updosing of second-generation H1-antihistamines (sgAHs, 97%) and omalizumab (96%) were reported to be the most effective treatment options in all regions of the world. Dapsone, montelukast and H2-antihistamines were considered effective drugs for treatment of CSU worldwide only by 17, 17 and 15% physicians, respectively. Less respondents from North America as compared to other regions of the world reported that sgAHs at standard dose and montelukast are highly effective (48% vs 60–76 and 9% vs 13–35%, respectively). Vice versa, more physicians from North America as compared to other countries reported that tricyclic antidepressants are highly effective (52% vs 15–33%). Physicians who follow a urticaria guideline showed higher rates of sgAHs administration as a first-line treatment of CSU (p = 0.001) and more frequently observed higher efficacy of treatment (or had more confidence that it would work, p < 0.019) as compared to those who do not follow the guidelines (Table 8). Guideline followers more frequently use higher than standard-dosed sgAHs and omalizumab as a second and third line treatment, respectively, and less frequently administer first generation antihistamines, tricyclic antidepressants and systemic corticosteroids in comparison to physicians who do not follow a urticaria guideline (Tables 9 and 10).
Table 8.
Differences in the approach to a first line treatment of CSU in physicians who do and do not follow the guidelines
| Treatment | Compared groups | n | % (n) of physicians, who follow the guidelines | % (n) of physicians, who don’t follow the guidelines | X2 | p |
|---|---|---|---|---|---|---|
| First-generation H1-antihistamines | Administer | 173 | 15.4 (160) | 14.8 (13) | 0.026 | 0.873 |
| No | 953 | 84.6 (878) | 85.2 (75) | |||
| Second-generation H1-antihistamines at standard dose | Administer | 704 | 64 (664) | 45.5 (40) | 11.868 | 0.001 |
| No | 422 | 36 (374) | 54.5 (48) | |||
| Updosed second-generation H1-antihistamines | Administer | 540 | 47.8 (496) | 50.0 (44) | 0.160 | 0.690 |
| No | 586 | 52.2 (542) | 50.0 (44) | |||
| H2-antihistamines (e.g. famotidine or ranitidine) | Administer | 224 | 19.7 (204) | 22.7 (20) | 0.481 | 0.488 |
| No | 902 | 80.3 (834) | 77.3 (68) | |||
| Ciclosporin | Administer | 15 | 1.4 (15) | 0 | 1.289 | 0.256 |
| No | 1111 | 98.6 (1023) | 100 (88) | |||
| Omalizumab | Administer | 32 | 3.0 (31) | 1.1 (1) | 1.006 | 0.316 |
| No | 1094 | 97.0 (1007) | 98.9 (87) | |||
| Montelukast | Administer | 150 | 13.2 (137) | 14.8 (13) | 0.174 | 0.676 |
| No | 976 | 86.8 (901) | 85.2 (75) | |||
| Dapsone | Administer | 7 | 0.6 (6) | 1.1 (1) | 0.409 | 0.522 |
| No | 1119 | 99.4 (1032) | 98.9 (87) | |||
| Systemic corticosteroids (for less than 10 days) | Administer | 215 | 18.7 (194) | 23.9 (21) | 1.406 | 0.236 |
| No | 911 | 81.3 (844) | 76.1 (67) | |||
| Systemic corticosteroids (for more than 10 days in a row) | Administer | 16 | 1.5 (16) | 0 | 1.376 | 0.241 |
| No | 1110 | 98.5 (1022) | 100 (88) | |||
| Tricyclic antidepressants (e.g. doxepin) | Administer | 35 | 3.0 (31) | 4.5 (4) | 0.655 | 0.418 |
| No | 1091 | 97.0 (1007) | 95.5 (84) |
Values marked in bold indicate a statistically significant difference (p < 0.05)
Table 9.
Differences in the approach to a second line treatment of CSU in physicians who do and do not follow the guidelines
| Treatment | Compared groups | n | % (n) of physicians, who follow the guidelines | % (n) of physicians, who don’t follow the guidelines | X2 | p |
|---|---|---|---|---|---|---|
| First-generation H1-antihistamines | Administer | 156 | 13.1 (136) | 22.7 (20) | 6.297 | 0.012 |
| No | 970 | 86.9 (902) | 77.3 (68) | |||
| Second-generation H1-antihistamines at standard dose | Administer | 127 | 11.3 (117) | 11.4 (10) | 0.001 | 0.979 |
| No | 999 | 88.7 (921) | 88.6 (78) | |||
| Updosed second-generation H1-antihistamines | Administer | 651 | 58.8 (610) | 46.6 (41) | 4.931 | 0.026 |
| No | 475 | 41.2 (428) | 53.4 (47) | |||
| H2-antihistamines (e.g. famotidine or ranitidine) | Administer | 308 | 27.1 (281) | 30.7 (27) | 0.532 | 0.466 |
| No | 818 | 72.9 (757) | 69.3 (61) | |||
| Ciclosporin | Administer | 76 | 6.8 (71) | 5.7 (5) | 0.173 | 0.678 |
| No | 1050 | 93.2 (967) | 94.3 (83) | |||
| Omalizumab | Administer | 163 | 14.5 (150) | 14.8 (13) | 0.007 | 0.934 |
| No | 963 | 85.5 (888) | 85.2 (75) | |||
| Montelukast | Administer | 391 | 35.5 (368) | 26.1 (23) | 3.106 | 0.078 |
| No | 735 | 64.5 (670) | 73.9 (65) | |||
| Dapsone | Administer | 39 | 3.2 (33) | 6.8 (6) | 3.213 | 0.073 |
| No | 1087 | 96.8 (1005) | 93.2 (82) | |||
| Systemic corticosteroids (for less than 10 days) | Administer | 265 | 23.8 (247) | 20.5 (18) | 0.503 | 0.478 |
| No | 861 | 76.2 (791) | 79.5 (70) | |||
| Systemic corticosteroids (for more than 10 days in a row) | Administer | 89 | 7.4 (77) | 13.6 (12) | 4.309 | 0.038 |
| No | 1037 | 92.6 (961) | 86.4 (76) | |||
| Tricyclic antidepressants (e.g. doxepin) | Administer | 137 | 11.6 (120) | 19.3 (17) | 4.568 | 0.033 |
| No | 989 | 88.4 (918) | 80.7 (71) |
Values marked in bold indicate a statistically significant difference (p < 0.05)
Table 10.
Differences in the approach to a third line treatment of CSU in physicians who do and do not follow the guidelines
| Treatment | Compared groups | n | % (n) of physicians, who follow the guidelines | % (n) of physicians, who don’t follow the guidelines | X2 | p |
|---|---|---|---|---|---|---|
| First-generation H1-antihistamines | Administer | 93 | 7.7 (80) | 14.8 (13) | 5.345 | 0.021 |
| No | 1033 | 92.3 (958) | 85.2 (75) | |||
| Second-generation H1-antihistamines at standard dose | Administer | 78 | 6.9 (72) | 6.8 (6) | 0.002 | 0.967 |
| No | 1048 | 93.1 (966) | 93.2 (82) | |||
| Updosed second-generation H1-antihistamines | Administer | 283 | 25.4 (264) | 21.6 (19) | 0.637 | 0.425 |
| No | 843 | 74.6 (774) | 78.4 (69) | |||
| H2-antihistamines (e.g. famotidine or ranitidine) | Administer | 206 | 18.3 (190) | 18.2 (16) | 0.001 | 0.977 |
| No | 920 | 81.7 (848) | 81.8 (72) | |||
| Ciclosporin | Administer | 254 | 23.1 (240) | 15.9 (14) | 2.416 | 0.120 |
| No | 872 | 76.9 (798) | 84.1 (74) | |||
| Omalizumab | Administer | 570 | 51.8 (538) | 36.4 (32) | 7.764 | 0.005 |
| No | 556 | 48.2 (500) | 63.6 (56) | |||
| Montelukast | Administer | 319 | 28.9 (300) | 21.6 (19) | 2.135 | 0.144 |
| No | 807 | 71.1 (738) | 78.4 (69) | |||
| Dapsone | Administer | 91 | 7.8 (81) | 11.4 (10) | 1.384 | 0.239 |
| No | 1035 | 92.2 (957) | 88.6 (78) | |||
| Systemic corticosteroids (for less than 10 days) | Administer | 227 | 20.1 (209) | 20.5 (18) | 0.005 | 0.943 |
| No | 899 | 79.9 (829) | 79.5 (70) | |||
| Systemic corticosteroids (for more than 10 days in a row) | Administer | 146 | 12.7 (132) | 15.9 (14) | 0.733 | 0.392 |
| No | 980 | 87.3 (906) | 84.1 (74) | |||
| Tricyclic antidepressants (e.g. doxepin) | Administer | 134 | 11.7 (121) | 14.8 (13) | 0.751 | 0.386 |
| No | 992 | 88.3 (917) | 85.2 (75) |
Values marked in bold indicate a statistically significant difference (p < 0.05)
Discussion
Several guidelines, consensus papers, and practice parameters have been developed for the management of chronic urticaria. Some studies have explored, on the national level, if physicians know these guidelines and implement them in their actual clinical practice [8, 9]. To our knowledge, our study is the first global report of how physicians approach CSU.
Most physicians know and use urticaria guidelines in their clinical practice
More than 90% of respondents stated to be aware and follow urticaria guidelines. However, there is inconsistency between our study and other studies. For example, most respondents from Latin America in our study (94%) followed any urticaria guideline with 78% followed the EAACI/GA2LEN/EDF/WAO urticaria guideline. In contrast, only 79 of 421 (19%) physicians from Ecuador reported to know the EAACI/GA2LEN/EDF/WAO urticaria guideline, but more than half of them (67%) were dermatologists and allergists [8]. In German-wide study, only one-third of all physicians participating in the survey were familiar with the EAACI/GA2LEN/EDF/WAO urticaria guideline [9]. In Italy, 56% of specialists knew the CSU guidelines and only 27% used them regularly [10]. The high rates of adherence to urticaria guidelines in our study can be explained by increase in guidelines awareness worldwide over time and the fact that most of the participants were allergists/clinical immunologists (88%).
Factors associated with adherence to guidelines
Young (< 40 years) and less experienced physicians (≤19 years in practice) more often follow guidelines and less often deviate from them than their older and more experienced colleagues. A similar tendency has been observed for other diseases, where low adherence rates to guidelines were also showed to be linked with the advanced age of the physicians [11–13]. For example, old age, male sex, and incomplete residency training were associated with disagreement with clinical practice guidelines for cancer screening [13]. In contrast, compared with physicians ≥50 years, younger physicians (< 50 years) reported a lower level of awareness of cholesterol guidelines [14].
We did not compare the adherence to urticaria guidelines between respondents of different specialties because most physicians in our study were allergists and many of them had several specialties. However, in previous studies the level of knowledge was highest for allergists and/or dermatologists [8, 9], and these physicians have significantly higher expertise in caring for patients with urticaria than other specialists [15]. An observational study from the UK showed that allergists follow the urticaria guidelines more regularly and consistently compared to dermatologists [16]. The results of this study should be evaluated with caution because of the fact that Allergology is recognized as a specialty in some countries (for example, in Russia) or as a subspecialty in others (for example, in Germany).
Impact of following the guidelines on the quality of care for CSU patients
According to the EAACI/GA2LEN/EDF/WAO guideline, only differential blood count and CRP or ESR are recommended as routine diagnostic tests for CSU patients [1]. The US practice parameters recommend limited laboratory testing including a CBC with differential, ESR and/or CRP, liver enzymes, and TSH measurement [7]. Expectedly, these diagnostic tests were performed more frequently by physicians who follow a urticaria guideline in our and other studies [8, 9].
Additional tests are indicated as an extended diagnostic program for identification of underlying causes or eliciting factors and for ruling out possible differential diagnoses if suggested based on history only [1]. For example, allergy is regarded as a very rare cause of CSU [1], and allergy testing is usually not cost-effective and does not lead to improved patient care outcomes [7]. However, some physicians reported to determine total serum IgE (43.8%) and to perform allergy skin prick testing in patients with CSU (28.0%). In a cross-sectional study from Latin America, total serum IgE was the most common diagnostic test (83.5%) [8]. Interestingly, 5–15% of respondents perform other less useful diagnostic tests, e.g. ECP and tryptase, in patients with CSU.
Idiopathic CSU was reported to be the most common type of CSU; this is in the line with other studies [9, 17]. In one study, allergists and dermatologists more frequently searched for CSU etiology as compared to general practitioners [8] in contrast to the results of other study [9]. Although IgE-mediated allergy is a rare cause of CSU [1, 18], IgE-mediated allergy is considered to be a common cause of CSU by 26% of respondents.
Up to 50% of CSU patients can have circulating functional IgG autoantibodies against IgE and high-affinity IgE receptors on mast cells and basophils [19]. Half of respondents reported autoimmune CSU as the most common cause of CSU and 16.9% of physicians (10.1–13.5% in other studies [8, 9]) carried out ASST as a screening method for the detection of autoantibodies [1]. ASST was applied more often by physicians who were aware of and/or follow the guidelines in our and another study [9], but not in all [8].
There is a universal agreement among urticaria guidelines [1, 7, 20] that second generation antihistamines (sgAHs) at a standard dose should be the first line therapy, which is effective in improving symptoms in about 40% of CSU patients [21]. Guideline followers, quite expectedly, use sgAHs at a standard dose as a first line therapy more frequently than non-followers, while the administration of other drugs was not different between the two groups. This has been proved in early national cross-sectional studies where sgAHs taken regularly were the most common drugs prescribed [10, 22]. It is consistent with the finding that more guidelines followers (67.4%) than non-followers (50%) feel that sgAHs are highly effective in CSU treatment.
As a second line therapy, the EAACI/GA2LEN/EDF/WAO urticaria guideline recommends the use of sgAHs in higher doses up to four times the standard dose. Physicians who use urticaria guidelines more frequently selected up-dosing for a second-line treatment in our and other studies [9, 10].
For non-respondents to sgAHs up-dosing, the EAACI/GA2LEN/EDF/WAO guideline recommends omalizumab, ciclosporin (step 4 in the US practice parameters) or montelukast (step 2 in the US practice parameters) as a third line treatment option [1, 7]. Guideline followers in our and one other study [8] more frequently used omalizumab as a third line treatment in comparison to physicians who do not follow a urticaria guideline.
Our and early studies [9] showed that physicians who are familiar with the guidelines are less likely to use first generation antihistamines as a second and/or third line treatment and systemic steroids (for more than 10 days in a row) as a second line therapy, indicating that guideline recommendations may improve the quality of care [9].
The treatment of CSU can depend on physician’s specialty. For example, Cherrez et al. showed that allergists and dermatologists in Ecuador prescribed significantly more sgAHs (regular doses) as compared to general practitioners [8].
Reasons for not following or deviation from the available urticaria guidelines
Almost one-third of physicians do not follow a guideline or deviate from it. The most frequent reasons given were reliance on their own clinical experience (44%) and an overly simplified approach to CSU management recommended by the guidelines (30%). Moreover, many physicians, especially those of 40 years or older and with clinical experience of > 19 years, follow guidelines but can deviate from them in some cases, e.g. in difficult-to-treat CSU. This may point to a need to better communicate to physicians, especially experienced physicians, the benefits of guideline adherence and to better engage them in the guideline development and review process. Also, more efforts appear to be needed to improve physician “buy-in” to guidelines by allowing for sufficient flexibility and by educating them that guidelines are meant to complement, rather than substitute for, clinical judgement.
One-fifth of physicians reported that some of the guidelines’ recommendations cannot be implemented in physician’s country of residency. It suggests that economic considerations are an important and often decisive factor influencing the choice of a treatment strategy. For example, omalizumab is unavailable in some countries or its cost is too high and health insurance programs do not cover it (for example, in Russia or Latin America [23]). Systemic steroids and first generation antihistamines are cheaper than sgAHs (for example, in Ecuador [8]) and this can prompt a physician’s decision to prescribe them. The cost-effectiveness of the treatment for CSU, especially in the developing and low-income countries, should be further investigated in future studies.
The EAACI/GA2LEN/EDF/WAO guideline is revised every four years by a global panel of well-known experts in the field. Interestingly, 8–10% of respondents did not agree with guidelines’ recommendations and conclusions or found guideline recommendations unclear or outdated. Again, this calls for the consideration of improvements in the development of guideline updates and revisions.
The recommendations given by all of urticaria guidelines are similar, although some differences exist. For example, in contrast to the EAACI/GA2LEN/EDF/WAO guideline US practice parameters recommend H2-antagonists and first generation antihistamines for treatment of urticaria as a second or third line therapy, respectively [24]. Only 4% of respondents named the discrepancy and/or disagreement between the guidelines as a reason not to follow them.
Taken together, reliance on own clinical experience, especially in older physicians, rather than economic reasons or unavailability of drugs, appears to be the most frequent reason for deviation from or not following the guidelines. This observation offers the opportunity for a debate on medicine based on experience and evidence-based medicine and highlights the need for continuous medical education for healthcare providers.
Limitations
The main limitations of our study are the bias of participant selection, the use of an online non-validated questionnaire and a low response rate (3.5%). The fact that most participants in our study were allergists, whereas CSU is often managed by dermatologists and general practitioners, could explain some differences between our findings and those from other studies [8, 9]. There is limited information in regards to CSU management in Africa/Middle-East (only 64 questionnaires were filled out). The most recent EAACI guideline [18] appeared after we performed our study agreeing on our observations.
Conclusion
The results of our study indicate that urticaria guideline recommendations contribute to a higher quality of patient care. Most physicians worldwide follow a guideline, however, one in five deviates from them. We speculate there are three major reasons for deviation that should be addressed in future research. Firstly, older physicians may be prone to disproportionate reliance on their clinical experience and unable to fully incorporate rapidly emerging evidence-based approaches in their routine clinical practice, which highlights the need for continuous medical education for healthcare providers regardless of their age group or occupying position. Secondly, the quality of CSU patient care may be, to a large degree, compromised by the financial constraints and insufficient level of training of the treating physicians in developing countries. It warrants more research into pharmacoeconomics and sustainability of up-to-date CSU treatments and further propagation of new knowledge about CSU etiopathogenesis and treatment among practicing physicians of different specialties and healthcare authorities in different countries. Finally, urticaria guidelines themselves can be a cause for suboptimal patient care (for example, unclear recommendations and discrepancies between the guidelines). Thus, on the one hand, urticaria guidelines should be flexible enough to allow a physician to tailor the treatment to the unique profile of each patient and circumstances specific to their country of residence; on the other hand, further standardization and dissemination of guidelines can increase adherence among physicians worldwide and result in better patient care.
Additional file
The web-based questionnaire. (PDF 180 kb)
Acknowledgements
The authors thank the World Allergy Organization for the resources to carry out and publish this paper.
Funding
The study was supported by WAO (the dissemination of the questionnaire via WAO online resources, the hosting of the questionnaire and data and funding the authors’ APC fees to the publisher of the WAO Journal).
Abbreviations
- AAAAI
American Academy of Allergy, Asthma & Immunology
- ASST
Autologous serum skin test
- CBC
Complete blood count
- CRP
C-reactive protein
- CSU
Chronic spontaneous urticaria
- ESR
Erythrocyte sedimentation rate
- JMG
Junior Member Group
- sgAHs
Second generation antihistamines
- TSH
Thyroid-stimulating hormone
- WAO
World Allergy Organization
Authors’ contributions
PK drafted the article and contributed to the conception and design of the study, analysis and interpretation of data. MM made substantial contributions to the conception and design of the study and revised the article critically for important intellectual content. PK, DP, RD, MC, LKT, DLP, AGE, DAA, VD and KW contributed substantially to the development of the questionnaire used for the online survey. MSB and IA made substantial contributions to the revisions of the manuscript. All authors contributed to the interpretation of the data, critical revisions, and approved the final version of the manuscript for submission.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests related to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s40413-018-0193-4) contains supplementary material, which is available to authorized users.
Contributor Information
Pavel Kolkhir, Phone: +7 (495) 609-14-00, Email: pavel.kolkhir@yandex.ru.
Dmitry Pogorelov, Email: rusmeddoc@gmail.com.
Razvigor Darlenski, Email: darlenski@gmail.com.
Marco Caminati, Email: ma.caminati@gmail.com.
Luciana Kase Tanno, Email: luciana.tanno@gmail.com.
Duy Le Pham, Email: drduypham87@gmail.com.
Alexei Gonzalez-Estrada, Email: gonzalez.alexei@gmail.com.
Darío Antolín-Amérigo, Email: dario.antolin@gmail.com.
Ves Dimov, Email: ves.dimov@gmail.com.
Karsten Weller, Email: karsten.weller@charite.de.
Mario Sánchez-Borges, Email: sanchezbmario@gmail.com.
Ignacio Ansotegui, Email: ignacioansotegui@gmail.com.
Marcus Maurer, Email: marcus.maurer@charite.de.
References
- 1.Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 2.Gaig P, Olona M, Munoz Lejarazu D, Caballero MT, Dominguez FJ, Echechipia S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14(3):214–220. [PubMed] [Google Scholar]
- 3.Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, et al. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174(5):996–1004. doi: 10.1111/bjd.14470. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell BF, Lawlor F, Simpson J, Morgan M, Greaves MW. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136(2):197–201. doi: 10.1111/j.1365-2133.1997.tb14895.x. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy. 2011;66(3):317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 6.Fine LM, Bernstein JA. Urticaria guidelines: consensus and controversies in the European and American guidelines. Curr Allergy Asthma Rep. 2015;15(6):30. doi: 10.1007/s11882-015-0535-z. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Cherrez A, Maurer M, Weller K, Calderon JC, Simancas-Racines D, Cherrez Ojeda I. Knowledge and management of chronic spontaneous urticaria in Latin America: a cross-sectional study in Ecuador. World Allergy Organ J. 2017;10(1):21. doi: 10.1186/s40413-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller K, Viehmann K, Brautigam M, Krause K, Siebenhaar F, Zuberbier T, et al. Management of chronic spontaneous urticaria in real life--in accordance with the guidelines? A cross-sectional physician-based survey study. J Eur Acad Dermatol Venereol. 2013;27(1):43–50. doi: 10.1111/j.1468-3083.2011.04370.x. [DOI] [PubMed] [Google Scholar]
- 10.Rimoldi M, Rossi O, Rota N. State of the art of chronic spontaneous urticaria in Italy: a multicentre survey to evaluate physicians’ and patients’ perspectives. BMJ Open. 2016;6(10):e012378. doi: 10.1136/bmjopen-2016-012378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adedeji AR, Tumbo J, Govender I. Adherence of doctors to a clinical guideline for hypertension in Bojanala district, north-West Province, South Africa. Afr J Prim Health Care Fam Med. 2015;7(1). Art. #776, 6 pages. [DOI] [PMC free article] [PubMed]
- 12.Kenefick H, Lee J, Fleishman V. In: Improving Physician Adherence to Clinical Practice Guidelines: Barriers and Strategies for Change. Everett W, editor. USA: New England Healthcare Institute; 2008. p. 55. [Google Scholar]
- 13.Tudiver F, Herbert C, Goel V. Why don't family physicians follow clinical practice guidelines for cancer screening? CMAJ. 1998;159(7):797–798. [PMC free article] [PubMed] [Google Scholar]
- 14.Christian AH, Mills T, Simpson SL, Mosca L. Quality of cardiovascular disease preventive care and physician/practice characteristics. J Gen Intern Med. 2006;21(3):231–237. doi: 10.1111/j.1525-1497.2006.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson RL, Jr, Fleischer AB, Jr, Feldman SR. Allergists and dermatologists have far more expertise in caring for patients with urticaria than other specialists. J Am Acad Dermatol. 2000;43(6):1084–1091. doi: 10.1067/mjd.2000.109305. [DOI] [PubMed] [Google Scholar]
- 16.Wu CH, Ardern-Jones MR, Eren E, Venter C. An observational study of the diagnosis and Management of Chronic Urticaria in the UK. Int Arch Allergy Immunol. 2015;167(1):1–8. doi: 10.1159/000430442. [DOI] [PubMed] [Google Scholar]
- 17.Maurice-Tison S, Pouyanne J, Doutre MS. General practitioners, dermatologists, allergists, and the management of chronic urticaria. Results of a practice survey. Ann Dermatol Venereol. 2003;130(1):1S160–1S173. [PubMed] [Google Scholar]
- 18.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al., The EAACI/GA(2)LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. The 2017 Revision and Update. Allergy. 2018. 10.1111/all.13397. [Epub ahead of print]. [DOI] [PubMed]
- 19.Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139(6):1772–1781. doi: 10.1016/j.jaci.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Ortonne JP. Chronic urticaria: a comparison of management guidelines. Expert Opin Pharmacother. 2011;12(17):2683–2693. doi: 10.1517/14656566.2011.629189. [DOI] [PubMed] [Google Scholar]
- 21.Guillen-Aguinaga S, Jauregui Presa I, Aguinaga-Ontoso E, Guillen-Grima F, Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. Br J Dermatol. 2016;175(6):1153–1165. doi: 10.1111/bjd.14768. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer M, Jauregui I, Bartra J, Davila I, del Cuvillo A, Montoro J, et al. Chronic urticaria: do urticaria nonexperts implement treatment guidelines? A survey of adherence to published guidelines by nonexperts. Br J Dermatol. 2009;160(4):823–827. doi: 10.1111/j.1365-2133.2008.08985.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilches P, Calderon JC, Cherrez A, Cherrez Ojeda I. Omalizumab for chronic urticaria in Latin America. World Allergy Organ J. 2016;9(1):36. doi: 10.1186/s40413-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck LA, Bernstein JA, Maurer M. A review of international recommendations for the diagnosis and management of chronic Urticaria. Acta Derm Venereol. 2017;97(2):149–158. doi: 10.2340/00015555-2496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The web-based questionnaire. (PDF 180 kb)


