Abstract
Atrophy of the brain grey matter (GM) is an accepted and important feature of multiple sclerosis (MS). However, its accurate measurement is hampered by various technical, pathological and physiological factors. As a consequence, it is challenging to investigate the role of GM atrophy in the disease process as well as the effect of treatments that aim to reduce neurodegeneration. In this paper we discuss the most important challenges currently hampering the measurement and interpretation of GM atrophy in MS. The focus is on measurements that are obtained in individual patients rather than on group analysis methods, because of their importance in clinical trials and ultimately in clinical care. We discuss the sources and possible solutions of the current challenges, and provide recommendations to achieve reliable measurement and interpretation of brain GM atrophy in MS.
Abbreviations: BET, brain extraction tool; CNS, central nervous system; CTh, cortical thickness; DGM, deep grey matter; DTI, diffusion tensor imaging; FA, fractional anisotropy; GM, grey matter; MRI, magnetic resonance imaging; MS, multiple sclerosis; TE, echo time; TI, inversion time; TR, repetition time; VBM, voxel-based morphometry; WM, white matter
Keywords: Multiple sclerosis, Brain atrophy, Grey matter, Magnetic resonance imaging
Highlights
-
•
Accurate measurement of brain GM atrophy in MS is hampered by various physiological, pathological and technical factors.
-
•
Challenges to achieve accuracy in quantification and interpretation of atrophy in MS are discussed.
-
•
Recommendations on how to achieve reliable measurement and interpretation of GM atrophy in MS are suggested.
1. Introduction
Multiple sclerosis (MS) is a disease of the central nervous system (CNS) that typically affects both the brain and the spinal cord (Compston and Coles, 2008). In addition to the well-known focal inflammatory, demyelinating lesions that are typically seen in the white matter (WM), MS also causes degeneration and consequent volume loss of grey matter (GM), which is often referred to as GM atrophy. The focus of the present paper will be on measurement of GM atrophy in the brain, which is currently widely accessible as opposed to spinal cord GM atrophy measurement. Nevertheless, GM atrophy in the spinal cord is an important topic, accurate measurement of which can contribute to better understanding MS and quantitatively characterizing its effects in individual patients in trials or clinical care. However, technical improvements are needed to obtain high resolution images at reduced scan times and to increase coverage of the spinal cord, before broader application is feasible (Kearney et al., 2015).
Brain GM atrophy is typically measured in vivo from standard 3D T1-weighted images acquired by magnetic resonance imaging (MRI), using automated analysis methods. Studies have demonstrated volume decrease of subcortical GM structures and volume or thickness decrease of cortical regions (Bergsland et al., 2012; Pareto et al., 2016). GM atrophy in the brain has been shown to be associated with cognitive impairment in MS (Nocentini et al., 2014; Riccitelli et al., 2011). Furthermore, atrophy of the deep GM (DGM) structures, such as thalamus, has been shown to be strongly related to clinical and cognitive decline (Bermel et al., 2003; Houtchens et al., 2007; Pagani et al., 2005).
This paper, after reviewing the measurement techniques, will discuss the most important challenges for reliable measurement and interpretation of brain GM atrophy in patients with MS. We divide these challenges into two categories. Challenges in the category “Pathology, physiology and treatment effects” are: 1) Unclear pathological substrate, 2) Evolution of GM atrophy, 3) Influence of physiological variability, and 4) Evaluation of treatment response. “Measurement challenges”, the second category, includes: 1) Influence of WM lesions, 2) Influence of atrophy itself, 3) Influence of other pathology, and 4) Technical variability. For each section, we provide specific recommendations (summarized in Box 1) to improve measurement and interpretation of GM atrophy in individual MS patients.
Box 1. Recommendations for improving measurement and interpretation of GM atrophy.
Pathology, physiology and treatment effects
-
➢
The prominent role of neuronal and neuro-axonal loss in GM atrophy in MS should be confirmed or refuted by acquiring more post mortem MRI- and histopathology data.
-
➢
Sensitivity of GM atrophy measurement techniques for different anatomical brain regions should be quantitatively evaluated, to allow reliable studies of the temporal evolution of GM atrophy across the MS brain.
-
➢
More data on the pathological substrate of cortical and subcortical grey matter atrophy in MS should be obtained.
-
➢
The relation between GM atrophy and other pathological changes in both WM and GM should be investigated longitudinally, both in treated and untreated patients.
-
➢
Next to other factors, the statistical analysis should be corrected also for the time of day of MRI scan.
-
➢
Contributions to the variability of GM atrophy measures due to other physiological factors such as smoking needs to be studied.
-
➢
The mechanism by which common MS medications impact GM atrophy in different brain regions should be elucidated.
Measurement challenges
-
➢
Publically available reference datasets with annotations are needed to serve as benchmarks for analysis software.
-
➢
To minimize the effect of WM lesions on GM atrophy measures, improved (multi-class) segmentation and/or lesion-filling methods are required.
-
➢
To minimize systematic differences related to scanners and sequence parameters, standardized imaging and image analysis pipelines are warranted.
-
➢
To improve image co-registration in the presence of pronounced brain atrophy, a multi-channel approach is recommended, but the precise implementation requires further study and validation.
-
➢
Research is needed on the influence of subtle and diffuse MS abnormalities on image analysis steps involved in GM atrophy measurement.
Alt-text: Box 1
2. Review of measurement techniques
Various methods have been developed to measure anatomical changes of the brain. Some of these techniques produce single-subject measurements while others, such as voxel-based morphometry and vertexwise analyses, provide group-based statistical tests. In the current paper we are predominantly interested in the application of brain GM atrophy measures in individual MS patients, because single-subject measures are the most relevant outcome measures in clinical care and clinical treatment trials. Therefore, this review discusses cortical and deep GM atrophy measurement techniques with an emphasis on methods that produce single-subject results, while briefly reviewing relevant aspects of methods for group-level analysis used in the literature.
2.1. Cortical atrophy measurement techniques
There is convincing evidence that MS involves structural cortical changes resulting in atrophy (Charil et al., 2007; Lansley et al., 2013; Narayana et al., 2012; Prinster et al., 2006; Ramasamy et al., 2009; Sailer et al., 2003). Current MRI technology provides a valuable tool for detecting and monitoring cortical atrophy early in the disease (Chard et al., 2002; Chard et al., 2004; Dalton et al., 2004; De et al., 2003; Sastre-Garriga et al., 2004; Sepulcre et al., 2006). For reproducible and accurate estimation of cortical thickness (CTh), a number of automated methods have been developed, such as FreeSurfer (Dale et al., 1999; Fischl and Dale, 2000) (http://surfer.nmr.mgh.harvard.edu/fswiki/), CIVET (Zijdenbos et al., 2002), and CLADA (Cortical Longitudinal Atrophy Detection Algorithm) (Nakamura et al., 2011). For measurement of cortical volume, other methods can be used such as SIENAX (Smith et al., 2002) (cross-sectional pipeline of SIENA; Structural Image Evaluation using Normalization of Atrophy), SIENAX multi-time-point (SIENAX-MTP) (Dwyer et al., 2014), and SPM (Statistical Parametric Mapping software) (Ashburner and Friston, 2005) or IBASPM (Individual Brain Atlas using SPM) (Alemán-Gómez et al., 2006).
Automated methods for measuring CTh in MRI may be categorized as surface-based, voxel-based, or a combination of the two categories (Hutton et al., 2008). Currently, the majority of published studies that employ automated methods for measuring CTh have been using surface-based techniques for which the reliability has been investigated (Han et al., 2006; Lerch and Evans, 2005). With these methods, the thickness is calculated at each point on the extracted cortical surface and surface-based smoothing is applied to the results. The benefit of this type of smoothing is that it prevents the problem of averaging across different banks of sulci and gyri (Hutton et al., 2009). However, this is only the case if the surface has been accurately extracted. In general, these methods are limited by the difficulty in obtaining homogeneous GM boundaries due to the variation of contrast within the image that results from MRI (B1) field inhomogeneity. This problem has been partly solved by using 3D T1-weighted images and by implementing tools for intensity non-uniformity correction.
FreeSurfer (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2002) calculates the CTh after inflation of the folded cortical surface using tools for the analysis and visualization of structural MRI data (Dale et al., 1999; Fischl and Dale, 2000). It contains a fully automated structural imaging stream, although visual inspection and manual editing (if necessary) are recommended at several stages in the pipeline. For analysis of changes over time, FreeSurfer has a longitudinal pipeline (Reuter et al., 2012).
CIVET (Zijdenbos et al., 2002) is a series of algorithms for corticometric analysis of MR images including the extraction and analysis of cortical surfaces from MR images, and other volumetric and corticometric functions (Zijdenbos et al., 2002). In contrast to other software such as FreeSurfer, CIVET is a fully automated method, which can be an advantage for the analysis of large MR data sets.
CLADA (Zijdenbos et al., 2002) measures changes in CTh using explicit deformable models. Like Freesurfer, CLADA allows the concurrent analysis of multiple time points, thereby improving quantification of within-patient change. The algorithm creates a deformable model of the cerebral cortex consisting of two explicit surfaces based on a combined image representing all time points, and then deforms the model for each individual time point (Nakamura et al., 2011). An advantage of CLADA compared to other methods is that it can be applied to 2D images with large slice thickness (3–5 mm), which can be advantageous for retrospective analysis of clinical or trial data.
Voxel-based cortical thickness (VBCT) measurements do not require the construction of a three-dimensional surface model (Han et al., 2006; Hutton et al., 2009). This approach is a complementary technique to the voxel-based morphometry (VBM) (Ashburner and Friston, 2000; Good et al., 2001), which uses a mass-univariate approach with voxel-wise comparisons of the local volume of GM across brain regions between two groups of subjects. VBCT maps may be particularly advantageous for the analysis of conditions that are associated with cortical thinning, because the local topography of the GM is used to assign an absolute metric to GM voxels (Hutton et al., 2009). In contrast, in VBM the local GM volume is confounded by the convolution of the brain in a given region. However, using both VBM and VBCT together might be a useful tool for understanding the topography and time-course of cortical atrophy in MS (Hutton et al., 2009).
Other methods such as SIENAX (Smith et al., 2002) (part of FSL, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki) and (IBA)SPM (Alemán-Gómez et al., 2006; Ashburner and Friston, 2005) primarily use intensity histogram based voxelwise GM partial volume estimation. These have been combined with anatomical atlases to separately quantify the total volume of GM in cortical regions (Dupuy et al., 2016; Horakova et al., 2008). SIENAX is the cross-sectional pipeline of the SIENA method (Smith et al., 2002). Based on voxel intensities it estimates partial volume fractions of GM, WM and cerebrospinal fluid (CSF) for each voxel. The longitudinal SIENA only quantifies overall brain volume change (based on the shift of the parenchyma-CSF border over time), and therefore does not measure GM or WM volume change separately. Two different extensions of SIENAX to perform direct longitudinal analysis of GM or WM atrophy have recently been developed, both of which combine cross-sectional and longitudinal modelling: SIENAX-MTP (Dwyer et al., 2014) and SIENA-XL (Battaglini et al., 2016).
SPM (http://www.fil.ion.ucl.ac.uk/spm/software/; (Penny et al., 2006)) is a MATLAB toolbox that performs segmentation providing probability maps for GM, WM, and CSF. Individual Brain Atlas using SPM (IBASPM, http://www.thomaskoenig.ch/Lester/ibaspm.htm) is an SPM extension that uses the SPM segmentation and then warps a standardized atlas to the individual scan to classify the image into different anatomical structures, in this case the cortical GM (Alemán-Gómez et al., 2006). The GM volume of each structure is then quantified. Both non-linear registration and GM segmentation processes are performed using SPM subroutines; SPM12 includes symmetric registration procedures (Ashburner and Ridgway, 2012).
Longitudinal GM or WM volume changes can also be quantified using Jacobian integration (Ashburner et al., 1998; Nakamura et al., 2014). This is done by quantifying the total net amount of contraction or expansion of a selected region in an image during an accurate non-linear registration between the images of the first and last time points. Jacobian integration applied to longitudinal GM atrophy analysis has shown to reduce variability related to measurement error compared to other commonly used methods, thus potentially requiring smaller sample sizes (Nakamura et al., 2014).
2.2. Deep GM atrophy measurement techniques
DGM atrophy has been shown to be associated with the development of definite MS and disability progression in early relapsing remitting MS (RRMS) (Mesaros et al., 2011; Rocca et al., 2010; Sepulcre et al., 2006; Zivadinov et al., 2013a; Zivadinov et al., 2013b). However, measurement of DGM atrophy remains challenging. To date, several available tools have been developed for segmenting DGM structures, some of which have been widely used in MS research. These methods are based on combinations of various approaches and techniques such as shape analysis (FIRST, FMRIB's Integrated Registration and Segmentation Tool (Patenaude et al., 2011) and TOADS, Topology-preserving Anatomy-Driven Segmentation, https://www.nitrc.org/projects/toads-cruise/), voxelwise classification (FreeSurfer, TOADS), voxelwise partial volume estimation (SPM/IBASPM), topological constraints (TOADS) and brain atlases (IBASPM, FreeSurfer, TOADS). Here we describe the principles of each method.
FIRST (part of the FSL toolbox) is a fully automated model-based segmentation software (Patenaude et al., 2011). The model was trained for 15 different structures using 336 manually-labelled T1-weighted MR images. FIRST models the outer surface of each DGM structure as a mesh using models derived from the reference images and the local intensity profiles around the mesh. Finally, while taking into account the presence of neighboring structures, it labels voxels to assign them to different DGM structures.
FreeSurfer (Dale et al., 1999; Fischl et al., 1999) includes volumetric segmentation of DGM structures. The processing briefly includes registration to the Talairach space and intensity normalization (Sled et al., 1998), skull-stripping (Segonne et al., 2004), and labeling predefined brain regions including DGM and deep WM using a probabilistic atlas (Fischl et al., 2002; Fischl et al., 2004). DGM volume change over time can be analyzed through the longitudinal stream (Reuter et al., 2012), which uses an unbiased within-subject template that is created using inverse consistent registration (Reuter et al., 2010), improving reliability of volume change measurements (Reuter et al., 2012).
TOADS is another automated brain segmentation tool that includes segmentation of DGM structures (Bazin and Pham, 2007; Shiee et al., 2010). TOADS uses a topological atlas to constrain not only the topology of each brain structure, but also the relations between the structures. In addition, it uses a shape atlas and intensity based tissue classification.
As described above for cortical regions, the methods based on estimating voxelwise partial volume or probability such as SPM or SIENAX, can be used together with anatomical atlases as in IBASPM to extract GM volumes of subcortical regions.
2.3. Group-level analysis methods
Two types of group-level analysis methods are important in the context of this work. Voxel-based morphometry (VBM) is an extension of voxelwise segmentation-based techniques such as SIENAX or SPM, in which GM segmentation maps are transformed into a common space. Similarly, individual vertexwise cortical thickness maps can be transformed into a common space, for example in FreeSurfer. One can then investigate differences between groups and correlations with other variables for each voxel or vertex in the common space separately (followed by appropriate corrections for multiple testing). A major strength of such methods is that they allow the study of anatomical patterns of atrophy without any a priori selection of regions of interest. Salient regions and anatomical patterns can, therefore, be detected from the data itself. A limitation of such techniques is that in order to detect an effect, e.g. a common GM atrophy pattern in MS patients, there has to be substantial overlap between the true GM atrophy patterns of individual patients in the study. For example, if a patient group has reduced mean global GM volume compared to controls but the locations of GM atrophy in the brain vary, the voxel-based and vertexwise group-level analysis methods may not detect any significant atrophy patterns. This limitation should be considered when interpreting such group-level VBM or vertexwise studies.
3. Challenges of pathology, physiology and treatment effects
3.1. Unclear pathological substrate
Despite the importance of GM atrophy in MS, the underlying pathological substrate is not well known. The extent and severity of neuronal loss is less severe in MS than in a ‘classical’ neurodegenerative disease such as Alzheimer's disease (Dutta et al., 2011; Haider et al., 2014; Peterson et al., 2001). Nevertheless, a recent post mortem MRI and histopathology study found that regional cortical atrophy is predominantly explained by a combination of (mild) neuro-axonal loss and neuronal shrinkage (Popescu et al., 2015). The cortical atrophy was not related to myelin loss (Popescu et al., 2015), despite (subpial) cortical demyelination being frequent and extensive in (progressive) MS (Geurts et al., 2005; Kutzelnigg and Lassmann, 2005). Another study found that neuronal and axonal density did not differ between subjects with normally myelinated GM and those with subpial cortical lesions (Klaver et al., 2015), suggesting that neurodegeneration in MS cerebral cortex is largely independent of cortical demyelination. It has also been shown that the observed cortical volume reduction of around 10% in MS patients is independent of the presence of cortical lesions (Pareto et al., 2016; Wegner et al., 2006). Although the work by Popescu et al., 2015 is valuable as a direct local association study between MRI atrophy measures and pathological characteristics, the number of patients is small and the results have to be confirmed independently. It is therefore imperative that more data is obtained on the pathological substrate of cortical and subcortical grey matter atrophy in MS.
3.2. Evolution of GM atrophy
It is not clear which brain regions are most likely to develop GM atrophy in the early phase of MS, whether the atrophic process is mainly primary or secondary, or to what extent its evolution differs between disease types (Bishop et al., 2017; Calabrese et al., 2007; Fisher et al., 2008). In part this arises from methodological issues such as unknown sensitivities of different measurement methods to atrophy in different GM regions. The concurrent evolution of focal lesions and other pathological changes, together with the varying and partly unknown effects of different treatments on GM atrophy, further complicate understanding the natural evolution of GM in MS.
In relapse-onset MS, GM atrophy has been observed already in the earliest phases of the disease (Bergsland et al., 2012; Calabrese et al., 2007). Moreover, GM atrophy may differ between disease types (Fisher et al., 2008; Sicotte et al., 2008), as well as between patients with and without evidence of disease activity (Nygaard et al., 2015). Some evidence also exists of early and eloquent GM atrophy in specific regions: for example, in primary progressive MS, the involvement of the cingulate cortex was found to occur throughout the disease course (Eshaghi et al., 2014). Also, significant GM volume loss occurred in the right precuneus in relapsing-remitting MS patients with progressive disability (Hofstetter et al., 2014). Moreover, atrophy of specific DGM structures, most notably the thalamus, also occurs in MS patients (Bishop et al., 2017; Houtchens et al., 2007; Schoonheim et al., 2015; Solomon et al., 2017). Atrophy of the thalamus appears to occur especially early and prominently, to be worse in men, and to be associated with cognitive decline (Schoonheim et al., 2015). However, it remains unclear to what extent these observations could be biased by the size and partially limited contrast with the neighboring WM of the thalamus. In general, more work is needed to validate the dynamic changes and anatomical patterns observed in previous studies. Specifically, the sensitivity of current techniques to measure atrophy of different anatomical regions should be quantitatively evaluated.
Although studies have shown that the relation between GM pathology and WM lesions is an important factor in MS, this relation remains to be fully elucidated (Geurts et al., 2012). Global WM lesion volumes are associated with both global GM atrophy (Charil et al., 2007; Roosendaal et al., 2011; Steenwijk et al., 2014) and local GM atrophy (Charil et al., 2007; Sailer et al., 2003). Furthermore, there are similarities between the average anatomical distribution of GM atrophy and the distributions of both existing WM lesions (Bodini et al., 2009; Muhlau et al., 2013; Riccitelli et al., 2012) and new WM lesions (Battaglini et al., 2009; Bendfeldt et al., 2010). The relation of local GM atrophy to lesional and non-lesional damage in connected WM tracts has only been partially explored (Bergsland et al., 2015; Louapre et al., 2016; Steenwijk et al., 2015) and further studies are needed to clarify this.
How GM atrophy is related to other pathological changes inside the GM has been less investigated. Longitudinal studies, in larger groups and other clinical disease sub-types, are needed to fully answer the questions about temporal and causal relations between GM atrophy and other MS pathology, both in natural evolution and on treatment.
3.3. Influence of physiological variability
Effects of physiological variability could affect measurement of GM atrophy; brain volumes have been shown to be affected by, among other, food intake (Roberto et al., 2011), steroids, (excess) body fat (Janowitz et al., 2015), alcohol abuse (Thayer et al., 2016), and tobacco smoking (Sutherland et al., 2016). One study (Sampat et al., 2010) showed that, when using whole-brain volume change measures in an MS trial, physiological fluctuations may increase required sample sizes by a factor of 5 compared to sample sizes based on scan-rescan reproducibility error only.
Different physiological sources of variability also have been investigated separately. Brain volumes decrease systematically during the day, and by correcting for these diurnal fluctuations, required sample sizes in a typical trial could be reduced by 2.6% (Nakamura et al., 2015). Brain volumes also differed significantly between hydrated and dehydrated states induced by 16 h of no fluid intake (Duning et al., 2005). Although such extreme situations seem unlikely in MS clinical practice, the effect of hydration status should be taken into account. In all, clear guidelines towards diminishing the impact of physiological variations are needed before GM atrophy measurements can be considered in clinical practice.
3.4. Evaluation of treatment response
The effect of disease modifying therapies may be different for WM than GM atrophy measures (Filippi et al., 2014; Fisher et al., 2016; Perez-Miralles et al., 2015). Moreover, currently available therapies predominantly target processes involved in formation of new WM lesions rather than atrophy. Therefore, tissue-specific brain volume monitoring could provide differential insights on treatment effects and response (De Stefano et al., 2014) and help in the development of new treatments. WM volume changes after starting treatment are heavily dependent on baseline inflammation (Vidal-Jordana et al., 2016; Vidal-Jordana et al., 2013). Therefore, immediate decreases in WM volume after therapy onset need to be interpreted with great caution. This initial pseudo-atrophy effect does not seem to occur for GM (Prinster et al., 2006). In addition, GM volume changes have been shown to be relatively insensitive to baseline inflammation (Fisher et al., 2016; Vidal-Jordana et al., 2016; Vidal-Jordana et al., 2013). Whether a whole-tissue or a region-specific approach should be preferred, remains to be elucidated and would depend on the acquisition of an a-priori knowledge of the pathological substrate as well as the potential effects of any given drug on the specific regions.
Another important aspect is the influence of pre-existing damage on GM atrophy development. As indicated above (“Evolution of GM atrophy”), there is currently in general insufficient data on the relation between lesions and other damage in the WM on the one hand, and (subsequent) atrophy of the GM on the other. Also, specifically whether or not GM atrophy resulting from pre-existing WM damage may be halted by treatment remains to be investigated. Studies should be performed to provide such data, because otherwise interpretation of data obtained in patients on treatment may be incorrect. If the secondary atrophy due to pre-existing damage cannot be halted, a situation may arise in which in a treated patient new GM atrophy is adequately suppressed by treatment, but GM atrophy still progresses merely as a late result of pre-existing WM lesions. In such a case, in clinical treatment trials, these effects of pre-existing WM damage on current GM atropy may lead to an underestimation of the effectiveness of the treatment under evaluation. Similarly, in a clinical setting, the treating physician may incorrectly conclude that the treatment is not effective on GM atrophy in that patient. It is therefore of great importance to obtain data to understand the relation between WM damage and GM atrophy and allowing, ultimately, the disentanglement of new GM atrophy from GM atrophy that results from pre-existing WM damage.
4. Measurement challenges
4.1. Influence of WM lesions
Several papers (Battaglini et al., 2012; Chard et al., 2010; Gonzalez-Villa et al., 2017; Nakamura and Fisher, 2009) have shown that WM lesions that are hypointense on T1-weighted images can affect the assessment of GM volumes, as illustrated in Fig. 1. One paper noted an artificial GM volume underestimation in the presence of lesions with an intensity between GM and WM, also when lesions were correctly reclassified as WM (Nakamura and Fisher, 2009). Another study, using synthetic data, found that the extent and the direction of the GM estimation error depended strongly on the lesion load, on the degree of hypointensity, and on the partial volume model used in the segmentation algorithm (Battaglini et al., 2012).
Fig. 1.
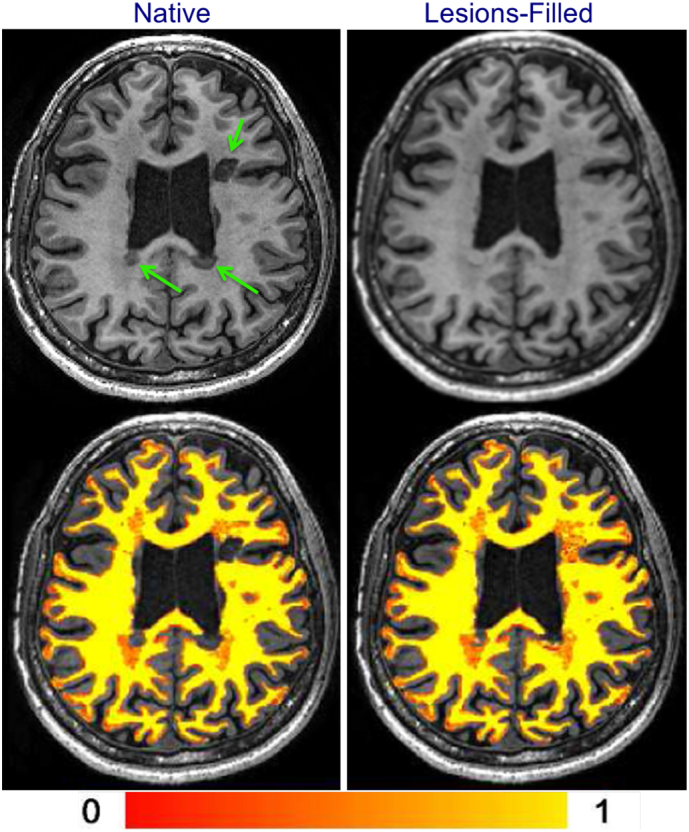
The effect of WM lesions on the segmentation using FSL-FAST software. Left column: The native (non-filled) T1-weighted image and its WM segmentation. Right column: T1-weighted image after lesion-filling and its WM segmentation. Arrows point to obvious WM lesions. Color bar indicates the WM partial volume estimate.
Reproduced in modified form with permission from Popescu et al. (2014).
The presence of T1 hypointense lesions, especially along tissue interfaces, severely affects non-linear registration of images (Di Perri et al., 2008; Sdika and Pelletier, 2009). In VBM-type experiments, these local distortions in registrations led to an overestimation of GM loss in the cortex and in the DGM structures (Ceccarelli et al., 2012).
The most commonly used solution to these WM lesion-induced problems is the so-called ‘lesion-filling’ or lesion ‘inpainting’, which replaces WM lesion voxels with intensities similar to normal WM, using parametric or numerical approaches to reproduce global or local WM intensity histograms (Battaglini et al., 2012; Chard et al., 2010; Gelineau-Morel et al., 2012; Prados et al., 2014; Sdika and Pelletier, 2009). Lesion-filling significantly reduces the error due to the presence of lesions in evaluating cortical and DGM volumes (Prados et al., 2014). Moreover, lesion-filling significantly improves the accuracy of local CTh estimation, thereby also improving the whole-brain average CTh measurement (Magon et al., 2014). A limitation of the lesion-filling approach is that it requires accurate high-resolution segmentation of the lesions. Nonetheless, even with low-resolution lesion segmentations it is possible to measure the whole-brain GM volume from lesion-filled images with good accuracy, although analysis of smaller GM regions may be hampered by the remaining lesion voxels (Popescu et al., 2014). Because of the importance of this issue, integrated segmentation algorithms by which not only WM, GM and CSF but also lesions can be segmented could be beneficial.
4.2. Influence of atrophy itself
Paradoxically, the presence of brain atrophy distorts measurement of that same brain atrophy, by affecting various steps in the analysis pipelines used.
Many analysis pipelines involve the removal of non-brain tissue when measuring atrophy; the commonly used BET software (part of FSL) was found to require fairly extreme parameter settings to perform accurately in images of MS patients compared to manual brain extraction (Popescu et al., 2012). Besides BET, other methods have also been proposed to achieve accurate non-brain tissue in atrophic patient scans including multi-atlas skull stripping (Doshi et al., 2013), OptiBET (Lutkenhoff et al., 2014), BrainMAPS (Leung et al., 2011), BEAST (Eskildsen et al., 2012), and STEPS (Cardoso et al., 2013).
The accuracy of image co-registration, another step used in almost all methods, also can be affected by the presence of brain atrophy (Pereira et al., 2010), as illustrated in Fig. 2. Proposed approaches to overcome this problem include multi-channel registration using fractional anisotropy (FA) maps from diffusion tensor imaging (DTI) alongside T1-weighted images (Ceritoglu et al., 2009; Roura et al., 2015), and inclusion of a-priori knowledge of ventricular shapes in the presence of atrophy (Djamanakova et al., 2013). These are expected to improve GM atrophy quantification by better matching of templates, priors, atlases and regions of interest; these approaches should be evaluated correctly for better use and understanding.
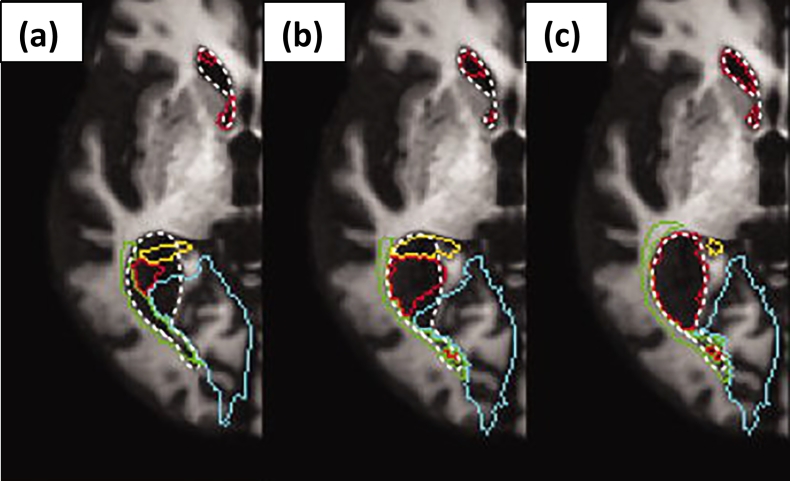
Fig. 2.
An example of image misregistration due to atrophy and a possible method for its improvement based on multi-channel mapping. Warped contours of lateral ventricles and surrounding regions are superimposed on T1-weighted image as color overlays. (a) The red outline indicates the contour of the lateral ventricle warped to the target image space by default parameters FNIRT. This registration compared to the actual location of the lateral ventricle (white dotted line) is poor. (b) Taking into account the large deformations necessary to co-register images in the presence of severe atrophy (method proposed by Djamanakova et al. (2013)) slightly improved the registration. (c) Using a dual-channel approach incorporating a coarse ventricle segmentation in the target image space improved the lateral ventricle registration substantially.
Reproduced in modified form with permission from Djamanakova et al. (2013).
As a very specific example, it has been shown that atrophy of the WM affects cortical surface curvature in MS (Deppe et al., 2014), which could lead to incorrect interpretations of GM atrophy metrics based on cortical surface reconstruction, depending on the method used.
4.3. Influence of other pathology
Beyond WM lesions and brain atrophy, focal GM lesions are prominent in MS (Geurts et al., 2005; Kutzelnigg et al., 2005). The presence of such GM lesions also could have a significant effect on GM volume estimations, as some of them may still be visible in the sequences frequently used for atrophy measurement such as MPRAGE (Bagnato et al., 2006; Nelson et al., 2014). There is also abundant evidence of diffuse GM and WM changes from post mortem and in vivo studies (Raz et al., 2010; Seewann et al., 2009; van Munster et al., 2015; Vrenken et al., 2010). The effect of these diffuse WM and GM abnormalities on quantification of atrophy measures, to the best of our knowledge, has not been investigated and could affect these measurements. First, it is interesting that even a small volume of WM lesions can have a substantial effect on GM partial volume estimates throughout the brain for segmentation techniques in which intensity distributions of different tissue classes are modelled (with or without priors), such as FSL-FAST (Popescu et al., 2014). Second, subtle age or disease related changes of the WM-GM contrast at the white matter – cortical border have been shown to influence cortical thickness measurement systematically in a way that can be corrected statistically, at least at group level (see Fig. 3) (Westlye et al., 2009). There is also evidence that diffuse GM pathological abnormalities are more widespread and possibly independent of atrophy (Khaleeli et al., 2007; Mallik et al., 2015). How these subtle GM and WM abnormalities affect segmentation and registration algorithms of the atrophy measurement pipelines is unknown and this warrants further research.
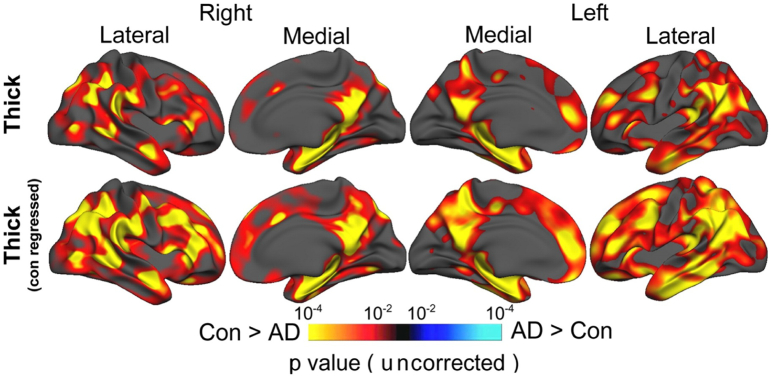
Fig. 3.
The effect of grey/white contrast on cortical thickness measurement using FreeSurfer. Statistical p maps thresholded at p < 10−2 superimposed on a template brain's semi-inflated surface showing the results from GLMs testing the difference between Alzheimer's disease (AD) and controls (Con). Warm colors denote areas with significantly thinner cortex in AD compared to controls. Adjusting for grey/white tissue contrast (bottom row) increases sensitivity to the AD-control differences in cortical thickness over large portions of the brain compared to results obtained when not adjusting for this contrast (top row).
Adopted with permission from Westlye et al. (2009).
4.4. Technical variability
MRI-derived GM atrophy measurements can exhibit large variability due to causes related only to the MR image acquisition and analysis; these problems are not specific to MS but occur equally in other diseases that cause GM atrophy.
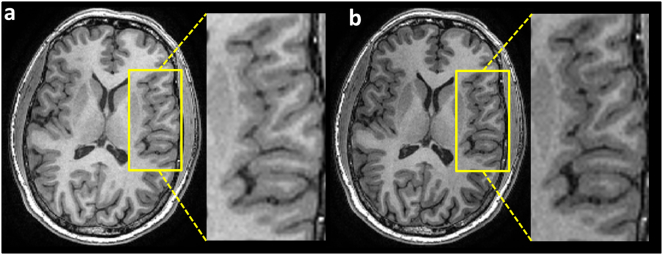
Concerning image acquisition, usually (near)isotropic 3D T1-weighted images with similar acquisition parameters are used to quantify brain atrophy. Nevertheless, remaining minor differences can systematically affect GM atrophy measurements. As an example, Fig. 4 shows how signal intensity and image contrast can change noticeably even when only a single sequence parameter (the inversion time) is slightly altered (from 450 to 400 ms). Similarly, different scanners can give different volumes or volume changes and this has been reported – this can occur even when using similar or even identical acquisition parameters (Durand-Dubief et al., 2012). Large systematic differences have been observed between scanners for CTh and GM volume measurements (Biberacher et al., 2016). Also, it has been shown that even with identical scanners and imaging protocols, systematic differences of GM volumes can occur (Takao et al., 2011).
Fig. 4.
MPRAGE T1-weighted images of a healthy volunteer to compare the image contrast and signal intensity by modifying only one imaging parameter, i.e. the inversion time (TI). (a) TI = 450 ms and (b) TI = 400 ms.
Focusing on image analysis reproducibility, using the ADNI1 data (http://adni.loni.usc.edu/), which contains a within-session rescan, i.e. without repositioning, the reproducibility error was non-negligible compared to typical atrophy rates for a commonly used whole-brain software, SIENA (Cover et al., 2011). Moreover, for both FSL-FIRST and FreeSurfer the within-session reproducibility error for measuring 1-year hippocampal volume change was of similar size as the typical atrophy rates (Mulder et al., 2014). A recent study (Cover et al., 2016) found that some image analysis methods exhibited better reproducibility of 1-year hippocampal volume change than others, leading to improved statistical power and smaller required sample sizes in treatment trials. Reproducibility of caudate nucleus, putamen, amygdala, globus pallidus, and thalamus atrophy rates were found to be about 1.5–3.5 times higher then the mean measured volume change across 500 Alzheimer's disease, mild cognitive impairment and healthy elderly subjects for both FreeSurfer and FSL-FIRST (Meijerman et al., 2018). In a smaller group in MS, 3 T imaging yielded slight improvements of subcortical volume reproducibility compared to 1.5 T by using FSL-FIRST (Chu et al., 2017).
With respect to differences between different image analysis methods, in a longitudinal study, robustness across sites was better for registration-based techniques than for segmentation-based techniques (Durand-Dubief et al., 2012), although the data suggests that the improved robustness across sites may come at the cost of a somewhat reduced sensitivity to the volume change between time points. The two most widely used methods for GM-VBM (FSL-VBM and SPM-VBM) gave substantially different results when comparing RRMS patients to healthy controls, which was especially noticeable when considering separate brain regions based on an anatomical atlas (Battaglini et al., 2009). For volume measures in individual MS patients, volumetric agreement between different analysis methods (FreeSurfer, SPM, and FSL) was only moderate to good for most DGM structures and cortical regions, and correlations with clinical and cognitive measures varied with the analysis method used (Popescu et al., 2016). Lastly, FreeSurfer GM volume and CTh measurements depend on operating system, FreeSurfer version and workstation type (Gronenschild et al., 2012).
A benchmark dataset is needed to establish and test the robustness of analysis methods in the context of repeated scans within or between scanners. Data acquired in MS patients is of special interest, because that would allow users to concurrently investigate the effects of lesions and other pathology occurring in MS on the GM atrophy measurement.
5. Conclusions
We reviewed the state of the art regarding the most urgent challenges in the measurement of GM atrophy in MS, and provided recommendations to overcome them (summarized in Box 1). Difficulties associated with physiological variability or with technical variability between scans and image analysis methods extend beyond MS. Therefore, the broader brain research community would benefit from addressing these challenges adequately. Some disease-specific technical challenges also exist: while solutions are available to minimize the influence of WM lesions on brain atrophy, the influence of diffuse damage and of GM lesions remains to be elucidated. Such improvements are needed to allow evaluation of GM atrophy independent of other MS pathology. This would be achieved when the evolution of GM in untreated and treated patients, as well as its relation to the other MS pathology can be properly understood. This understanding and the technical improvements are also prerequisites to the use of GM atrophy measures in the clinical routine.
Acknowledgements
This paper is the outcome of the MAGNIMS workshop “Measuring cortical and deep gray matter atrophy in MS”, which was kindly supported by the Multiple Sclerosis International Federation (www.msif.org), the Dutch MS Research Foundation (www.msresearch.nl), the journal “Nederlands Tijdschrift voor Geneeskunde” (www.ntvg.nl), the Neuroscience Campus Amsterdam (www.neurosciencecampus-amsterdam.nl), and the VU University medical center. Frederik Barkhof is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
References
- Alemán-Gómez Y., Melie-García L., Valdés-Hernandez P. IBASPM: toolbox for automatic parcellation of brain structures 12th annual meeting of the Organization for human brain mapping, June 11–15, 2006, Florence, Italy. Available on CD-rom in. NeuroImage. 2006;27(1) [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Ridgway G.R. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front. Neurosci. 2012;6:197. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Hutton C., Frackowiak R., Johnsrude I., Price C., Friston K. Identifying global anatomical differences: deformation-based morphometry. Hum. Brain Mapp. 1998;6:348–357. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<348::AID-HBM4>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato F., Butman J.A., Gupta S., Calabrese M., Pezawas L., Ohayon J.M., Tovar-Moll F., Riva M., Cao M.M., Talagala S.L., McFarland H.F. In vivo detection of cortical plaques by MR imaging in patients with multiple sclerosis. AJNR Am. J. Neuroradiol. 2006;27:2161–2167. [PMC free article] [PubMed] [Google Scholar]
- Battaglini M., Giorgio A., Stromillo M.L., Bartolozzi M.L., Guidi L., Federico A., De Stefano N. Voxel-wise assessment of progression of regional brain atrophy in relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2009;282:55–60. doi: 10.1016/j.jns.2009.02.322. [DOI] [PubMed] [Google Scholar]
- Battaglini M., Jenkinson M., De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum. Brain Mapp. 2012;33:2062–2071. doi: 10.1002/hbm.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglini M., Jenkinson M., De Stefano N. Paper Presented at: 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2016. SIENA-XL for the assessment of gray matter and white matter volume changes in clinical studies of patients with multiple sclerosis. [Google Scholar]
- Bazin P.L., Pham D.L. Topology-preserving tissue classification of magnetic resonance brain images. IEEE Trans. Med. Imaging. 2007;26:487–496. doi: 10.1109/TMI.2007.893283. [DOI] [PubMed] [Google Scholar]
- Bendfeldt K., Blumhagen J.O., Egger H., Loetscher P., Denier N., Kuster P., Traud S., Mueller-Lenke N., Naegelin Y., Gass A., Hirsch J., Kappos L., Nichols T.E., Radue E.W., Borgwardt S.J. Spatiotemporal distribution pattern of white matter lesion volumes and their association with regional grey matter volume reductions in relapsing-remitting multiple sclerosis. Hum. Brain Mapp. 2010;31:1542–1555. doi: 10.1002/hbm.20951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland N., Horakova D., Dwyer M.G., Dolezal O., Seidl Z.K., Vaneckova M., Krasensky J., Havrdova E., Zivadinov R. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR Am. J. Neuroradiol. 2012;33:1573–1578. doi: 10.3174/ajnr.A3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland N., Lagana M.M., Tavazzi E., Caffini M., Tortorella P., Baglio F., Baselli G., Rovaris M. Corticospinal tract integrity is related to primary motor cortex thinning in relapsing-remitting multiple sclerosis. Mult. Scler. 2015;21:1771–1780. doi: 10.1177/1352458515576985. [DOI] [PubMed] [Google Scholar]
- Bermel R.A., Innus M.D., Tjoa C.W., Bakshi R. Selective caudate atrophy in multiple sclerosis: a 3D MRI parcellation study. Neuroreport. 2003;14:335–339. doi: 10.1097/00001756-200303030-00008. [DOI] [PubMed] [Google Scholar]
- Biberacher V., Schmidt P., Keshavan A., Boucard C.C., Righart R., Samann P., Preibisch C., Frobel D., Aly L., Hemmer B., Zimmer C., Henry R.G., Muhlau M. Intra- and interscanner variability of magnetic resonance imaging based volumetry in multiple sclerosis. NeuroImage. 2016;142:188–197. doi: 10.1016/j.neuroimage.2016.07.035. [DOI] [PubMed] [Google Scholar]
- Bishop C.A., Newbould R.D., Lee J.S., Honeyfield L., Quest R., Colasanti A., Ali R., Mattoscio M., Cortese A., Nicholas R., Matthews P.M., Muraro P.A., Waldman A.D. Analysis of ageing-associated grey matter volume in patients with multiple sclerosis shows excess atrophy in subcortical regions. Neuroimage. Clin. 2017;13:9–15. doi: 10.1016/j.nicl.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodini B., Khaleeli Z., Cercignani M., Miller D.H., Thompson A.J., Ciccarelli O. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum. Brain Mapp. 2009;30:2852–2861. doi: 10.1002/hbm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese M., Atzori M., Bernardi V., Morra A., Romualdi C., Rinaldi L., McAuliffe M.J., Barachino L., Perini P., Fischl B., Battistin L., Gallo P. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J. Neurol. 2007;254:1212–1220. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- Cardoso M.J., Leung K., Modat M., Keihaninejad S., Cash D., Barnes J., Fox N.C., Ourselin S. Alzheimer's Disease Neuroimaging Initiative. STEPS: similarity and truth estimation for propagated segmentations and its application to hippocampal segmentation and brain parcelation. Med. Image Anal. 2013;17:671–684. doi: 10.1016/j.media.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A., Jackson J.S., Tauhid S., Arora A., Gorky J., Dell'Oglio E., Bakshi A., Chitnis T., Khoury S.J., Weiner H.L., Guttmann C.R., Bakshi R., Neema M. The impact of lesion in-painting and registration methods on voxel-based morphometry in detecting regional cerebral gray matter atrophy in multiple sclerosis. AJNR Am. J. Neuroradiol. 2012;33:1579–1585. doi: 10.3174/ajnr.A3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceritoglu C., Oishi K., Li X., Chou M.C., Younes L., Albert M., Lyketsos C., van Zijl P.C., Miller M.I., Mori S. Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. NeuroImage. 2009;47:618–627. doi: 10.1016/j.neuroimage.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard D.T., Griffin C.M., Parker G.J., Kapoor R., Thompson A.J., Miller D.H. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- Chard D.T., Griffin C.M., Rashid W., Davies G.R., Altmann D.R., Kapoor R., Barker G.J., Thompson A.J., Miller D.H. Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult. Scler. 2004;10:387–391. doi: 10.1191/1352458504ms1050oa. [DOI] [PubMed] [Google Scholar]
- Chard D.T., Jackson J.S., Miller D.H., Wheeler-Kingshott C.A. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J. Magn. Reson. Imaging. 2010;32:223–228. doi: 10.1002/jmri.22214. [DOI] [PubMed] [Google Scholar]
- Charil A., Dagher A., Lerch J.P., Zijdenbos A.P., Worsley K.J., Evans A.C. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. NeuroImage. 2007;34:509–517. doi: 10.1016/j.neuroimage.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Chu R., Hurwitz S., Tauhid S., Bakshi R. Automated segmentation of cerebral deep gray matter from MRI scans: effect of field strength on sensitivity and reliability. BMC Neurol. 2017;17:172. doi: 10.1186/s12883-017-0949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cover K.S., van Schijndel R.A., van Dijk B.W., Redolfi A., Knol D.L., Frisoni G.B., Barkhof F., Vrenken H., neuGrid, Alzheimer's Disease Neuroimaging, I Assessing the reproducibility of the SienaX and Siena brain atrophy measures using the ADNI back-to-back MP-RAGE MRI scans. Psychiatry Res. 2011;193:182–190. doi: 10.1016/j.pscychresns.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Cover K.S., van Schijndel R.A., Versteeg A., Leung K.K., Mulder E.R., Jong R.A., Visser P.J., Redolfi A., Revillard J., Grenier B., Manset D., Damangir S., Bosco P., Vrenken H., van Dijk B.W., Frisoni G.B., Barkhof F., Alzheimer's Disease Neuroimaging Initiative, n Reproducibility of hippocampal atrophy rates measured with manual, FreeSurfer, AdaBoost, FSL/FIRST and the MAPS-HBSI methods in Alzheimer's disease. Psychiatry Res. 2016;252:26–35. doi: 10.1016/j.pscychresns.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dalton C.M., Chard D.T., Davies G.R., Miszkiel K.A., Altmann D.R., Fernando K., Plant G.T., Thompson A.J., Miller D.H. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–1107. doi: 10.1093/brain/awh126. [DOI] [PubMed] [Google Scholar]
- De Stefano N., Airas L., Grigoriadis N., Mattle H.P., O'Riordan J., Oreja-Guevara C., Sellebjerg F., Stankoff B., Walczak A., Wiendl H., Kieseier B.C. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs. 2014;28:147–156. doi: 10.1007/s40263-014-0140-z. [DOI] [PubMed] [Google Scholar]
- De S.N., Matthews P.M., Filippi M., Agosta F., De L.M., Bartolozzi M.L., Guidi L., Ghezzi A., Montanari E., Cifelli A., Federico A., Smith S.M. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- Deppe M., Marinell J., Kramer J., Duning T., Ruck T., Simon O.J., Zipp F., Wiendl H., Meuth S.G. Increased cortical curvature reflects white matter atrophy in individual patients with early multiple sclerosis. Neuroimage. Clin. 2014;6:475–487. doi: 10.1016/j.nicl.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Perri C., Battaglini M., Stromillo M.L., Bartolozzi M.L., Guidi L., Federico A., De Stefano N. Voxel-based assessment of differences in damage and distribution of white matter lesions between patients with primary progressive and relapsing-remitting multiple sclerosis. Arch. Neurol. 2008;65:236–243. doi: 10.1001/archneurol.2007.51. [DOI] [PubMed] [Google Scholar]
- Djamanakova A., Faria A.V., Hsu J., Ceritoglu C., Oishi K., Miller M.I., Hillis A.E., Mori S. Diffeomorphic brain mapping based on T1-weighted images: improvement of registration accuracy by multichannel mapping. J. Magn. Reson. Imaging. 2013;37:76–84. doi: 10.1002/jmri.23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi J., Erus G., Ou Y., Gaonkar B., Davatzikos C. Multi-atlas skull-stripping. Acad. Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duning T., Kloska S., Steinstrater O., Kugel H., Heindel W., Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- Dupuy S.L., Tauhid S., Hurwitz S., Chu R., Yousuf F., Bakshi R. The effect of dimethyl fumarate on cerebral gray matter atrophy in multiple sclerosis. Neurol. Ther. 2016;2:215–229. doi: 10.1007/s40120-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand-Dubief F., Belaroussi B., Armspach J.P., Dufour M., Roggerone S., Vukusic S., Hannoun S., Sappey-Marinier D., Confavreux C., Cotton F. Reliability of longitudinal brain volume loss measurements between 2 sites in patients with multiple sclerosis: comparison of 7 quantification techniques. AJNR Am. J. Neuroradiol. 2012;33:1918–1924. doi: 10.3174/ajnr.A3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R., Chang A., Doud M.K., Kidd G.J., Ribaudo M.V., Young E.A., Fox R.J., Staugaitis S.M., Trapp B.D. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 2011;69:445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer M.G., Bergsland N., Zivadinov R. Improved longitudinal gray and white matter atrophy assessment via application of a 4-dimensional hidden Markov random field model. NeuroImage. 2014;90:207–217. doi: 10.1016/j.neuroimage.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Eshaghi A., Bodini B., Ridgway G.R., Garcia-Lorenzo D., Tozer D.J., Sahraian M.A., Thompson A.J., Ciccarelli O. Temporal and spatial evolution of grey matter atrophy in primary progressive multiple sclerosis. NeuroImage. 2014;86:257–264. doi: 10.1016/j.neuroimage.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen S.F., Coupe P., Fonov V., Manjon J.V., Leung K.K., Guizard N., Wassef S.N., Ostergaard L.R., Collins D.L., Alzheimer's Disease Neuroimaging, I BEaST: brain extraction based on nonlocal segmentation technique. NeuroImage. 2012;59:2362–2373. doi: 10.1016/j.neuroimage.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A., Pagani E., De S.N., Jeffery D., Kappos L., Montalban X., Boyko A.N., Comi G. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J. Neurol. Neurosurg. Psychiatry. 2014;85:851–858. doi: 10.1136/jnnp-2013-306132. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J., Makris N., Segonne F., Quinn B.T., Dale A.M. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl. 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fisher E., Lee J.C., Nakamura K., Rudick R.A. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann. Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- Fisher E., Nakamura K., Lee J.C., You X., Sperling B., Rudick R.A. Effect of intramuscular interferon beta-1a on gray matter atrophy in relapsing-remitting multiple sclerosis: a retrospective analysis. Mult. Scler. 2016;22:668–676. doi: 10.1177/1352458515599072. [DOI] [PubMed] [Google Scholar]
- Gelineau-Morel R., Tomassini V., Jenkinson M., Johansen-Berg H., Matthews P.M., Palace J. The effect of hypointense white matter lesions on automated gray matter segmentation in multiple sclerosis. Hum. Brain Mapp. 2012;33:2802–2814. doi: 10.1002/hbm.21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts J.J., Bo L., Pouwels P.J., Castelijns J.A., Polman C.H., Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am. J. Neuroradiol. 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- Geurts J.J., Calabrese M., Fisher E., Rudick R.A. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 2012;11:1082–1092. doi: 10.1016/S1474-4422(12)70230-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Villa S., Valverde S., Cabezas M., Pareto D., Vilanova J.C., Ramio-Torrenta L., Rovira A., Oliver A., Llado X. Evaluating the effect of multiple sclerosis lesions on automatic brain structure segmentation. Neuroimage. Clin. 2017;15:228–238. doi: 10.1016/j.nicl.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gronenschild E.H., Habets P., Jacobs H.I., Mengelers R., Rozendaal N., van O.J., Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L., Simeonidou C., Steinberger G., Hametner S., Grigoriadis N., Deretzi G., Kovacs G.G., Kutzelnigg A., Lassmann H., Frischer J.M. Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry. 2014;85:1386–1395. doi: 10.1136/jnnp-2014-307712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hofstetter L., Naegelin Y., Filli L., Kuster P., Traud S., Smieskova R., Mueller-Lenke N., Kappos L., Gass A., Sprenger T., Penner I.K., Nichols T.E., Vrenken H., Barkhof F., Polman C., Radue E.W., Borgwardt S.J., Bendfeldt K. Progression in disability and regional grey matter atrophy in relapsing-remitting multiple sclerosis. Mult. Scler. 2014;20:202–213. doi: 10.1177/1352458513493034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horakova D., Cox J.L., Havrdova E., Hussein S., Dolezal O., Cookfair D., Dwyer M.G., Seidl Z., Bergsland N., Vaneckova M., Zivadinov R. Evolution of different MRI measures in patients with active relapsing-remitting multiple sclerosis over 2 and 5 years: a case-control study. J. Neurol. Neurosurg. Psychiatry. 2008;79:407–414. doi: 10.1136/jnnp.2007.120378. [DOI] [PubMed] [Google Scholar]
- Houtchens M.K., Benedict R.H., Killiany R., Sharma J., Jaisani Z., Singh B., Weinstock-Guttman B., Guttmann C.R., Bakshi R. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–1223. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- Hutton C., De V.E., Ashburner J., Deichmann R., Turner R. Voxel-based cortical thickness measurements in MRI. NeuroImage. 2008;40:1701–1710. doi: 10.1016/j.neuroimage.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C., Draganski B., Ashburner J., Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz D., Wittfeld K., Terock J., Freyberger H.J., Hegenscheid K., Volzke H., Habes M., Hosten N., Friedrich N., Nauck M., Domanska G., Grabe H.J. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. NeuroImage. 2015;122:149–157. doi: 10.1016/j.neuroimage.2015.07.086. [DOI] [PubMed] [Google Scholar]
- Kearney H., Miller D.H., Ciccarelli O. Spinal cord MRI in multiple sclerosis–diagnostic, prognostic and clinical value. Nat. Rev. Neurol. 2015;11:327–338. doi: 10.1038/nrneurol.2015.80. [DOI] [PubMed] [Google Scholar]
- Khaleeli Z., Cercignani M., Audoin B., Ciccarelli O., Miller D.H., Thompson A.J. Localized grey matter damage in early primary progressive multiple sclerosis contributes to disability. NeuroImage. 2007;37:253–261. doi: 10.1016/j.neuroimage.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Klaver R., Popescu V., Voorn P., Galis-de Graaf Y., van der Valk P., de Vries H.E., Schenk G.J., Geurts J.J. Neuronal and axonal loss in normal-appearing gray matter and subpial lesions in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2015;74:453–458. doi: 10.1097/NEN.0000000000000189. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Lassmann H. Cortical lesions and brain atrophy in MS. J. Neurol. Sci. 2005;233:55–59. doi: 10.1016/j.jns.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Lucchinetti C.F., Stadelmann C., Bruck W., Rauschka H., Bergmann M., Schmidbauer M., Parisi J.E., Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Lansley J., Mataix-Cols D., Grau M., Radua J., Sastre-Garriga J. Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci. Biobehav. Rev. 2013;37:819–830. doi: 10.1016/j.neubiorev.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Lerch J.P., Evans A.C. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Leung K.K., Barnes J., Modat M., Ridgway G.R., Bartlett J.W., Fox N.C., Ourselin S., Alzheimer's Disease Neuroimaging, I Brain MAPS: an automated, accurate and robust brain extraction technique using a template library. NeuroImage. 2011;55:1091–1108. doi: 10.1016/j.neuroimage.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Govindarajan S.T., Gianni C., Cohen-Adad J., Gregory M.D., Nielsen A.S., Madigan N., Sloane J.A., Kinkel R.P., Mainero C. Is the relationship between cortical and white matter pathologic changes in multiple sclerosis spatially specific? A multimodal 7-T and 3-T MR imaging study with surface and tract-based analysis. Radiology. 2016;278:524–535. doi: 10.1148/radiol.2015150486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhoff E.S., Rosenberg M., Chiang J., Zhang K., Pickard J.D., Owen A.M., Monti M.M. Optimized brain extraction for pathological brains (optiBET) PLoS One. 2014;9 doi: 10.1371/journal.pone.0115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magon S., Gaetano L., Chakravarty M.M., Lerch J.P., Naegelin Y., Stippich C., Kappos L., Radue E.W., Sprenger T. White matter lesion filling improves the accuracy of cortical thickness measurements in multiple sclerosis patients: a longitudinal study. BMC Neurosci. 2014;15:106. doi: 10.1186/1471-2202-15-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik S., Muhlert N., Samson R.S., Sethi V., Wheeler-Kingshott C.A., Miller D.H., Chard D.T. Regional patterns of grey matter atrophy and magnetisation transfer ratio abnormalities in multiple sclerosis clinical subgroups: a voxel-based analysis study. Mult. Scler. 2015;21:423–432. doi: 10.1177/1352458514546513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerman A., Amiri H., Steenwijk M.D., Jonker M.A., van Schijndel R.A., Cover K.S., Vrenken H., Alzheimer's Disease Neuroimaging, I Reproducibility of deep gray matter atrophy rate measurement in a large multicenter dataset. AJNR Am. J. Neuroradiol. 2018;39:46–53. doi: 10.3174/ajnr.A5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesaros S., Rocca M.A., Pagani E., Sormani M.P., Petrolini M., Comi G., Filippi M. Thalamic damage predicts the evolution of primary-progressive multiple sclerosis at 5 years. AJNR Am. J. Neuroradiol. 2011;32:1016–1020. doi: 10.3174/ajnr.A2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlau M., Buck D., Forschler A., Boucard C.C., Arsic M., Schmidt P., Gaser C., Berthele A., Hoshi M., Jochim A., Kronsbein H., Zimmer C., Hemmer B., Ilg R. White-matter lesions drive deep gray-matter atrophy in early multiple sclerosis: support from structural MRI. Mult. Scler. 2013;19:1485–1492. doi: 10.1177/1352458513478673. [DOI] [PubMed] [Google Scholar]
- Mulder E.R., de Jong R.A., Knol D.L., van Schijndel R.A., Cover K.S., Visser P.J., Barkhof F., Vrenken H., Alzheimer's Disease Neuroimaging, I Hippocampal volume change measurement: quantitative assessment of the reproducibility of expert manual outlining and the automated methods FreeSurfer and FIRST. NeuroImage. 2014;92:169–181. doi: 10.1016/j.neuroimage.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Fisher E. Segmentation of brain magnetic resonance images for measurement of gray matter atrophy in multiple sclerosis patients. NeuroImage. 2009;44:769–776. doi: 10.1016/j.neuroimage.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Fox R., Fisher E. CLADA: cortical longitudinal atrophy detection algorithm. NeuroImage. 2011;54:278–289. doi: 10.1016/j.neuroimage.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Guizard N., Fonov V.S., Narayanan S., Collins D.L., Arnold D.L. Jacobian integration method increases the statistical power to measure gray matter atrophy in multiple sclerosis. Neuroimage. Clin. 2014;4:10–17. doi: 10.1016/j.nicl.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Brown R.A., Narayanan S., Collins D.L., Arnold D.L., Alzheimer's Disease Neuroimaging, I Diurnal fluctuations in brain volume: statistical analyses of MRI from large populations. NeuroImage. 2015;118:126–132. doi: 10.1016/j.neuroimage.2015.05.077. [DOI] [PubMed] [Google Scholar]
- Narayana P.A., Govindarajan K.A., Goel P., Datta S., Lincoln J.A., Cofield S.S., Cutter G.R., Lublin F.D., Wolinsky J.S. Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. Neuroimage. Clin. 2012;2:120–131. doi: 10.1016/j.nicl.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson F., Poonawalla A., Datta S., Wolinsky J., Narayana P. Is 3D MPRAGE better than the combination DIR/PSIR for cortical lesion detection at 3T MRI? Mult. Scler. Relat. Disord. 2014;3:253–257. doi: 10.1016/j.msard.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Nocentini U., Bozzali M., Spano B., Cercignani M., Serra L., Basile B., Mannu R., Caltagirone C., De Luca J. Exploration of the relationships between regional grey matter atrophy and cognition in multiple sclerosis. Brain Imaging Behav. 2014;8:378–386. doi: 10.1007/s11682-012-9170-7. [DOI] [PubMed] [Google Scholar]
- Nygaard G.O., Celius E.G., de Rodez Benavent S.A., Sowa P., Gustavsen M.W., Fjell A.M., Landro N.I., Walhovd K.B., Harbo H.F. A longitudinal study of disability, cognition and gray matter atrophy in early multiple sclerosis patients according to evidence of disease activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E., Rocca M.A., Gallo A., Rovaris M., Martinelli V., Comi G., Filippi M. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am. J. Neuroradiol. 2005;26:341–346. [PMC free article] [PubMed] [Google Scholar]
- Pareto D., Sastre-Garriga J., Aymerich F.X., Auger C., Tintore M., Montalban X., Rovira A. Lesion filling effect in regional brain volume estimations: a study in multiple sclerosis patients with low lesion load. Neuroradiology. 2016;58:467–474. doi: 10.1007/s00234-016-1654-5. [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W., Friston K., Ashburner J., Kiebel S., Nichols T.E. Academic Press; 2006. Statistical Parametric Mapping: The Analysis of Functional Brain Images. [Google Scholar]
- Pereira J.M., Xiong L., Acosta-Cabronero J., Pengas G., Williams G.B., Nestor P.J. Registration accuracy for VBM studies varies according to region and degenerative disease grouping. NeuroImage. 2010;49:2205–2215. doi: 10.1016/j.neuroimage.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Perez-Miralles F.C., Sastre-Garriga J., Vidal-Jordana A., Rio J., Auger C., Pareto D., Tintore M., Rovira A., Montalban X. Predictive value of early brain atrophy on response in patients treated with interferon beta. Neurol. Neuroimmunol. Neuroinflamm. 2015;2 doi: 10.1212/NXI.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J.W., Bo L., Mork S., Chang A., Trapp B.D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Popescu V., Battaglini M., Hoogstrate W.S., Verfaillie S.C., Sluimer I.C., van Schijndel R.A., van Dijk B.W., Cover K.S., Knol D.L., Jenkinson M., Barkhof F., de Stefano N., Vrenken H., Group, M.S. Optimizing parameter choice for FSL-brain extraction tool (BET) on 3D T1 images in multiple sclerosis. NeuroImage. 2012;61:1484–1494. doi: 10.1016/j.neuroimage.2012.03.074. [DOI] [PubMed] [Google Scholar]
- Popescu V., Ran N.C., Barkhof F., Chard D.T., Wheeler-Kingshott C.A., Vrenken H. Accurate GM atrophy quantification in MS using lesion-filling with co-registered 2D lesion masks. Neuroimage. Clin. 2014;4:366–373. doi: 10.1016/j.nicl.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu V., Klaver R., Voorn P., Galis-de G.Y., Knol D.L., Twisk J.W., Versteeg A., Schenk G.J., V Van D., Barkhof F., de Vries H.E., Vrenken H., Geurts J.J. What drives MRI-measured cortical atrophy in multiple sclerosis? Mult. Scler. 2015;21:1280–1290. doi: 10.1177/1352458514562440. [DOI] [PubMed] [Google Scholar]
- Popescu V., Schoonheim M.M., Versteeg A., Chaturvedi N., Jonker M., Xavier de Menezes R., Gallindo Garre F., Uitdehaag B.M., Barkhof F., Vrenken H. Grey matter atrophy in multiple sclerosis: clinical interpretation depends on choice of analysis method. PLoS One. 2016;11 doi: 10.1371/journal.pone.0143942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados F., Cardoso M.J., MacManus D., Wheeler-Kingshott C.A., Ourselin S. A modality-agnostic patch-based technique for lesion filling in multiple sclerosis. Med. Image Comput. Comput. Assist. Interv. 2014;17:781–788. doi: 10.1007/978-3-319-10470-6_97. [DOI] [PubMed] [Google Scholar]
- Prinster A., Quarantelli M., Orefice G., Lanzillo R., Brunetti A., Mollica C., Salvatore E., Morra V.B., Coppola G., Vacca G., Alfano B., Salvatore M. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. NeuroImage. 2006;29:859–867. doi: 10.1016/j.neuroimage.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Ramasamy D.P., Benedict R.H., Cox J.L., Fritz D., Abdelrahman N., Hussein S., Minagar A., Dwyer M.G., Zivadinov R. Extent of cerebellum, subcortical and cortical atrophy in patients with MS: a case-control study. J. Neurol. Sci. 2009;282:47–54. doi: 10.1016/j.jns.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Raz E., Cercignani M., Sbardella E., Totaro P., Pozzilli C., Bozzali M., Pantano P. Gray- and white-matter changes 1 year after first clinical episode of multiple sclerosis: MR imaging. Radiology. 2010;257:448–454. doi: 10.1148/radiol.10100626. [DOI] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccitelli G., Rocca M.A., Pagani E., Rodegher M.E., Rossi P., Falini A., Comi G., Filippi M. Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum. Brain Mapp. 2011;32:1535–1543. doi: 10.1002/hbm.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccitelli G., Rocca M.A., Pagani E., Martinelli V., Radaelli M., Falini A., Comi G., Filippi M. Mapping regional grey and white matter atrophy in relapsing-remitting multiple sclerosis. Mult. Scler. 2012;18:1027–1037. doi: 10.1177/1352458512439239. [DOI] [PubMed] [Google Scholar]
- Roberto C.A., Mayer L.E., Brickman A.M., Barnes A., Muraskin J., Yeung L.K., Steffener J., Sy M., Hirsch J., Stern Y., Walsh B.T. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int. J. Eat. Disord. 2011;44:406–411. doi: 10.1002/eat.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Mesaros S., Pagani E., Sormani M.P., Comi G., Filippi M. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology. 2010;257:463–469. doi: 10.1148/radiol.10100326. [DOI] [PubMed] [Google Scholar]
- Roosendaal S.D., Bendfeldt K., Vrenken H., Polman C.H., Borgwardt S., Radue E.W., Kappos L., Pelletier D., Hauser S.L., Matthews P.M., Barkhof F., Geurts J.J. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult. Scler. 2011;17:1098–1106. doi: 10.1177/1352458511404916. [DOI] [PubMed] [Google Scholar]
- Roura E., Schneider T., Modat M., Daga P., Muhlert N., Chard D., Ourselin S., Llado X., Gandini Wheeler-Kingshott C. Multi-channel registration of fractional anisotropy and T1-weighted images in the presence of atrophy: application to multiple sclerosis. Funct. Neurol. 2015;30:245–256. doi: 10.11138/FNeur/2015.30.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer M., Fischl B., Salat D., Tempelmann C., Schonfeld M.A., Busa E., Bodammer N., Heinze H.J., Dale A. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–1744. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- Sampat M.P., Healy B.C., Meier D.S., Dell'Oglio E., Liguori M., Guttmann C.R. Disease modeling in multiple sclerosis: assessment and quantification of sources of variability in brain parenchymal fraction measurements. NeuroImage. 2010;52:1367–1373. doi: 10.1016/j.neuroimage.2010.03.075. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J., Ingle G.T., Chard D.T., Ramio-Torrenta L., Miller D.H., Thompson A.J. Grey and white matter atrophy in early clinical stages of primary progressive multiple sclerosis. NeuroImage. 2004;22:353–359. doi: 10.1016/j.neuroimage.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Schoonheim M.M., Hulst H.E., Brandt R.B., Strik M., Wink A.M., Uitdehaag B.M., Barkhof F., Geurts J.J. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology. 2015;84:776–783. doi: 10.1212/WNL.0000000000001285. [DOI] [PubMed] [Google Scholar]
- Sdika M., Pelletier D. Nonrigid registration of multiple sclerosis brain images using lesion inpainting for morphometry or lesion mapping. Hum. Brain Mapp. 2009;30:1060–1067. doi: 10.1002/hbm.20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewann A., Vrenken H., van der Valk P., Blezer E.L., Knol D.L., Castelijns J.A., Polman C.H., Pouwels P.J., Barkhof F., Geurts J.J. Diffusely abnormal white matter in chronic multiple sclerosis: imaging and histopathologic analysis. Arch. Neurol. 2009;66:601–609. doi: 10.1001/archneurol.2009.57. [DOI] [PubMed] [Google Scholar]
- Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sepulcre J., Sastre-Garriga J., Cercignani M., Ingle G.T., Miller D.H., Thompson A.J. Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study. Arch. Neurol. 2006;63:1175–1180. doi: 10.1001/archneur.63.8.1175. [DOI] [PubMed] [Google Scholar]
- Shiee N., Bazin P.L., Ozturk A., Reich D.S., Calabresi P.A., Pham D.L. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. NeuroImage. 2010;49:1524–1535. doi: 10.1016/j.neuroimage.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte N.L., Kern K.C., Giesser B.S., Arshanapalli A., Schultz A., Montag M., Wang H., Bookheimer S.Y. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A., De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Solomon A.J., Watts R., Dewey B.E., Reich D.S. MRI evaluation of thalamic volume differentiates MS from common mimics. Neurol. Neuroimmunol. Neuroinflamm. 2017;4 doi: 10.1212/NXI.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwijk M.D., Daams M., Pouwels P.J., Balk L.J., Tewarie P.K., Killestein J., Uitdehaag B.M., Geurts J.J., Barkhof F., Vrenken H. What explains gray matter atrophy in long-standing multiple sclerosis? Radiology. 2014;272:832–842. doi: 10.1148/radiol.14132708. [DOI] [PubMed] [Google Scholar]
- Steenwijk M.D., Daams M., Pouwels P.J., Balk J., Tewarie P.K., Geurts J.J., Barkhof F., Vrenken H. Unraveling the relationship between regional gray matter atrophy and pathology in connected white matter tracts in long-standing multiple sclerosis. Hum. Brain Mapp. 2015;36:1796–1807. doi: 10.1002/hbm.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M.T., Riedel M.C., Flannery J.S., Yanes J.A., Fox P.T., Stein E.A., Laird A.R. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav. Brain Funct. 2016;12:16. doi: 10.1186/s12993-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao H., Hayashi N., Ohtomo K. Effect of scanner in longitudinal studies of brain volume changes. J. Magn. Reson. Imaging. 2011;34:438–444. doi: 10.1002/jmri.22636. [DOI] [PubMed] [Google Scholar]
- Thayer R.E., Hagerty S.L., Sabbineni A., Claus E.D., Hutchison K.E., Weiland B.J. Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Hum. Brain Mapp. 2016;37:2276–2292. doi: 10.1002/hbm.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Munster C.E., Jonkman L.E., Weinstein H.C., Uitdehaag B.M., Geurts J.J. Gray matter damage in multiple sclerosis: impact on clinical symptoms. Neuroscience. 2015;303:446–461. doi: 10.1016/j.neuroscience.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Vidal-Jordana A., Sastre-Garriga J., Perez-Miralles F., Tur C., Tintore M., Horga A., Auger C., Rio J., Nos C., Edo M.C., Arevalo M.J., Castillo J., Rovira A., Montalban X. Early brain pseudoatrophy while on natalizumab therapy is due to white matter volume changes. Mult. Scler. 2013;19:1175–1181. doi: 10.1177/1352458512473190. [DOI] [PubMed] [Google Scholar]
- Vidal-Jordana A., Sastre-Garriga J., Perez-Miralles F., Pareto D., Rio J., Auger C., Tintore M., Rovira A., Montalban X. Brain volume loss during the first year of interferon-Beta treatment: baseline inflammation and tissue-specific volume dynamics. J. Neuroimaging. 2016;5:532–538. doi: 10.1111/jon.12337. [DOI] [PubMed] [Google Scholar]
- Vrenken H., Seewann A., Knol D.L., Polman C.H., Barkhof F., Geurts J.J. Diffusely abnormal white matter in progressive multiple sclerosis: in vivo quantitative MR imaging characterization and comparison between disease types. AJNR Am. J. Neuroradiol. 2010;31:541–548. doi: 10.3174/ajnr.A1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner C., Esiri M.M., Chance S.A., Palace J., Matthews P.M. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–967. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Espeseth T., Reinvang I., Raz N., Agartz I., Greve D.N., Fischl B., Fjell A.M. Increased sensitivity to effects of normal aging and Alzheimer's disease on cortical thickness by adjustment for local variability in gray/white contrast: a multi-sample MRI study. NeuroImage. 2009;47:1545–1557. doi: 10.1016/j.neuroimage.2009.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos A.P., Forghani R., Evans A.C. Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Bergsland N., Dolezal O., Hussein S., Seidl Z., Dwyer M.G., Vaneckova M., Krasensky J., Potts J.A., Kalincik T., Havrdova E., Horakova D. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. AJNR Am. J. Neuroradiol. 2013;34:1931–1939. doi: 10.3174/ajnr.A3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Havrdova E., Bergsland N., Tyblova M., Hagemeier J., Seidl Z., Dwyer M.G., Vaneckova M., Krasensky J., Carl E., Kalincik T., Horakova D. Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology. 2013;268:831–841. doi: 10.1148/radiol.13122424. [DOI] [PubMed] [Google Scholar]