Abstract
Next-generation sequencing (NGS) technology has led to the ability to test for multiple cancer susceptibility genes simultaneously without significantly increasing cost or turnaround time. With growing usage of multigene testing for inherited cancer, ongoing education for nurses and other health-care providers about hereditary cancer screening is imperative to ensure appropriate testing candidate identification, test selection, and posttest management. The purpose of this review article is to (1) provide an overview of how NGS works to detect germline mutations, (2) summarize the benefits and limitations of multigene panel testing, (3) describe risk categories of cancer susceptibility genes, and (4) highlight the counseling considerations for patients pursuing multigene testing.
Keywords: inherited cancer, multigene panel, next-generation sequencing, hereditary cancer testing
The field of genetic testing for inherited cancer is rapidly evolving. Identification of BRCA1 and BRCA2 paved the way for personalized medicine and created a new paradigm for hereditary breast and ovarian cancer (HBOC) syndrome diagnosis and prevention (Easton, Ford, & Bishop, 1995; Ford et al., 1998). Likewise, discovery of the molecular basis of Lynch syndrome led to a clearer definition of the syndrome’s clinical spectrum and improved our ability to identify individuals at high risk of hereditary colon and endometrial cancers (Espenschied et al., 2017). Identification of mutation carriers is critical, as it enables the administration of interventions that are proven to confer significant survival benefits, particularly for highly penetrant genetic mutations (Domchek, Friebel, et al., 2010).
Beyond these two well-known syndromes, numerous other genes associated with hereditary cancer syndromes have been identified in recent years. Concurrently, advances in next-generation sequencing (NGS) technology have made it possible to test multiple genes simultaneously. Inherited cancer testing is now being offered by a variety of specialists in a multitude of clinical settings for both affected and unaffected individuals (Bellcross et al., 2011).
Genetic counseling and testing is often performed by health-care providers with specialized training in clinical genetics. These providers include board-certified genetic counselors, board-certified clinical geneticists, and advanced practice genetic nurses who are certified by the American Nursing Credentialing Center based on minimum practice hours within the specialty and continuing education hours (www.nursecredentialing.org). However, because of their consistent and sustained interactions with patients, nurses of various backgrounds are well positioned to educate, support, and advocate for patients throughout the genetic testing process by effectively obtaining family histories, identifying test candidates, helping patients and families understand results, and incorporating genetic test results into ongoing care (Calzone et al., 2010). All registered nurses have received broad training in genetics, while master’s-level nurses achieve a more rigorous set of genetic and genomic proficiencies (www.ncsbn.org), but given the rapid changes in this field, ongoing education is imperative. This article reviews how multigene testing is performed using NGS technology and highlights counseling considerations associated with multigene hereditary cancer testing.
Overview of Sequencing Technologies
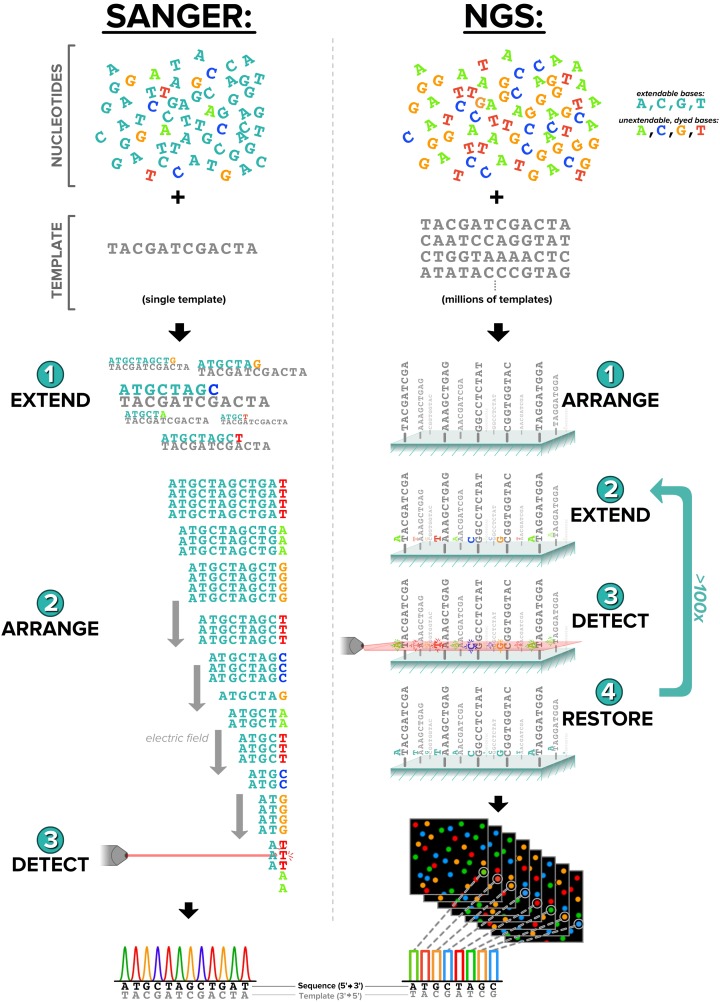
NGS refers to a collection of technologies that allow for the parallel sequencing of millions of DNA fragments. With the previous standard, Sanger sequencing, one molecule is sequenced at a time (Sanger, Nicklen, & Coulson, 1977). Despite their stark differences in throughput, Sanger sequencing and NGS share a similar molecular foundation: Both utilize the cell’s own DNA-copying process to elucidate the sequence of a targeted portion of the genome (Figure 1). A single cycle of NGS involves (1) single-base extension (such that every piece of DNA is now fluorescent at its terminus with a color corresponding to the terminal base), (2) imaging of the fluorescence color at every position on the glass slide, and (3) recycling of the terminal bases such that they are no longer fluorescent and can undergo extension again. NGS sequencers can perform hundreds of such cycles, yielding millions of DNA-fragment sequences, each up to several hundred bases in length. Although at the molecular level NGS and Sanger technologies subtly differ, this difference makes NGS clinically groundbreaking. NGS throughput and quality has greatly decreased both the cost and time involved in sequencing, allowing patients access to testing for multiple genes at a fraction of the cost of traditional single-gene testing.
Figure 1.
Traditional Sanger sequencing compared with next-generation sequencing technology. In each method, dyed, unextendable bases are utilized to create a fluorescent signal that can be translated into a sequence of nucleotides. Subtle differences in the two methods lead to vast differences in throughput. This image was reproduced from figure 1 in Muzzey, Evans, and Lieber (2015). It is licensed under Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Depth of Coverage on NGS
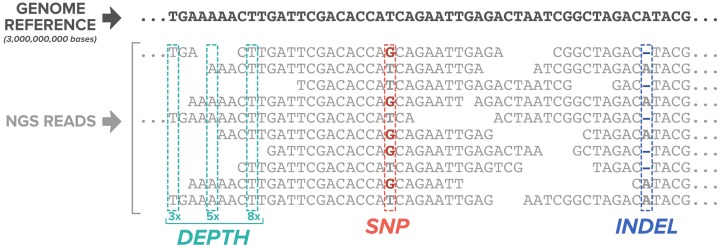
The goal of genetic testing is to resolve the sequence of a patient’s genes such that any pathogenic mutations can be identified. Therefore, it is important to sequence molecules originating from each chromosome many times over to have confidence in the result. Depth, or coverage, refers to the number of sequenced fragments that are generated from a given site in the genome or simply the number of times a certain base was sequenced. In general, as depth increases, so does the confidence that the identified mutation is real. Most commercial laboratories establish a minimum depth between 20× and 50× for targeted inherited cancer panels, which means that at each genomic position, a base is read at least 20–50 times (Chong et al., 2014; Judkins et al., 2015; Lincoln et al., 2015; Vysotskaia et al., 2017).
Variant Identification and Classification
Pathogenic mutations can occur when nucleotides, varying from a single base to thousands, are altered, inserted, or deleted (Figure 2). Any sequencing method used for clinical testing must be able to identify this wide variety of mutation types that can lead to human disease. Once a genetic alteration is identified, a laboratory must then determine the biological significance of that alteration through the process of variant curation. The American College of Medical Genetics and Genomics (ACMG) sets standards for classifying genetic alterations into five categories: pathogenic, likely pathogenic, uncertain significance, likely benign, and benign. These classifications are based on multiple lines of evidence including public and private databases as well as population, computational, functional, segregation, de novo, and allelic data (Richards et al., 2015). Because of the difficulty of developing rigid guidelines that encompass all nuances of genetic variation, there is the potential for differences in classifications between laboratories, and based on analysis of public database submissions, such differences in classification have occurred (Gradishar, Johnson, Brown, Mundt, & Manley, 2017). However, authors report overall high interlaboratory concordance for hereditary cancer results when the clinical actionability of a variant and quality of a database submission is considered (Lincoln et al., 2017). Concordance will continue to increase with ongoing data sharing efforts among researchers and commercial laboratories. Genomic data sharing, through contribution to public databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar), is supported by ACMG as a crucial practice in improving genomic health care (ACMG Board of Directors, 2017).
Figure 2.
Next-generation sequencing reads aligned to a reference genome demonstrate two mutation types: a single-nucleotide polymorphism where a guanine has replaced a thymine and, further downstream, a deletion of an adenine. Depth of coverage of 3×, 5×, and 8× indicates the number of reads at each position. This image was adapted from figure 2 in Muzzey, Evans, and Lieber (2015). It is licensed under Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Modifications were made to include only a portion of the original image.
Confirmation of NGS Findings
In its early usage, NGS was limited by low depth of coverage and error rates that were unsuitable for routine clinical testing. Confirmation of results through traditional methods, such as Sanger sequencing, was common laboratory practice. Although NGS can now be optimized for low error rates and high depth of coverage, some laboratories still rely on orthogonal confirmation of positive findings (Chong et al., 2014; Judkins et al., 2015; Lincoln et al., 2015; Vysotskaia et al., 2017). In a recent study, authors analyzed data from 20,000 clinical samples tested at one commercial laboratory and claimed the necessity of utilizing Sanger sequencing to confirm variants detected by NGS (Mu, Lu, Chen, Li, & Elliott, 2016). However, many other reports have demonstrated that NGS alone produces results with high sensitivity and specificity and that the need for orthogonal confirmation is dependent on the specific NGS assay and is not a general limitation of all NGS protocols (Beck, Mullikin, & Biesecker, 2016; Lincoln et al., 2015; Vysotskaia et al., 2017). Nonetheless, the purpose of orthogonal confirmation is to reduce false positives. It is important, therefore, for laboratories to establish a protocol for addressing the potential for such results, especially in difficult-to-sequence regions of the genome, and to demonstrate the efficacy of these protocols in published validation studies (Mu et al., 2016).
Assessing Quality of NGS Testing
The Center for Medicare and Medicaid Services Clinical Laboratory Improvement Amendment program stipulates standards for analytic validity of NGS assays but does not address the accuracy of individual aspects of these methods such as depth of coverage, bioinformatics for variant calling, results interpretation, or reporting (Robson et al., 2015). Most commercially available assays report greater than 99% sensitivity, specificity, and accuracy but use varying methods to achieve this standard (Chong et al., 2014; Judkins et al., 2015; Lincoln et al., 2015; Vysotskaia et al., 2017). Experts call for clinicians to carefully select a laboratory for testing but give little guidance for how to assess laboratory quality (Fecteau, Vogel, Hanson, & Morrill-Cornelius, 2014). Laboratories can demonstrate their test performance through a published validation study. Analytical sensitivity, analytic specificity, repeatability, and reproducibility are all characteristics that a test validation should establish (Rehm et al., 2013).
Overview of Multigene Testing
Multigene testing for cancer susceptibility became commercially available in 2012 (Dalton & Thompson, 2015). Although many laboratories now offer these tests using similar NGS technologies, panel design (e.g., panel size, how genes are selected for inclusion, design of syndrome-specific panels) can vary greatly between laboratories (Cragun et al., 2014; Domchek, Bradbury, Garber, Offit, & Robson, 2013; Hall et al., 2016; Slavin et al., 2015). The National Comprehensive Cancer Network (NCCN) recommends consideration of multigene testing when a patient’s personal and/or family history is suggestive of an inherited cancer syndrome that could be caused by more than one gene or when an individual has tested negative for a single syndrome, but their personal and/or family history remains suggestive of an inherited cause (NCCN, 2017). However, due to decreasing cost and turnaround time, multigene panels are more often being used as a first-line test for any patient suspected to have an inherited cancer syndrome.
Although BRCA1 and BRCA2 are the most recognized hereditary cancer genes, mutations in these genes only account for 50% of all hereditary breast cancer (Kapoor et al., 2015). Multiple studies have demonstrated that multigene testing identifies more individuals with hereditary breast cancer than testing for BRCA1/2 alone. For individuals suspected of having hereditary breast cancer who previously tested negative for BRCA1/2, testing for additional genes results in a positive result in 2.9–11.4% of cases (Desmond et al., 2015; Kapoor et al., 2015; Kurian et al., 2014; Maxwell et al., 2015; Thompson et al., 2016; Tung, Lin, et al., 2016; Yorczyk, Robinson, & Ross, 2015).
Another common indication for hereditary cancer testing is a personal or family history of colon cancer. In the first large clinical series of patients tested for inherited colorectal cancer (CRC) with multigene panel testing, 10% of high-risk patients tested positive for a mutation in 1 of the 14 genes associated with nine CRC syndromes; the majority of these results were expected to change clinical management (Cragun et al., 2014). In a larger, subsequent series, 1,260 patients with suspected Lynch syndrome were tested using a 25-gene panel, and 185 (14.6%) were found to have a pathogenic mutation; 38% of mutations were in non-Lynch syndrome genes (Yurgelun et al., 2015).
Overlapping phenotypes and complex guidelines may lead to patients being missed with the traditional single-gene testing approach. In a study of 9,000 individuals referred for hereditary cancer testing, 30% of patients tested for Lynch syndrome also met criteria for HBOC testing, and inversely, 7% of patients sent for HBOC testing also met criteria for Lynch syndrome testing (Saam et al., 2015). Multigene tests may offer a simplified and efficient option for clinicians needing to select appropriate genes for a given patient. A growing body of evidence also suggests that adhering to a guidelines-based approach, testing solely for a gene or syndrome for which a patient meets criteria, can lead to missed mutations. In a study of 475 patients referred for genetic counseling and testing at an academic center, 15.6% were positive for a mutation identified through multigene testing (Ricker et al., 2016). Based on provider-recorded differential diagnoses, the authors determined that nearly half of these mutations (47.3%) would have been missed with a single-gene, stepwise approach. The potential missed mutations included those in high-penetrance genes with atypical presentations as well as those in moderate-penetrance genes where the phenotype is less well defined. Other studies have reported similar findings, with 6.6–17% of individuals referred for multigene testing being found to carry a pathogenic mutation in at least one gene (LaDuca et al., 2014; Selkirk et al., 2014; Slavin et al., 2015; Susswein et al., 2016).
Despite the advantages of multigene testing, some argue that new DNA testing technology is outpacing evidence for clinical utility and consideration of proper implementation. Multigene tests may include genes with ill-defined lifetime cancer risks, unclear clinical management guidance, and increased rates of results with uncertain significance (Axilbund, 2016; Domchek et al., 2013; Robson et al., 2015). Further carefully designed studies with large sample sizes are needed to demonstrate the clinical validity and clinical utility of multigene testing (Easton et al., 2015).
Categories of Risk
Cancer predisposition genes included on multigene tests can be grouped into three categories of disease penetrance: high, moderate, and low. There is also a growing category of genes that have been associated with risk of cancer, but the magnitude of this risk is unknown due to limited available data.
The likelihoods of the development of cancer due to genetic mutations can be discussed in terms of relative risk (RR) or lifetime risk (LTR). RR, also called a risk ratio, is the likelihood of an event’s occurrence in one group compared with that in another group. For example, the RR of developing breast cancer for a PALB2 mutation carrier is 5.3 (Easton et al., 2015), meaning that a woman with a pathogenic PALB2 mutation is 5.3 times more likely to develop breast cancer compared to a woman in the general population. Absolute risk refers to the probability of an event happening over a defined period of time, for example, the likelihood of breast cancer manifestation in the next 5 years or over a lifetime.
Calculations of lifetime cancer risks are influenced by risk factors, such as family history, environmental exposures, or hormonal factors, and can overestimate risk of genes where risk ratio may decrease as carriers age (Easton et al., 2015). However, absolute risks may be more relevant in clinical practice since guidelines cite 5-year risk and LTR in recommendations for interventions such as chemoprevention and magnetic resonance imaging (MRI) screening for breast cancer (Saslow et al., 2007). Table 1 displays a list of genes commonly found on multigene cancer tests and respective lifetime cancer risks. It is important to note that individuals who test negative for a genetic mutation may still have an elevated risk of cancer based on personal or family history. Multiple risk models are available to calculate breast cancer risk in families who do not harbor an identifiable mutation (e.g., Claus model, Claus, Risch, & Thompson, 1994, Tyrer–Cuzick model). However, current risk models have limitations, and it is important to understand the specific limitations of each model when calculating risk of a given patient (Amir, Freedman, Seruga, & Evans, 2010).
Table 1.
Genes Commonly Included on Multigene Hereditary Cancer Tests and Their Associated LTRs of Cancer.
Note. LTR = lifetime risk.
aRisk estimates based on limited data. bHighest risk based on the mutation 7271T > G. cMost data utilized for estimating risks based on common 1100delC mutation. dMost data utilized for estimating risks based on Slavic mutation 657del5.
Although risk categories are somewhat arbitrary, high penetrance describes genes conferring an RR greater than 4 times the risk of the general population (Easton et al., 2015). Examples include BRCA1 (RR 11.4, LTR 46–87%) and PALB2 (RR 5.3, LTR 17–58%). Moderate-penetrance genes confer an RR 2–4 times the general population risk. Examples include CHEK2 (RR 3.0, LTR 26–56%) and ATM (RR 2.8, 7–52%; Easton et al., 2015). Genes that confer cancer risks less than 2 times the general population risk may be referred to as low-penetrance genes or risk alleles. Most commercially available multigene tests include only moderate- and high-penetrance genes. However, not all mutations within a moderate- or high-penetrance gene confer equal risks of cancer. For example, a common mutation in CHEK2, 1100delC, confers an approximate 3-fold RR of breast cancer (Weischer, Bojesen, Ellervik, Tybjærg-Hansen, & Nordestgaard, 2008), but another well-documented mutation in the same gene, I157T, is associated with only a 1.58-fold RR of breast cancer (Han, Guo, & Liu, 2013).
Although it is controversial, many commercially available inherited cancer panels also include genes with limited or conflicting evidence for association with cancer risk and the magnitude of that risk. For example, the Mre11-Rad50-Nbs1 (MRN) complex, which includes the genes MRE11A, RAD50, and NBN, is involved in double-strand break repairs. Data have shown that mutations in genes that make up the MRN complex confer either a moderately increased risk of breast cancer (Damiola et al., 2014) or no increased risk (Couch et al., 2017). All three genes are included on many multigene cancer tests, but currently, only the NBN gene has corresponding consensus guidelines for management (NCCN, 2017). More studies are needed to establish the appropriate evidence threshold for inclusion of a specific gene in clinical testing.
Counseling Considerations
Although NGS technology has brought significant benefit to clinical genetics, genetic counseling in the era of panel testing can be more complex than for single-gene testing. These complexities come in the form of new challenges but more commonly stem from traditional challenges in genetic counseling that are amplified by testing for many genes simultaneously. It is important that all clinicians who order hereditary cancer panels are equipped with the knowledge to navigate these complexities, stay up to date with rapidly changing guidelines, and maintain a network of genetics professionals to refer patients to when required.
Pretest Education and Informed Consent Considerations
The informed consent process for genetic testing, whether for single-gene testing or multigene testing, requires the same basic elements: description of its purpose, general information about the genes being tested, potential test results, accuracy, financial considerations, potential for genetic discrimination, confidentiality, actionability of test results for patient and family members, psychological implications of a test result, and alternatives to genetic testing (Riley et al., 2012). Health-care providers or genetics professionals may want to consider modifying this traditional approach to informed consent in the case of multigene testing to ensure that patients have a high-level understanding of the genes for which they are being tested as well as a heightened awareness of the potential to find an unexpected, uncertain, or unclear result (Robson et al., 2015).
Pretest counseling for single-gene or single-syndrome testing historically included a comprehensive discussion of the hereditary cancer gene(s) for which the patient is being tested. For example, a candidate for Lynch syndrome testing may have formerly received a detailed explanation of each of the genes associated with Lynch syndrome, the cancer risks associated with a positive result in each gene, as well as the medical management guidelines for carriers. In order to remain effective, pretest counseling for multigene tests requires modification since it is not feasible to have a comprehensive discussion about each gene on a large panel (Robson et al., 2015). Instead, bucketing genes into categories of risk can provide the necessary education without information overload (Table 2; Fecteau et al., 2014).
Table 2.
Examples of Risk Categories to Aid in Simplification of Pretest Counseling in the Case of Multigene Testing.
| Patient Concern | High-Penetrance Genes | Moderate-Penetrance Genes | Limited Data/Low-Risk Genes |
|---|---|---|---|
| Cancer risk | High cancer risks, likely explains cancer in family | Moderate cancer risks, may explain cancer in family | Unknown cancer risk, may explain cancer in the family |
| Medical-management options | Many options, which may include increased screening, preventative surgery, and chemopreventiona | Options generally involve increased screening beginning at younger ages | Established guidelines not yet available; clinician will make recommendations based on current data and the patient’s personal and family medical history |
| Implications for family members | Recommend testing to all blood relatives. Negative results are considered “true negative” results | Family members should consider genetic testing; family members with negative results may still have increased risk of cancer based on the family history | Unknown implications for family members |
aIt is important to discuss limitations in cancer screening and prevention. It is not possible to effectively screen for all cancer risks conferred by a high-penetrance gene. For example, TP53 mutations cause risk of many cancer types, and screening options are of unknown efficacy.
Discussing the potential for uncertain findings has been an integral part of pretest education and informed consent since the beginning of clinical testing for hereditary cancer (Petrucelli, Lazebnik, Huelsman, & Lazebnik, 2002). In 2002, the variant of uncertain significance (VUS) rate for BRCA1 and BRCA2 alone was as high as 12.8%, but it decreased to 2.1% as knowledge accumulated on the effects of specific genetic alterations (Eggington et al., 2014). The likelihood for a VUS to be identified on a multigene cancer test varies based on many factors including the number of genes tested, the quantity of the region of interest being sequenced (i.e., how much of the introns, or noncoding region, is included), and the ethnic diversity of the population being tested, but it can range from 19.7% to 42% (Selkirk et al., 2014; Slavin et al., 2015). Preparing patients for the possibility of an uncertain result prior to testing may help to normalize this result and alleviate patient anxiety when a VUS is identified. It is important to recognize that, as labs and clinicians collaborate and participate in broad data sharing practices, VUS rates will continue to decrease, which will benefit patient care in genetics (ACMG Board of Directors, 2017).
The potential for genetic discrimination is another aspect of pretest counseling that is not new but has become a more important issue as more individuals, particularly those who have not had cancer, gain access to genetic testing services. Multigene tests are more likely to return a positive result than single-gene tests and therefore may introduce more potential for genetic discrimination. The Genetic Information Nondiscrimination Act (GINA) is a federal law passed in 2008 that provides protections against genetic discrimination in health insurance and employment. The law disallows use of genetic information by a health insurer to make determinations about premiums and states that health insurers may not require subscribers undergo genetic testing. The law does have some limitations in its protections. For example, GINA does not apply to life insurance, long-term care insurance, or disability insurance. It also does not apply to employers with fewer than 15 employees, members of the U.S. military, veterans accessing health care through the U.S. Department of Veterans Affairs, or federal employees enrolled in the Federal Employees Health Benefits program (The GINA, 2008). The Affordable Care Act, the Health Insurance Portability and Accountability Act, and numerous state laws provide additional protections against genetic discrimination in health insurance (National Human Genome Research Institute, 2017). It is important to discuss the protections, and the limitations in protections, with patients prior to their undergoing genetic testing.
Posttest Counseling Considerations
Posttest, a discussion with a patient on results includes sensitivity, specificity, and limitations of the test; the patient’s cancer risks based on the result; medical management recommendations; and implications for family members. Referral to other health-care providers as well as assessment of psychological impacts and provision of emotional support can also be vital parts of this process (Riley et al., 2012). Similar to pretest counseling, posttest counseling with multigene testing is at the core very similar to that for single-gene testing, but it does pose a few unique challenges.
Identifying unexpected results
While rare, “unexpected findings” occur when individuals test positive for a mutation in a gene that is not associated with their personal or family history. For example, pathogenic mutations in CDH1 are associated with increased risk of developing diffuse gastric cancer and lobular breast cancer (Pharoah, Guilford, Caldas, & the International Gastric Cancer Linkage Consortium, 2001), and experts recommend genetic testing in families that have multiple cases of diffuse gastric cancer, diffuse gastric cancer diagnosed in an individual younger than 40 years of age, or individuals with a personal history of both diffuse gastric cancer and lobular breast cancer (Fitzgerald et al., 2010). However, since pan-cancer multigene tests have become available, there have been multiple case reports of patients found to carry pathogenic mutations in CDH1 with no reported personal or family history of diffuse gastric cancer (Huynh & Laukaitis 2016). Risks of cancer and guidelines for management of individuals with CDH1 mutations were developed based on data from families with significant histories of gastric cancer. It is unclear whether CDH1 carriers without a significant history have the same risks and should be managed in the same way. The conservative approach, guided by studies of BRCA mutations identified in low-risk families (Gabai-Kapara et al., 2014), assumes the same level of cancer risk of any CDH1 mutation carrier, but more studies are needed to better understand the appropriate management of these individuals and individuals in other families with unexpected findings from multigene testing.
Pathogenic mutations in multiple genes
Multigene testing introduces the possibility of identifying pathogenic mutations in multiple genes in one individual. In a cohort of more than 2,000 patients, investigators found that 2.9% carried two pathogenic mutations (LaDuca et al., 2014). Although this finding suggests that individuals with known familial mutations identified by single-gene testing may still benefit from a multigene test, limited data exist to guide the management of individuals with more than one inherited cancer predisposition syndrome. Large, prospective studies are needed to define cancer risks in individuals who carry mutations in two or more genes, so that appropriate management recommendations can be developed. In the meantime, management of both syndromes according to their independent guidelines is a reasonable approach.
Management and moderate-penetrance genes
Many moderate-penetrance genes, like CHEK2, were discovered long before clinical testing of these genes was common (Meijers-Heijboer et al., 2002). With single-gene testing, limited value was outweighed by incremental cost. NGS technology allows these genes to be included in multigene tests with little additional cost. While the addition of moderate-penetrance genes to multigene cancer tests preceded the existence of clinical management guidelines, experts have now established a framework for counseling carriers about their risk (NCCN, 2017; Tung, Domchek, et al., 2016). However, while guidelines for managing carriers develop, establishment of cancer risks of family members remains challenging. Prior studies support that when a family member tests negative for a known familial high-penetrance mutation, like a BRCA1 or BRCA2 mutation, they are considered to be a “true negative” and their risk of cancer approaches that of the general population regardless of family history (Domchek, Gaudet, et al., 2010). It may not be appropriate to apply this same approach to moderate-penetrance genes. Moderate-penetrance genes, like ATM, may not account for all cancer risk in a family, as gene/gene and gene/environment interactions may also contribute (NCCN, 2017). For this reason, family members who test negative for a familial moderate-penetrance mutation may still have an increased risk of cancer based on their personal and family history and should be counseled accordingly (Tung, Domchek, et al., 2016).
Inclusion of genes with limited or conflicting evidence of cancer risk can pose additional challenges to multigene testing. Consensus guidelines state that genetic testing is most appropriate when results of testing will have a direct impact on the medical management of the patient or their family members (NCCN, 2017). Therefore, genetic testing with a guidelines-based panel is a reasonable approach. Genes that have uncertain clinical utility should be included in clinical testing with caution, and the involvement of a provider with expertise in cancer genetics and risk assessment is important in these circumstances (Robson et al., 2015).
Counseling about variants of uncertain significance
Rates for VUS identified on multigene hereditary cancer panels range from 19.7% to 42% (Selkirk et al., 2014; Slavin et al., 2015), with one report of a VUS rate of 88% for a 42-gene panel (Kurian et al., 2014). Although multigene panels have higher VUS rates than traditional single-gene testing, posttest counseling and management of patients with VUS identified with either technology are similar. Because most VUS results that are reclassified are found to be benign, VUS results should not be used to alter clinical management (Easton et al., 2007). Instead, a patient with a VUS should receive individualized recommendations based on their personal and family history (NCCN, 2017). Providers can encourage patients to enroll in research studies that are working collaboratively toward improved interpretation of genetic variants such as ClinGen (www.clinicalgenome.org), PROMPT (www.promptstudy.info), ENIGMA (www.enigmaconsortium.org), or InSIGHT (www.insight-group.org).
Counseling for reproductive risks
Discussion of reproductive risks with carriers is another complex issue that is not unique to, but may be increasing in frequency because of, multigene testing. Many genes cause increased risk of cancer when carriers inherit one mutation from one parent but lead to a different genetic syndrome when they inherit two mutations, one from each parent. Reproductive risks should be discussed with carriers of mutations in the genes in Table 3, and carriers should be made aware of the availability of partner testing to clarify the risk of conceiving a child with a clinically distinct genetic condition.
Table 3.
Genes Commonly Found on Multigene Cancer Panels That Are Also Associated With Other Phenotypes in Individuals With Two Mutations.
| Gene(s) | Heterozygous | Homozygous or Compound Heterozygous |
|---|---|---|
| ATM | ATM-associated hereditary cancer | Ataxia telangiectasia |
| BRCA2 | Hereditary breast and ovarian cancer syndrome | Fanconi anemia (complementation group D1—FANCd1) |
| BRIP1 | BRIP1-associated hereditary cancer | Fanconi anemia (complementation group J—FANCJ) |
| MMR genes | Lynch syndrome | Constitutional mismatch repair deficiency |
| NBN | NBN-associated hereditary breast cancer | Nijmegen breakage syndrome |
| PALB2 | PALB2-associated hereditary cancer | Fanconi anemia (complementation group N—FANCN) |
| RAD51C | RAD51C-associated hereditary cancer | Fanconi anemia (complementation group O—FANCO) |
| RAD51D | RAD51D-associated hereditary cancer | Fanconi anemiaa |
Note. Counseling for these syndromes should include discussion about reproductive risks. MMR = mismatch repair.
a RAD51D is involved in the Fanconi anemia pathway, but there are no reported cases of Fanconi anemia with mutations in RAD51D.
Ongoing Communication
Providers ordering hereditary cancer testing of any type must be aware of changing guidelines, and this imperative is especially true for multigene panel testing. Patients’ family histories, guidelines for testing criteria, interpretation of variants, and management recommendations for mutation carriers are all evolving. Clinicians must establish a protocol to ensure that patients have access to current recommendations surrounding their hereditary cancer risk.
Conclusions
Multigene testing allows for increased detection of hereditary cancer syndromes by utilizing the benefits of high-throughput NGS. Genetic counseling complexities may arise on a more frequent basis with panel testing; however, these challenges are not novel to counseling for inherited cancer. Nurses of all levels and specialties can play an integral role in identifying, testing, and managing patients with inherited risk of cancer. All health-care professionals who offer inherited cancer testing must engage in ongoing education as the field is continuously evolving as new data become available. Future research opportunities are many in this field and include analysis of clinical utility for moderate-penetrance genes, delineation of cancer risks and management for individuals positive for mutations in multiple genes, development of robust standards to assess lab quality, and data collection to further refine cancer risks conferred by more newly described genes, especially in diverse populations. While these data will undoubtedly improve upon the usefulness of multigene testing, the current landscape represents an opportunity to expand the number of individuals who can receive timely and appropriate clinical guidance.
Supplementary Material
Acknowledgment
The authors would like to acknowledge Dale Muzzey, PhD, who provided guidance for the next-generation sequencing technologies portion of this review.
Footnotes
Author Contribution: K. Price contributed to conception, design, data acquisition, data analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. A. Svenson contributed to conception, design, data acquisition, data analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. E. King contributed to design, critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. K. Ready contributed to design, critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. G. Lazarin contributed to conception and design, critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors, with the exception of Ms. King, are employees of Counsyl, a laboratory providing inherited cancer screening.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This study was funded by Counsyl, a laboratory providing inherited cancer screening.
ORCID iD: Gabriel A. Lazarin, MS, CGC  http://orcid.org/0000-0001-5061-8595
http://orcid.org/0000-0001-5061-8595
Supplemental Material: Supplementary material is available for this article online.
References
- American College of Medical Genetics and Genomics (ACMG) Board of Directors. (2017). Laboratory and clinical genomic data sharing is crucial to improving genetic health care: A position statement of the American College of Medical Genetics and Genomics. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 19, 721–722. doi:10.1038/gim.2016.196 [DOI] [PubMed] [Google Scholar]
- Amir E., Freedman O. C., Seruga B., Evans D. G. (2010). Assessing women at high risk of breast cancer: A review of risk assessment models. Journal of the National Cancer Institute, 102, 680–691. [DOI] [PubMed] [Google Scholar]
- Antoniou A. C., Casadei S., Heikkinen T., Barrowdale D., Pylkäs K., Roberts J.…Tischkowitz M. (2014). Breast-cancer risk in families with mutations in PALB2. The New England Journal of Medicine, 371, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axilbund J. E. (2016). Panel testing is not a panacea. Journal of Clinical Oncology, 34, 1433–1435. [DOI] [PubMed] [Google Scholar]
- Beck T. F., Mullikin J. C. (on behalf of the NISC Comparative Sequencing Program), Biesecker L. G. (2016). Systematic evaluation of Sanger validation of next-generation sequencing variants. Clinical Chemistry, 62, 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellcross C. A., Kolor K., Goddard K. A., Coates R. J., Reyes M., Khoury M. J. (2011). Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. American Journal of Preventive Medicine, 40, 61–66. [DOI] [PubMed] [Google Scholar]
- Bellido F., Pineda M., Aiza G., Valdés-Mas R., Navarro M., Puente D.A.…Valle L. (2015). POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: Review of reported cases and recommendations for genetic testing and surveillance. Genetics in Medicine, 18, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. L., Teraoka S., Southey M. C., Jenkins M. A., Andrulis I. L., Knight J. A.…Concannon P. (2006). Population-based estimates of breast cancer risks associated with ATM gene variants c.7271T> G and c.1066-6T> G (IVS10-6T> G) from the Breast Cancer Family Registry. Human Mutation, 27, 1122. [DOI] [PubMed] [Google Scholar]
- Bisgaard M. L., Fenger K., Bülow S., Niebuhr E., Mohr J. (1994). Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Human Mutation, 3, 121–125. [DOI] [PubMed] [Google Scholar]
- Bonadona V., Bonaïti B., Olschwang S., Grandjouan S., Huiart L, Longy M.…French Cancer Genetics Network. (2011). Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. The Journal of the American Medical Association, 305, 2304–2310. [DOI] [PubMed] [Google Scholar]
- Bubien V., Bonnet F., Brouste V., Hoppe S., Barouk-Simonet E., David A.…French Cowden Disease Network. (2013). High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. Journal of Medical Genetics, 50, 255–263. [DOI] [PubMed] [Google Scholar]
- Burt R. W., Leppert M. F., Slattery M. L., Samowitz W. S., Spirio L. N., Kerber R. A.…White R. L. (2004). Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology, 127, 444–451. [DOI] [PubMed] [Google Scholar]
- Calzone K. A., Cashion A., Feetham S., Jenkins J., Prows C. A., Williams J. K., Wung S. F. (2010). Nurses transforming health care using genetics and genomics. Nursing Outlook, 58, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei S., Norquist B. M., Walsh T., Stray S., Mandell J. B., Lee M. K.…King M. C. (2011). Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Research, 71, 2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong H. K., Wang T., Lu H. M., Seidler S., Lu H., Keiles S.…Elliott A. M. (2014). The validation and clinical implementation of BRCAplus: A comprehensive high-risk breast cancer diagnostic assay. PloS One, 9, e97408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus E. B., Risch N., Thompson W. D. (1994). Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer, 73, 643–651. [DOI] [PubMed] [Google Scholar]
- Concannon P. (2002). ATM heterozygosity and cancer risk. Nature Genetics, 32, 89–90. [DOI] [PubMed] [Google Scholar]
- Couch F. J., Shimelis H., Hu C., Hart S. N., Polley E. C., Na J.…Dolinsky J. S. (2017). Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncology, 3, 1190–1196. doi:10.1001/jamaoncol.2017.0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragun D., Radford C., Dolinsky J. S., Caldwell M., Chao E., Pal T. (2014). Panel-based testing for inherited colorectal cancer: A descriptive study of clinical testing performed by a US laboratory. Clinical Genetics, 86, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski C., Wokołorczyk D., Kluźniak W., Jakubowska A., Górski B., Gronwald J.…Polish Hereditary Prostate Cancer Consortium. (2013). An inherited NBN mutation is associated with poor prognosis prostate cancer. British Journal of Cancer, 108, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton E., Thompson J. (2015). Overview of multi-gene panels for hereditary cancer. Annals of Translational Medicine, 3, AB054. [Google Scholar]
- Damiola F., Pertesi M., Oliver J., Le Calvez-Kelm F., Voegele C., Young E. L.…Tavtigian S. V. (2014). Rare key functional domain missense substitutions in MRE11A, RAD50, and NBN contribute to breast cancer susceptibility: Results from a Breast Cancer Family Registry case-control mutation-screening study. Breast Cancer Research, 16, R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond A., Kurian A. W., Gabree M., Mills M. A., Anderson M. J., Kobayashi Y.…Ellisen L. W. (2015). Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncology, 1, 943–951. [DOI] [PubMed] [Google Scholar]
- Domchek S. M., Bradbury A., Garber J. E., Offit K., Robson M. E. (2013). Multiplex genetic testing for cancer susceptibility: Out on the high wire without a net? Journal of Clinical Oncology, 31, 1267–1270. [DOI] [PubMed] [Google Scholar]
- Domchek S. M., Friebel T. M., Singer C. F., Evans D. G., Lynch H. T., Isaacs C.…Rebbeck T. R. (2010). Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. Journal of the American Medical Association, 304, 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domchek S. M., Gaudet M. M., Stopfer J. E., Fleischaut M. H., Powers J., Kauff N.…Robson M. (2010). Breast cancer risks in individuals testing negative for a known family mutation in BRCA1 or BRCA2. Breast Cancer Research and Treatment, 119, 409–414. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Deffenbaugh A. M., Pruss D., Frye C., Wenstrup R. J., Allen-Brady K.…Goldgar D. E. (2007). A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. American Journal of Human Genetics, 81, 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Ford D., Bishop D. T. (1995). Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. American Journal of Human Genetics, 56, 265–271. [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Pharoah P. D. P., Antoniou A. C., Tischkowitz M., Tavtigian S. V., Nathanson K. L.…Foulkes W. D. (2015). Gene-panel sequencing and the prediction of breast-cancer risk. New England Journal of Medicine, 372, 2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggington J. M., Bowles K. R., Moyes K., Manley S., Esterling L., Sizemore S.…Wenstrup R. J. (2014). A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clinical Genetics, 86, 229–237. [DOI] [PubMed] [Google Scholar]
- Espenschied C. R., LaDuca H., Li S., McFarland R., Gau C.-L., Hampel H. (2017). Multigene panel testing provides a new perspective on Lynch syndrome. Journal of Clinical Oncology, 35, 2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington S. M., Tenesa A., Barnetson R., Wiltshire A., Prendergast J., Porteous M.…Dunlop M. G. (2005). Germline susceptibility to colorectal cancer due to base-excision repair gene defects. American Journal of Human Genetics, 77, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau H., Vogel K. J., Hanson K., Morrill-Cornelius S. (2014). The evolution of cancer risk assessment in the era of next generation sequencing. Journal of Genetic Counseling, 23, 633–639. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. C., Hardwick R., Huntsman D., Carneiro F., Guilford P., Blair V.…International Gastric Cancer Linkage Consortium. (2010). Hereditary diffuse gastric cancer: Updated consensus guidelines for clinical management and directions for future research. Journal of Medical Genetics, 47, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Stratton M., Narod S., Goldgar D., Devilee P.…Zelada-Hedman M. (1998). Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. American Journal of Human Genetics, 62, 676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai-Kapara E., Lahad A., Kaufman B., Friedman E., Segev S., Renbaum P.…Levy-Lahad E. (2014). Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proceedings of the National Academy of Sciences of the United States of America, 111, 14205–14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic Information Nondiscrimination Act, 2 U.S.C. §§ 201-213 (2008).
- Giardiello F. M., Brensinger J. D., Tersmette A. C., Goodman S. N., Petersen G. M., Booker S. V.…Offerhaus J. A. (2000). Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology, 119, 1447–1453. [DOI] [PubMed] [Google Scholar]
- Gradishar W., Johnson K., Brown K., Mundt E., Manley S. (2017). Clinical variant classification: A comparison of public databases and a commercial testing laboratory. Oncologist, 22, 797–803. doi:10.1634/theoncologist.2016-0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P., Blair V., More H., Humar B. (2007). A short guide to hereditary diffuse gastric cancer. Hereditary Cancer in Clinical Practice, 5, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. J., Obeid E. I., Schwartz S. C., Mantia-Smaldone G., Forman A. D., Daly M. B. (2016). Genetic testing for hereditary cancer predisposition: BRCA1/2, Lynch syndrome, and beyond. Gynecologic Oncology, 140, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F. F., Guo C. L., Liu L. H. (2013). The effect of CHEK2 variant I157T on cancer susceptibility: Evidence from a meta-analysis. DNA and Cell Biology, 32, 329–335. [DOI] [PubMed] [Google Scholar]
- Hearle N., Schumacher V., Menko F. H., Olschwang S., Boardman L. A., Gille J. P.…Houlston R. S. (2006). Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clinical Cancer Research, 12, 3209–3215. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Mitros F. A., Summers R. W. (1998). The risk of gastrointestinal carcinoma in familial juvenile polyposis. Annals of Surgical Oncology, 5, 751–756. [DOI] [PubMed] [Google Scholar]
- Huynh J. M., Laukaitis C. M. (2016). Panel testing reveals nonsense and missense CDH1 mutations in families without hereditary diffuse gastric cancer. Molecular Genetics & Genomic Medicine, 4, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Ragone A., Lubinski J., Lynch H. T., Moller P., Ghadirian P.,…Hereditary Breast Cancer Study Group. (2012). The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. British Journal of Cancer, 107, 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger E., Leedham S., Lewis A., Segditsas S., Becker M., Cuadrado P. R.…Tomlinson I. (2012). Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nature Genetics, 44, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judkins T., Leclair B., Bowles K., Gutin N., Trost J., McCulloch J.…Timms K. (2015). Development and analytical validation of a 25-gene next generation sequencing panel that includes the BRCA1 and BRCA2 genes to assess hereditary cancer risk. BMC Cancer, 15, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor N. S., Curcio L. D., Blakemore C. A., Bremner A. K., McFarland R. E., West J. G., Banks K. C. (2015). Multigene panel testing detects equal rates of pathogenic BRCA1/2 mutations and has a higher diagnostic yield compared to limited BRCA1/2 analysis alone in patients at risk for hereditary breast cancer. Annals of Surgical Oncology, 22, 3282–3288. [DOI] [PubMed] [Google Scholar]
- Kempers M. J. E., Kuiper R. P., Ockeloen C. W., Chappuis P. O., Hutter P., Rahner N.…Ligtenberg M. J. L. (2011). Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. The Lancet Oncology, 12, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Schäuble B., zur Hausen A., Estève J., Ohgaki H. (1997). Tumors associated with p53 germline mutations: a synopsis of 91 families. The American Journal of Pathology, 150, 1–13. [PMC free article] [PubMed] [Google Scholar]
- Kote-Jarai Z., Leongamornlert D., Saunders E., Tymrakiewicz M., Castro E., Mahmud N.…Eeles R. (2011). BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. British Journal of Cancer, 105, 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian A. W., Hare E. E., Mills M. A., Kingham K. E., McPherson L., Whittemore A. S.…Ford J. M. (2014). Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. Journal of Clinical Oncology, 32, 2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDuca H., Stuenkel A. J., Dolinsky J. S., Keiles S., Tandy S., Pesaran T.…Chao E. (2014). Utilization of multigene panels in hereditary cancer predisposition testing: Analysis of more than 2,000 patients. Genetics in Medicine, 16, 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leongamornlert D., Mahmud N., Tymrakiewicz M., Saunders E., Dadaev T., Castro E.…Kote-Jarai Z. (2012). Germline BRCA1 mutations increase prostate cancer risk. British Journal of Cancer, 106, 1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg M. J. L., Kuiper R. P., Geurts van Kessel A., Hoogerbrugge N. (2013). EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Familial Cancer, 12, 169–174. [DOI] [PubMed] [Google Scholar]
- Lim W., Olschwang S., Keller J. J., Westerman A. M., Menko F. H., Boardman L. A.…Houlston R. S. (2004). Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology, 126, 1788–1794. [DOI] [PubMed] [Google Scholar]
- Lincoln S. E., Kobayashi Y., Anderson M. J., Yang S., Desmond A. J., Mills M. A.…Ellisen L. W. (2015). A systematic comparison of traditional and multigene panel testing for hereditary breast and ovarian cancer genes in more than 1000 patients. Journal of Molecular Diagnostics, 17, 533–544. [DOI] [PubMed] [Google Scholar]
- Lincoln S. E., Yang S., Cline M. S., Kobayashi Y., Zhang C., Topper S.…Nussbaum R. L. (2017). Consistency of BRCA1 and BRCA2 variant classifications among clinical diagnostic laboratories. JCO Precision Oncology, 1, 1–10. doi:10.1200/PO.16.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday C., Turnbull C., Ruark E., Xicola R. M. M., Ramsay E., Hughes D.…Rahman N. (2012). Germline RAD51C mutations confer susceptibility to ovarian cancer. Nature Genetics, 44, 475. [DOI] [PubMed] [Google Scholar]
- Lubbe S. J., Di Bernardo M. C., Chandler I. P., Houlston R. S. (2009). Clinical implications of the colorectal cancer risk associated with MUTYH mutation. Journal of Clinical Oncology, 27, 3975–3980. [DOI] [PubMed] [Google Scholar]
- Mavaddat N., Peock S., Frost D., Ellis S., Platte R., Fineberg E.…EMBRACE. (2013). Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. Journal of the National Cancer Institute, 105, 812–822. [DOI] [PubMed] [Google Scholar]
- Maxwell K. N., Wubbenhorst B., D’Andrea K., Garman B., Long J. M., Powers J.…Nathanson K. L. (2015). Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genetics in Medicine, 17, 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers-Heijboer H., van den Ouweland A., Klijn J., Wasielewski M., de Snoo A.…Oldenburg R. CHEK2-Breast Cancer Consortium. (2002). Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nature Genetics, 31, 55–59. [DOI] [PubMed] [Google Scholar]
- Mu W., Lu H. M., Chen J., Li S., Elliott A. M. (2016). Sanger confirmation is required to achieve optimal sensitivity and specificity in next-generation sequencing panel testing. Journal of Molecular Diagnostics, 18, 923–932. [DOI] [PubMed] [Google Scholar]
- Muzzey D., Evans E. A., Lieber C. (2015). Understanding the basics of NGS: From mechanism to variant calling. Current Genetic Medicine Reports, 3, 158–165. doi:10.1007/s40142-015-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2017). Genetic/familial high-risk assessment: Breast and ovarian (Version 2.2017). Retrieved from https://www.nccn.org/professionals/physician_gls/default.aspx#detection
- National Human Genome Research Institute. (2017, April 17). Genetic discrimination and other laws. Retrieved October 2, 2017, from https://www.genome.gov/27568503/genetic-discrimination-and-other-laws/
- Olivier M., Goldgar D. E., Sodha N., Ohgaki H., Kleihues P., Hainaut P., Eeles R. A. (2003). Li-Fraumeni and related syndromes: Correlation between tumor type, family structure, and TP53 genotype. Cancer Research, 63, 6643–6650. [PubMed] [Google Scholar]
- Pelttari L. M., Heikkinen T., Thompson D., Kallioniemi A., Schleutker J., Holli K.…Nevanlinna H. (2011). RAD51C is a susceptibility gene for ovarian cancer. Human Molecular Genetics, 20, 3278–3288. [DOI] [PubMed] [Google Scholar]
- Petrucelli N., Lazebnik N., Huelsman K. M., Lazebnik R. S. (2002). Clinical interpretation and recommendations for patients with a variant of uncertain significance in BRCA1 or BRCA2: A survey of genetic counseling practice. Genetic Testing, 6, 107–113. [DOI] [PubMed] [Google Scholar]
- Pharoah P. D., Guilford P., Caldas C. , & the International Gastric Cancer Linkage Consortium. (2001). Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology, 121, 1348–1353. [DOI] [PubMed] [Google Scholar]
- Pilarski R., Burt R., Kohlman W., Pho L., Shannon K. M., Swisher E. (2013). Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. Journal of the National Cancer Institute, 105, 1607–1616. [DOI] [PubMed] [Google Scholar]
- Provenzale D., Gupta S., Ahnen D. J., Bray T., Cannon J. A., Cooper G.…Darlow S. (2016). Genetic/familial high-risk assessment: Colorectal version 1.2016, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 14, 1010–1030. [DOI] [PubMed] [Google Scholar]
- Rafnar T., Gudbjartsson D. F., Sulem P., Jonasdottir A., Sigurdsson A., Jonasdottir A.…Stefansson, K. (2011). Mutations in BRIP1 confer high risk of ovarian cancer. Nature Genetics, 43, 1104–1107. [DOI] [PubMed] [Google Scholar]
- Rahman N., Seal S., Thompson D., Kelly P., Renwick A., Elliott A.…Stratton M. R. (2007). PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nature Genetics, 39, 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus S. J., Song H., Dicks E., Tyrer J. P., Rosenthal A. N., Intermaggio M. P.…Gayther S. A. (2015). Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. Journal of the National Cancer Institute, 107 doi:10.1093/jnci/djv214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H. L., Bale S. J., Bayrak-Toydemir P., Berg J. S., Brown K. K., Deignan J. L.…Working Group of the ACMG Laboratory Quality Assurance Committee. (2013). ACMG clinical laboratory standards for next-generation sequencing. Genetics in Medicine, 15, 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick A., Thompson D., Seal S., Kelly P., Chagtai T., Ahmed M.…Rahman N. (2006). ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nature Genetics, 38, 873–875. [DOI] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J.…ACMG Laboratory Quality Assurance Committee. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17, 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker C., Culver J. O., Lowstuter K., Sturgeon D., Sturgeon J. D., Chanock C. R.…Gruber S. B. (2016). Increased yield of actionable mutations using multi-gene panels to assess hereditary cancer susceptibility in an ethnically diverse clinical cohort. Cancer Genetics, 209, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley B. D., Culver J. O., Skrzynia C., Senter L. A., Peters J. A., Costalas J. W.…Trepanier A. M. (2012). Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 21, 151–161. [DOI] [PubMed] [Google Scholar]
- Robson M. E., Bradbury A. R., Arun B., Domchek S. M., Ford J. M., Hampel H. L.…Lindor N. M. (2015). American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. Journal of Clinical Oncology, 33, 3660–3667. [DOI] [PubMed] [Google Scholar]
- Rozen P., Samuel Z., Brazowski E. (2003). A prospective study of the clinical, genetic, screening, and pathologic features of a family with hereditary mixed polyposis syndrome. The American Journal of Gastroenterology, 98, 2317–2320. [DOI] [PubMed] [Google Scholar]
- Saam J., Arnell C., Theisen A., Moyes K., Marino I., Roundy K. M., Wenstrup R. J. (2015). Patients tested at a laboratory for hereditary cancer syndromes show an overlap for multiple syndromes in their personal and familial cancer histories. Oncology, 89, 288–293. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Jones S., Dolwani S., Cheadle J. P. (2005). MutYH (MYH) and colorectal cancer. Biochemical Society Transactions, 33, 679–683. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 74, 5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow D., Boetes C., Burke W., Harms S., Leach M. O., Lehman C. D.…American Cancer Society Breast Cancer Advisory Group. (2007). American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA: Cancer Journal for Clinicians, 57, 75–89. [DOI] [PubMed] [Google Scholar]
- Seal S., Thompson D., Renwick A., Elliott A., Kelly P., Barfoot R.…Rahman N. (2006). Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nature Genetics, 38, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Selkirk C. G., Vogel K. J., Newlin A. C., Weissman S. M., Weiss S. M., Wang C. H., Hulick P. J. (2014). Cancer genetic testing panels for inherited cancer susceptibility: The clinical experience of a large adult genetics practice. Familial Cancer, 13, 527–536. [DOI] [PubMed] [Google Scholar]
- Senter L., Clendenning M., Sotamaa K., Hampel H., Green J., Potter J. D.…de la Chapelle A. (2008). The clinical phenotype of Lynch syndrome due to germline PMS2 mutations. Gastroenterology, 135, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber O. M., Lipton L., Crabtree M., Heinimann K., Fidalgo P., Phillips R. K. S.…Tomlinson I. P. M. (2003). Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. The New England Journal of Medicine, 348, 791–799. [DOI] [PubMed] [Google Scholar]
- Slavin T. P., Niell-Swiller M., Solomon I., Nehoray B., Rybak C., Blazer K. R., Weitzel J. N. (2015). Clinical application of multigene panels: Challenges of next-generation counseling and cancer risk management. Frontiers in Oncology, 5, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier I., Holzapfel S., Altmüller J., Zhao B., Horpaopan S., Vogt S.…Stienen D. (2015). Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. International Journal of Cancer, 137, 320–331. [DOI] [PubMed] [Google Scholar]
- Steffen J., Varon R., Mosor M., Maneva G., Maurer M., Stumm M.…Sperling K. (2004). Increased cancer risk of heterozygotes with NBS1 germline mutations in Poland. International Journal of Cancer, 111, 67–71. [DOI] [PubMed] [Google Scholar]
- Susswein L. R., Marshall M. L., Nusbaum R., Vogel Postula K. J., Weissman S. M., Yackowski L.…Chung W. K. (2016). Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genetics in Medicine, 18, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M. H., Mester J. L., Ngeow J., Rybicki L. A., Orloff M. S., Eng C. (2012). Lifetime cancer risks in individuals with germline PTEN mutations. Clinical Cancer Research, 18, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Broeke S. W., Brohet R. M., Tops C. M., van der Klift H. M., Velthuizen M. E., Bernstein I.…Wijnen J. T. (2015). Lynch syndrome caused by germline PMS2 mutations: Delineating the cancer risk. Journal of Clinical Oncology, 33, 319–325. [DOI] [PubMed] [Google Scholar]
- Thompson E. R., Rowley S. M., Li N., McInerny S., Devereux L., Wong-Brown M. W.…Campbell I. G. (2016). Panel testing for familial breast cancer: Calibrating the tension between research and clinical care. Journal of Clinical Oncology, 34, 1455–1459. [DOI] [PubMed] [Google Scholar]
- Tung N., Domchek S. M., Stadler Z., Nathanson K. L., Couch F., Garber J. E.…Robson M. E. (2016). Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nature Reviews Clinical Oncology, 13, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N., Lin N. U., Kidd J., Allen B. A., Singh N., Wenstrup R. J.…Garber J. E. (2016). Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. Journal of Clinical Oncology, 34, 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutlewska K., Lubinski J., Kurzawski G. (2013). Germline deletions in the EPCAM gene as a cause of Lynch syndrome—Literature review. Hereditary Cancer in Clinical Practice, 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asperen C. J., Brohet R. M., Meijers-Heijboer E. J., Hoogerbrugge N., Verhoef S., Vasen H. …Netherlands Collaborative Group on Hereditary Breast Cancer (HEBON). (2005). Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. Journal of Medical Genetics, 42, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vysotskaia V. S., Hogan G. J., Gould G. M., Wang X., Robertson A. D., Haas K. R.…Haque I. S. (2017). Development and validation of a 36-gene sequencing assay for hereditary cancer risk assessment. PeerJ, 5, e3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischer M., Bojesen S. E., Ellervik C., Tybjærg-Hansen A., Nordestgaard B. G. (2008). CHEK2* 1100delC genotyping for clinical assessment of breast cancer risk: Meta-analyses of 26,000 patient cases and 27,000 controls. Journal of Clinical Oncology, 26, 542–548. [DOI] [PubMed] [Google Scholar]
- Yorczyk A., Robinson L. S., Ross T. S. (2015). Use of panel tests in place of single gene tests in the cancer genetics clinic. Clinical Genetics, 88, 278–282. [DOI] [PubMed] [Google Scholar]
- Yurgelun M. B., Allen B., Kaldate R. R., Bowles K. R., Judkins T., Kaushik P.…Syngal S. (2015). Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology, 149, 604–613.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Beeghly-Fadiel A., Long J., Zheng W. (2011). Genetic variants associated with breast-cancer risk: Comprehensive research synopsis, meta-analysis, and epidemiological evidence. The Lancet Oncology, 12, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.