Abstract
Context
Low serum adiponectin (Ad) level is an important risk factor for the development of type 2 diabetes mellitus (T2DM).
Objective
To determine whether the changes in Ad in subjects with low baseline serum Ad levels can reduce the rate of development of T2DM.
Design/Setting/Participants
We performed a large-scale longitudinal study of 7052 healthy Japanese men who underwent general health checkups more than twice between April 2007 and May 2015 at the Physical Check up Center, Sumitomo Hospital. The participants were divided into quartile groups according to baseline Ad level. Subjects of the lowest baseline Ad group (≤5.2 μg/mL) were subdivided into quartile subgroups according to the percent change in Ad (%ΔAd) and into two subgroups according to endpoint Ad (>5.2 and ≤5.2 μg/mL).
Main Outcome Measures
The cumulative incidence rate of T2DM.
Results
The cumulative incidence rate of T2DM of the lowest baseline Ad group (≤5.2 μg/mL) was significantly higher than the other quartile groups. The cumulative incidence rates of T2DM were significantly lower in the largest (≥21.5%) and the second largest (9.3% to 21.4%) %ΔAd-increased subgroups compared with the %ΔAd-decreased subgroup (P < 0.001 and P = 0.005, respectively). The cumulative incidence rates of T2DM were significantly lower in the endpoint Ad >5.2 μg/mL subgroup than in the ≤5.2 μg/mL subgroup (P < 0.001).
Conclusions
Increases in serum Ad levels of at least ~10% or >5.2 μg/mL can potentially reduce the risk of development of T2DM in Japanese men with low baseline Ad levels who are at a high risk of developing T2DM.
Keywords: adiponectin, type 2 diabetes mellitus
Increases in serum Ad levels of ∼10% or >5.2 μg/mL in Japanese men with low baseline serum Ad levels can reduce the risk of development of T2DM.
Adipose tissue is not only a storage organ of excessive energy but is also an endocrine organ secreting many bioactive substances, namely adipocytokines. Adiponectin (Ad) is an adipose-derived, collagen-like protein that has antiartherogenic, antidiabetic, and anti-inflammatory functions [1–3]. Therefore, Ad plays an important role in the development of obesity-related disorders, such as diabetes mellitus, hypertension, dyslipidemia, and metabolic syndrome. Several studies have reported the association of hypoadiponectinemia and an increased risk of type 2 diabetes mellitus (T2DM) [1, 4–8]. We reported that a 3% to 5% reduction in waist circumference (WC) in nondiabetic Japanese men with abdominal obesity can reduce the risk of development of T2DM [9] and found that baseline serum Ad concentration is a significant factor in the onset of T2DM based on a stepwise backward selection procedure. The result highlighted the strong negative relationship between Ad levels and the development of T2DM. Increases in Ad levels were also reported to correlate significantly with a reduction in the rate of progression to T2DM [10].
It is not clear whether the change in Ad level can alleviate the risk of T2DM in subjects with low Ad levels who are presumed to be at a higher risk than those with high Ad levels. Moreover, the critical percent change of increase in serum Ad or the critical cutoff value of Ad levels to reduce the risk of T2DM remains unknown.
The present large-scale longitudinal study of Japanese men was designed to determine the effect of baseline Ad level on the risk of T2DM and whether the changes in Ad in subjects with low baseline serum Ad levels who are at a high risk of developing T2DM can prevent the onset of T2DM. The study also investigated whether the percent change in Ad (%ΔAd) or the absolute level of Ad is important in reducing the risk of T2DM.
1. Materials and Methods
A. Subjects
We retrospectively examined 16,395 men who underwent health checkups at the Physical Check up Center, Sumitomo Hospital, between April 2007 and May 2015, as reported previously [9]. The health checkup program for Japanese citizens is designed to detect diseases or risk factors at early stages. Participants often undergo the checkups spontaneously, and none had any serious diseases. The eligibility criteria for enrolment in the current study included male subjects who had undergone at least two checkup examinations between April 2007 and May 2015, with fasting plasma glucose (FPG) <126 mg/dL, hemoglobin A1c (HbA1c) <6.5% (48 mmol/mol), and 2-hour plasma glucose (PG) on 75 g of oral glucose tolerance test (OGTT) of <200 mg/dL, if available, at baseline. FPG ≥126 mg/dL or 2-hour PG on OGTT ≥200 mg/dL was used to diagnose T2DM according to the criteria for the diagnosis of T2DM in Japan. Based on the clinical data, participants who were at high risk for the development of T2DM in association with visceral fat accumulation were advised to improve their dietary habits and engage in adequate aerobic exercise to avoid the potential development of T2DM. Subjects who used glucose-lowering agents or who had regular visits to the hospital or family physician for T2DM were excluded from the study. We also excluded participants with missing serum Ad levels of baseline or endpoint. Using these criteria, 7052 men were included in the current study.
The study was approved by the human ethics committees of Sumitomo Hospital and Osaka University and conducted according to the principles of the Declaration of Helsinki. Informed consent to provide medical information and blood samples was obtained before the checkup examinations from all participants, and all subjects had the right to refuse the use of their results.
B. Clinical and Laboratory Assessment
Clinical and laboratory data were assessed as described in detail previously [9]. Briefly, physical examination to determine height, body weight (BW), WC, and blood pressure was conducted after overnight fasting. WC was measured at the level of the umbilicus based on the recommendation of the Japan Society for the Study of Obesity [11]. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Information about the use of medications, family history of diabetes, and regular visits to the hospital or family physician was obtained by questionnaires. Blood samples were obtained for measurement of FPG, fasting serum immunoreactive insulin (F-IRI), HbA1c, lipid profile, uric acid (UA), and creatinine (Cr). The reported value for HbA1c (%) represented the National Glycohemoglobin Standardization Program (NGSP). The formula used for conversion of HbA1c (Japan Diabetes Society) to HbA1c (NGSP) was as follows: HbA1c (NGSP) (%) = 1.02 × HbA1c (Japan Diabetes Society) (%) + 0.25% [12, 13]. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as F-IRI (μU/mL) × FPG (mg/dL)/405. Serum total Ad level was measured by the latex particle-enhanced turbidimetric immunoassay (Human Adiponectin Latex Kit; Otsuka Pharmaceutical Co., Tokyo, Japan). The within-run and total coefficient of variation of this measurement were 0.8% to 1.9% and 1.1% to 2.0%, respectively, and the results correlated highly with those obtained by enzyme-linked immunosorbent assay (r = 0.99) [14]. %ΔAd was estimated at the end of the observation period and was calculated as follows: (Ad at endpoint − Ad at baseline)/Ad at baseline. Changes in BW (%ΔBW) and WC (%ΔWC) were calculated in a manner similar to that applied for %ΔAd calculation.
C. Endpoints and Categorization
Each participant was followed from the first visit to Physical Check up Center in our hospital to the diagnosis of T2DM or the last visit for checkup by May 2015. In this study, T2DM was set at HbA1c ≥6.5% (48 mmol/mol), FPG ≥126 mg/dL, or 2-hour PG on OGTT ≥200 mg/dL if conducted. The mean follow-up period was 3.9 ± 2.2 years (range, 0.4 to 8.1 years).
We compared the cumulative incidence rates of T2DM according to baseline serum Ad levels to identify the group of subjects with baseline Ad levels associated with the highest risk of developing T2DM. We divided the participants into quartile groups according to the baseline level of serum Ad.
We selected the group with the lowest Ad (baseline serum Ad ≤5.2 μg/mL) as the high-risk group and examined whether the increase in serum Ad level could alleviate the risk of T2DM using %ΔAd and endpoint serum Ad level. Thus, the lowest baseline Ad quartile (≤5.2 μg/mL) was subdivided into four subgroups according to %ΔAd: the %ΔAd-decreased group and the tertile of the %ΔAd-increased groups. A multivariate analysis was applied to investigate whether %ΔAd is an independent factor that can influence the development of T2DM.
We also assessed the effect of endpoint serum Ad level. Subjects with the lowest baseline serum Ad levels (≤5.2 μg/mL) were divided into two subgroups according to the serum Ad level at endpoint: endpoint Ad >5.2 and ≤5.2 μg/mL.
D. Statistical Analysis
Data are presented as mean ± standard deviation or number/percentage of participants. First, the cumulative incidence rates of T2DM of each baseline Ad group were compared by using the Kaplan-Meier product limit method and the log-rank test. Second, the variables of %ΔAd subgroups were compared using analysis of variance. We analyzed post hoc multiple comparisons by Tukey test. We compared the cumulative incidence rates of T2DM among the four subgroups according to %ΔAd using the Kaplan-Meier product limit method and log-rank test. The cumulative incidence rate for each %ΔAd-increased tertile was compared with that of the %ΔAd-decreased group. Cox proportional hazard analysis was used to estimate the multivariable adjusted hazard ratio (HR) and 95% confidence interval (CI) of the %ΔAd subgroups. Factors associated with the development of T2DM were evaluated using multivariate Cox proportional hazards regression models. We selected the covariates by the Pearson product moment correlation coefficient procedure and excluded factors that were significantly associated with the change in Ad level. The multivariable models included the covariates of BMI, HbA1c, F-IRI, triglyceride, Cr, UA, and %ΔAd subgroups. We then used the stepwise backward selection procedure to identify those variables that independently and significantly influenced the development of T2DM based on the P value.
Variables of two endpoint Ad subgroups (endpoint Ad >5.2 and ≤5.2 μg/mL) were compared using the Mann-Whitney U test. We compared the cumulative incidence rates of T2DM between the two endpoint Ad subgroups using the Kaplan-Meier product limit method and log-rank test. The cumulative incidence rate was compared between the two endpoint Ad subgroups. Cox proportional hazard analysis was also used to estimate the multivariable adjusted HR and 95% CI of the endpoint Ad subgroups. The covariates were selected by the Pearson product moment correlation coefficient procedure, and factors that were significantly associated with the endpoint Ad level were excluded. The multivariable models included the covariates of BMI, HbA1c, total cholesterol, Cr, UA, and endpoint Ad subgroups. We then used the stepwise backward selection procedure to identify variables that independently and significantly influenced the development of T2DM based on the P value.
We performed the Kruskal-Wallis test and confirmed that there were no significant differences in the time periods from the previous visits to endpoints between each group. All statistical analyses were performed using SPSS software version 23 for Windows (SPSS Inc., Chicago, IL). A P value <0.05 was considered statistically significant.
2. Results
A. Cumulative Incidence Rates of T2DM According to Baseline Serum Ad Level
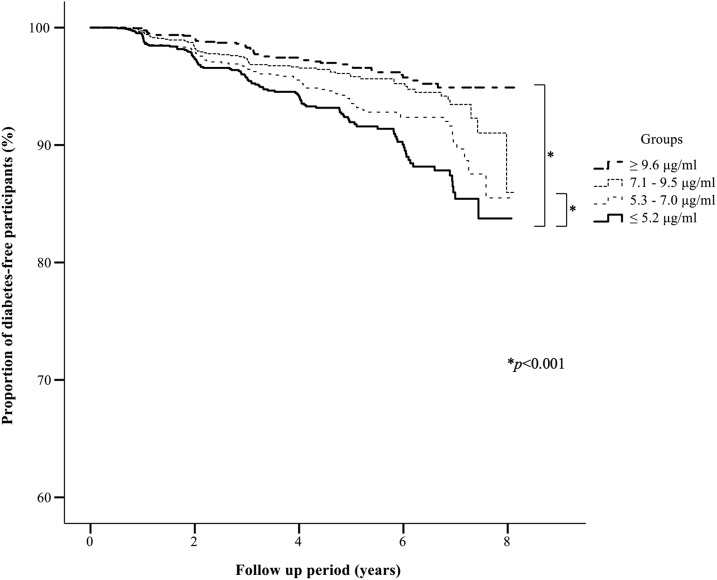
The cumulative incidence rate of T2DM was significantly higher in the lowest baseline serum Ad quartile (≤5.2 μg/mL) than in the highest quartile (≥9.6 μg/mL) and the second highest (7.1 to 9.5 μg/mL) baseline quartile (Fig. 1).
Figure 1.
Kaplan-Meier curves of the proportion of diabetes-free participants stratified according to serum Ad level at baseline. The cumulative incidence rates of T2DM in individuals of the baseline Ad quartiles (highest, ≥9.6 μg/mL; second highest, 7.1 to 9.5 μg/mL; third highest, 5.3 to 7.0 μg/mL; lowest, ≤5.2 μg/mL) were 2.7%, 3.6%, 5.1%, and 6.7%, respectively. The cumulative incidence rate of subjects of the lowest Ad quartile was significantly higher than that of subjects of the highest and second highest Ad quartiles (P < 0.001 and P < 0.001, respectively).
B. Cumulative Incidence Rates of T2DM According to Change in Ad of the Lowest Baseline Ad Group
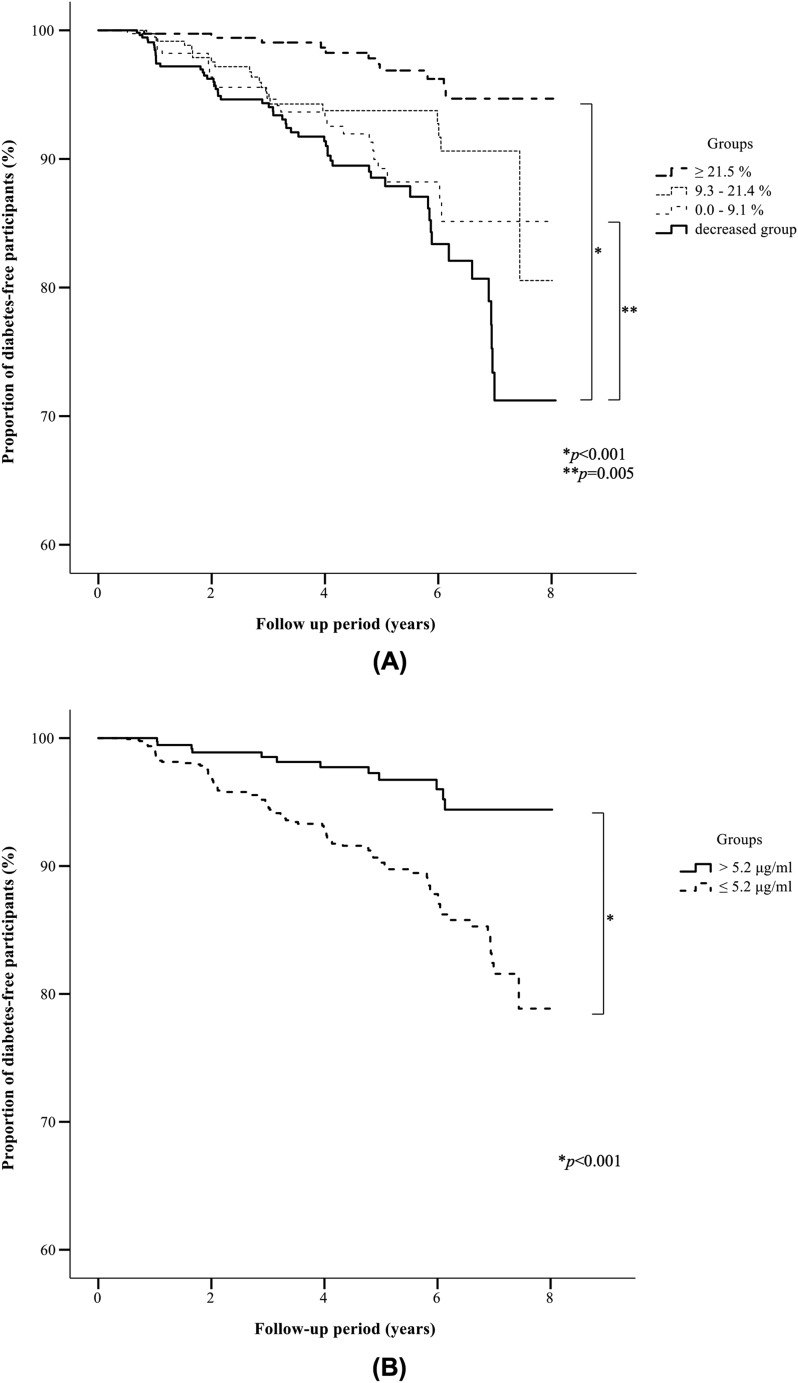
The lowest baseline Ad quartile (≤5.2 μg/mL) was subdivided into four subgroups according to %ΔAd. The baseline clinical characteristics of the patients of the four subgroups are summarized in Table 1. There were no significant differences among the subgroups in BMI, WC, BW, FPG, HbA1c, UA, lipid profile, F-IRI, HOMA-IR, and the proportion of participants with family history of diabetes. In each subgroup, %ΔBW and %ΔWC were significantly inversely associated with %ΔAd. Furthermore, there were significant differences in age of subjects of the four subgroups but no significant differences by Turkey test between the %ΔAd-increased subgroups and the %ΔAd-decreased subgroup. We also analyzed the data after adjustment for age at baseline, and similar results were obtained for the cumulative incidence rate and for the proportion of diabetes-free participants (data not shown). The results also showed significant differences in baseline serum Ad levels among the %ΔAd subgroups. However, there was no specific increase or decrease tendency in those parameters in each subgroup. Figure 2A shows the cumulative incidence rate and the proportion of diabetes-free subjects for each subgroup. The cumulative incidence rates of the %ΔAd-increased subgroups (largest, ≥21.5%; second largest, 9.3% to 21.4%; third largest, ≤9.1%) and of the %ΔAd-decreased subgroup were 2.9%, 5.5%, 7.0%, and 9.9%, respectively. The cumulative incidence rates were significantly lower in the largest (≥21.5%) and the second largest (9.3% to 21.4%) %ΔAd-increased subgroups than in the %ΔAd-decreased subgroup (P < 0.001 and P = 0.005, respectively). There was no significant difference in the cumulative incidence rate between the third largest %ΔAd-increased subgroup (≤9.1%) and the %ΔAd-decreased subgroup.
Table 1.
Baseline Clinical Characteristics of Subjects of the %ΔAd Subgroups and Endpoint Ad Subgroups
| %ΔAd Subgroups |
Endpoint Ad Subgroups |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Decreased Group | ≥21.5% Group | 21.4%–9.3% Group | 9.1%–0.0% Group | P Value (ANOVA) | >5.2 μg/mL | ≤5.2 μg/mL | P Valuea | |
| n | 1698 | 544 | 382 | 384 | 388 | 404 | 1294 | ||
| Age, y | 49 ± 10 | 48 ± 10 | 50 ± 10 | 50 ± 10 | 49 ± 10 | 0.037 | 51 ± 10 | 49 ± 10 | 0.002 |
| BMI, kg/m2 | 24.6 ± 2.9 | 24.6 ± 2.8 | 24.8 ± 3.0 | 24.7 ± 3.0 | 24.5 ± 2.9 | 0.693 | 24.5 ± 3.0 | 24.7 ± 2.9 | 0.213 |
| BW, kg | 72.0 ± 10.3 | 71.7 ± 9.4 | 72.7 ± 11.2 | 72.4 ± 10.6 | 71.5 ± 10.2 | 0.710 | 72.1 ± 11.1 | 72.0 ± 10.0 | 0.803 |
| WC, cm | 87.9 ± 7.7 | 87.6 ± 7.5 | 88.7 ± 8.2 | 88.1 ± 7.6 | 87.5 ± 7.3 | 0.258 | 88.0 ± 8.0 | 87.9 ± 7.6 | 0.838 |
| SBP, mm Hg | 126 ± 14 | 125 ± 15 | 127 ± 15 | 125 ± 13 | 125 ± 13 | 0.152 | 127 ± 14 | 126 ± 14 | 0.055 |
| DBP, mm Hg | 79 ± 9 | 79 ± 10 | 80 ± 10 | 79 ± 10 | 79 ± 9 | 0.153 | 80 ± 10 | 79 ± 9 | 0.013 |
| Total cholesterol, mg/dL | 213 ± 33 | 216 ± 34 | 210 ± 33 | 210 ± 32 | 212 ± 32 | 0.074 | 209 ± 31 | 214 ± 33 | 0.041 |
| HDL cholesterol, mg/dL | 53 ± 12 | 54 ± 12 | 53 ± 13 | 54 ± 12 | 53 ± 10 | 0.440 | 54 ± 12 | 53 ± 12 | 0.722 |
| LDL cholesterol, mg/dL | 132 ± 31 | 134 ± 31 | 129 ± 30 | 131 ± 30 | 133 ± 31 | 0.185 | 131 ± 30 | 133 ± 31 | 0.486 |
| TG, mg/dL | 176 ± 135 | 184 ± 135 | 180 ± 158 | 169 ± 129 | 169 ± 114 | 0.100 | 160 ± 106 | 182 ± 143 | 0.001 |
| UA, mg/dL | 6.5 ± 1.2 | 6.5 ± 1.2 | 6.5 ± 1.2 | 6.4 ± 1.2 | 6.5 ± 1.2 | 0.339 | 6.4 ± 1.1 | 6.5 ± 1.2 | 0.222 |
| FPG, mg/dL | 97 ± 9 | 97 ± 9 | 97 ± 9 | 97 ± 10 | 97 ± 9 | 0.955 | 97 ± 9 | 97 ± 10 | 0.892 |
| HbA1c, % | 5.2 ± 0.4 | 5.3 ± 0.4 | 5.2 ± 0.4 | 5.3 ± 0.4 | 5.3 ± 0.4 | 0.451 | 5.2 ± 0.4 | 5.3 ± 0.4 | 0.056 |
| Cr, mg/dL | 0.83 ± 0.13 | 0.83 ± 0.13 | 0.84 ± 0.16 | 0.84 ± 0.13 | 0.83 ± 0.12 | 0.476 | 0.83 ± 0.13 | 0.83 ± 0.13 | 0.750 |
| HOMA-IR | 1.9 ± 1.1 | 1.9 ± 1.1 | 2.0 ± 1.2 | 1.9 ± 1.2 | 1.8 ± 1.0 | 0.521 | 1.8 ± 1.0 | 2.0 ± 1.1 | 0.003 |
| F-IRI. μU/mL | 7.9 ± 4.4 | 8.0 ± 4.1 | 8.1 ± 4.9 | 7.9 ± 4.5 | 7.6 ± 4.0 | 0.370 | 7.4 ± 4.2 | 8.1 ± 4.4 | 0.003 |
| Baseline Ad, μg/mL | 4.1 ± 0.8 | 4.2 ± 0.8 | 4.1 ± 0.8b | 4.0 ± 0.8b | 4.2 ± 0.7 | <0.001 | 4.7 ± 0.4 | 4.0 ± 0.8 | <0.001 |
| Ad at endpoint, μg/mL | 4.5 ± 1.2 | 3.8 ± 0.8 | 5.7 ± 1.4b | 4.6 ± 0.9b | 4.4 ± 0.8b | <0.001 | 6.2 ± 0.9 | 4.0 ± 0.8 | <0.001 |
| %ΔAd, % | 9.7 ± 22.4 | −11.4 ± 8.0 | 40.4 ± 22.1b | 15.1 ± 3.5b | 3.8 ± 3.0b | <0.001 | 31.9 ± 25.6 | 2.8 ± 16.0 | <0.001 |
| %ΔBW, % | −0.5 ± 4.8 | 1.5 ± 4.2 | −3.8 ± 5.6b | −0.9 ± 4.2b | 0.4 ± 3.7b | <0.001 | −2.8 ± 5.7 | 0.2 ± 4.3 | <0.001 |
| %ΔBMI, % | −0.2 ± 4.8 | 1.6 ± 4.2 | −3.4 ± 5.6b | −0.6 ± 4.2b | 0.6 ± 3.7b | <0.001 | −2.4 ± 5.6 | 0.4 ± 4.3 | <0.001 |
| %ΔWC, % | −0.4 ± 4.7 | 1.2 ± 4.2 | −3.1 ± 5.3b | −0.7 ± 4.3b | 0.4 ± 4.0b | <0.001 | −2.3 ± 5.4 | 0.2 ± 4.3 | <0.001 |
| Family history of diabetes, n (%) | 412 (24.2) | 143 (26.3) | 96 (25.1) | 74 (19.3) | 99 (25.5) | 0.075 | 85(21.0) | 327 (25.3) | 0.083 |
Abbreviations: ANOVA, analysis of variance; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; TG, triglyceride; %ΔAd, percent change in serum adiponectin; %ΔBW, percent change in body weight; %ΔWC, percent change in waist circumference.
Data are mean ± standard deviation or number of subjects.
P < 0.05 compared with the decreased group by the Mann-Whitney U test.
P < 0.05 compared with the decreased group by post hoc multiple comparisons (Turkey test).
Figure 2.
Kaplan-Meier curves of the proportion of diabetes-free participants of the lowest baseline Ad group. (A) Kaplan-Meier curves of the proportion of diabetes-free participants of the %ΔAd-increased subgroups of the lowest baseline Ad group. The cumulative incidence rates of T2DM for subjects of the %ΔAd-increased subgroups (largest, ≥21.5%; second largest, 9.3% to 21.4%; third largest, ≤9.1%) and the %ΔAd-decreased subgroup were 2.9%, 5.5%, 7.0%, and 9.9%, respectively. The cumulative incidence rate was significantly lower in subjects of the largest (≥21.5%) and second largest (9.3% to 21.4%) %ΔAd-increased subgroups than in the %ΔAd-decreased subgroup (P < 0.001 and P = 0.005, respectively). (B) Kaplan-Meier curves of the proportion of diabetes-free participants of the endpoint Ad subgroups of the lowest baseline Ad group. The cumulative incidence rates of T2DM for subjects of the endpoint Ad subgroups (endpoint Ad >5.2 and ≤5.2 μg/mL) were 3.0% and 7.8%, respectively. The cumulative incidence rate was significantly lower in the endpoint Ad >5.2 μg/mL subgroup than in the endpoint Ad ≤5.2 μg/mL subgroup (P < 0.001).
Table 2 shows the results of multivariate Cox proportional hazards regression analyses. Baseline HbA1c, F-IRI, UA, Cr, and %ΔAd were identified as significant factors in the development of T2DM. The results of stepwise backward selection procedure are also shown in Table 2. Baseline HbA1c, F-IRI, UA, Cr, and %ΔAd were the most significant factors.
Table 2.
Hazard Ratios for the Development of T2DM (%ΔAd)
| HR | 95% CI | P Value | |
|---|---|---|---|
| BMI | 1.057 | 0.907–1.132 | NS |
| HbA1c | 19.126 | 11.799–31.002 | <0.001 |
| F-IRI | 1.051 | 1.009–1.095 | 0.018 |
| TG | 1.000 | 0.999–1.002 | NS |
| Cr | 0.424 | 0.119–1.503 | 0.014 |
| UA | 0.833 | 0.717–0.975 | 0.022 |
| %ΔAd | |||
| Largest subgroup (≥21.5%) | 0.196 | 0.102–0.377 | <0.001 |
| Second largest subgroup (9.3%–21.4%) | 0.381 | 0.225–0.645 | <0.001 |
| Third largest subgroup (≤9.1%) | 0.753 | 0.472–1.200 | NS |
| Stepwise backward selection procedure | |||
| HbA1c | 19.977 | 12.320–32.392 | <0.001 |
| F-IRI | 1.071 | 1.036–1.107 | <0.001 |
| Cr | 0.145 | 0.029–0.716 | 0.010 |
| UA | 0.852 | 0.731–0.992 | 0.039 |
| %ΔAd | |||
| Largest subgroup (≥21.5%) | 0.201 | 0.105–0.386 | <0.001 |
| Second largest subgroup (9.3%–21.4%) | 0.381 | 0.226–0.642 | <0.001 |
| Third largest subgroup (≤9.1%) | 0.761 | 0.478–1.211 | 0.249 |
Abbreviations: NS, not significant; TG, triglyceride; %ΔAd, percent change in serum adiponectin level.
C. Cumulative Incidence Rates of T2DM According to Ad at Endpoint of the Lowest Baseline Ad Group
The group of subjects with the lowest baseline Ad (≤5.2 μg/mL) was divided into two subgroups according to serum Ad level at endpoint: endpoint Ad >5.2 and ≤5.2 μg/mL. The baseline clinical characteristics of subjects of the two subgroups are summarized in Table 1. For each endpoint Ad subgroup, there were no significant differences between the subjects in BMI, WC, BW, FPG, HbA1c, UA, Cr, and the proportion of participants with family history of diabetes. There were significant differences in age, %ΔBW, and %ΔWC of each endpoint Ad subgroup. Similar results of cumulative incidence rate and proportion of diabetes-free participants were obtained after adjustment for age at baseline (data not shown). We also found significant differences in baseline Ad, HOMA-IR, and F-IRI of endpoint Ad subgroups. Figure 2B shows the cumulative incidence rate and proportion of diabetes-free subjects for the two endpoint Ad subgroups. The cumulative incidence rates of the endpoint Ad subgroups were 3.0% and 7.8% for the endpoint adiponectin >5.2 μg/mL and endpoint adiponectin ≤5.2 μg/mL groups, respectively. The cumulative incidence rate was significantly lower in the former than in the latter subgroup (P < 0.001). We also performed multivariate Cox proportional hazards regression analysis and the stepwise backward selection procedure in a manner similar to that applied for %ΔAd to evaluate the significance of Ad at endpoint. Ad at endpoint was a significant factor that reduced the risk of the development of T2DM (Table 3).
Table 3.
Hazard Ratios for the Development of T2DM (Endpoint Ad)
| HR | 95% CI | P Value | |
|---|---|---|---|
| BMI | 1.073 | 1.014–1.136 | 0.015 |
| HbA1c | 19.907 | 12.311–32.189 | <0.001 |
| Total cholesterol | 0.998 | 0.993–1.004 | NS |
| Cr | 0.105 | 0.022–0.512 | 0.005 |
| UA | 0.909 | 0.780–1.059 | NS |
| Endpoint Ad >5.2 μg/mL subgroup | 0.274 | 0.149–0.503 | <0.001 |
| Stepwise backward selection procedure | |||
| HbA1c | 19.662 | 12.195–31.702 | <0.001 |
| BMI | 1.062 | 1.007–1.121 | 0.026 |
| Cr | 0.087 | 0.018–0.410 | 0.002 |
| Endpoint Ad >5.2 μg/mL subgroup | 0.275 | 0.150–0.504 | <0.001 |
Abbreviation: NS, not significant.
D. Results After Adjusting for the Change in BMI
Linear regression model analysis revealed that %ΔAd and %ΔBMI had significant correlation (P < 0.001). We then performed Cox proportional hazard analysis and stepwise backward selection procedure, which are similar to %ΔAd, to assess the influence of %ΔBMI. The multivariable models included the covariates of %ΔBMI, HbA1c, Cr, and LDL-cholesterol. %ΔBMI was the significant factor (HR, 1.086; 95% CI, 1.048 to 1.126; P < 0.001). Therefore, we selected the subjects from the group with the lowest Ad (baseline serum Ad ≤5.2 μg/mL) whose %ΔBMI increased and adjusted the mean of %ΔBMI. The cumulative incidence rate was significantly lower in the %ΔAd-increased subgroup (≥11.4%) than in the %ΔAd-decreased subgroup (P = 0.002). Moreover, the cumulative incidence rate was significantly lower in the endpoint Ad >5.2 μg/mL subgroup than in the endpoint Ad ≤5.2 μg/mL subgroup (P = 0.008). The results were consistent after adjusting for the change in BMI.
3. Discussion
Results from this large-scale longitudinal study suggest that an increase in Ad levels could reduce the risk of T2DM in Japanese men with low baseline Ad levels. Moreover, this study shows that an increase of Ad levels of at least ~10% or >5.2 μg/mL in Japanese men with low Ad levels is potentially beneficial to prevent the onset of T2DM.
Our study has several important features. This study examined the longitudinal effect of increase in Ad in subjects with low Ad levels on the development of T2DM. Serum Ad concentrations correlated strongly with insulin sensitivity, suggesting that hypoadiponectinemia is associated with insulin resistance [15]. In our study, the risk of development of T2DM was significantly higher in subjects with baseline serum Ad of ≤5.2 μg/mL compared with those with ≥7.1 μg/mL. This result is consistent with previous studies that reported the association between hypoadiponectinemia and increased risk of T2DM [1, 4–8]. Furthermore, a meta-analysis of past reports showed the consistency of the association across studies with diverse populations [7]. Previous studies indicated that serum Ad levels in Japanese subjects were significantly inversely associated with the incidence of T2DM, with odds ratios of 0.79 (Ad level of 5.2 to 6.8 μg/mL), 0.60 (6.9 to 9.5 μg/mL), and 0.40 (≥9.6 μg/mL) compared with the group with an Ad level of <5.2 μg/mL [16]. These baseline Ad quartiles are similar to those used in our study, although the past studies included both male and female subjects. Although it has been reported that low serum Ad level is an important risk factor for T2DM, our study focused on changes in serum Ad in subjects with low baseline Ad. A healthier dietary pattern was confirmed to be positively and independently associated with total and high-molecular-weight plasma Ad concentrations [17]. Another study showed that a 1 μg/mL increase in serum Ad level was associated with a 16% reduction in the rate of progression to T2DM in the lifestyle intervention group [10]. However, there is little information on the effect of change in serum Ad level in subjects with low Ad levels on the development of T2DM. The current study showed that an increase in serum Ad levels could prevent the onset of T2DM in Japanese men with low baseline Ad levels who are at high risk of developing T2DM.
The current study also suggested that the risk of development of T2DM can be reduced by a ≥10% increase in serum Ad or an increase in Ad level of >5.2 μg/mL in Japanese men with low baseline serum Ad level. Our study suggested that visceral fat reduction aiming at increasing Ad levels might be useful in preventing the onset of T2DM. The changes in BW and WC were found to be significantly inversely associated with the change in serum Ad levels. Accumulation of visceral fat leads to the development of T2DM, dyslipidemia, hypertension, and arteriosclerosis. The prevalence of T2DM is known to be high in subjects with visceral fat area of ≥100 cm2 [18]. Another study showed that an increase in WC is significantly associated with a 9-year incidence of T2DM, with a standardized odds ratio of 1.79 (95% CI, 1.45 to 2.21) [19]. Therefore, a reduction of visceral fat is expected to improve or prevent metabolic disorders, including T2DM. Several studies have reported that a decrease in visceral fat improved glucose tolerance [20–22]. We have also demonstrated that WC reduction, which parallels visceral fat reduction, of at least ~3% in young (<55 years) men and ~5% in elderly (≥55 years) nondiabetic Japanese men with abdominal obesity can reduce the chance of developing T2DM [9]. It is widely known that hypoadiponectinemia induced by visceral fat accumulation is closely associated with T2DM [1, 4–8]. In our previous study, we also found that baseline serum Ad concentration is an important risk factor for the onset of T2DM based on results of a stepwise backward selection procedure, suggesting a strong relationship between Ad and visceral fat accumulation [9]. Another study concluded that serum Ad levels correlate negatively with visceral fat accumulation, as measured by CT scan at the level of umbilicus [23]. In this regard, the change in Ad noted in the current study may be due to a change in visceral fat accumulation, which corresponds to WC. Based our results, the change in serum Ad level can be a useful marker of risk of development of T2DM through a change in visceral fat accumulation.
However, using the stepwise backward selection procedure in our study, we demonstrated that Ad at endpoint reduced the risk of the development of T2DM. In addition, the results of cumulative incidence rates of T2DM according to Ad at endpoint of the lowest baseline Ad group were consistent after adjusting for the change in BMI. These results suggested that the level of Ad could be recognized as not only the marker of change in visceral fat accumulation but also as one of the risk factors of developing T2DM and that there would be an absolute level of Ad to reduce the risk. In other words, hypoadiponectinemia might be the risk factor of developing T2DM. The effect of hypoadiponectinemia toward developing T2DM might be independent of visceral fat accumulation. The mechanisms responsible for the antidiabetic effect of high Ad level remain unclear. Ad is known to accumulate in the heart, vascular endothelium, and skeletal muscles through interaction with T-cadherin, and this interaction is essential for Ad-mediated cardiovascular protection [24–26]. It has been reported that skeletal muscle mass is inversely associated with insulin resistance [27]. Similar to the cardiovascular-protective effect of the interaction of Ad and T-cadherin, its antidiabetic effect may be mediated through the interaction of Ad and T-cadherin, which is present in large concentrations in skeletal muscles. Also, it has been suggested that Ad enhances insulin sensitivity by activating insulin-receptor substrate 1–associated phosphatidylinositol 3 kinase, accelerates FFA clearance by enhancing fatty-acid transport protein 1 mRNA expression, and ameliorates TNF-α–mediated suppression of these parameters. Furthermore, Ad and TNF-α were found to induce local and reciprocal suppression in adipose tissue [28]. Another report showed that Ad increased phosphorylation and the activity of 5′-AMP–activated protein kinase and simultaneously increased glucose uptake in skeletal muscles and reduced the expression of molecules involved in gluconeogenesis in the liver [29]. Moreover, the Ad receptors AdipoR1 and AdipoR2 are thought to be downregulated in obesity-linked insulin resistance [30, 31]. A critical level of Ad might be required to produce the antidiabetic effects through the above-mentioned mechanisms. If the absolute levels of Ad that prevent T2DM could be determined, the treatment of hypoadiponectinemia might be a good therapeutic strategy to prevent T2DM.
The age at baseline of subjects of the %ΔAd-increased subgroups was significantly different from that of the %ΔAd-decreased subgroup, although no significant differences were found among the subgroups with respect to baseline age when we performed post hoc analysis with Turkey tests. We also analyzed the data after adjustment for age at baseline, and similar results of cumulative incidence rate and proportion of diabetes-free participants were obtained. Therefore, we think that it is not necessary to take the difference into consideration.
The stepwise backward selection procedure in our study showed that baseline HbA1c, F-IRI, UA, and Cr were important factors in the development of T2DM. HbA1c is used to diagnose diabetes, and it is reasonable that HbA1c is an important factor in the development of T2DM. An increase of F-IRI concentration suggests the existence of insulin resistance. It is also reasonable that F-IRI is an important factor in the development of T2DM. In addition, the amount of serum Cr depends on the amount of skeletal muscle tissue. It is suggested that higher muscle mass is associated with the reduced risk of developing T2DM. Moreover, the loss of body weight is known to cause a temporary increase in serum UA levels [32]. The risk of developing T2DM is suggested to be lowered by a decrease in visceral fat that is associated with increase in serum UA.
It has been reported that plasma Ad levels could be changed in statin therapy [33, 34]. We performed the same analysis after excluding the subjects who were using lipid-lowering agents at the endpoint. Similar results of cumulative incidence rate and proportion of diabetes-free participants were obtained.
Our study has several limitations. First, the inclusion of subjects who underwent health checkups may imply selection bias toward highly health-conscious people. The baseline serum Ad level or the extent of change in serum Ad might be different in other cohorts, but we think that our population is appropriate for investigating the relationship between baseline and change in serum Ad level and the development of T2DM. Second, we advised the participants regarding dietary habits and exercise, but we did not evaluate whether such dietary and exercise habits improved or not. Third, this study included only male subjects because the majority of participants who underwent health checkups were male. Therefore, the results of our study cannot be applied to women.
In summary, our study demonstrated that increases in serum Ad levels can reduce the risk T2DM in Japanese men with low baseline Ad levels. Moreover, the target level of Ad increase (at least ~10% or >5.2 μg/mL) in Japanese men with low serum Ad levels is potentially beneficial in the prevention of T2DM.
Acknowledgments
We thank Ayumi Shintani from the Department of Clinical Epidemiology and Biostatistics, Graduate School of Medicine, Osaka University, Suita, Japan, for advice on statistical analysis.
Author Contributions: R.K., Y.Y., and H.I. conceived and designed the study, analyzed the data, and wrote the manuscript. Y.I., Y.M., T.S., R.I., K.S., S.T., K.Y., T.W., T.F., and Y.M. contributed to the discussion and reviewed the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Ad
adiponectin
- BMI
body mass index
- BW
body weight
- CI
confidence interval
- Cr
creatinine
- F-IRI
fasting serum immunoreactive insulin
- FPG
fasting plasma glucose
- HbA1c
hemoglobin A1c
- HOMA-IR
homeostasis model assessment of insulin resistance
- HR
hazard ratio
- NGSP
National Glycohemoglobin Standardization Program
- OGTT
oral glucose tolerance test
- PG
plasma glucose
- T2DM
type 2 diabetes mellitus
- UA
uric acid
- WC
waist circumference
References and Notes
- 1. Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes. 2008;32(Suppl 7):S83–S92. [DOI] [PubMed] [Google Scholar]
- 2. Matsuzawa Y. Adiponectin: identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler Suppl. 2005;6(2):7–14. [DOI] [PubMed] [Google Scholar]
- 3. González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3:17034. [DOI] [PubMed] [Google Scholar]
- 4. Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360(9326):57–58. [DOI] [PubMed] [Google Scholar]
- 5. Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, Ohnuma H, Igarashi M, Tominaga M, Kato T; Funagata study . Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care. 2003;26(7):2015–2020. [DOI] [PubMed] [Google Scholar]
- 6. Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361(9353):226–228. [DOI] [PubMed] [Google Scholar]
- 7. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–188. [DOI] [PubMed] [Google Scholar]
- 8. Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16(17):1896–1901. [DOI] [PubMed] [Google Scholar]
- 9. Kashiwagi R, Iwahashi H, Yamada Y, Sakaue T, Okita T, Kawachi Y, Iwamoto R, Saisho K, Tamba S, Yamamoto K, Watanabe T, Fujimoto T, Matsuzawa Y. Effective waist circumference reduction rate necessary to avoid the development of type 2 diabetes in Japanese men with abdominal obesity. Endocr J. 2017;64(9):881–894. [DOI] [PubMed] [Google Scholar]
- 10. Mather KJ, Funahashi T, Matsuzawa Y, Edelstein S, Bray GA, Kahn SE, Crandall J, Marcovina S, Goldstein B, Goldberg R; Diabetes Prevention Program . Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008;57(4):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66(11):987–992. [DOI] [PubMed] [Google Scholar]
- 12. Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K; Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus . Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1(5):212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H; Committee on the Standardization of Diabetes Mellitus‐Related Laboratory Testing of Japan Diabetes Society . International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3(1):39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2006;371(1-2):163–168. [DOI] [PubMed] [Google Scholar]
- 15. Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, Youngren JF, Havel PJ, Pratley RE, Bogardus C, Tataranni PA. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51(6):1884–1888. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto S, Matsushita Y, Nakagawa T, Hayashi T, Noda M, Mizoue T. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diabetes. 2014;4(8):e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fargnoli JL, Fung TT, Olenczuk DM, Chamberland JP, Hu FB, Mantzoros CS. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr. 2008;88(5):1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryo M, Funahashi T, Nakamura T, Kihara S, Kotani K, Tokunaga K, Matsuzawa Y, Shimomura I. Fat accumulation and obesity-related cardiovascular risk factors in middle-aged Japanese men and women. Intern Med. 2014;53(4):299–305. [DOI] [PubMed] [Google Scholar]
- 19. Gautier A, Roussel R, Ducluzeau PH, Lange C, Vol S, Balkau B, Bonnet F; DESIR Study Group . Increases in waist circumference and weight as predictors of type 2 diabetes in individuals with impaired fasting glucose: influence of baseline BMI: data from the DESIR study [published correction appears in Diabetes Care. 2010;33(10):2294–2294]. Diabetes Care. 2010;33(8):1850–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okauchi Y, Iwahashi H, Okita K, Funahashi T, Kishida K, Noguchi M, Ohira T, Nakamura T, Imagawa A, Shimomura I. Weight reduction is associated with improvement of glycemic control in Japanese men, whose hemoglobin A1C is 5.6-6.4%, with visceral fat accumulation, but not without visceral fat accumulation. J Diabetes Investig. 2013;4(5):454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanai H, Tokunaga K, Fujioka S, Yamashita S, Kameda-Takemura KK, Matsuzawa Y. Decrease in intra-abdominal visceral fat may reduce blood pressure in obese hypertensive women. Hypertension. 1996;27(1):125–129. [DOI] [PubMed] [Google Scholar]
- 22. Okauchi Y, Nishizawa H, Funahashi T, Ogawa T, Noguchi M, Ryo M, Kihara S, Iwahashi H, Yamagata K, Nakamura T, Shimomura I, Matsuzawa Y. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007;30(9):2392–2394. [DOI] [PubMed] [Google Scholar]
- 23. Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad, Ser B, Phys Biol Sci. 2010;86(2):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukuda S, Kita S, Obata Y, Fujishima Y, Nagao H, Masuda S, Tanaka Y, Nishizawa H, Funahashi T, Takagi J, Maeda N, Shimomura I. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J Biol Chem. 2017;292(19):7840–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120(12):4342–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288(34):24886–24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. [DOI] [PubMed] [Google Scholar]
- 28. Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–737. [DOI] [PubMed] [Google Scholar]
- 29. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. [DOI] [PubMed] [Google Scholar]
- 30. Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17(2):185–196. [DOI] [PubMed] [Google Scholar]
- 31. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielsen SM, Bartels EM, Henriksen M, Wæhrens EE, Gudbergsen H, Bliddal H, Astrup A, Knop FK, Carmona L, Taylor WJ, Singh JA, Perez-Ruiz F, Kristensen LE, Christensen R. Weight loss for overweight and obese individuals with gout: a systematic review of longitudinal studies. Ann Rheum Dis. 2017;76(11):1870–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chruściel P, Sahebkar A, Rembek-Wieliczko M, Serban MC, Ursoniu S, Mikhailidis DP, Jones SR, Mosteoru S, Blaha MJ, Martin SS, Rysz J, Toth PP, Lip GY, Pencina MJ, Ray KK, Banach M; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group . Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208. [DOI] [PubMed] [Google Scholar]
- 34. Koh KK, Oh PC, Sakuma I, Lee Y, Han SH, Shin EK. Rosuvastatin dose-dependently improves flow-mediated dilation, but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Int J Cardiol. 2016;223:488–493. [DOI] [PubMed] [Google Scholar]