Abstract
Context
Controlled, blinded studies of sex-hormone replacement in postmenopausal women using natural estradiol (E2) and native progesterone (P) are few.
Objective
To delineate the effect of E2 alone or with P on lipids and inflammatory markers.
Design
A placebo-controlled, double-masked, prospectively randomized study of 40 healthy, postmenopausal volunteers assigned to four treatment groups: placebo, intramuscular E2, and/or micronized oral P for 23 (±2) days.
Results
Treatment with E2 alone compared with placebo lowered total cholesterol (TC; P = 0.006), non–high-density lipoprotein cholesterol (nonHDL-C; P = 0.004), low-density lipoprotein cholesterol (LDL-C; P = 0.012), and apolipoprotein B (Apo B; P = 0.02) levels, and raised HDL-C levels (P = 0.03 vs the 3 other groups). Conversely, addition of P to E2 reduced HDL-C levels (P = 0.015). Triglyceride concentrations manifested no effect on E2 or P. High-sensitivity C-reactive protein (hsCRP) level was highest in women with E2 and P replacement (P = 0.018 vs placebo). Leptin and IL-6 concentrations did not vary. P treatment decreased adiponectin levels (P = 0.019). Serum E2 levels correlated linearly with TC, LDL-C, nonHDL-C, Apo B (all negatively), and SHBG (positively) concentrations. P level correlated negatively with TC (P = 0.029), HDL-C (P = 0.002), and adiponectin (P = 0.002) levels.
Conclusion
In this study, there were individual and interactive effects of E2 and P on key lipids in postmenopausal individuals.
Keywords: human, inflammation, lipids, sex hormone, women
Estradiol given parenterally modifies TC, LDL-C, apo B, and HDL-C, whereas progesterone regulates TC, HDL and adiponectin. Neither hormone alters Lp(a), leptin or IL-6.
Menopause greatly affects mental and physical health, due to associated hot flushes, urinary symptoms, anxiety, depression, insomnia, osteoporosis, and cardiovascular disease [1]. Whereas premenopausal women are protected from coronary artery disease compared with age-matched men, this advantage rapidly declines after the onset of menopause [2]. Indeed, menopause is associated with the emergence of a less favorable lipid profile, including increased concentrations of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), and decreased levels of high-density lipoprotein cholesterol (HDL-C). In particular, the premenopausal sex-steroid milieu is considered more favorable to lipids [3]. Moreover, in postmenopausal individuals, estrogen replacement may improve certain aspects of the lipid profile, depending on dose, duration, and route of administration (i.e., oral, transdermal, other), and choice of estrogen and/or accompanying progestin [4].
Unopposed estrogen administration in women who have not undergone hysterectomy is undesirable in view of the risk of endometrial hyperplasia and possible carcinoma, prompting the addition of various synthetic progestins. However, synthetic progestins have variable intrinsic androgenicity and may oppose otherwise favorable estrogen effects [5]. Progestins derived from 19-nortestosterone have androgenic properties and thus commonly suppress HDL [6, 7]. On the other hand, natural progesterone (P) and 17-hydroxyprogesterone derivatives have low intrinsic androgenicity. Despite these confounding concerns, few studies have evaluated the lipid effect of natural P administered to mimic physiologic sex-steroid variations [8, 9]. Likewise, few investigations have used IM delivery of natural estradiol (E2), which would circumvent the first pass effect of oral E2, which may exert distinct effects on blood clotting, lipids, and inflammatory markers [10–12].
We measured concentrations of lipids, lipoproteins, and inflammatory markers to appraise the actions of native gonadal hormone on cardiovascular risk motifs without confounding by synthetic progestins and nonphysiological oral or transdermal estrogen delivery. The end points of this study were the influence of E2 on the lipid profile and inflammatory markers, and the modulating effect of P.
1. Material and Methods
A. Subjects
A total of 40 healthy, ambulatory, community-dwelling postmenopausal women, clinically defined by E2 level <50 pg/mL and FSH >30 IU/L, within the allowable age range of 50 to 80 years participated in the overnight clinical research unit (CRU)-based study. Volunteers were recruited by newspaper advertisements, local posters, the Mayo Clinical Trials Center Web page, and community (general and minority) bulletin boards. The design of the study was a placebo-controlled, double-masked, prospectively randomization assignment to four hormone treatment groups (1) saline placebo IM and oral placebo, (2) saline placebo IM and oral micronized P, (3) E2 valerate IM and oral placebo, and (4) E2 valerate IM and oral micronized P. The treatment paradigm comprised the following: day 1, IM E2 valerate 2.4 mg or saline placebo 0.12 mL administration during an outpatient visit; day 10 (±2 days), IM E2 valerate 5 mg or saline placebo, plus oral micronized P 100 mg or oral placebo three times daily for 14 days (±2 days). At day 23 (±2 days), subjects were admitted to the CRU at 5:00 pm and received a prescribed metabolic study meal consisting of 8 kcal/kg of 50% carbohydrate, 20% protein, and 30% fat at 1900 hours. They remained fasting until the end of the study at 10:00 am the following morning. Breakfast was offered after 10:00 am before discharge from the CRU. Volunteers were allowed to sleep during the overnight sampling window. This comprised blood sampling every 10 minutes during 12 hours overnight, starting at 10:00 pm.
The study was designed to address whether E2 and/or P amplifies GH secretion and affects lipid concentrations and inflammatory markers. Hormones and peptides were measured in the fasting blood sample obtained at 8:00 am. The GH results will be reported elsewhere. At the end of the CRU study, subjects in the E2-only group received medroxyprogesterone acetate 5 mg capsules (in blinded fashion) taken orally for 10 days, whereas women enrolled in the other groups received placebo oral capsules for 10 days.
The protocol was approved by Mayo Institutional Review Board. Witnessed voluntary written informed consent was obtained before study enrollment. A complete medical history, physical examination, and results of screening tests of hematological, renal, hepatic, metabolic, and endocrine function were normal. Subjects underwent a single-slice CT of the abdomen, level L3-4, as an exploratory test of the affect of relative visceral obesity on GH and lipid responses. E2 valerate was obtained from PharmaForce (New Albany, OH) and micronized P from Akorn (Lake Forest, IL).
B. Exclusion Criteria
Exclusion criteria were acute or chronic systemic diseases; HIV positivity by medical history; anemia; endocrine disorders (except hypothyroid subjects who were biochemically euthyroid on replacement therapy); psychiatric illness; alcohol or drug abuse; deep-venous or arterial thromboses; cancer of any type (except localized basal or squamous cell cancer of the skin treated surgically without recurrence); recent use (within 2 months) of estrogen, progestin, anabolic steroids or glucocorticoids; history of stroke, myocardial infarction, or angina; clinically significant ECG abnormality as determined by study team physicians; allergy to medications used in the study; significant recent weight change (defined as loss or gain of ≥6 pounds over 6 weeks); transmeridian travel (exceeding three time zones within the preceding 3 weeks); current or recent night-shift work; abnormal renal, hepatic, or hematologic function; concomitant sex-hormone replacement; and unwillingness to provide written informed consent.
C. Assays
TC, triglyceride (TG), and HDL-C levels were measured using the Roche Cobas c311 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) with interassay coefficients of variance (CVs) of 2.2%, 0.8%, and 0.6% at 249 mg/dL, 178 mg/dL, and 51 mg/dL, respectively. LDL-C was calculated by the Friedewald equation. Apolipoprotein B (Apo B) was measured immunoturbimetrically on the Hitachi Chemistry Analyzer (Hitachi High-Technologies, Tokyo, Japan) using the DiaSorin Apo B SPQ II Reagent Set (DiaSorin, Stillwater, MN) with an interassay CV of 4% at 94 mg/dL. Lipoprotein α [Lp(a)] was measured by a turbidmetric immunoassay on the Roche Cobas c311 Chemistry Analyzer. The interassay CV was 4.3% at 21 mg/dL. IL-6 was measured by a high-sensitivity, two-site, enzyme-linked immunoassay from R&D Systems (Minneapolis, MN). The interassay CV was 3.6% at 3.88 pg/mL. Leptin and adiponectin were measured by specific immunoassays kits (Linco Research, St Louis, MO). The interassay CVs were 11% and 4.7% at 20.4 ng/mL and 29.9 ng/mL, respectively. High-sensitivity C-reactive protein (hsCRP) was measured by a high-sensitivity immunoturbimetric assay on the Roche Cobas c311 Chemistry Analyzer. The interassay CV was 1.7% at 0.17 mg/dL.
The following hormones and peptides were measured in the 8:00 am fasting blood specimen: estrone, E2, and total testosterone, using liquid chromatography-tandem mass spectrometry (Agilent Technologies, Santa Clara, CA). Intraassay CVs were as follows: estrone, 12% at 0.25 pg/mL and 7.4% at 30 pg/mL; E2, 10.8% at 0.29 pg/mL and 5.1% at 32 pg/mL; testosterone, 8.9% at 0.69 ng/dL, 4.0% at 45 ng/dL, and 3.5% at 841 ng/dL. P was measured by a two-site immunoenzymatic sandwich assay on the Roche Cobas e411 (Roche Diagnostics). Intraassay CVs were 4.8%, 2.2%, and 1.8% at 0.474, 8.26, and 28.5 ng/mL, respectively. SHBG was quantified by solid-phase chemiluminescent assay on the Siemens Immulite 2000 Automated Immunoassay System (Siemens Healthcare Diagnostics, Deerfield, IL). Intraassay CVs for SHBG were 4.0% at 5.4 nmol/L and 5.9% at 74 nmol/L. Insulin was measured by a two-site immunoenzymatic sandwich assay on the Roche e411. Intraassay CVs were 3.3%, 2.8%, and 2.5% at 18, 61, and 172 mU/L, respectively. Prolactin, FSH, and LH were measured by two-site chemiluminescent sandwich immunoassays on a DXL 800 automated immunoassay system (Beckman Instruments, Chaska, MN). For prolactin, the intraassay CVs were 3.7%, 2,1%, and 4.8% at 6.1, 16.4, and 34.5 µg/L, respectively. For FSH, the interassay CVs were 3.6%, 3.2%, and 4.7% at 6.5, 16.7, and 58.0 IU/L, respectively. For LH, CVs were 9.3%, 6.0%, and 6.0% at 1.4, 15.6, and 48.8 IU/L, respectively.
D. Statistics
Data were analyzed by ANOVA for the four randomly assigned treatment groups. Post hoc testing was mainly restricted to comparisons between E2(+) and E2(−) subjects, and between P(+) and P(−) subjects as prestated hypotheses. Age and visceral fat mass were covariates in all statistical analyses. In case of nonnormal distribution, the Kruskal-Wallis test was used. This was followed by the Dwass-Steel-Critchlow test for pairwise comparisons. Linear regression analysis was applied to identify concentration-dependent effects of P and/or E2 after correcting for age and visceral fat area. Calculations were performed with Systat 13 (Systat Software, San Jose, CA). P < 0.05 was considered statistically significant for the overall study.
2. Results
Demographic and hormonal data of the four groups of women at the screening visit are listed in Table 1. By ANOVA, the four groups were strictly comparable, including parameters of body composition, mean age, and serum concentrations of hormones and binding proteins. Table 2 lists the effects of E2 and/or P and/or placebo treatment on hormone levels. Differences in estrone, LH, FSH, and prolactin concentrations were related to E2 replacement only (P values between E2 (+) vs E2 (−) subjects all <0.0001). P had no additional effect on these measures. No effects were observed on glucose homeostasis parameters.
Table 1.
Demographic Data at the Start of the Study
| Plac + Plac | Plac + P | E2 + Plac | E2 + P | ANOVA P Value | |
|---|---|---|---|---|---|
| Age, y | 61 ± 2 | 63 ± 2 | 63 ± 2 | 65 ± 1 | 0.48 |
| BMI, kg/m2 | 25.6 ± 1.5 | 26.8 ± 1.7 | 25.5 ± 0.9 | 24.9 ± 1.3 | 0.81 |
| Visceral fat, cm2 | 89 ± 18 | 99 ± 25 | 90 ± 15 | 86 ± 13 | 0.97 |
| Total fat, cm2 | 313 ± 51 | 311 ± 60 | 325 ± 47 | 300 ± 33 | 0.99 |
| E2, pg/mL | 23.6 ± 1.4 | 23.3 ± 1.7 | 23.5 ± 1.5 | 23.5 ± 1.5 | 0.99 |
| P, ng/mL | 0.32 ± 0.04 | 0.27 ± 0.03 | 0.39 ± 0.06 | 0.26 ± 0.03 | 0.19 |
| Testosterone, ng/dL | 13.7 ± 1.7 | 14.5 ± 1.6 | 17.5 ± 1.6 | 23.7 ± 1.8 | 0.34 |
| LH, mU/L | 37 ± 4 | 32 ± 5 | 32 ± 5 | 31 ± 3 | 0.77 |
| FSH, mU/L | 74 ± 5 | 88 ± 12 | 78 ± 7 | 73 ± 7 | 0.56 |
| SHBG, nmol/L | 55 ± 7 | 54 ± 7 | 61 ± 7 | 72 ± 8 | 0.30 |
| TSH, mU/L | 3.66 ± 0.69 | 3.58 ± 0.47 | 2.75 ± 0.39 | 2.92 ± 0.31 | 0.48 |
| Cortisol, µg/dL | 12.6 ± 1.3 | 10.9 ± 0.7 | 12.6 ± 0.9 | 12.5 ± 1.2 | 0.64 |
| Prolactin, µg/L | 9.1 ± 1.1 | 10.7 ± 1.7 | 10.5 ± 1.2 | 9.8 ± 1.9 | 0.78 |
| IGF-I, µg/L | 124 ± 8 | 123 ± 8 | 133 ± 12 | 110 ± 18 | 0.78 |
| IGFBP1, µg/L | 3.02 ± 0.47 | 2.36 ± 0.37 | 3.05 ± 0.47 | 3.93 ± 0.90 | 0.35 |
| IGFBP3, mg/L | 4.05 ± 0.25 | 3.98 ± 0.33 | 4.03 ± 0.19 | 3.41 ± 0.25 | 0.25 |
| TGs, mg/dL | 116 ± 14 | 87 ± 10 | 97 ± 13 | 96 ± 12 | 0.43 |
Data are given as mean ± SEM.
Abbreviations: BMI, body mass index; IGFBP, like growth factor-binding protein; Plac, placebo.
Table 2.
Selected Hormone Levels During the Experiment
| Plac + Plac | Plac + P | E2 + Plac | E2 + P | ANOVA | |
|---|---|---|---|---|---|
| LH, mU/L | 27 ± 2 | 22 ± 2 | 6.0 ± 1.0 | 3.5 ± 1.0 | <0.0001 |
| FSH, mU/L | 65 ± 5 | 69 ± 6 | 18 ± 2 | 9.0 ± 2.1 | <0.0001 |
| E1, pg/mL | 16 ± 3 | 12 ± 2 | 50 ± 6 | 33.8 ± 5.4 | <0.0001 |
| E2, pg/mL | 3.8 ± 0.7 | 3.3 ± 0.6 | 99 ± 16 | 88 ± 13 | <0.0001 |
| P, ng/mL | 0.2 ± 0.0 | 10.1 ± 1.0 | 0.2 ± 0.0 | 20.3 ± 3.1 | <0.0001 |
| Prolactin, µg/L | 9.7 ± 0.7 | 10.4 ± 1.3 | 19.5 ± 2.4 | 17.3 ± 2.1 | 0.0001 |
| Cortisol, µg/dL | 13.0 ± 1.1 | 11.3 ± 1.2 | 12.5 ± 1.3 | 11.1 ± 1.6 | 0.67 |
| SHBG, nmol/L | 46 ± 6 | 47 ± 7 | 85 ± 9 | 93 ± 10 | <0.0001 |
| Insulin, mU/L | 6.2 ± 1.0 | 6.8 ± 1.1 | 5.2 ± 0.8 | 5.3 ± 1.2 | 0.56 |
| Glucose, mg/dL | 89 ± 2 | 91 ± 2 | 88 ± 4 | 87 ± 3 | 0.66 |
| HOMA-IR | 1.58 ± 0.27 | 1.96 ± 0.39 | 1.34 ± 0.23 | 1.81 ± 0.43 | 0.49 |
Data are given as mean ± SEM. The effects on E1, LH, FSH, prolactin, and SHBG are related to E2 administration. P < 0.0001 for comparison of E2 (+) vs E2 (−) for these five hormones.
Abbreviations: E1, estrone; Plac, placebo.
P < 0.05.
The effects of hormone treatment on lipids and inflammation markers are summarized in Table 3. TG concentrations were comparable in the four groups without any effect of IM E2 and/or oral P. E2 caused a decrease in TC (P = 0.006). HDL-C concentration was highest in the E2-only treatment group (P = 0.03 vs the three other groups). Addition of P to E2 caused a significant decrease in HDL-C level (P = 0.015). Apo B level was lower in women treated with E2 than in those with unreplaced E2 (P = 0.02). The concentration of hsCRP was highest in women with E2 plus P replacement compared with women treated with double placebo (P = 0.031). Leptin, Lp(a), and IL-6 concentrations were similar among the four groups. The addition of P with or without E2 decreased adiponectin levels (P = 0.019).
Table 3.
Lipid, Lipoprotein, and Inflammation Markers in Postmenopausal Volunteers
| Plac + Plac | Plac + P | E2 + Plac | E2 + P | Treatment P Value | |
|---|---|---|---|---|---|
| Adiponectin, ng/mL | 13,400 ± 1190 | 10,530 ± 1170 | 12,520 ± 1400 | 10,270 ± 770 | 0.089 |
| Leptin, ng/mL | 31.7 ± 8.2 | 36.4 ± 9.5 | 35.2 ± 6.7 | 44.9 ± 8.8 | 0.30 |
| TGs, mg/dL | 154 ± 19 | 148 ± 14 | 126 ± 12 | 129 ± 20 | 0.53 |
| TC, mg/dL | 199 ± 9 | 192 ± 8 | 180 ± 7 | 164 ± 7.9 | 0.02 |
| HDL-C, mg/dL | 56 ± 4 | 50 ± 3.7 | 62 ± 5 | 47 ± 4 | 0.06 |
| LDL-C, mg/dL | 112 ± 8 | 112 ± 8 | 92 ± 7 | 91 ± 8 | 0.08 |
| NonHDL-C, mg/dL | 143 ± 10 | 142 ± 8 | 117 ± 7 | 116 ± 8 | 0.04 |
| Apo B, mg/dL | 0.90 ± 0.05 | 0.93 ± 0.05 | 0.77 ± 0.05 | 0.80 ± 0.07 | 0.13 |
| Lp(a), mg/dL | 17.7 ± 7.6 | 22.3 ± 5.5 | 29.5 ± 8.5 | 19.3 ± 8.3 | 0.60 |
| hsCRP, mg/dL | 0.16 ± 0.08 | 0.079 ± 0.024 | 0.186 ± 0.036 | 0.333 ± 0.114 | 0.018a |
| IL-6, pg/mL | 3.7 ± 0.9 | 2.9 ± 0.6 | 4.5 ± 1.0 | 5.4 ± 1.2 | 0.46 |
Data are mean ± SEM. In the analysis of covariance, age and visceral mass were covariates.TC, HDL-C, LDL-C, and nonHDL-C were lower in women receiving E2 treatment than in those without E2 replacement (post hocP = 0.006, P = 0.01, P = 0.012, and P = 0.004, respectively). The addition of P to E2 decreased HDL-C concentration (P = 0.019). Adiponectin levels were lower during P treatment (P = 0.019).
Abbreviation: Plac, placebo.
Because of nonnormality of hsCRP data, the Kruskal-Wallis test was used with post hoc contrasts. The hsCRP concentration in women treated with E2 and P was higher than in placebo-treated women (P = 0.018).
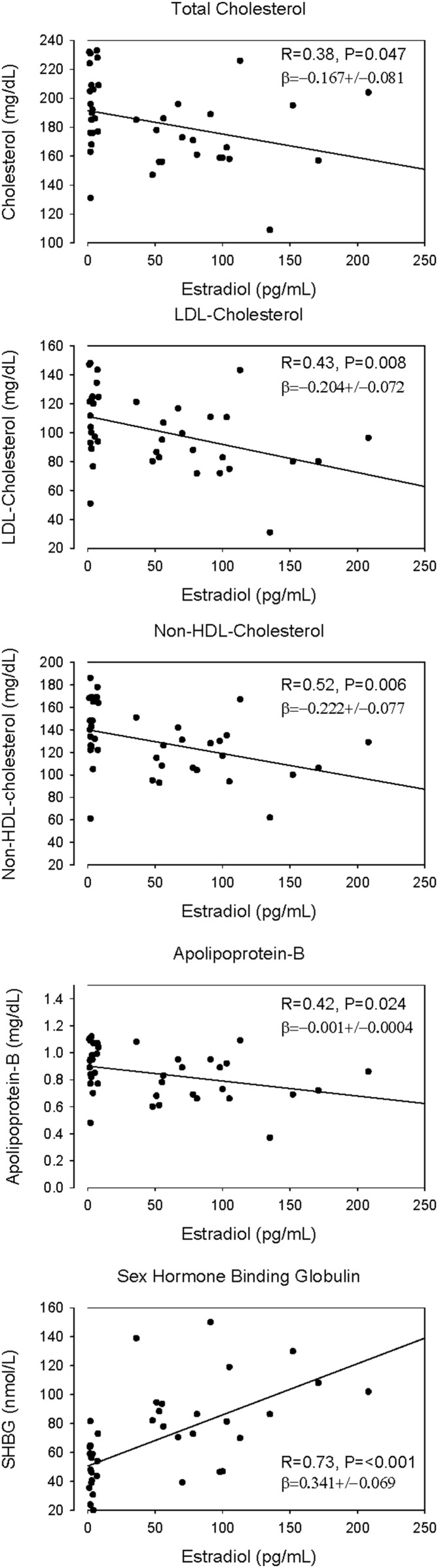
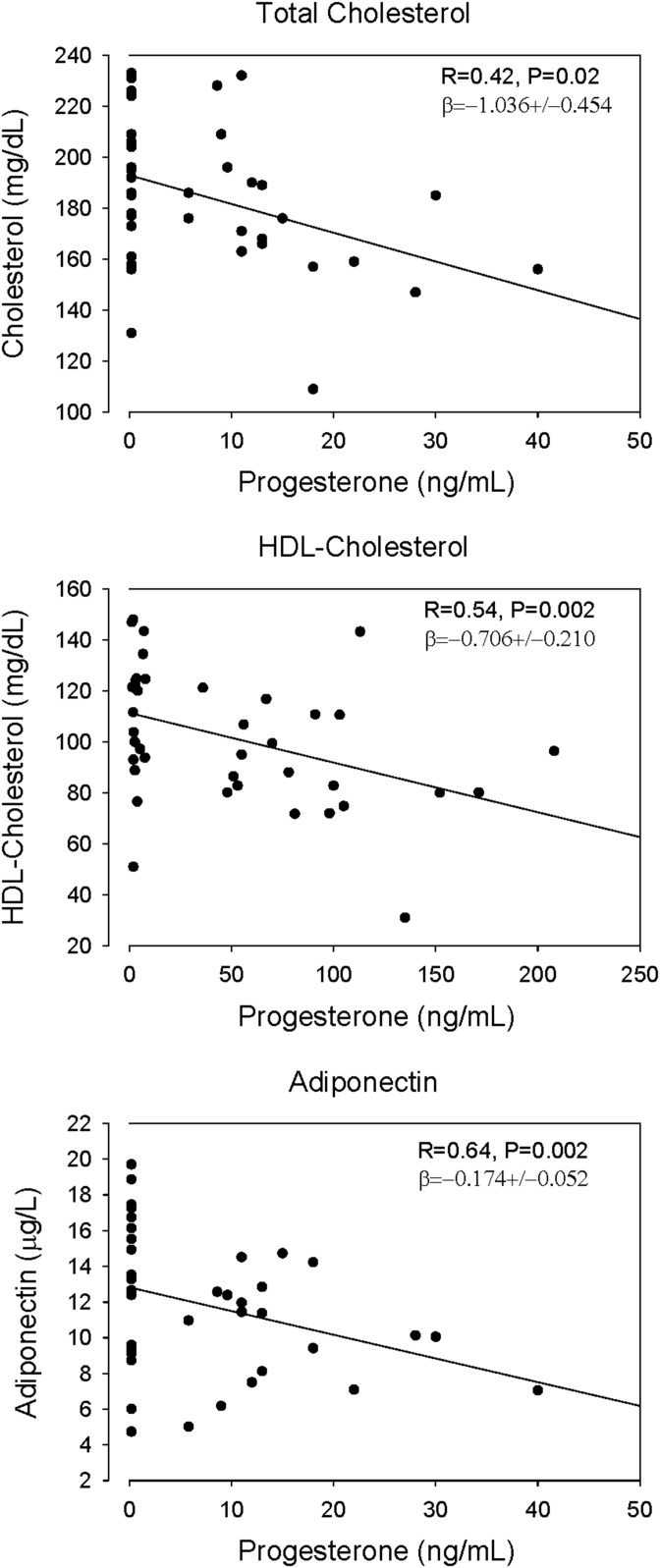
In regression analyses, E2 level correlated negatively with TC, Apo B, LDL-C, and nonHDL-C levels, but positively with that of SHBG (Fig. 1). Serum P concentrations correlated negatively with those of TC (P = 0.029), HDL-C (P = 0.002), and adiponectin (P = 0.002; Fig. 2). In addition, adiponectin correlated negatively with abdominal visceral fat [R = −0.45; β = −31.0 ± 10.0 (mean ± SD)], but not with total fat (R = 0.19; P = 0.25), E2 (R = 0.006; P = 0.97), or SHBG (R = 0.27; P = 0.09). No correlations were present between concentrations of E2 and TGs (R = 0.22), Lp(a) (R = 0.07), Apo B (R = 0.19), leptin (R = 0.01), IL-6 (R = 0.06), and hsCRP (R = 0.18). NonHDL-C concentration correlated positively with that of leptin (P = 0.013; Supplemental Table 1). Homeostatic model assessment of insulin resistance (HOMA-IR) correlated with body fat and SHBG, specifically visceral fat [β = 0.008 ± 0.00 (mean ± SD); P < 0.0001] and SHBG [β = −0.008 ± 0.003 (mean ± SD); P = 0.011], but not with lipids in these healthy women.
Figure 1.
Linear regressions of TC, LDL-C, Apo B, and nonHDL-C on serum E2 concentrations. Linear regressions were calculated with the inclusion of visceral fat area and age as variables. P and β values are adjusted for these covariates.
Figure 2.
Linear regressions of TC, HDL-C, and adiponectin on serum P concentrations in 40 postmenopausal women. Linear regressions were calculated with the inclusion of visceral fat area and age as variables. P and β values are adjusted for these covariates.
3. Discussion
In this placebo-controlled, prospectively randomized, and doubly masked study, we investigated the separate and combined effects of parenteral E2, natural P, and/or placebo on lipids, lipoproteins, adipokines (i.e., leptin and adiponectin), inflammatory markers (i.e., hsCRP and IL-6), and several basic liver-derived, estrogen-responsive proteins in postmenopausal women. A comparable study design has not been used before to our knowledge. There were no dropouts among or serious adverse effects in the volunteers. Sex-hormone doses successfully mimicked E2 and P concentrations in the luteal phase of the menstrual cycle in healthy young women [13]. Furthermore, and essential to the study design, baseline demographic, lipid, and endocrine data prior to intervention were quantifiably comparable among the four subject cohorts. E2 supplementation led to well-known effects on serum SHBG, prolactin, insulin-like growth factor-binding protein 3, and gonadotropin concentrations (positive controls). This establishes adequacy of E2 administration to regulate well-established physiological end points.
In accordance with earlier reports, E2 treatment (given IM in this study to avoid first-pass liver effects) decreased TC concentration [8, 9, 14–17]. The addition of natural P in a daily oral dose of 300 mg did not block the TC-lowering effect of E2, in line with earlier findings [17–20]. In other studies, P did not attenuate the favorable effect of E2 on endothelium-dependent vasodilatation [21]. The E2 effect in our study was corroborated by the negative linear relation between serum E2 and TC (as well as LDL, nonHDL-C, and Apo B) concentrations. However, data indicate that when other progestins are used, the cholesterol-lowering effect of E2 may be blocked, putatively depending on the intrinsic androgenic properties of the P analog [6, 7]. Parental E2 supplementation caused the well-established increase in HDL-C concentration. This effect seems common to all estrogens, but the degree of effect depends on the dose, the chemical structure of the estrogen, and its route of administration, being generally greater after orally administered estrogens [6, 7]. Natural P in our study achieved serum concentrations typically found in the luteal phase of the normal menstrual cycle [13]. Under these conditions, P caused a concentration-dependent decrease in serum HDL-C concentrations, as several other scattered reports suggested [6, 9, 22], albeit not in two other studies [9, 23].
Although the molecular mechanisms of E2 action on liver lipid metabolism have been investigated, those of P are not well studied. Estrogen receptor is widely expressed in many tissues, but nuclear receptors for P are limited to female reproductive organs, the pituitary gland, and brain. Such data do not necessarily exclude effects of P on lipids and inflammatory markers via membrane-associated receptors, which are also preset in nonreproductive tissues, including the liver [24]. The reported nonspecific effects of synthetic progestins, on the other hand, are likely mediated via the androgen, glucocorticoid, and/or mineralocorticoid receptors [7].
Although TGs tended to be lower in women treated parenterally with E2, the differences were not significant, and there was no influence of P on TGs in either women with deprived of or supplemented with E2. Earlier studies of TG changes during combined sex-hormone treatment found either no effect, decreases [25], or increases in TG concentrations [8, 14]. A common feature of those studies was that the estrogen (either conjugated equine estrogen or E2) was given orally. One study did not report the medication route [26]. In contrast, continuous transdermal E2 administration, with or without medroxyprogesterone, decreased TGs in another study [27]. Overall, these results suggest that the route of estrogen administration in part determines TG levels [6].
Important independent predictors of cardiovascular disease are Apo B and Lp(a) [22]. In the present study, there was a clear negative linear relation between serum Apo B and E2 concentrations. During combined treatment with estrogen and synthetic progestins, several but not all prior studies reported decreased Apo B levels [25, 28, 29]. Lp(a) was statistically similar in the four studied groups. However, previous studies using oral estrogens generally described a decrease in Lp(a) levels on treatment [30]. This was not the case in studies using intranasal or transdermal routes [31, 32]. Unlike IM E2 injection used in our study, the oral route exposes the liver to massively increased estrogen concentrations, which are not typical of normal physiology of ovarian estrogen secretion into systemic blood.
E2 treatment was accompanied by increased levels of the nonspecific inflammatory marker hsCRP. The rise in hsCRP levels was mainly evident after addition of P to E2. This liver-derived protein is an independent risk marker, albeit not a casual factor, for coronary artery disease [33]. Similar results were noted in other studies when E2 was administered orally, but not transdermally [12]. The discrepant behavior of several inflammatory markers during oral estrogen replacement in postmenopausal women has raised the question whether change in hsCRP concentration in this context may be less an acute phase response than a metabolic liver response [12]. In these cases, as well as in our study, IL-6 levels did not increase. Concentrations of several other liver-derived inflammation markers have been shown to decline during oral estrogen administration [10, 12, 34].
Leptin, adiponectin, and resistin are all proteins synthesized and secreted by adipose tissue. Low serum adiponectin and high resistin levels are associated with prediabetes, diabetes, and/or atherosclerosis [35]. During gonadal-hormone changes in the menstrual cycle, adiponectin levels remain constant, in contrast to leptin and resistin levels, which, in one study, rose during the luteal phase [36]. Another study reported a 15% decrease in hormone replacement–associated adiponectin levels, which correlated negatively with the change of the waist-hip ratio [37]. This was not observed in an independent investigation [38]. The change in body composition is a potential caveat in the interpretation of direct steroid effects on adiponectin. Indeed, there is a positive correlation between waist girth and adiponectin [39]. In the present study, we found a strong correlation between CT-estimated visceral fat and adiponectin concentrations, explaining the mechanism of the waist effect.
As surrogate for insulin resistance, we used HOMA-IR. There was no deleterious effect of E2 or P on this measure. In agreement with other data, there were strong linear correlations between HOMA-IR and serum SHBG levels (inverse) and visceral fat (positive) in the present cohort of 40 subjects.
NonHDL-C is a strong epidemiological marker of atherosclerosis risk, even in familial hypercholesterolemia [40]. To our knowledge, the present analysis is the first to report an E2 concentration–dependent reduction of nonHDL-C in humans. We also found no opposing effect of native P on the E2 effect. Our data allow the hypothesis that a late follicular phase decrease of nonHDL-C concentration could be sustained across the luteal-phase rise in P level in normal younger women after the preovulatory rise in E2 level [13]. This hypothesis remains to be confirmed or refuted, because it was not evaluated in early menstrual-cycle studies [3]. Likewise, whether recurrent lowering of nonHDL-C level summed across menstrual cycles contributes to partial protection of younger women compared with men from early atherosclerotic cardiovascular disease is not known, although it is suggested in the analogous case of LDL-C lowering before and after ovulation. Indeed, if verified, menstrual cycle–related changes in nonHDL-C concentration would need to be considered in screening and monitoring nonHDL-C concentrations in health and disease.
The design of this study included an intentionally relatively short-term (i.e., 23-day) hormone replacement because we wished to approach the follicular and luteal phase lengths of about 14 days and we were particularly interested in more acute effects of sex steroids on lipids and inflammation markers. In uncontrolled epidemiological studies, long-term effects of sex-steroid administration over months can introduce confounding factors (e.g., changes in lifestyle, body weight, physical activity, and/or body composition), which would secondarily alter concentrations of lipoproteins and inflammatory markers. The present design limits the interpretative confounding otherwise expected by significant shifts in body composition.
Limitations of the study include the relatively small number of subjects, which could have limited statistical power, although the groups were balanced in clinical characteristics. In this study, we relied on the differences in lipid and inflammation marker concentrations between randomly and prospectively assigned treatment groups and not on the magnitude in change from baseline levels. Although the latter approach could have strengthened weaker outcomes of the study, it was not required here to see strong effects of both estrogen and P on major lipids. In addition, in light of the results, the inclusion of additional circulating inflammatory markers could have strengthened this study.
Finally, in the design of the study, the IM route of E2 was chosen, which is rarely used in clinical practice. Therefore, a head-to-head comparison with published clinical epidemiological studies would have limitations. However, the physiological hormones chosen and the significant results obtained (e.g., by regression of circulating E2 concentrations on LDL-C concentrations in 20 women) should be quite useful for future sex-hormone studies. Strong points of this study are the placebo-controlled, blinded, prospectively randomized design and the use of physiological sex hormones at physiological concentrations, thus avoiding liver exposure to extremely high estrogen concentrations and/or systemic effects of synthetic progestins.
In summary, near-physiological E2 concentrations decreased TC, Apo B, nonHDL-C, and SHBG levels, but increased those of HDL-C and hsCRP and did not effect Lp(a), IL-6 and a surrogate measure of insulin resistance. P treatment resulted in increased adiponectin and hsCRP concentrations while decreasing those of TC and HDL-C. The P effect on HDL occurred in the face of unchanged E2 concentrations, indicating a strong interaction.
Supplementary Material
Acknowledgments
We thank Jill Smith for support of manuscript preparation, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Financial Support: This work was supported in part by the National Institutes of Health [Grants R01 AG029362 (to J.D.V.), R01 AG031763 (to J.D.V.), and P30 DK050456 (to Metabolic Studies Core of the Minnesota Obesity Center)]; the National Center for Advancing Translational Sciences (Grant UL1 TR000135); and the National Institute of Standards and Technology (Grant 60NANB10D005Z). Contents are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Apo B
apolipoprotein B
- CRU
clinical research unit
- CV
coefficient of variance
- E2
natural estradiol
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostatic model assessment of insulin resistance
- hsCRP
high-sensitivity C-reactive protein
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
lipoprotein α
- P
natural progesterone
- TC
total cholesterol
- TG
triglyceride
References and Notes
- 1. Gartlehner G, Patel SV, Feltner C, Weber RP, Long R, Mullican K, Boland E, Lux L, Viswanathan M. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;318(22):2234–2249. [DOI] [PubMed] [Google Scholar]
- 2. Cifkova R, Krajcoviechova A. Dyslipidemia and cardiovascular disease in women. Curr Cardiol Rep. 2015;17(7):609. [DOI] [PubMed] [Google Scholar]
- 3. Muesing RA, Forman MR, Graubard BI, Beecher GR, Lanza E, McAdam PA, Campbell WS, Olson BR. Cyclic changes in lipoprotein and apolipoprotein levels during the menstrual cycle in healthy premenopausal women on a controlled diet. J Clin Endocrinol Metab. 1996;81(10):3599–3603. [DOI] [PubMed] [Google Scholar]
- 4. Jensen J. Effects of sex steroids on serum lipids and lipoproteins. Baillieres Clin Obstet Gynaecol. 1991;5(4):867–887. [DOI] [PubMed] [Google Scholar]
- 5. Rijpkema AH, van der Sanden AA, Ruijs AH. Effects of post-menopausal oestrogen-progestogen replacement therapy on serum lipids and lipoproteins: a review. Maturitas. 1990;12(3):259–285. [DOI] [PubMed] [Google Scholar]
- 6. Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974-2000. Fertil Steril. 2001;75(5):898–915. [DOI] [PubMed] [Google Scholar]
- 7. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34(2):171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bolaji II, Grimes H, Mortimer G, Tallon DF, Fottrell PF, O’Dwyer EM. Low-dose progesterone therapy in oestrogenised postmenopausal women: effects on plasma lipids, lipoproteins and liver function parameters. Eur J Obstet Gynecol Reprod Biol. 1993;48(1):61–68. [DOI] [PubMed] [Google Scholar]
- 9. Cuadros JL, Fernández-Alonso AM, Chedraui P, Cuadros AM, Sabatel RM, Pérez-López FR. Metabolic and hormonal parameters in post-menopausal women 10 years after transdermal oestradiol treatment, alone or combined to micronized oral progesterone. Gynecol Endocrinol. 2011;27(3):156–162. [DOI] [PubMed] [Google Scholar]
- 10. Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: a review article. Climacteric. 2005;8(4):317–326. [DOI] [PubMed] [Google Scholar]
- 11. Rachoń D, Suchecka-Rachoń K, Hak Ł, Myśliwska J. Effects of intranasal 17beta-estradiol administration on serum bioactive interleukin-6 and C-reactive protein levels in healthy postmenopausal women. Menopause. 2006;13(5):840–845. [DOI] [PubMed] [Google Scholar]
- 12. Silvestri A, Gebara O, Vitale C, Wajngarten M, Leonardo F, Ramires JA, Fini M, Mercuro G, Rosano GM. Increased levels of C-reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation. 2003;107(25):3165–3169. [DOI] [PubMed] [Google Scholar]
- 13. Evans WS, Sollenberger MJ, Booth RA Jr, Rogol AD, Urban RJ, Carlsen EC, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak-detection algorithms. II. The paradigm of the luteinizing hormone pulse signal in women. Endocr Rev. 1992;13(1):81–104. [DOI] [PubMed] [Google Scholar]
- 14. Xue W, Deng Y, Wang YF, Sun AJ. Effect of half-dose and standard-dose conjugated equine estrogens combined with natural progesterone or dydrogesterone on components of metabolic syndrome in healthy postmenopausal women: a randomized controlled trial. Chin Med J (Engl). 2016;129(23):2773–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Kraker AT, Kenemans P, Smolders RG, Kroeks MV, van der Mooren MJ. The effects of 17 beta-oestradiol plus dydrogesterone compared with conjugated equine oestrogens plus medroxyprogesterone acetate on lipids, apolipoproteins and lipoprotein(a). Maturitas. 2004;49(3):253–263. [DOI] [PubMed] [Google Scholar]
- 16. Christodoulakos GE, Lambrinoudaki IV, Panoulis CP, Papadias CA, Kouskouni EE, Creatsas GC. Effect of hormone replacement therapy, tibolone and raloxifene on serum lipids, apolipoprotein A1, apolipoprotein B and lipoprotein(a) in Greek postmenopausal women. Gynecol Endocrinol. 2004;18(5):244–257. [DOI] [PubMed] [Google Scholar]
- 17. Cheung AP. Acute effects of estradiol and progesterone on insulin, lipids and lipoproteins in postmenopausal women: a pilot study. Maturitas. 2000;35(1):45–50. [DOI] [PubMed] [Google Scholar]
- 18. Lee JY, Hyun HS, Park HG, Seo JH, Lee EY, Lee JS, Lee DY, Choi DS, Yoon BK. Effects of hormone therapy on serum lipid levels in postmenopausal Korean women. J Menopausal Med. 2015;21(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ottosson UB, Johansson BG, von Schoultz B. Subfractions of high-density lipoprotein cholesterol during estrogen replacement therapy: a comparison between progestogens and natural progesterone. Am J Obstet Gynecol. 1985;151(6):746–750. [DOI] [PubMed] [Google Scholar]
- 20. Ottosson UB. Oral progesterone and estrogen/progestogen therapy. Effects of natural and synthetic hormones on subfractions of HDL cholesterol and liver proteins. Acta Obstet Gynecol Scand Suppl. 1984;127(s127):1–37. [DOI] [PubMed] [Google Scholar]
- 21. Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MA. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98(12):1158–1163. [DOI] [PubMed] [Google Scholar]
- 22. Jacobson TA. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88(11):1294–1311. [DOI] [PubMed] [Google Scholar]
- 23. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gynecol. 1987;156(1):66–71. [DOI] [PubMed] [Google Scholar]
- 24. Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76(1-2):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendoza S, Velazquez E, Osona A, Hamer T, Glueck CJ. Postmenopausal cyclic estrogen-progestin therapy lowers lipoprotein[a]. J Lab Clin Med. 1994;123(6):837–841. [PubMed] [Google Scholar]
- 26. Patrelli TS, Gizzo S, Franchi L, Berretta R, Pedrazzi G, Volpi L, Lukanovic A, Zanni GC, Modena AB. A prospective, case-control study on the lipid profile and the cardiovascular risk of menopausal women on oestrogen plus progestogen therapy in a northern Italy province. Arch Gynecol Obstet. 2013;288(1):91–97. [DOI] [PubMed] [Google Scholar]
- 27. Bhathena RK, Anklesaria BS, Ganatra AM, Pinto R. The influence of transdermal oestradiol replacement therapy and medroxyprogesterone acetate on serum lipids and lipoproteins. Br J Clin Pharmacol. 1998;45(2):170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez-Alemán F, Torres JM, Cuadros JL, Ruiz E, Ortega E. Effect of estrogen-progestin replacement therapy on plasma lipids and lipoproteins in postmenopausal women. Endocr Res. 2000;26(2):263–273. [DOI] [PubMed] [Google Scholar]
- 29. Lussier-Cacan S, Xhignesse M, Desmarais JL, Davignon J, Kafrissen ME, Chapdelaine A. Cyclic fluctuations in human serum lipid and apolipoprotein levels during the normal menstrual cycle: comparison with changes occurring during oral contraceptive therapy. Metabolism. 1991;40(8):849–854. [DOI] [PubMed] [Google Scholar]
- 30. Anagnostis P, Galanis P, Chatzistergiou V, Stevenson JC, Godsland IF, Lambrinoudaki I, Theodorou M, Goulis DG. The effect of hormone replacement therapy and tibolone on lipoprotein (a) concentrations in postmenopausal women: a systematic review and meta-analysis. Maturitas. 2017;99:27–36. [DOI] [PubMed] [Google Scholar]
- 31. Bukowska H, Stanosz S, Zochowska E, Millo B, Sieja K, Chełstowski K, Naruszewicz M. Does the type of hormone replacement therapy affect lipoprotein (a), homocysteine, and C-reactive protein levels in postmenopausal women? Metabolism. 2005;54(1):72–78. [DOI] [PubMed] [Google Scholar]
- 32. Kurdoglu M, Yildirim M, Kurdoglu Z, Erdem A, Erdem M, Bilgihan A, Goktas B. Cardiovascular risk assessment with oxidised LDL measurement in postmenopausal women receiving intranasal estrogen replacement therapy. Gynecol Endocrinol. 2011;27(8):551–557. [DOI] [PubMed] [Google Scholar]
- 33. Rifai N, Buring JE, Lee IM, Manson JE, Ridker PM. Is C-reactive protein specific for vascular disease in women? Ann Intern Med. 2002;136(7):529–533. [DOI] [PubMed] [Google Scholar]
- 34. Shifren JL, Rifai N, Desindes S, McIlwain M, Doros G, Mazer NA. A comparison of the short-term effects of oral conjugated equine estrogens versus transdermal estradiol on C-reactive protein, other serum markers of inflammation, and other hepatic proteins in naturally menopausal women. J Clin Endocrinol Metab. 2008;93(5):1702–1710. [DOI] [PubMed] [Google Scholar]
- 35. Lacut K, Oger E, Le Gal G, Blouch MT, Abgrall JF, Kerlan V, Scarabin PY, Mottier D; SARAH Investigators . Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C-reactive protein. Thromb Haemost. 2003;90(1):124–131. [PubMed] [Google Scholar]
- 36. Asimakopoulos B, Milousis A, Gioka T, Kabouromiti G, Gianisslis G, Troussa A, Simopoulou M, Katergari S, Tripsianis G, Nikolettos N. Serum pattern of circulating adipokines throughout the physiological menstrual cycle. Endocr J. 2009;56(3):425–433. [DOI] [PubMed] [Google Scholar]
- 37. Kunnari A, Santaniemi M, Jokela M, Karjalainen AH, Heikkinen J, Ukkola O, Kesäniemi YA. Estrogen replacement therapy decreases plasma adiponectin but not resistin in postmenopausal women. Metabolism. 2008;57(11):1509–1515. [DOI] [PubMed] [Google Scholar]
- 38. Sumino H, Takahashi T, Itoh T, Kusaka K, Yamakawa J, Ichikawa S, Kurabayashi M, Kanda T. Plasma adiponectin levels in post-menopausal women receiving hormone replacement therapy. J Int Med Res. 2004;32(6):639–645. [DOI] [PubMed] [Google Scholar]
- 39. Laughlin GA, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes. 2007;31(3):457–465. [DOI] [PubMed] [Google Scholar]
- 40. Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS. Familial hypercholesterolaemia. Nat Rev Dis Primers. 2017;3:17093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.