Abstract
Background:
Auditory Neuropathy Spectrum Disorder (ANSD) is manifested as impairment of auditory nerve activity but preservation of the outer hair cell function.
Objective:
This study was to detect the disease-causing gene and variant(s) in a Chinese ANSD family.
Methods:
A four-generation consanguineous Chinese ANSD family and 200 unrelated healthy controls were enrolled. Exome sequencing and Sanger sequencing were applied to identify the genetic basis for ANSD in this family.
Results:
Exome sequencing detected a c.1236delC variant of the otoferlin gene in an apparently homozygous state. Sanger sequencing confirmed that the variant co-segregating with the phenotype of hearing impairments in this family. The variant was not detected in 200 healthy controls. The c.1236delC alteration may result in a truncated otoferlin missing the C2C-C2F domains and the C-terminal transmembrane domain, and thus severely damages Ca2+-dependent synaptic vesicle fusion and targeting function of the otoferlin.
Conclusion:
Our study suggested that the c.1236delC alteration in the otoferlin gene may be the disease-causing variant in this family, and also shed new light on genetic counseling to this ANSD family.
Keywords: Auditory neuropathy spectrum disorder, Exome sequencing, Hearing loss, Variant, The OTOF gene, ANSD family
1. INTRODUCTION
Auditory Neuropathy Spectrum Disorder (ANSD), also termed as Auditory Neuropathy (AN) or Auditory Dyssynchrony (AD), is manifested as impairment of auditory nerve activity but preservation of the Outer Hair Cell (OHC) function [1, 2]. It is clinically diagnosed by the absent or abnormal Auditory Brainstem Responses (ABR) while preserved otoacoustic emissions (OAE) and/or Cochlear Microphonics (CM) [2]. ANSD is a disorder of the auditory pathway caused by the lesions in the auditory nerve, Inner Hair Cells (IHC), or synapses between auditory nerve and IHC [3]. El-Badry and McFadden [4] stated that ANSD might be caused by any pathological mechanism that damages temporal encoding and neural synchrony. Patients with ANSD generally present with various levels of hearing impairments and poor language comprehension in disproportion to the levels of hearing impairments [5].
ANSD occurs in approximately 11-17.3% of infants with hearing loss [6]. About 42% of ANSD cases are caused by genetic factors. Hyperbilirubinemia, anoxia, infection, prematurity, demyelination, and drug reaction are also associated with ANSD [7]. ANSD may occur either as an isolated disorder of the auditory nerve (nonsyndromic ANSD) or as a manifestation of other syndromes, such as Charcot-Marie-Tooth disease [3]. Previous reports suggested that most forms of ANSD were nonsyndromic. Nonsyndromic ANSD displays autosomal dominant, autosomal recessive or X-linked inheritance pattern [1].
To date, at least five genes, including the otoferlin gene (OTOF) [5], the pejvakin gene (PJVK) [8], the connexin 26 gene (GJB2) [9], the mitochondrial 12S ribosomal RNA gene (MTRNR1) [10], and the drosophila diaphanous homolog 3 gene (DIAPH3) [11] have been identified as the disease-causing genes of nonsyndromic ANSD. Variants in the TBC1 domain family member 24 gene (TBC1D24) might also be associated with ANSD [12]. Hearing aids offer little help to patients with ANSD [5], while cochlear implantation offers good auditory outcomes to patients with severe to profound hearing impairments due to variants in the OTOF or GJB2 gene. Thus identification of pathogenic gene variants in ANSD families may be useful in guiding the choice of optimal treatment and assisting prenatal diagnosis [13].
It is a challenge to detect pathogenic gene variants for nonsyndromic ANSD by regular Sanger sequencing due to its high genetic heterogeneity. As an effective alternative strategy, exome sequencing has been suggested [14]. This study detected an apparently homozygous OTOF frameshift variant in a consanguineous Chinese family with nonsyndromic ANSD.
2. MATERIALS AND METHODS
2.1. Subjects
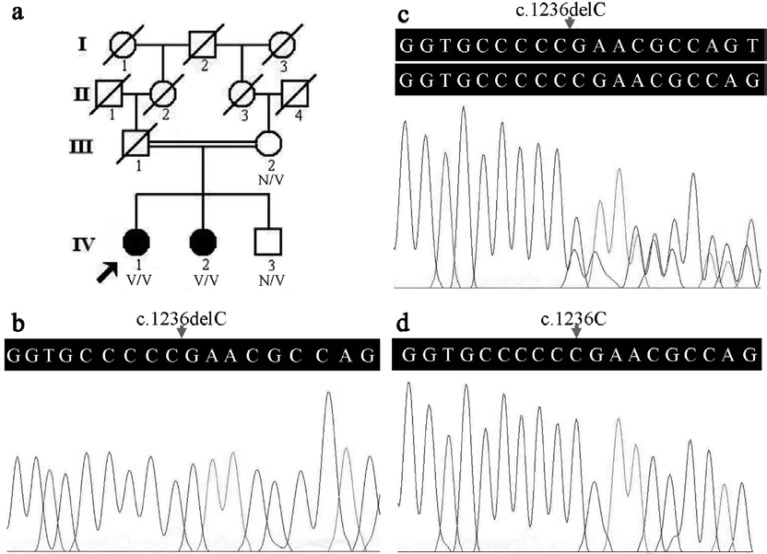
A four-generation consanguineous Han Chinese family with ANSD (Fig. 1a) was enrolled. Two patients (IV:1 and IV:2) and two unaffected individuals (III:2 and IV:3) in this family were involved in this study. Both the proband (IV:1), a 40-year-old female, and her affected sister (IV:2), a 35-year-old female, suffered from bilateral prelingual hearing loss with poor speech discrimination, not accompanied with other clinical features. The two sisters were not offered hearing aids or cochlear implantation during their childhood. Their first-cousin parents (III:1 and III:2) and younger brother (IV:3) had normal hearing and speech abilities. Two hundred unrelated healthy subjects (aged 38.5±7.8 years) matched by gender and ethnicity were enlisted as controls. Informed consent forms were signed by all the participants or their guardians, and this study was reviewed and approved by the Institutional Review Board of the Third Xiangya Hospital, Central South University, China.
Fig. (1).
Pedigree and sequence analysis in a consanguineous Chinese family with ANSD. a) Pedigree of the ANSD family. N: normal, V: the OTOF c.1236delC variant. The arrow indicates the proband. b) The homozygous OTOF c.1236delC variant in the IV:1 patient. c) The heterozygous OTOF c.1236delC variant in the unaffected individual (IV:3). d) The OTOF gene sequences of a healthy control. The arrows in Figs. b, c, and d indicate the position of nucleotide 1236 in the OTOF gene.
2.2. Clinical Evaluation
Subjects in this ANSD family had clinical and audiological assessments carried out in the Third Xiangya Hospital, Changsha. Pure Tone Audiometry (PTA), acoustic immitance measurement, ABR, OAE and Magnetic Resonance Imaging (MRI) were performed. Hearing degree was divided into normal (<20 dB), mild (20-40 dB), moderate (41-70 dB), severe (71-95 dB), and profound (>95 dB) hearing impairments [15]. OAE was evaluated by GN otometrics-Madsen capella™, and the f1 and f2 parameters of DPOAE were set as 65 dB and 55 dB, respectively. Subjects who had preservation of OAE but absence of ABR were diagnosed with ANSD.
2.3. Exome Sequencing
Venous blood samples of the participants were used for obtaining genomic DNA (gDNA) with the saturated phenol-chloroform extraction method [16]. No less than 1.5 µg of gDNA from the IV:1 patient was applied to exome sequencing at Novogene Bioinformatics Institute, Beijing, China. The process of exome sequencing and variant analysis was described in detail in our earlier publication [15]. Exome Aggregation Consortium (ExAC) data (http://exac.broadinsti tute.org/) was further used for allele frequency of the potential causative variants in the general population.
2.4. Sanger Sequencing
Sanger sequencing was applied to identify the presence of the candidate alterations in this family, and then whether the candidate variants co-segregated with the phenotype of ANSD was evaluated [17]. Two primer sequences for the OTOF candidate alteration were designed and synthesized as follows: Forward, 5′-ACCCAGGAGTGTGTAGATGC-3′ and reverse, 5′-CTTGTGTTCATACGGGGCAG-3′. The functional prediction of the candidate variant was performed by MutationTaster (http://www.mutationtaster.org/).
3. RESULTS
3.1. Clinical and Audiological Assessments
The two patients were born at full term without history of either hyperbilirubinemia or infection. Hearing impairments were manifested in the first year of their lives. PTA revealed bilateral profound sensorineural hearing impairments with thresholds at 100 dB, and acoustic immitance measurement showed the type A tympanometric curve and absent acoustic reflex in the two patients. Bilateral ABR and Transient Evoked OAE (TEOAE) were absent, while distortion product OAE (DPOAE) at 500 or 1000 Hz was elicited in bilateral ears of the affected sister (IV:2) and the left ear of the proband, and low amplitude DPOAE at 8000 Hz was present in the right ear of the proband. MRI did not detect any inner ear malformation. The two patients present DPOAE but lack ABR, consistent with the diagnostic criteria of ANSD. The clinical information of the family is summarized in Table 1.
Table 1.
The phenotype and genotype of the ANSD family members.
| Individuals | Age | Hearing Loss | DPOAE | ABR | AR | OTOF c.1236delC Variant |
|---|---|---|---|---|---|---|
| III:2 | 57 y | - | Bil (+) | Bil (+) | Normal | Heterozygous |
| IV:1 | 40 y | Bil profound | Bil (+) | Bil (-) | Absence | Homozygous |
| IV:2 | 35 y | Bil profound | Bil (+) | Bil (-) | Absence | Homozygous |
| IV:3 | 31 y | - | Bil (+) | Bil (+) | Normal | Heterozygous |
ABR: Auditory Brainstem Responses, ANSD: Auditory Neuropathy Spectrum Disorder, AR: Acoustic Reflex, Bil: Bilateral, DPOAE: Distortion Product Otoacoustic Emissions, OTOF: the otoferlin gene, y: years, +: presence, -: absence.
3.2. Exome Sequencing
A total of 22,981,608 paired reads with an average length of 125 bp were generated by exome sequencing of the proband. About 94.16% (21,639,545) of raw reads were checked to fulfill the quality evaluation and 99.91% of clean reads were located to the human reference genome. After filtering out common variants and nonpathogenic variants, we obtained a total of 215 candidate variants. Then a homozygous OTOF c.1236delC variant was identified to be the potential pathogenic variant in the proband, and other alterations in the known pathogenic genes for congenital hearing loss were not found.
3.3. Identification of Disease-causing Variant
The OTOF c.1236delC alteration in an apparently homozygous state was confirmed in the proband (Fig. 1b) and her affected sister by Sanger sequencing, and the heterozygous variant was detected in her unaffected mother and brother (Fig. 1c). The variant was not detected in 200 healthy controls (Fig. 1d). The OTOF c.1236delC variant was predicted to be disease causing and result in a reading frame shift and truncated polypeptides p.(E413Nfs*9) by MutationTaster. The allele frequency of the OTOF c.1236delC variant was very low (less than 0.0001) in the total population, while it was absent in the East Asian population by the ExAC database.
4. DISCUSSION
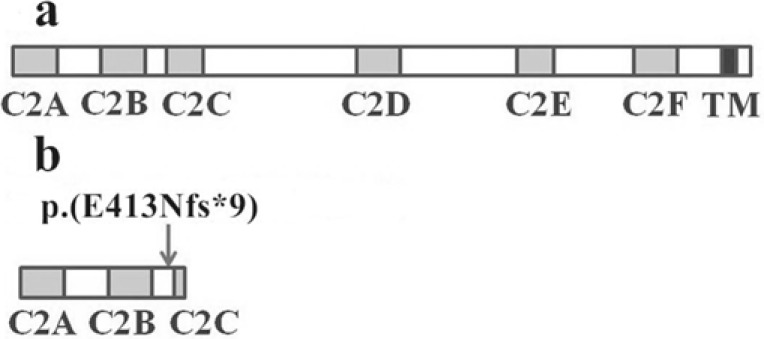
The OTOF gene encodes a membrane-anchored cytosolic protein with multiple isoforms. The long isoforms involve 48 coding exons, including six C2 domains (C2A-C2F) and a transmembrane domain (TM). The C2A and C2B domains of otoferlin are incomplete and lack some of the five calcium-binding aspartates, while the last four C2 domains (C2C-C2F) are full C2 domains with calcium-binding capability [18]. The otoferlin, primarily located in the cochlear IHC and vestibular sensory hair cells [19], is involved in Ca2+-dependent synaptic vesicle-plasma membrane fusion and neurotransmitter release [20].
Otoferlin deficient (Otof-/-) mice lacking Otof exons 14 and 15 displayed profound hearing loss, normal OHC function and absence of detectable ABR. Defective Ca2+-dependent synaptic vesicle fusion at the IHC synapse was proposed to be responsible for the hearing impairments in these Otof-/- mice [20]. The same phenotype was also reported in mice with ENU-induced Otof p.I318N missense variant [21].
This study identified the homozygous OTOF c.1236delC alteration in the two patients and the heterozygous alteration in the two unaffected individuals. The c.1236delC alteration co-segregated with the phenotype of ANSD in this family and was not present in healthy controls. Thus the alteration might be the pathogenic variant in the ANSD family. Additionally, the OTOF compound heterozygous state (a gross deletion involving in exon 14 and a heterozygous c.1236delC variant) should also be considered for the two patients due to the unavailable genotype of the deceased father (III:1) and limitation of the experimental methods employed in this study. However, the homozygous OTOF c.1236delC variant was most likely to be disease-causing alteration in this family due to the consanguineous marriage of the patients’ parents and rarity of gross deletion involving in exon 14. The OTOF c.1236delC variant (rs397515581) was previously reported in a Spanish infant with hearing loss [22]. Both the Spanish infant and the two patients in our family showed presence of bilateral profound hearing impairments but absence of TEOAE. The Spanish infant with compound heterozygous variants (p.Q829X and c.1236delC) of the OTOF gene was further diagnosed with autosomal recessive nonsyndromic hearing loss (ARNSHL). However, the two Chinese patients with homozygous OTOF c.1236delC variant were diagnosed with ANSD due to bilateral DPOAE elicited at partial frequencies but absence of bilateral ABR. The low allele frequency in the total population and absence in the East Asian population of the OTOF c.1236delC variant in ExAC database further supported its association with the disease in this family.
The C2C-C2F domains are full C2 domains with calcium-binding capability and contribute to the Ca2+-dependent synaptic vesicle-plasma membrane fusion [18, 20]. The C2C and C2F domains are involved in targeting otoferlin to the presynapse [23], and the transmembrane domain at C-terminus may target the otoferlin to diverse cell compartments and/or interact with diverse ligands [18]. The c.1236delC variant in the exon 14 of the OTOF gene, located in the region between C2B domain and C2C domain, was predicted to result in a reading frame shift and truncated polypeptides p.(E413Nfs*9). Thus the OTOF c.1236delC p.(E413Nfs*9) variant results in a truncated otoferlin missing the C2C-C2F domains and the C-terminal transmembrane domain (Fig. 2), and severely damages Ca2+-dependent synaptic vesicle-plasma membrane fusion and targeting function of the otoferlin. The two patients in our family had bilateral profound prelingual hearing loss with poor speech discrimination and social communication, consistent with the findings from previous studies that patients with the homozygous OTOF truncating variants or frameshift variants often have more severe hearing loss than those with non-truncating variants or compound heterozygous variants [24].
Fig. (2).
The schematic diagram of the otoferlin and the truncated polypeptides p.(E413Nfs*9). a) The schematic diagram of the human otoferlin. b) The schematic diagram of the truncated polypeptides p.(E413Nfs*9) due to the OTOF c.1236delC variant. The arrow indicates the position 413 in otoferlin encoded by the OTOF gene.
TEOAE and DPOAE might diminish with age in patients with ANSD [5, 25]. The oldest patient with OTOF variant and preservation of OAE was 18 years old [26], while the 40-year-old proband was by far the oldest reported OTOF-associated patients with partial preservation of DPOAE. In our study, preservation of bilateral DPOAE and absence of TEOAE in the two patients support the hypothesis that DPOAE might be a more sensitive measure than TEOAE for OHC function in adult patients with ANSD [27].
In summary, the homozygous OTOF c.1236delC variant was identified in our Chinese family with ANSD. Our study demonstrated that exome sequencing is an efficient strategy for identifying the genetic cause of nonsyndromic ANSD, a disorder with high genetic heterogeneity. This advanced technique will be also widely applied to genetic diagnosis of monogenic disorders with a few affected members [28].
CONCLUSION
In conclusion, the homozygous c.1236delC variant of the OTOF gene may be the pathogenic variant in our consanguineous Chinese family with ANSD. Our findings may also shed new light on clinical management and genetic counseling to this family.
LIST OF ABBREVIATIONS
All abbreviations have been defined within the text where first used.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was reviewed and approved by the Institutional Review Board of the Third Xiangya Hospital, Central South University (Changsha, China).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are base of this research. All human research procedures followed were in accordance with the World Medical Association Declaration of Helsinki, revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants or their guardians.
ACKNOWLEDGEMENTS
This work was supported by grants from National Natural Science Foundation of China (81271921 and 81441033), Natural Science Foundation of Hunan Province, China (2015JJ4088 and 2016JJ2166), Hunan Provincial Innovation Foundation for Postgraduate, China (CX2014B109), the Foster Key Subject of the Third Xiangya Hospital Clinical Laboratory Diagnostics (H. Deng), and Zhishan Lead Project of the Third Xiangya Hospital (H. Deng).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Manchaiah V.K., Zhao F., Danesh A.A., Duprey R. The genetic basis of auditory neuropathy spectrum disorder (ANSD). Int. J. Pediatr. Otorhinolaryngol. 2011;75(2):151–158. doi: 10.1016/j.ijporl.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Zeng F.G., Kong Y.Y., Michalewski H.J., Starr A. Perceptual consequences of disrupted auditory nerve activity. J. Neurophysiol. 2005;93(6):3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- 3.Starr A., Picton T.W., Sininger Y., Hood L.J., Berlin C.I. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 4.El-Badry M.M., McFadden S.L. Evaluation of inner hair cell and nerve fiber loss as sufficient pathologies underlying auditory neuropathy. Hear. Res. 2009;255(1-2):84–90. doi: 10.1016/j.heares.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga R., Kelley P.M., Keats B.J., Starr A., Leal S.M., Cohn E., Kimberling W.J. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J. Med. Genet. 2003;40(1):45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngo R.Y., Tan H.K., Balakrishnan A., Lim S.B., Lazaroo D.T. Auditory neuropathy/auditory dys-synchrony detected by universal newborn hearing screening. Int. J. Pediatr. Otorhinolaryngol. 2006;70(7):1299–1306. doi: 10.1016/j.ijporl.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Starr A., Sininger Y.S., Pratt H. The varieties of auditory neuropathy. J. Basic Clin. Physiol. Pharmacol. 2000;11(3):215–230. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- 8.Delmaghani S., del Castillo F.J., Michel V., Leibovici M., Aghaie A., Ron U., Van Laer L., Ben-Tal N., Van Camp G., Weil D., Langa F., Lathrop M., Avan P., Petit C. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 2006;38(7):770–778. doi: 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X., Li L., Brashears S., Morlet T., Ng S.S., Berlin C., Hood L., Keats B. Connexin 26 variants and auditory neuropathy/dys-synchrony among children in schools for the deaf. Am. J. Med. Genet. A. 2005;139(1):13–18. doi: 10.1002/ajmg.a.30929. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q., Li R., Zhao H., Peters J.L., Liu Q., Yang L., Han D., Greinwald J.H., Jr, Young W.Y., Guan M.X. Clinical and molecular characterization of a Chinese patient with auditory neuropathy associated with mitochondrial 12S rRNA T1095C mutation. Am. J. Med. Genet. A. 2005;133A(1):27–30. doi: 10.1002/ajmg.a.30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoen C.J., Emery S.B., Thorne M.C., Ammana H.R., Sliwerska E., Arnett J., Hortsch M., Hannan F., Burmeister M., Lesperance M.M. Increased activity of Diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc. Natl. Acad. Sci. USA. 2010;107(30):13396–13401. doi: 10.1073/pnas.1003027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman A.U., Santos-Cortez R.L., Morell R.J., Drummond M.C., Ito T., Lee K., Khan A.A., Basra M.A., Wasif N., Ayub M., Ali R.A., Raza S.I., Nickerson D.A., Shendure J., Bamshad M., Riazuddin S., Billington N., Khan S.N., Friedman P.L., Griffith A.J., Ahmad W., Riazuddin S., Leal S.M., Friedman T.B. Mutations in TBC1D24, a gene associated with epilepsy, also cause nonsyndromic deafness DFNB86. Am. J. Hum. Genet. 2014;94(1):144–152. doi: 10.1016/j.ajhg.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang M.Y., Choi B.Y. Strategy for the customized mass screening of genetic sensorineural hearing loss in Koreans. Korean J. Audiol. 2014;18(2):45–49. doi: 10.7874/kja.2014.18.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Horta O., Duman D., Foster J., II, Sirmaci A., Gonzalez M., Mahdieh N., Fotouhi N., Bonyadi M., Cengiz F.B., Menendez I., Ulloa R.H., Edwards Y.J., Zuchner S., Blanton S., Tekin M. Whole-exome sequencing efficiently detects rare mutations in autosomal recessive nonsyndromic hearing loss. PLoS One. 2012;7(11):e50628. doi: 10.1371/journal.pone.0050628. http://journals.plos.org/plosone/ article?id=10.1371/journal.pone.0050628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia H., Huang X., Guo Y., Hu P., He G., Deng X., Xu H., Yang Z., Deng H. 2015 http:// journals.plos.org/plosone/article?id=10.1371/journal.pone.01363
- 16.Zheng W., Deng X., Liang H., Song Z., Gao K., Yang Y., Deng H. Genetic analysis of the fused in sarcoma gene in Chinese Han patients with essential tremor. Neurobiol. Aging. 2013;34(8):2078.e3–2078.e4. doi: 10.1016/j.neurobiolaging.2013.03.001. http://www.neurobiologyofaging.org/ article/S0197-4580(13)00101-2/fulltext [DOI] [PubMed] [Google Scholar]
- 17.Yuan L., Wu S., Xu H., Xiao J., Yang Z., Xia H., Liu A., Hu P., Lu A., Chen Y., Xu F., Deng H. Identification of a novel PHEX mutation in a Chinese family with X-linked hypophosphatemic rickets using exome sequencing. Biol. Chem. 2015;396(1):27–33. doi: 10.1515/hsz-2014-0187. [DOI] [PubMed] [Google Scholar]
- 18.Yasunaga S., Grati M., Chardenoux S., Smith T.N., Friedman T.B., Lalwani A.K., Wilcox E.R., Petit C. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am. J. Hum. Genet. 2000;67(3):591–600. doi: 10.1086/303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasunaga S., Grati M., Cohen-Salmon M., El-Amraoui A., Mustapha M., Salem N., El-Zir E., Loiselet J., Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 1999;21(4):363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- 20.Roux I., Safieddine S., Nouvian R., Grati M., Simmler M.C., Bahloul A., Perfettini I., Le Gall M., Rostaing P., Hamard G., Triller A., Avan P., Moser T., Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127(2):277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Longo-Guess C., Gagnon L.H., Bergstrom D.E., Johnson K.R. A missense mutation in the conserved C2B domain of otoferlin causes deafness in a new mouse model of DFNB9. Hear. Res. 2007;234(1-2):21–28. doi: 10.1016/j.heares.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Ballesteros M., Reynoso R., Olarte M., Villamar M., Morera C., Santarelli R., Arslan E., Meda C., Curet C., Volter C., Sainz-Quevedo M., Castorina P., Ambrosetti U., Berrettini S., Frei K., Tedin S., Smith J., Cruz Tapia M., Cavalle L., Gelvez N., Primignani P., Gomez-Rosas E., Martin M., Moreno-Pelayo M.A., Tamayo M., Moreno-Barral J., Moreno F., del Castillo I. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum. Mutat. 2008;29(6):823–831. doi: 10.1002/humu.20708. [DOI] [PubMed] [Google Scholar]
- 23.Padmanarayana M., Hams N., Speight L.C., Petersson E.J., Mehl R.A., Johnson C.P. Characterization of the lipid binding properties of Otoferlin reveals specific interactions between PI(4,5)P2 and the C2C and C2F domains. Biochemistry. 2014;53(30):5023–5033. doi: 10.1021/bi5004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsunaga T., Mutai H., Kunishima S., Namba K., Morimoto N., Shinjo Y., Arimoto Y., Kataoka Y., Shintani T., Morita N., Sugiuchi T., Masuda S., Nakano A., Taiji H., Kaga K. A prevalent founder mutation and genotype-phenotype correlations of OTOF in Japanese patients with auditory neuropathy. Clin. Genet. 2012;82(5):425–432. doi: 10.1111/j.1399-0004.2012.01897.x. [DOI] [PubMed] [Google Scholar]
- 25.Chiu Y.H., Wu C.C., Lu Y.C., Chen P.J., Lee W.Y., Liu A.Y., Hsu C.J. Mutations in the OTOF gene in Taiwanese patients with auditory neuropathy. Audiol. Neurotol. 2010;15(6):364–374. doi: 10.1159/000293992. [DOI] [PubMed] [Google Scholar]
- 26.Yildirim-Baylan M., Bademci G., Duman D., Ozturkmen-Akay H., Tokgoz-Yilmaz S., Tekin M. Evidence for genotype-phenotype correlation for OTOF mutations. Int. J. Pediatr. Otorhinolaryngol. 2014;78(6):950–953. doi: 10.1016/j.ijporl.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botelho C.T., Carvalho S.A., Silva I.N. Increased prevalence of early cochlear damage in young patients with type 1 diabetes detected by distortion product otoacoustic emissions. Int. J. Audiol. 2014;53(6):402–408. doi: 10.3109/14992027.2013.879341. [DOI] [PubMed] [Google Scholar]
- 28.Ng S.B., Buckingham K.J., Lee C., Bigham A.W., Tabor H.K., Dent K.M., Huff C.D., Shannon P.T., Jabs E.W., Nickerson D.A., Shendure J., Bamshad M.J. Exome sequencing identifies the cause of a mendelian disorder. Nat. Genet. 2010;42(1):30–35. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]