Abstract
The present study investigated the effects of nicorandil on cerebral injury following cardiopulmonary resuscitation (CPR) in a swine model of cardiac arrest. CPR was performed on swine following 4 min induced ventricular fibrillation. Surviving animals were randomly divided into 3 groups: A nicorandil group (n=8), a control group (n=8) and a sham group (n=4). The sham group underwent the same surgical procedure to imitate cardiac arrest, but ventricular fibrillation was not induced. When the earliest observable return of spontaneous circulation (ROSC) was detected, the nicorandil and control groups received injections of nicorandil and saline, respectively. Swine serum was collected at baseline and 5 min, 0.5, 3 and 6 h following ROSC. Serum levels of neuron-specific enolase (NSE), S100β, tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) were measured using ELISA. Animals were euthanized and brain tissue samples were collected and assessed using light and electron microscopy 6 h following ROSC. The expression of aquaporin-4 (AQP-4) in the brain tissue was measured using western blotting. Malondialdehyde (MDA) and glutathione (GSH) levels in the brain tissue were determined using thiobarbituric acid and thiobenzoic acid colorimetric methods, respectively. Serum NSE and S100β were significantly higher in the nicorandil and control groups following CPR, compared with baseline (P<0.05). Additionally, NSE and S100β levels were significantly lower in the nicorandil group compared with the control (P<0.05). Pathological examinations and electron microscopy indicated that nicorandil reduced brain tissue damage. TNF-α and IL-6 levels were significantly decreased in the nicorandil group compared with the control group (P<0.05). Furthermore, AQP-4 expression in brain tissue 6 h following ROSC was significantly lower in the nicorandil group compared with the control group (P<0.05). MDA and GSH levels in swine brain tissue decreased and increased, respectively, in the nicorandil group compared with the control group (P<0.05). The results of the present study demonstrate that nicorandil exerts a protective effect against brain injury following cardiac arrest by reducing oxidative damage, inflammatory responses and brain edema post-ROSC.
Keywords: nicorandil, cerebral injury, cardiopulmonary resuscitation, swine model
Introduction
Cardiac arrest is a common event in clinical practice and is caused by a number of different factors (1). Cardiac arrest can occur within and external to the hospital. According to statistics, the yearly incidence of out-of-hospital cardiac arrest is ~38/10 million, while the incidence of in-hospital cardiac arrest is ~1–5/1,000 (2).
Ischemic heart disease resulting from coronary heart disease is the most common cause of cardiac arrest (3). Cardiopulmonary resuscitation (CPR) is an efficient method to improve the survival rate of patients with cardiac arrest. At present, the success rate of cardiopulmonary resuscitation is ~23.8% (4). However, many patients admitted to hospital following successful resuscitation, die of post-cardiac arrest syndrome prior to discharge (5).
The incidence of clinical brain death following cardiac arrest and the sustained return of spontaneous circulation (ROSC) ranges from 8–16% (6). Only a minority of patients experience optimal neurological and functional recovery following treatment. The primary reason for this outcome is the development of sustained brain injury. Cardiac arrest and subsequent cardiopulmonary resuscitation is a complex process of ischemia-reperfusion. Brain damage can occur during ischemic and reperfusion periods, leading to severe neurological dysfunction in patients following ROSC (7). Patients with brain injury exhibit coma, epilepsy, muscle spasms, varying degrees of recognition dysfunction and brain death (8–10). These symptoms often occur within h to a days following ROSC (11,12). Recovery of brain function is an indicator of the success rate of CPR and therefore methods of evaluating this and the degree of brain damage following CPR are important for critical care practitioners. Injury and mitochondrial ATP-sensitive potassium channels (mitoKATPs) have received increasing attention due to advancements into understanding the effects of ischemia and hypoxia in the heart and brain. Nicorandil is a nitrate ester capable of opening mitoKATPs, which is currently used to treat angina (13). MitoKATPs are able to protect against myocardial ischemia and reperfusion injury by reducing oxidative stress, preventing calcium overload, maintaining mitochondrial function and structural integrity, and inhibiting apoptosis (14–16). Previous studies have revealed that neuronal cells express 6- to 7-fold more mitoKATPs as the myocardium and it has been demonstrated that mitoKATPs exist in the brain tissue (17,18). Studies assessing the protective effects of nicorandil in the brain have demonstrated that its protective function is mediated by the activation of mitoKATPs (19,20). It has also been determined that premedication with nicorandil induces a cerebroprotective effect in patients receiving liver transplants (21). However, the protective effect of nicorandil in brain tissue following CPR remains unknown. Therefore, the present study assessed whether nicorandil exhibits a protective effect against cerebral injury in a swine model of cardiac arrest.
Materials and methods
Ethical approval
All trials were conducted with the approval of the Animal Care and Use Committee of Shandong Province Hospital Affiliated to Shandong University (Shandong, China). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (22).
Animal preparation
Healthy male inbred landrace domestic pigs were obtained from Shandong laboratory animal center (Jinan, China). A total of 20 pigs, (age, ~2 months; weight, 25±2 kg) were divided into three groups as follows: The sham group (n=4), the control group (n=8) and the nicorandil group (n=8). All animals were housed in the same environment for 1 week in which the room temperature was set to 26°C with a relative humidity of 50±10% and a 12 h light/dark cycle and all allowed free access to food and water.
The pigs were fasted overnight with ad libitum access to water prior to surgery. Pigs then received an intramuscular injection of midazolam (0.5 mg/kg, Jiangsu Nhwa Pharmaceutical Co., Ltd., Jiangsu, China.) followed by an intravenous (i.v.) injection of 3% pentobarbital sodium (8 mg/kg, cat. no. P3761; Sigma Chemical Co.; Sigma-Aldrich, Darmstadt, Germany) in the ear vein. Anesthesia was maintained with pentobarbital (8 mg/kg/h; i.v.), which was also administered into the ear vein.
A cuffed 7 mm endotracheal tube was inserted into the trachea and animals were mechanically ventilated using a volume-controlled ventilator (8417801–22; Dräger Medical GmbH, Lübeck, Germany) at a tidal volume of 12 ml/kg, with the apparatus set for synchronized intermittent mandatory and pressure support ventilation. Pressure support ventilation included 10 cm H2O and 21% FiO2. End-tidal CO2 partial pressure was monitored continuously using an infrared capnograph. Respiratory frequency was adjusted to maintain an end-tidal CO2 pressure of 35–40 mmHg. Lead II of the surface electrocardiograph was monitored continuously throughout the present study using a Philips monitor (M8003A; Philips Medizin Systeme Böeblingen GmbH, Böeblingen, Germany).
The right femoral artery was dissected to insert a fluid-filled catheter to measure mean arterial blood pressure using a pressure transducer. Cardiac output was measured by thermodilution using a pulse contour cardiac output monitor. The right external jugular vein was dissected to insert a 5F catheter for the insertion of bipolar pacing electrodes into the right ventricle to induce ventricular fibrillation (VF).
Experimental procedure
Following surgery, animals were allowed to stabilize for 30 min to achieve constant resting levels. Bipolar pacing electrodes were inserted into the right ventricle and set at the esophageal output S1S2 mode (300/200 ms), 8:1 ratio and 10-ms step continuous electrical stimulation until VF was induced using an external programmable electrical stimulator (DF-5A; KaiFeng Qingtianweiye Flow Instrument Co., Ltd., Kaifeng, Henan, China). VF was identified by an abrupt drop in arterial blood pressure and from the appearance of a VF waveform on the electrocardiograph. VF was maintained for 4 min and mechanical ventilation was attenuated during VF.
Following 4 min untreated VF, manual chest compressions were performed for at least 2 min at a frequency of 100 beats/min. Compression and relaxation times were divided evenly and the compression depth was 25% of the anterior/posterior diameter of the chest. Following 2 min compressions, electric defibrillation was performed (150-J biphasic shocks) using a defibrillator (LIFEPAK20; Medtronic, Inc., Minneapolis, MN, USA). If VF waves persisted, CPR was continued for a further 2 min, followed by a second electrical defibrillation (200-J biphasic shocks). This continued until ROSC was observed, which was defined as a mean systolic arterial pressure >60 mmHg lasting for ≥10 min without pharmacological support or manual chest compressions. Pig mortality was determined if no observable ROSC was measured following 15 min.
Groups and drug delivery methods
After successful resuscitation, animals underwent intensive care for 6 h and mechanical ventilation was resumed using pre-cardiac arrest settings. Following successful resuscitation, 16 pigs achieved ROSC and were divided randomly into nicorandil and control treatment groups (n=8 per group). Pigs in the nicorandil group received an i.v. infusion of nicorandil (150 µg/kg; Beijing Pharmaceutical Co., Ltd., Beijing, China) at the onset of ROSC, followed by a second 3 µg/kg/min infusion until reperfusion was achieved. While pigs in the control group received an equivalent volume of 0.9% physiological saline solution following ROSC. A total of 4 pigs were placed into a sham group and received surgery without VF induction. All experimental animals were euthanized with i.v. pentobarbital (150 mg/kg) 6 h following ROSC.
Biochemical assay
Venous blood samples were collected at 0, 5 min, 0.5, 3 and 6 h following ROSC. Clotted blood was centrifuged at 4°C for 10 min at a speed of 1,760 × g. The resulting serum was removed and stored at −80°C for subsequent analysis. ELISA kits were utilized at room temperature to determine the serum concentrations of neuron-specific enolase (NSE; cat. no. EIA06130P), S100β (cat. no. EIA06308P), tumor necrosis factor α (TNF-α; cat. no. EIA06460P) and interleukin 6 (IL-6; cat. no. EIA05884P) in 96-well plates. All ELISA kits were sourced from Bio-swamp Life Science Lab (Shanghai, China). The results were calibrated with hemoglobin values as follows: Calibrated value=(analytic result ×100)/hemoglobin value (g/l).
Histopathological and ultrastructural observations of cerebral cortex
Following the euthanasia of pigs, the cerebral cortex was immediately removed. Samples (~1.5×1.5×0.3 cm3) were removed and quickly fixed in 4% paraformaldehyde for 24 h at room temperature. After being processed by routine histological procedures, the samples were cut into 4 µm sections. Sections were then stained using hematoxylin for 5 min at 40°C and eosin solution for 1 min at room temperature. The slides were then observed using an optical microscope at a magnification of 100×. Additionally, Brain tissue was sliced into a tissue block of 1 mm3. Samples were then placed in 2% glutaraldehyde overnight for fixation at 4°C, rinsed 3 times with 0.2 mol/l phosphate buffer and fixed with 2% osmium acid for 2 h, Samples were further rinsed with 0.2 mol/l phosphate buffer 3 times. Degradation using a gradient of ethanol and embedded in epoxy resin. The tissues were then cut into 50 nm slices. Sliced sections were double stained with uranium lead (3% uranyl acetate for 15 min and lead lemon solution for 10 min) at room temperature and then observed and photographed using a transmission electron microscope (JEM-1200EX; JEOL Ltd., Tokyo, Japan). Western blotting determination of aquaporin-4 (AQP-4) content. Segments of brain tissue were homogenized in 10 µl/mg lysis solution and centrifuged at 4°C at a speed of 12,000 × g for 30 min. A small quantity of supernatant was removed for protein quantification using a BCA protein assay kit (P0012 BCA; Beyotime Institute of Biotechnology, Haimen, China). A total of 100 µg protein was loaded per lane and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were electrically transferred to a polyvinylidene fluoride membrane. The membranes were then blocked with 5% skimmed milk with Tris-buffered saline containing Tween-20 (TBST) for 2 h at room temperature. These were then incubated with primary antibodies (AQP-4; 1:1,000; cat. no. ab46182; Abcam, Cambridge, UK and GAPDH; 1:1,000; cat. no. ab22555; Abcam) at 4°C for 13 h and washed three times in Tris-buffered saline with Tween 20. Proteins were then incubated with a secondary antibody (horseradish peroxidase-labeled goat anti-rabbit; 1:5,000; cat. no. ZB-2301; OriGene Technologies, Inc., Beijing, China) at room temperature for 1 h. The membranes were then washed and signals were detected using an enhanced chemiluminescence (ECL-Plus) reagent (EMD Millipore, Billerica, MA, USA). Band images were scanned and densitometric analysis of the western blots was performed (Tanon-5200; Tanon Science & Technology Co., Ltd., Shanghai, China). Quantitative analysis of western blotting was performed using ImageJ 1.41 software (National Institutes of Health, Bethesda, MD, USA).
Determination of malondialdehyde (MDA) and glutathione (GSH) levels in brain tissue
Animals were euthanized 6 h post-ROSC and the cerebral cortex was immediately removed. MDA levels were subsequently determined using the thiobarbituric acid colorimetric method (MDA assay kit; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and GSH content was determined using the thiobenzoic acid colorimetric method (GSH peroxidase assay kit; Nanjing Jiancheng Bioengineering Institute).
Statistical analysis
Data were analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). All data are expressed as the mean ± standard deviation. Comparisons between multiple groups and times were made using one-way analysis of variance, followed by post-hoc least significant difference tests. P<0.05 was considered to indicate a statistically significant result.
Results
CPR and basic characteristics
The baseline characteristics of the 3 groups prior to ROSC were compared (Table I). There were no significant differences in body weight, heart rate, mean aortic blood pressure, cardiac output or body temperature among the 3 groups. Additionally, there were no significant differences in the number of electrical defibrillations or doses of epinephrine administered during CPR prior to ROSC between the nicorandil and control groups (Table II).
Table I.
Baseline characteristics.
| Characteristic | Nicorandil (n=8) | Control (n=8) | Sham (n=4) | F | P-value |
|---|---|---|---|---|---|
| Heart rate (BPM) | 125.60±6.54 | 124.80±6.42 | 120.67±7.02 | 0.564 | 0.586 |
| Weight (kg) | 25.80±0.84 | 25.00±1.41 | 25.00±1.00 | 0.048 | 0.953 |
| Temperature (°C) | 37.96±0.29 | 38.06±0.31 | 37.90±0.30 | 0.293 | 0.752 |
| Cardiac output (l/min) | 4.49±0.20 | 4.48±0.09 | 4.52±0.09 | 0.057 | 0.944 |
| MAP (mmHg) | 115.20±5.45 | 115.80±5.31 | 111.33±4.16 | 0.769 | 0.489 |
Results are presented as the mean ± standard deviation. BPM, beats per minute; MAP, mean arterial pressure; F, Statistics of one-way analysis of variance.
Table II.
Resuscitation outcomes.
| Characteristic | Nicorandil (n=8) | Control (n=8) |
|---|---|---|
| Total adrenaline dose (mg) | 0.40±0.042 | 0.30±0.045 |
| Time to ROSC (min) | 2.40±1.41 | 2.20±1.30 |
| Energy of defibrillation (J) | 185.00±78.26 | 220.00±95.85 |
| Number of defibrillations | 1.20±0.45 | 1.40±0.55 |
Results are presented as the mean ± standard deviation. ROSC, return of spontaneous circulation.
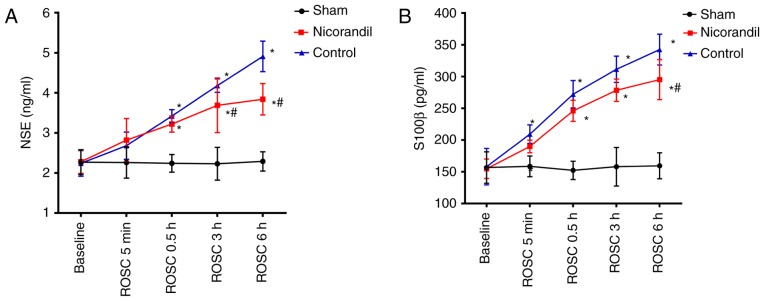
Serum NSE and S100β levels
The serum levels of NSE and S100β were compared among the 3 groups at different times to determine the effects of nicorandil on brain injury (Fig. 1). There were no significant differences in the serum levels of NSE or S100β among the three groups at baseline. However, NSE and S100β levels in the nicorandil and control group significantly increased at 0.5, 3 and 6 h following ROSC, compared with the sham group (P<0.05). Control groups were significantly increased compared with the sham group at 5 min 0.5, 3 and 6 h following ROSC (P<0.05). Serum NSE levels in the nicorandil group were significantly lower than that of the control group (P<0.05) at 3 and 6 h following ROSC. Furthermore, serum levels of S100β were significantly decreased in the nicorandil group compared with the control group 6 h following ROSC (P<0.05).
Figure 1.
Serum concentrations of (A) NSE and (B) S100β in the cardiac arrest swine model following ROSC. Drugs were administered intravenously at the time of ROSC. *P<0.05 vs. sham group; #P<0.05 vs. control group. NSE, neuron-specific enolase; ROSC, return of spontaneous circulation.
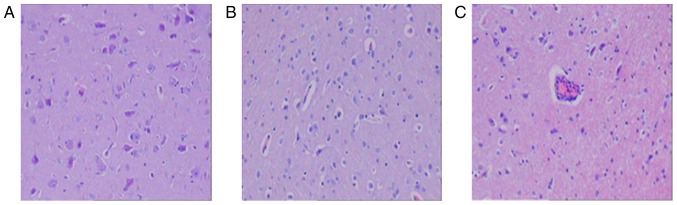
H&E staining and brain ultramicrostructure
Pathological changes in brain structures were examined by performing H&E staining (Fig. 2). At 6 h following ROSC, neuronal structures in the sham group were normal (Fig. 2A); however, cells from the control group exhibited lymphocyte infiltration around the blood vessels and swelling (Fig. 2C). Cells from the nicrorandil group also exhibited swelling but to a lesser extent than cells from the control group (Fig. 2B).
Figure 2.
Light microscopy of brain tissue 6 h following the return of spontaneous circulation. Light microscopic images of the (A) sham, (B) nicorandil and (C) control groups (magnification, 100×). Neuron structures were normal in the sham group and swollen in the nicorandil group. The control group exhibited increased swelling and lymphocyte infiltration surrounding the blood vessels.
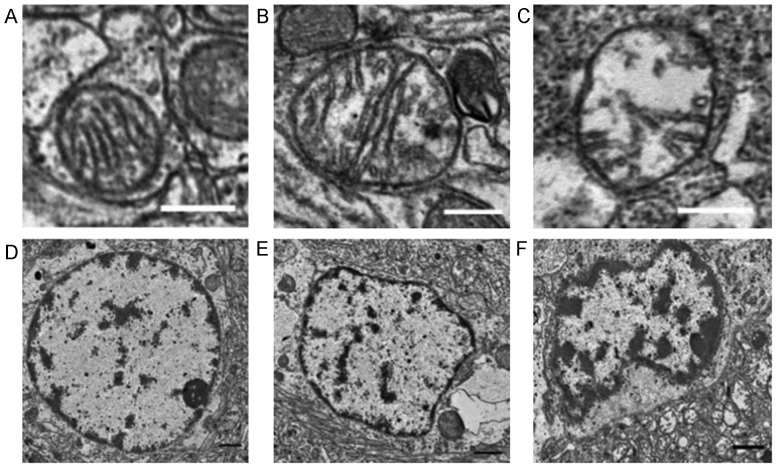
The ultrastructure of the cerebral cortex was normal in the sham group 6 h following ROSC, as determined by electron transmission microscopy (Fig. 3). Nuclei in the nicorandil and control groups exhibited partial dissolution, condensation and nuclear membrane depression, although this was exhibited to a greater extent in the control group. The nicorandil group also exhibited mild mitochondrial edema, with clear, visible cristae ridges that were less characteristic of necrosis. The control group exhibited a greater severity of mitochondrial edema than the nicorandil group; this included the loss of the mitochondrial bilayer and cristae ridges, as well as evidence of vacuolar changes.
Figure 3.
Transmission electron microscopy of the brain tissue ultrastructure 6 h following the return of spontaneous circulation. Electron microscopic images of mitochondria in the (A) sham, (B) nicorandil and (C) control groups. The sham group exhibited an intact mitochondrial structure with clear cristae ridges and the nicorandil group exhibited mitochondrial swelling with internal structural disorder. Marked swelling was observed in the control group, with damage to cristae ridges. Scale bars=0.4 µm. Electron microscope images of nuclei in the (D) sham, (E) nicorandil and (F) control groups. Nuclear structures were normal in the sham group. The nicorandil and control groups exhibited chromatin condensation, but this was more evident in the control group. Scale bars=1 µm.
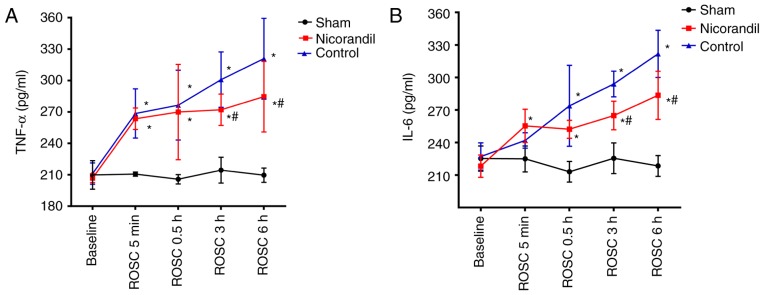
Serum TNF-α and IL-6 concentrations
There were no significant differences in TNF-α and IL-6 levels in the cerebral cortex among the 3 groups at baseline (Fig. 4). Levels of TNF-α were significantly higher in the nicorandil and control groups, compared with the sham group (P<0.05) at 5 min and 0.5, 3 and 6 h following ROSC (Fig. 4A). IL-6 levels were significantly higher in the control and nicorandil groups 0.5, 3 and 6 h following ROSC compared with the sham group (Fig. 4B). However, TNF-α and IL-6 levels in the nicorandil group were significantly lower than those in the control group 3 and 6 h post-ROSC (P<0.05).
Figure 4.
Levels of the inflammatory markers (A) TNF-α and (B) IL-6 in the cardiac arrest swine model following ROSC. Drugs were administered intravenously at the time of ROSC. *P<0.05 vs. sham group, #P<0.05 vs. control group. TNF-α, tumor necrosis factor α; IL-6, interleukin-6; ROSC, return of spontaneous circulation.
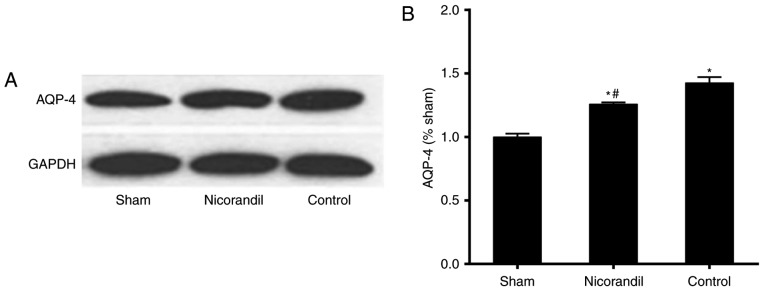
AQP-4 levels in brain tissue
Levels of AQP-4 in brain tissue were significantly higher in the nicorandil and control groups compared with the sham group 6 h following ROSC (Fig. 5). However, AQP-4 levels were significantly lower in the nicorandil group than in the control group 6 h following ROSC (P<0.05).
Figure 5.
Effect of nicorandil on AQP-4 expression in the cerebral cortex of swine in the cardiac arrest model 6 h following ROSC. (A) Western blot analysis and (B) quantification of western blot results. Drugs were administered intravenously at the time of ROSC. *P<0.05 vs. sham group, #P<0.05 vs. control group. AQP-4, aquaporin-4; ROSC, return of spontaneous circulation.
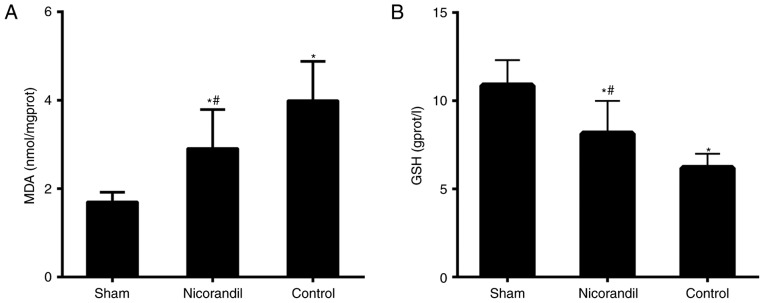
MDA and GSH levels in the cerebral cortex
MDA levels in the cerebral cortex were significantly higher in the nicorandil and control groups compared with the sham group 6 h following ROSC (P<0.05; Fig. 6A). However, MDA levels were significantly lower in the nicorandil group compared with the control group. GSH levels were significantly lower in the control and nicorandil groups, compared with the sham group (P<0.05; Fig. 6B). Furthermore, GSH levels in the nicorandil group were significantly higher than in the control group (P<0.05).
Figure 6.
Effect of nicorandil on (A) MDA and (B) GSH levels in the cerebral cortex of swine, 6 h following ROSC. Drugs were administered intravenously at the time of ROSC. *P<0.05 vs. sham group, #P<0.05 vs. control group. MDA, malondialdehyde; GSH, glutathione; ROSC, return of spontaneous circulation.
Discussion
The results of the present study demonstrated that cardiac arrest followed by CPR causes significant damage to swine brain tissue. Electron microscopy and pathological examinations of the cerebral cortex and cellular ultrastructure revealed that nerve cell mitochondria were damaged and that NSE and S100β levels were significantly increased. However, treatment with nicorandil attenuated the increase in NSE and S100β following CPR and reduced the degree of mitochondrial swelling, indicating that nicorandil induces a protective effect following brain injury.
Serum NSE and S100β levels were assessed to determine their potential as neurobiochemical markers of brain damage. NSE is localized in the neuronal and neuroendocrine cell cytoplasm; however, it is also present in small quantities in serum and cerebrospinal fluid. In the event of neuronal necrosis, cells become leaky, leading to an increase in NSE serum levels (23). S100β is an EF-hand-type calcium-binding protein with a low molecular weight and is abundant in brain tissue (24). In certain types of brain injury involving damage to the blood-brain barrier, S100β may enter the cerebrospinal fluid and bloodstream. Various microproteins, including S100β and NSE can therefore be detected in the blood (25,26). Rundgren et al (27) identified that NSE and S100β predict the severity of brain injury following CPR. The results of the current study demonstrated that nicorandil reduces serum levels of NSE and S100β following CPR, indicating that it serves a protective role against brain damage.
Cardiac arrest affects the central nervous system and results in whole-brain structural injury and functional damage, the severity of which directly affects patient prognosis. Despite the implementation of CPR, further brain damage may occur in the reperfusion phase of cardiac arrest, due to the accumulation of free radicals, the systemic inflammatory response and mitochondrial damage (28,29).
The results of the present study demonstrated that TNF-α and IL-6 levels in pigs increased rapidly following resuscitation in VF cardiac arrest and remained elevated during the initial 6 h following ROSC. TNF-α and IL-6 are upregulated following cerebral ischemia and it has been demonstrated that transient global ischemia, induced by cardiac arrest, increases IL-6 and TNF-α levels in rat hippocampi (30). In the current study, the serum levels of these pro-inflammatory cytokines were significantly reduced following treatment with nicorandil, indicating that it exhibits protective effects against brain injury.
AQPs are channels located in the cell membrane that transport water to regulate intra- and extracellular environments. Intracerebral AQP-4 is primarily expressed in capillary endothelial cells and in the foot process of astrocytes located on the blood-brain barrier (BBB). Previous studies have therefore speculated that AQP-4 may serve an important role in mediating the transport of water across the BBB (31,32). It has been demonstrated that AQP-4 expression is upregulated following cardiac arrest and CPR in rats (33). Furthermore, a positive association between cerebral edema and AQP-4 expression has been identified, indicating that AQP-4 is involved in ischemic brain damage and edema formation (34). The results of the present study demonstrated that nicorandil reduces AQP-4 expression in brain tissue, which may have protected the brain by reducing the degree of edema following CPR.
The brain is a lipid-rich organ, in which oxidation and antioxidation are maintained in a balanced state. However, ischemia, hypoxia or ischemia-reperfusion brain injury produces large quantities of oxygen free radicals, which induce oxidative stress and brain damage (35). MDA is a major metabolite of lipid peroxidation that is induced by reactive oxygen species and therefore may indicate the degree of oxidative stress present. GSH is also an important antioxidant and scavenger of free radicals (36). In the present study, levels of GSH and MDA in the 3 experimental groups were assessed. It was demonstrated that nicorandil increases GSH and decreases MDA levels, indicating that it reduces oxidative damage and thus exerts a protective effect in the brain.
Neurons contain two types of ATP-sensitive potassium channels: MitoKATPs and intima-ATP-sensitive potassium channels (18,37). MitoKATPs maintain the mitochondrial K+ balance, thus controlling volume changes in the mitochondrial matrix (18). It is also involved in K+ reuptake, compensating for the charge transfer in oxidative phosphorylation generated by proton pumps, thus maintaining the stability of the pH gradient and transmissive potential difference (38). Nicorandil is a selective mitoKATP agonist (13). It has been demonstrated in vitro that nicorandil protects the brain from oxidative stress by activating mitoKATPs (19). In addition, nicorandil has been revealed to function as a nitrate ester (39). Therefore, its protective effects may be associated with its vasodilator activity in the brain.
The present study demonstrated that nicorandil exerts a protective effect against brain injury by reducing oxidative damage, the inflammatory response and brain edema in a swine model of cardiac arrest. However, there were several limitations of the present study. There are numerous physiological differences between healthy experimental animals and human patients, making it difficult to extrapolate the results of this swine model to the relevant clinical situations. Furthermore, the results of the present study were limited by the small quantity of animals utilized. Therefore, large-scale studies in animal models and human subjects are required for future studies.
In conclusion, the results of the present study demonstrated that nicorandil exerts a protective effect against brain injury following cardiac arrest by reducing the oxidative damage, the inflammatory response and brain edema that occur post-ROSC.
Acknowledgements
The authors of the present study give thanks to Mr. Jinsen Huang and Mr Bao Zhao (Shandong Provincial Hospital Affiliated to Shandong University, China) for technical assistance.
Funding
The present study was supported by the Chinese National Natural Science Foundation (grant no. 81471833) and the 2012 Jinan Science and Technology Development Plan (grant no. 201219007).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DS and XJ designed and directed the experiment. FZ, YZ, ZH, HH, LL, JC and QC performed the experiments. XZ performed the statistical analysis. FZ and XZ wrote the manuscript. DS and XJ reviewed and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All trials were conducted with the approval of the Animal Care and Use Committee of Shandong Province Hospital Affiliated to Shandong University (Shandong, China). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sadaka F, Doerr D, Hindia J, Lee KP, Logan W. Continuous electroencephalogram in comatose postcardiac arrest syndrome patients treated with therapeutic hypothermia: Outcome prediction study. J intensive care med. 2015;30:292–296. doi: 10.1177/0885066613517214. [DOI] [PubMed] [Google Scholar]
- 2.Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, Perkins GD. European resuscitation council guideline for resuscitation 2010 section 4. Adult advanced life support. Resuscitation. 2010;81:1305–1352. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Pell JP, Sirel JM, Marsden AK, Ford I, Walker NL, Cobbe SM. Presentation, management and outcome of out of hospital cardiopulmonary arrest: Comparison by underlying aetiology. Heart. 2003;89:839–842. doi: 10.1136/heart.89.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan JP, Soar J, Wenzel V, Paal P. Cardiopulmonary resuscitation andmanagement of cardiac arrest. Nat Rev Cardiol. 2012;9:499–511. doi: 10.1038/nrcardio.2012.78. [DOI] [PubMed] [Google Scholar]
- 5.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment and prognostication. A consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, Inter American Heart Foundation, Resuscitation Council of Asia and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, Haouache H, Saleh M, Memain N, Laurent I, Thuong M, Dargues L, Geurrini P, Monchi M. An underrecognized source of organ donors: Patients with brain death after successfully resuscitated cardiac arrest. Intensive Care Med. 2008;34:132–137. doi: 10.1007/s00134-007-0885-7. [DOI] [PubMed] [Google Scholar]
- 7.Safar P, Behringer W, Bottiger BW, Sterz F. Cerebral resuscitation potentials for cardiac arrest. Crit Care Med. 2002;30(4 Suppl):S140–S144. doi: 10.1097/00003246-200204001-00004. [DOI] [PubMed] [Google Scholar]
- 8.Krumholz A, Stern BJ, Weiss HD. Outcome from coma after cardiopulmonary resuscitation: Relation to seizures and myoclonus. Neurology. 1988;38:401–405. doi: 10.1212/WNL.38.3.401. [DOI] [PubMed] [Google Scholar]
- 9.Pusswald G, Fertl E, Faltl M, Auff E. Neurological rehabilitation of severely disabled cardiac arrest survivors, part II: life situation of patients and families after treatment. Resuscitation. 2000;47:241–248. doi: 10.1016/S0300-9572(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 10.Groswasser Z, Cohen M, Costeff H. Rehabilitation outcome after anoxic brain damage. Arch Phys Med Rehabil. 1989;70:186–188. [PubMed] [Google Scholar]
- 11.Taraszewska A, Zelman IB, Ogonowska W, Chrzanowska H. The pattern of irreversible brain changes after cardiac arrest in humans. Folia Neuropathol. 2002;40:133–141. [PubMed] [Google Scholar]
- 12.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 13.Horinaka S. Use of nicorandil in cardiovascular disease and its optimization. Drugs. 2011;71:1105–1119. doi: 10.2165/11592300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Ye M, Yang J, Ding J, Yang J, Dong W, Wang X. Nicorandil protects the heart from ischemia/reperfusion injury by attenuating endoplasmic reticulum response-induced apoptosis through PI3K/Akt signaling pathway. Cell Physiol Biochem. 2015;35:2320–2332. doi: 10.1159/000374035. [DOI] [PubMed] [Google Scholar]
- 15.Nagata K, Obata K, Odashima M, Yamada A, Somura F, Nishizawa T, Ichihara S, Izawa H, Iwase M, Hayakawa A, et al. Nicorandil inhibits oxidative stress-induced apoptosis in cardiac myocytes through activation of mitochondrial ATP-sensitive potassium channels and a nitrate-like effect. J Mol Cell Cardiol. 2003;35:1505–1512. doi: 10.1016/j.yjmcc.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Raveaud S, Verdetti J, Faury G. Nicorandil protects ATP-sensitive potassium channels against oxidation-induced dysfunction in cardiomyocytes of aging rats. Biogerontology. 2009;10:537–547. doi: 10.1007/s10522-008-9196-9. [DOI] [PubMed] [Google Scholar]
- 17.Lacza Z, Snipes JA, Kis B, Szabó C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res. 2003;994:27–36. doi: 10.1016/j.brainres.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- 19.Teshima Y, Akao M, Baumgartner WA, Marbán E. Nicorandil prevents oxidative stress-induced apoptosis in neurons by activating mitochondrial ATP-sensitive potassium channels. Brain Res. 2003;990:45–50. doi: 10.1016/S0006-8993(03)03383-3. [DOI] [PubMed] [Google Scholar]
- 20.Kurihara J, Ochiai N, Kato H. Protection by nicorandil against the dysfunction of the central vagal baroreflex system following transient global cerebral ischemia in dogs. Br J Pharmacol. 1993;109:1263–1267. doi: 10.1111/j.1476-5381.1993.tb13758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia YF, Wang ZP, Zhou YC, Yan T, Li ST. Cerebral protective effect of nicorandil premedication on patients undergoing liver transplantation. Hepatobiliary Pancreat Dis Int. 2012;11:132–136. doi: 10.1016/S1499-3872(12)60137-4. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council of The National Academies: Guide for the Care and Use of Laboratory Animals. 8th edition. The National Academies Press; Washington, DC: 2010. [Google Scholar]
- 23.Marangos PJ, Schmechel DE, Parma AM, Goodwin FK. Developmental profile of neuron-specific (NSE) and non-neuronal (NNE) enolase. Brain Res. 1980;190:185–193. doi: 10.1016/0006-8993(80)91168-3. [DOI] [PubMed] [Google Scholar]
- 24.Heizmann CW, Fritz G, Schäfer BW. S100 proteins: Structure, functions and pathology. Front Biosci. 2002;7:d1356–d1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 25.Cavus E, Bein B, Dörges V, Stadlbauer KH, Wenzel V, Steinfath M, Hanss R, Scholz J. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2006;71:97–106. doi: 10.1016/j.resuscitation.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE, S-100, IL-8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation. 2007;75:219–228. doi: 10.1016/j.resuscitation.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80:784–789. doi: 10.1016/j.resuscitation.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007;38:1578–1584. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing J, Lu J. HIF-1α activation attenuates IL-6 and TNF-α pathways in hippocampus of rats following transient global ischemia. Cell Physiol Biochem. 2016;39:511–520. doi: 10.1159/000445643. [DOI] [PubMed] [Google Scholar]
- 31.Kleffner I, Bungeroth M, Schiffbauer H, Schäbitz WR, Ringelstein EB, Kuhlenbäumer G. The role of aquaporin-4 polymorphisms in the development of brain edema after middle cerebral artery occlusion. Stroke. 2008;39:1333–1335. doi: 10.1161/STROKEAHA.107.500785. [DOI] [PubMed] [Google Scholar]
- 32.Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, Miercke LJ, Stroud RM. Crystal structure of human aquaporin 4 at 1.8 Å and its mechanism of conductance. Proc Natl Acad Sci. 2009;106:7437–7442. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao F, Arnold TC, Zhang S, Brown C, Alexander JS, Carden DL, Conrad SA. Cerebral cortical aquaporin-4 expression in brain edema following cardiac arrest in rats. Acad Emerg Med. 2004;11:1001–1007. doi: 10.1197/j.aem.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Wang X, Guo Q. The correlation between DTI parameters and levels of AQP-4 in the early phases of cerebral edema after hypoxic-ischemic/reperfusion injury in piglets. Pediatr Radiol. 2012;42:992–999. doi: 10.1007/s00247-012-2373-7. [DOI] [PubMed] [Google Scholar]
- 35.Nunes C, Barbosa RM, Almeida L, Laranjinha J. Nitric oxide and DOPAC-induced cell death: from GSH depletion to mitochondrial energy crisis. Mol Cell Neurosci. 2011;48:94–103. doi: 10.1016/j.mcn.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Radak D, Resanovic I, Isenovic ER. Link between oxidative stress and acute brain ischemia. Angiology. 2014;65:667–676. doi: 10.1177/0003319713506516. [DOI] [PubMed] [Google Scholar]
- 37.Dunn-Meynell AA, Rawson NE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/S0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- 38.Garlid KD. Mitochondrial potassium transport: The K(+) cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/S0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 39.Tarkin J M, Kaski JC. Vasodilator therapy: Nitrates and nicorandil. Cardiovasc Drugs Ther. 2016;30:367–378. doi: 10.1007/s10557-016-6668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.