The relative infectiousness of chronic malaria infections determines the likelihood of success of different malaria elimination strategies.
Keywords: relative infectivity, chronic infections, Plasmodium falciparum, malaria elimination
Abstract
Assessing the importance of targeting the chronic Plasmodium falciparum malaria reservoir is pivotal as the world moves toward malaria eradication. Through the lens of a mathematical model, we show how, for a given malaria prevalence, the relative infectivity of chronic individuals determines what intervention tools are predicted be the most effective. Crucially, in a large part of the parameter space where elimination is theoretically possible, it can be achieved solely through improved case management. However, there are a significant number of settings where malaria elimination requires not only good vector control but also a mass drug administration campaign. Quantifying the relative infectiousness of chronic malaria across a range of epidemiological settings would provide essential information for the design of effective malaria elimination strategies. Given the difficulties obtaining this information, we also provide a set of epidemiological metrics that can be used to guide policy in the absence of such data.
In human feeding experiments, the measured infectivity of patent infections to mosquitoes seems to fluctuate significantly over time and not be linearly correlated with peripheral blood gametocyte counts [1]. In fact, several mosquito blood-feeding assays have shown that low gametocyte counts in humans do not preclude infection of mosquitoes, with a significant proportion of submicroscopic gametocytemias resulting in successful oocyst development in mosquitoes [2–6]. The data available suggest a sigmoidal relationship between human infectivity to mosquitoes and gametocyte density, with significant variation in infectivity levels at low gametocyte densities [7, 8]. Several estimates of the contribution of these submicroscopic gametocyte carriers to overall transmission reinforce that these infections are, in fact, nonnegligible [4, 5, 9, 10]. An analytical study that examined these infectivity trials has found that for low levels of transmission, submicroscopic infections could potentially be the source of 20%–50% of all human-to-mosquito transmissions [11].
Another study shows that gametocyte density can be larger in asymptomatic individuals than in some symptomatic patients [12]. That study also reported increased infectivity of asymptomatic gametocyte carriers not correlated with gametocyte densities, suggesting that other factors such as gametocyte maturity and/or human blood factors might influence their infectivity [13–15]. This might help explain why high gametocyte densities do not necessarily result in mosquito infections [2, 6, 10]. One recent analysis of longitudinal data from Dielmo, Senegal, showed that gametocyte densities increased as malaria prevalence decreased from 70% to 20% [16], further highlighting how factors determining the sexual commitment of the malaria parasite can confound the assumptions of how asexual parasitemia translates into human infectivity to mosquitoes [17]. In summary, there is no consensus on the relative infectivity of chronic malaria infections compared with clinical infections, owing to sparseness of data, difficulties in measuring individuals’ infectivity over time, and the variable relationship between asexual parasitemia, gametocytemia, and infectivity.
EFFECT OF HETEROGENEOUS INFECTIVITY ON MALARIA TRANSMISSION
Accepting that clinical malaria infections are, on average, considerably shorter in duration owing to treatment, and that chronic infections can extend up to several months, it is evident that, if clinical and chronic infections had similar infectivity, chronic infections would be the main contributors to overall malaria transmission. This has been explored theoretically in the past [18] and has helped in determining the limits for malaria control in low-transmission areas. That study presented a mathematical malaria transmission model that was used to fit age profiles of clinical malaria in 8 sub-Saharan Africa (SSA) regions of varying endemicity, to make inferences on key transmission parameters, such as region-specific entomological inoculation rates (EIRs), as well as setting independent parameters, such as the mean duration of clinical and chronic infections.
This allowed the extrapolation of the relative infectiousness of chronic malaria infections, given specific relative infectivity (ϕ) values. The estimated parameters suggested that chronic infections were 6 times longer in duration than clinical ones. Assuming ϕ to be 1, infectiousness would be 6 times higher in chronic infections. This means that in a clinically immune population, a single index case would generate 6 times more secondary infections than in a completely susceptible population, because the majority of infections would be chronic in the former and clinical in the latter. This difference translates into a phenomenon termed bistability, characterized by the existence of 2 stable equilibria for a given parameter set.
Published estimates of infectiousness period length were derived from African data sets, with chronic infections thought to last about 150 days on average [18–20]. Although these estimates have accounted for differences in exposure across populations in Africa, they are not necessarily applicable to the Greater Mekong Subregion (GMS). Preliminary unpublished data exploring the duration of chronic carriage in a low-transmission setting in Vietnam suggest that infections in the GMS are slightly shorter in duration (approximately 100 days). This difference could be driven by disparities in parasite population structures. Parasite genetic diversity has been described as lower in Southeast Asia than in African settings with the same transmission intensity [21]. Although this fits with the suggested lower gene flow in Southeast Asia [22, 23], genetic factors are only a single determinant among many that could account for differences in the period of infectiousness across malaria-endemic areas.
Another well-documented significant difference between SSA and the GMS is their markedly distinct vector bionomics. Whereas the most abundant vectors in Africa are endophilic and antropophilic, in Southeast Asia vectors most commonly bite outdoors and do not preferentially bite humans [24–26]. This has major implications for the effectiveness of any vector-based control strategy, specifically insecticide-treated bed nets (ITNs) [27, 28] and indoor residual spraying (IRS).
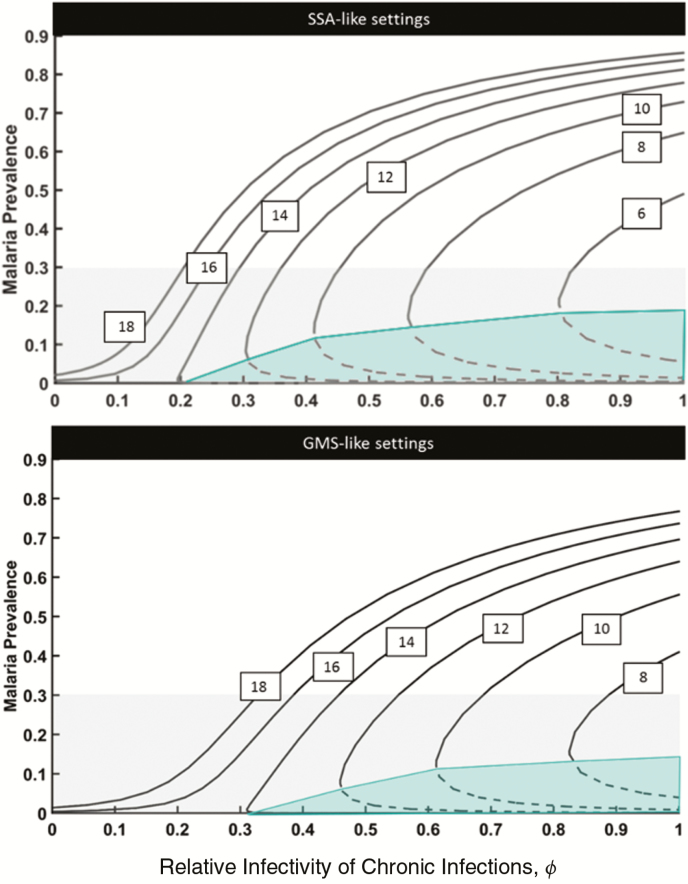
In this article we use a previously developed theoretical framework [18] to explore the predicted malaria prevalence at equilibrium across a range of relative infectivity values (ϕ) and vectorial capacities (VCs) (Figure 1). We do so for 2 distinct settings meant to characterize the generic features of malaria transmission in SSA and GMS settings. The 2 prototypical settings differ only in the assumed length of chronic infectiousness and vector control effectiveness, with SSA-like settings having an infectiousness period of 165 days versus 100 days for GMS-like settings. As mentioned above, vector control is much more “leaky” in the GMS, translating into an implemented 25% reduction in infection risk for the modeled vector control packages in the GMS, compared with an 80% reduction for the SSA simulations.
Figure 1.
Malaria equilibrium prevalence for different combinations of vectorial capacity (VC) and relative infectivity of chronic infections. Curves from left to right represent decreasing values of VC (18, 16, 14, 12, 10, 8, and 6 infectious bites per year) as indicated in the text boxes. Dashed lines reflect unstable equilibrium solutions (ie, elimination thresholds; solid lines, stable equilibrium solutions). The grey area illustrates the lower end of the malaria transmission spectrum which is usually deemed “controllable.” The green area delimits areas of unstable malaria transmission where malaria is expected to be eliminated over time without additional control measures. Abbreviations: GMS, Greater Mekong Subregion; SSA, sub-Saharan Africa.
The model shows, as expected, that for a fixed value of ϕ malaria prevalence is predicted to increase as VC increases. Of more relevance, however, is that low levels of endemicity (ie, <30% prevalence) could occur as a result of a very large number of combinations of both VC and ϕ values. This means that a 20% malaria prevalence, for example, can result from a very large VC with the assumption that chronic infections are virtually noninfectious, much lower VC with chronic infections being the main infection reservoir, or a range of intermediate scenarios. Details on all used model variables and key definitions mentioned throughout the article can be found in Table 1.
Table 1.
Key Definitions
| Term | Definition |
|---|---|
| VC | Vectorial capacity (the average number of infectious bites arising from mosquitoes originally infected by a single infectious person per day), formally defined as follows: where m is the number of mosquitoes per person; a, daily mosquito biting rate; and n, duration of the mosquito sporogony cycle; and g, mosquito life expectancy in days |
| EIR | Entomological inoculation rate (average number of infectious mosquito bites per person per year), formally defined as follows: where p is the proportion of infections that result in clinical malaria; and c, per-bite probability of infectious individuals with clinical manifestations to infect mosquitoes (commonly referred to as infectivity of humans to mosquitoes). |
| ϕ | Relative infectivity of chronic compared with clinical infections |
| Infectiousness | Overall transmissibility of 1 malaria infection over the course of the time when the infected person is infective to mosquitoes |
| R 0 | Basic reproduction number, formally defined as follows: where b is the per-bite probability that an infectious mosquito infects a susceptible human; Dc, mean duration of infectiousness for clinical infections; and Da, mean duration of infectiousness in asymptomatic individuals |
| ContrA | Contribution of chronic infections to overall malaria transmission; defined as follows: |
| AgeR | Ratio between malaria prevalence in children aged <10 y and all-age malaria prevalence |
| ITN | Insecticide-treated bed net |
| IRS | Indoor residual spraying |
| MDA | Mass drug administration in the form of 3 rounds of artemisinin combination therapy treatment |
| Bistability | Characterized by the existence of 2 stable equilibria for a given parameter set; the dynamics of the system converge to either the disease-free or the endemic equilibrium depending on the initial conditions (namely, initial prevalence); if the endemic state is perturbed past the unstable equilibrium solution (eg, through MDA), the system can converge to the disease-free equilibrium over time |
USE OF OTHER EPIDEMIOLOGICAL METRICS TO DETERMINE THE CONTRIBUTION OF CHRONIC INFECTIONS TO MALARIA TRANSMISSION
The relative infectivity of chronic malaria infections remains largely unknown. There is a pressing need for high-quality data that can inform such a crucial driver of malaria transmission, which will require longitudinal follow-up studies in both clinical and chronic infections, with serial mosquito-feeding assays. As we approach the elimination targets set by some GMS countries (as early as 2020), we need to make use of other more easily accessible data to advise national malaria control programs’ elimination strategies. Supplementary Figure S1 shows how different sets of VC and ϕ pairs lead to different predicted values for EIR, proportion of clinical malaria cases treated, all-age prevalence, percentage contribution of chronic infections to overall transmission, and the ratio of malaria prevalence in children <10 years old to all-age malaria prevalence. These data (or at least some of them) are routinely collected and could circumvent the gap in our knowledge of what value ϕ should take. If we know the EIR, the proportion of clinical malaria cases treated, and the ratio of malaria prevalence in children <10 years old to all-age malaria prevalence in any specific setting, we can easily place that setting on our VC-ϕ coordinate system.
OPTIMAL CONTROL STRATEGIES FOR A GIVEN TRANSMISSION INTENSITY
The prospects for malaria control has been thoroughly explored analytically elsewhere [18], and its predictions have since been revisited [29]. In essence, assuming ϕ to be 1, there is a range of VC values for which stable elimination can be reached solely through the administration of effective antimalarial drugs to asymptomatic individuals, without the need for any additional vector control efforts. This is illustrated by the green-shaded areas in Figure 1. The dashed black line represents the elimination threshold, that is, the malaria prevalence that must be achieved through treatment of both asymptomatic and symptomatic individuals. after which control measures can be relaxed and elimination is still achieved over time. If chronic infections are not transmissible (), however, treatment of asymptomatic parasite carriers is completely ineffective, and vector control strategies (and potentially vaccination) emerge as the only means to halt transmission (left-most curves in Figure 1).
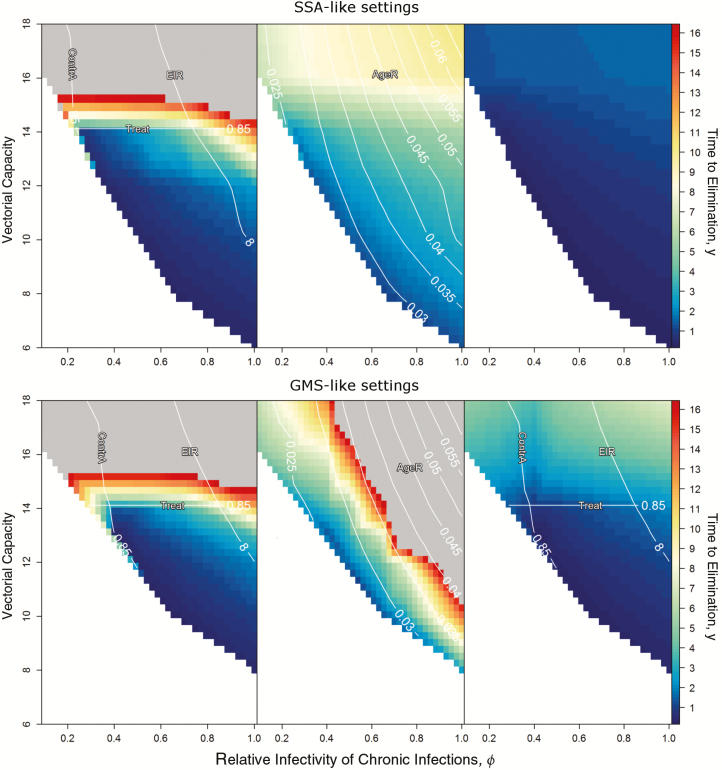
Figure 2 shows the timelines for elimination by deploying mass drug administration (MDA), vector control or MDA plus vector control strategies, assuming different combinations of VC and ϕ for the 2 explored settings. These MDA elimination strategies consist of annual MDA campaigns of 3 monthly rounds each, whereas vector control strategies consist of integrated packages, including annual ITN distribution and IRS deployment campaigns that reduce the VC by 80% in SSA-like settings and by 25% in GMS-like settings. It is clear that integrated control programs with both MDA and vector control components, are predicted to be by far the most efficient in reducing transmission to the point of elimination in a reasonable time frame for any parameter set. Although drug-centric approaches aimed at reducing the pool of chronic parasitemia can be the most effective strategy for a wide range of VC-ϕ combinations, for parameter combinations of high VC and low ϕ, the bistability region is lost, and elimination is rendered impossible with drug-centric approaches only. As expected, vector control–centric approaches have much less impact in GMS-like setting simulations and are predicted to be insufficient to eliminate malaria by themselves for high values of both VC and ϕ. Broadly, a few patterns emerge for potential operational programs considering different elimination horizons.
Figure 2.
Surfaces illustrating the minimum time required for elimination to be reached for a given combination of vectorial capacity and relative infectivity (ϕ). Colors reflect the time to elimination in years (the minimum time a given intervention must be sustained for elimination to be reached); this does not reflect the time it takes for malaria to be eliminated, because an intervention could be required to be sustained for only 1 year, and then relaxed, for example, with elimination following after another 3 months. The contour lines indicate epidemiological metrics useful to determine the feasibility of control strategies in the simulated settings. Abbreviations: AgeR, ratio between malaria prevalence in children aged <10 y and all-age malaria prevalence; ContrA, contribution of chronic infections to overall malaria transmission; EIR, entomological inoculation rate; GMS, Greater Mekong Subregion; SSA, sub-Saharan Africa; Treat, proportion of clinical malaria cases treated.
<5 Years
In SSA settings, control programs could choose either MDA or vector control for the lower end of EIRs to meet their elimination goal, because there is no combination of VC and ϕ values for which MDA outperforms vector control. For GMS-like settings, stand-alone vector control approaches are viable only for very low EIRs, but vector control is still needed in combination with MDA to enable malaria elimination at high VC values.
≥5 Years
Combined control strategies are only required for highest values of VC and ϕ in any setting. In GMS-like settings, MDA outperforms vector control for combinations of low VC and high ϕ. Sustaining MDA campaigns for more than a couple of years is not realistic owing to logistical considerations and concerns about the emergence or spread of drug resistance. We have thus focused on the potential impact of elimination strategies consisting of 2 annual MDA campaigns of 3 artemisinin combination therapy (ACT) rounds each and vector control intervention packages consisting of annual ITN and IRS campaigns (Figure 3).
Figure 3.
Time dynamics of interventions for the combinations of vectorial capacity and relative infectivity highlighted 1–8 in Figure 2. The panels on the left illustrate 2 MDA campaigns (3 rounds each) in consecutive years; those on the right, annual insecticide-treated bed net and indoor residual spraying campaigns over a period of 3 years. Colors reflect those of the contour plots in Figure 2, indicating the predicted time to elimination. Abbreviations: GMS, Greater Mekong Subregion; MDA, mass drug administration; SSA, sub-Saharan Africa.
We chose parameters sets that illustrate the 3 possible outcomes of elimination driven intervention strategies: successful elimination (lines 2 and 4) with the 2 annual MDA campaigns or 3 years of sustained vector control (note that elimination might only occur a few years after intervention has been halted); failure to eliminate where elimination is theoretically possible (lines 3 and 5) because the intervention effort was halted too soon, with resurgence following after a few years; and elimination impossible (lines 1 and 7), with malaria returning to baseline prevalence levels soon after the intervention stops.
One important factor to consider here is the speed at which MDA and vector control strategies reduce malaria prevalence. By definition, MDA campaigns consist of administering antimalarial drugs to as many individuals as possible, regardless of their infection status, producing a dramatic effect on malaria prevalence over a short period of time. If elimination is not achieved in the 12 months after MDA, then it is very unlikely to ever happen. Vector control, on the other hand, has a more diluted impact on transmission by affecting the likelihood that existing infections generate other infections. However, it has the advantage of being able to decrease the basic reproduction number (R0) past the R0 = 1 threshold, thus bringing the system to the bistability region for VC values >14 and potentially making elimination viable for a wider spectrum of VC values.
By overlaying the epidemiological metrics displayed in Supplementary Figure S1 onto the elimination surfaces in Figure 2, we get very convenient measures of elimination feasibility that can guide national malaria control programs in the absence of reliable data on the relative infectivity of chronic infections. These are shown as white lines in Figure 2 and help delimit the parameter space in which elimination can occur within a reasonable time frame (blue-colored surface).
For MDA approaches, elimination can be achieved with 2 annual MDA campaigns if (1) baseline clinical case management is very good, with 85% of all clinical cases getting treatment; (2) EIR is <8; and (3) chronic infections are the main contributors to malaria transmission, with 85% of all infections originating in asymptomatically infected individuals. Strikingly, this is consistent in both SSA-like and GMS-like settings. For vector control approaches, elimination can be achieved with a 3 year blanket intervention package of ITN and IRS if (1) baseline clinical case management is very good, with 85% of all clinical cases getting treatment, and (2) children <10 years old sustain ≤3.5% of all infections for SSA-like settings, or ≤2.6% for GMS-like settings. Of the tipping points presented here, the baseline clinical management coverage emerges as the most promising parameter for national control programs to adjust in order to increase their likelihood of success.
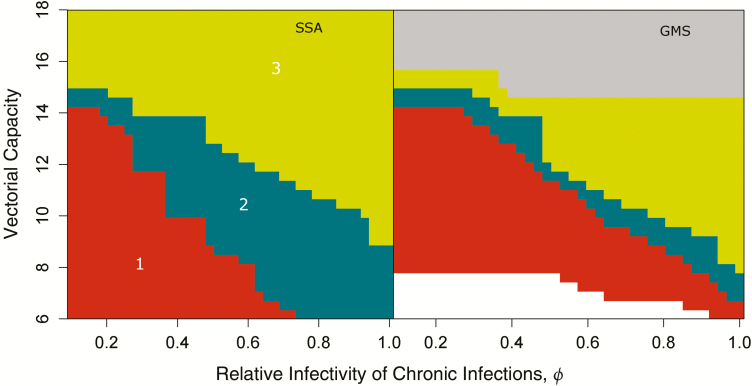
Supplementary Figure S2 shows how elimination can become feasible for higher VC settings by increasing the baseline treatment rates. It reflects the minimum treatment rate one would need to implement 1 year before an elimination strategy consisting of 2 annual MDAs, for elimination to be achieved. This highlights how good malaria case management should be the foundation of any malaria control program. If we consider areas where case management rates are poor (<50% of cases receiving treatment), with little to no vector control, we can explore 3 hierarchical intervention strategies: (1) increasing case management rates to 85%, (2) strategy 1 plus implementation of vector control packages decreasing VC by 80% in SSA and 25% in the GMS, and (3) strategy 2 plus deployment of 1 MDA campaign (3 ACT rounds).
Model predictions indicate that improved case management alone (strategy 1) could lead to elimination in a significant range of VC-ϕ parameter combinations (Figure 4). In fact, elimination can be achieved without the need for any MDA campaign in about half the settings where malaria is deemed “controllable.” The lower efficiency imposed on simulated vector control strategies for the GMS is evidenced by how narrow region 2 in Figure 4 is, but also by the large gray area, depicting the parameter region where even strategy 3 is predicted to be insufficient to reach elimination. Novel vector control tools that circumvent efficiency gaps in currently existing tools could then potentially expand both areas 2 and 3 in the right panel in Figure 4.
Figure 4.
Minimum strategy required for elimination to be reached. Strategies are hierarchical, starting with improved case management (1-red), and ultimately also including vector control (2-teal) and mass drug administration campaigns (3-green). Gray represents parameter sets for which no intervention package is successful in achieving elimination. Abbreviations: GMS, Greater Mekong Subregion; SSA, sub-Saharan Africa.
DISCLAIMER
Here we provide a qualitative analysis of how the predicted success of elimination strategies in SSA or GMS settings is extremely sensitive to a biological parameters for which we have no rigorous estimate. We emphasize that the quantitative aspects of these simulations do not necessarily reflect what would be seen in the field due to region-specific factors, such as spatial transmission heterogeneity, individual infection risk heterogeneity, and intervention logistical constraints (none of which are taken into account here). The significant tradeoff between complexity and tractability renders it impossible to analytically solve sophisticated models that take into account vector and human population dynamics with integrated within host dynamics.
The simple model presented here has several limitations, mainly with respect to how it fails to capture heterogeneity. The main source of heterogeneity lacking in the simple model pertains to the transmission network describing the human-mosquito interface. Infection risk is known to be heterogeneous across individuals and is likely to be clustered (depending on mosquito habitat dispersal). These factors are well known to have an impact on dynamical systems’ behavior, but their impact on the likelihood and timeline of elimination is complex. The VC numbers presented throughout the article can be translated into a mean risk of infection (which is the same for each individual in the simple model explored here).
If we consider a realistic scenario, wherein some individuals are at greater risk and others can be at no risk, keeping the same mean risk of infection, we decrease the likelihood of reaching elimination, because transmission can be sustained by the individuals with a greater than mean risk of infection. Supplementary Figure S3 shows the results of a very sophisticated individual-based model that assumes a skewed log-normal distribution of risk, wherein the majority of individuals are at little to no risk but some are constantly receiving infections, thus providing a more realistic but simultaneously “worst case” scenario. In this extreme example, the elimination parameter space is quite contracted, but the relative space attributable to each strategy is maintained. In this article, we intend not to make direct recommendations to national malaria control programs but rather to demonstrate how any rigorous recommendations considering detailed country-specific data are dependent on our assumptions about the duration of the infectious period and the relative infectivity of chronic infections.
CONCLUSIONS
The relative infectivity of chronic infections has severe consequences for malaria elimination prospects. Reliable estimates to inform that parameter will require longitudinal follow-up studies of sufficient duration in both clinical and chronic infections, with serial mosquito-feeding assays. Although we appreciate the time-consuming efforts involved and the significant logistical burden (specifically on the insectary) required to perform such a study, we consider those concerns to be greatly outweighed by the dramatic expected impact on malaria elimination policy. In the absence of such data, the presented model offers guidance as to what other more easily measurable epidemiological data might best inform elimination strategies’ likelihood of success. We also highlight how good case management should be the foundation of any malaria control strategy, having the potential to lead to elimination by itself in some settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funding sources did not influence the contents of the manuscript in any way.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (gatesfoundation.org/) (grant BMGF OPP1110500) and was part of the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme, funded by the Wellcome Trust of Great Britain (wellcome.ac.uk/) (grant 101148/Z/13/Z).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Collins WE, Jeffery GM. A retrospective examination of mosquito infection on humans infected with Plasmodium falciparum. Am J Trop Med Hyg 2003; 68:366–71. [PubMed] [Google Scholar]
- 2. Bousema T, Dinglasan RR, Morlais I, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One 2012; 7:e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 2011; 24:377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman RE, Kumpitak C, Ponlawat A, et al. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol 2004; 41:201–8. [DOI] [PubMed] [Google Scholar]
- 5. Ouédraogo AL, Bousema T, Schneider P, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 2009; 4:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schneider P, Bousema JT, Gouagna LC, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg 2007; 76:470–4. [PubMed] [Google Scholar]
- 7. Churcher TS, Bousema T, Walker M, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife 2013; 2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence?Trends Parasitol 2014; 30:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 1993; 48:700–6. [DOI] [PubMed] [Google Scholar]
- 10. Gaye A, Bousema T, Libasse G, et al. Infectiousness of the human population to Anopheles arabiensis by direct skin feeding in an area hypoendemic for malaria in Senegal. Am J Trop Med Hyg 2015; 92:648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okell LC, Bousema T, Griffin JT, et al. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 2012; 3:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gouagna LC, Ferguson HM, Okech BA, et al. Plasmodium falciparum malaria disease manifestations in humans and transmission to Anopheles gambiae: a field study in Western Kenya. Parasitology 2004; 128:235–43. [DOI] [PubMed] [Google Scholar]
- 13. Ouedraogo AL, Bousema T, de Vlas SJ, et al. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar J 2010; 9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nacher M. Does the shape of Plasmodium falciparum gametocytes have a function?Med Hypotheses 2004; 62:618–9. [DOI] [PubMed] [Google Scholar]
- 15. Pichon G, Awono-Ambene HP, Robert V. High heterogeneity in the number of Plasmodium falciparum gametocytes in the bloodmeal of mosquitoes fed on the same host. Parasitology 2000; 122:115–20. [DOI] [PubMed] [Google Scholar]
- 16. Churcher TS, Trape JF, Cohuet A. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat Commun 2015; 6:6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Josling GA, Llinás M. Sexual development in Plasmodium parasites: knowing when it’s time to commit. Nat Rev Microbiol 2015; 13:573–87. [DOI] [PubMed] [Google Scholar]
- 18. Aguas R, White LJ, Snow RW, Gomes MGM. Prospects for malaria eradication in sub-Saharan Africa. PLoS One 2008; 3:e1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bretscher MT, Maire N, Felger I, Owusu-Agyei S, Smith T. Asymptomatic Plasmodium falciparum infections may not be shortened by acquired immunity. Malar J 2015; 14:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felger I, Maire M, Bretscher MT, et al. The dynamics of natural Plasmodium falciparum infections. PLoS One 2012; 7:e45542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson TJC, Haubold B, Williams JT, et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol 2000; 17:1467–82. [DOI] [PubMed] [Google Scholar]
- 22. Campino S, Auburn S, Kivinen K, et al. Population genetic analysis of Plasmodium falciparum parasites using a customized Illumina GoldenGate genotyping assay. PLoS One 2011; 6:e20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barry AE, Schultz L, Buckee CO, Reeder JC. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One 2009; 4:e8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Massey NC, Garrod G, Wiebe A, et al. A global bionomic database for the dominant vectors of human malaria. Sci Data 2016; 3:160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinka ME, Bangs MJ, Manguin S, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors 2011; 4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinka ME, Bangs MJ, Manguin S, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors 2011; 4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. In: Cochrane Database Syst Rev 2004. doi:10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 28. Smithuis FM, Kyaw MK, Phe UO, et al. The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J 2013; 12:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith DL, Cohen JM, Chiyaka C, et al. A sticky situation: the unexpected stability of malaria elimination. Philos Trans R Soc Lond B Biol Sci 2013; 368:20120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.