The Taiwan MDR-TB Consortium operates under a strong collaboration between the public health authority and clinical teams. It has achieved a high treatment success rate and low loss to follow-up rate in the management of multidrug-resistant tuberculosis.
Keywords: tuberculosis, multidrug resistance, MDR, outcome
Abstract
Background
The proportion of treatment success among patients with multidrug-resistant tuberculosis (MDR-TB) enrolled between 1992 and 1996 was 51.2%, and that among patients enrolled between 2000 and April 2007 was 61%. To address the challenge of MDR-TB, the Taiwan MDR-TB Consortium (TMTC) was established in May 2007. To assess the performance of the TMTC, we analyzed the data of patients enrolled in its first 5 years.
Methods
Comprehensive care was provided at no cost to patients, who were usually hospitalized for 1 month initially. Treatment regimens consisted of 4–5 drugs and the duration of treatment was 18–24 months. A case manager and a directly observed therapy provider were assigned to each patient. Psychosocial support was provided to address emotional stress and stigma. Financial support was offered to avoid the financial hardship faced by patients and their families. We assessed treatment outcomes at 30 months using internationally recommended outcome definitions.
Results
Of the 692 MDR-TB patients, 570 (82.4%) were successfully treated, 84 (12.1%) died, 18 (2.6%) had treatment failure, and 20 (2.9%) were lost to follow-up. Age ≥65 years (adjusted odds ratio [aOR], 6.78 [95% confidence interval {CI}, 3.14–14.63]), cancer (aOR, 11.82 [95% CI, 5.55–25.18]), and chronic kidney disease (aOR, 3.62 [95% CI, 1.70–7.71]) were significantly associated with death. Resistance to fluoroquinolone (aOR, 10.89 [95% CI, 3.97–29.88]) was significantly associated with treatment failure.
Conclusions
The TMTC, which operates under a strong collaboration between the public health authority and clinical teams, has been a highly effective model of care in the management of MDR-TB.
The World Health Organization (WHO) reported that an estimated 3.9% of new tuberculosis (TB) cases and 21% of previously treated TB cases worldwide were multidrug-resistant TB (MDR-TB) or rifampicin-resistant TB (RR-TB), and the estimated number of incident cases of MDR-/RR-TB was 580000 (range, 520000–640000) in 2015 [1]. Management of MDR-/RR-TB is challenging because it involves the use of second-line drugs that cause a high frequency of adverse drug reactions and because the treatment is lengthy. Globally, the proportion of MDR-/RR-TB patients in the 2013 cohort who successfully completed treatment was only 52%, which was due to a high proportion of death (17%), treatment failure (9%), or loss to follow-up (22%). The outcomes of extensively drug-resistant tuberculosis (XDR-TB) were even worse; the proportion of patients who were successfully treated was only 28% [1].
The treatment outcomes of MDR-TB in Taiwan have been previously reported. Of the 299 pulmonary MDR-TB patients enrolled in treatment between 1992 and 1996, 51.2% were cured, 10.4% experienced treatment failure, 9.4% died, and 29.1% had a treatment interruption of ≥2 months [2]. The proportion of treatment success among MDR-TB patients enrolled between 2000 and April 2007 increased slightly to 61% [3]. To address the challenge of MDR-TB, the Taiwan MDR-TB Consortium (TMTC), which is funded by the Taiwan Centers for Disease Control (TCDC), was established in May 2007. It provides comprehensive patient-centered care at no cost to MDR-TB patients, with the aim of achieving a high proportion of treatment success.
An assessment of the performance of TMTC in its early phase [3] and on a limited scale [4] revealed that >80% of MDR-TB in TMTC achieved treatment success. To better assess the performance of TMTC and to analyze factors associated with the outcome of MDR-TB treatment in TMTC, we analyzed the data of patients enrolled in its first 5 years. The findings of the assessment are reported.
METHODS
Study Settings
The notification rate of all forms of TB in Taiwan was 72.5 per 100000 population in 2005, which decreased to 45.7 per 100000 population in 2015 (https://monitor.cdc.gov.tw/). The TMTC consists of 5 drug-resistant TB (DR-TB) management groups that provide services to the whole country. Each DR-TB management group had a lead hospital: Taipei Medical University–Wan Fang Hospital, Taipei; Tao-Yuan General Hospital, Department of Health, Tao-Yuan; Chang-Hua Hospital, Department of Health, Chang-Hua; Chest Hospital, Department of Health, Tainan; and Buddhist Tzu Chi General Hospital, Tzu Chi University, Hualien. Each management group was organized by a senior pulmonologist in charge who organized a management team of nurses and directly observed therapy (DOT) supporters and invited a few supportive hospitals to form a network of case management; the focal person at each supportive hospital in the network was a pulmonologist or an infectious disease specialist. Patients diagnosed with MDR-TB at any healthcare facility in Taiwan that was not part of the TMTC were strongly encouraged to be referred to the TMTC [5].
The diagnosis and treatment service of TB was fully covered by the National Health Insurance program, which was supplemented by additional funding from the TCDC. Drug susceptibility testing (DST) of first-line anti-TB drugs was performed at quality-assured laboratories that participated in a proficiency testing program organized by the Reference Laboratory of Mycobacteriology of the TCDC [6, 7]. Notification of TB was mandatory by law and reinforced by administrative and financial measures [8, 9]. It is mandatory to send isolates of MDR-TB to the Reference Laboratory of Mycobacteriology of the TCDC to confirm the diagnosis of MDR-TB. DST of the second-line anti-TB drugs for all patients was performed at the Chest Hospital, Department of Health, Tainan or at the Reference Laboratory of Mycobacteriology using proportion method with 7H10 medium (Becton, Dickinson and Company, Sparks, Maryland) and the GenoType MTBDRsltest (Hain Lifescience GmbH, Nehren, Germany) [10–12].
The anti-TB drugs used in the treatment of MDR-/XDR-TB, including rifabutin, kanamycin, capreomycin, amikacin, streptomycin, ofloxacin, levofloxacin, moxifloxacin, prothionamide, cycloserine, terizidone, para-aminosalicylic acid, linezolid, clofazimine, meropenem, and amoxicillin-clavulanate, were centrally procured by the TCDC and provided directly to the TMTC. The treatment was individualized by taking treatment history and results of DST into account. Patients were usually admitted at the initiation of the MDR-TB treatment for 1 month and discharged once they could tolerate the regimens. A case manager and a DOT provider were assigned to each patient in the TMTC to ensure that barriers in adherence to treatment were addressed effectively and in a timely manner. A few mobile DOT teams (including a nurse on each team) were organized in each DR-TB management group for delivering community-based DOT consistently throughout the whole treatment course. Supportive face-to-face DOT was strictly provided for at least 5 days per week; a limited number of patients had DOT using video mobile phones. Psychosocial support was provided to address emotional stress and stigma. Financial support, including enablers and incentives totaling about US$200 (range, US$0–600) per month, was offered to avoid the financial hardship faced by MDR-TB patients and their families. Radiography, sputum examinations, and blood tests were conducted regularly at no cost to the patients. Adverse drug reactions identified during day-to-day contact of DOT supporters and patients were immediately reported to clinicians. Ancillary drugs for adverse drug reactions, surgeries, and hospitalizations were also provided at no cost to patients. An expert committee meeting of the TMTC was organized on a quarterly basis and every MDR-TB case with an unsatisfactory response to treatment was reviewed to assess the need for modification of regimens and the need for surgical intervention.
The operation of the TMTC was funded by the TCDC; case management costs were approximately US$25000–$30000 per patient per year, excluding the costs of drugs [5].
Study Population
Our study population includes all MDR-TB patients who were referred to the TMTC between 1 May 2007 and 30 April 2012. However, patients treated in the health facilities that were not part of the TMTC may have been managed in a conventional manner that was less satisfactory; therefore, those who had been treated for 3 or more months for their current episode of MDR-TB before being referred to the TMTC were excluded from the analysis. Furthermore, patients who were <20 years old, patients treated with only first-line drugs, and patient with only extrapulmonary TB were also excluded.
The data collected included age, sex, body mass index, smear, cavitary lesions on the chest radiograph, alcohol use, comorbidity (diabetes, cancer, chronic liver disease, hepatitis B surface antigen positive, anti–hepatitis C antibody positive, hypertension, and cardiovascular disease), a history of treatment with anti-TB drugs, type of case registration, the results of first-line and second-line DST, anti-TB drugs used for the current episode of treatment, surgical intervention, and the outcome of treatment (cured, treatment completed, treatment failure, died, lost to follow-up). Because DST to ofloxacin, levofloxacin, moxifloxacin, and gatifloxacin was not available for all patients, those with resistance to any of these 4 fluoroquinolones (FQs) were classified as FQ-resistant MDR-TB; similarly, patients with any resistance to kanamycin, amikacin, or capreomycin were classified as second-line injectable (SLI)–resistant MDR-TB. MDR-TB patients with resistance to any FQ and any SLI were classified as XDR-TB.
Patients were usually treated with at least 4 anti-TB drugs, including an injectable agent and an FQ, following the recommendations of the WHO [13]. The treatment duration was 18–24 months, which accounted for the timing of sputum conversion. We assessed treatment outcomes at 30 months after the initiation of MDR-TB treatment to classify the patients as cured, treatment completed, treatment failed, died, or lost to follow-up using the international recommendations for outcome definitions [14]. Cured and treatment completed were further categorized as a treatment success.
Stata version 12 (Stata Corp LP, College Station, Texas) was used for statistical analyses. Categorical data were analyzed using the Pearson χ2 test. The treatment outcome was dichotomized into successful (treatment success) and unfavorable (died, treatment failed, and lost to follow-up). Logistic regression models were constructed to assess the factors associated with success, death, treatment failure, and loss to follow-up. We used the logistic command in Stata to fit the maximum-likelihood logit models. Variables significantly associated with outcome on univariate analysis by χ2 test were entered into a multivariate model; P < .05 was applied as threshold value of backward elimination and a final fitted model was determined by using the likelihood ratio test. The final models were checked by using the goodness-of-fit test to assess the model fit. A P value <.05 was considered statistically significant.
Ethics
This study was approved by the Joint Institute Review Board of Taipei Medical University.
RESULTS
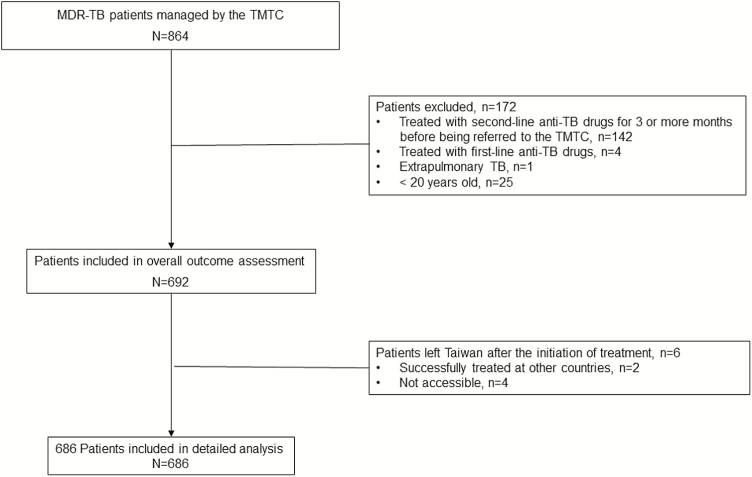
Between 1 May 2007 and 30 April 2012, a total of 864 MDR-TB patients were managed by the TMTC and accounted for more than 80% of the MDR-TB patients diagnosed during that period in Taiwan. Of the 864 MDR-TB patients, 692 adult patients were included in this study and 172 were excluded: 142 of those patients were excluded because they had been treated with second-line anti-TB drugs for ≥3 months before being referred to the TMTC; 4 patients were excluded because they were only treated with first-line anti-TB drugs but not second-line drugs; 1 patient with extrapulmonary TB was excluded because there was no pulmonary involvement; and 25 patients were excluded because they were <20 years old (Figure 1).
Figure 1.
Flow diagram of study population. Abbreviations: MDR, multidrug-resistant; TB, tuberculosis; TMTC, Taiwan MDR-TB Consortium.
Table 1 shows the treatment outcomes of 692 patients included in this study. Of the 692 patients, 6 patients left Taiwan after the initiation of treatment of MDR-TB; 2 were successfully treated at other countries and 4 were not accessible (classified as lost to follow-up). These 6 patients were not included in further analysis as detailed information of these patients was lacking.
Table 1.
Treatment Outcomes of Patients With Multidrug-Resistant Tuberculosis
| Outcome | Overall (N = 692)a |
Patients Treated in Taiwan (N = 686) |
|---|---|---|
| Treatment success | 570 (82.4) | 568 (82.8) |
| Death | 84 (12.1) | 84 (12.2) |
| Treatment failure | 18 (2.6) | 18 (2.6) |
| Lost to follow-up | 20 (2.9) | 16 (2.3) |
Data are presented as No. (%).
aSix patients left Taiwan after initiation of treatment: 2 were successfully treated at other countries and 4 were not accessible (classified as lost to follow-up).
Of the 686 patients included in detailed analysis, their mean age was 52.9 (interquartile range, 41–91) years; 351 (51.2%) were new patients, 189 (27.6%) relapse, 17 (2.5%) treatment after loss to follow-up, and 129 (18.8%) treatment after failure; 354 (51.6%) were smear negative at the initiation of MDR-TB treatment; 236 (34.4%) had diabetes mellitus and 97 (14.1%) had liver disease (Table 2).
Table 2.
Clinical and Demographic Characteristics (N = 686)
| Characteristic | No. (%) |
|---|---|
| Age, y (n = 686) | |
| <45 | 224 (32.7) |
| 45–64 | 294 (42.9) |
| ≥65 | 168 (24.5) |
| Sex (n = 686) | |
| Female | 186 (27.1) |
| Male | 500 (72.9) |
| BMI, kg/m2 (n = 685) | |
| <18.5 | 143 (20.9) |
| 18.5–23.9 | 394 (57.5) |
| ≥24 | 148 (21.6) |
| Smear (n = 686) | |
| Negative | 354 (51.6) |
| Positive | 332 (48.4) |
| Cavitary lesions on chest radiograph (n = 686) | |
| No | 441 (64.3) |
| Yes | 245 (35.7) |
| Alcohol use (n = 686) | |
| No | 587 (85.6) |
| Yes | 99 (14.4) |
| Diabetes mellitus (n = 686) | |
| No | 450 (65.6) |
| Yes | 236 (34.4) |
| Cancer (n = 684) | |
| No | 643 (94.0) |
| Yes | 41 (6.0) |
| Chronic kidney disease (n = 680) | |
| No | 634 (93.2) |
| Yes | 46 (6.8) |
| Liver disease (n = 679) | |
| No | 582 (85.7) |
| Yes | 97 (14.3) |
| Hypertension (n = 686) | |
| No | 524 (76.4) |
| Yes | 162 (23.6) |
| Cardiovascular disease (n = 680) | |
| No | 622 (91.5) |
| Yes | 58 (8.5) |
| History of anti-TB treatment (n = 686) | |
| No | 309 (45.0) |
| First-line drugs | 324 (47.2) |
| Second-line drugs | 53 (7.7) |
| Type of case registration (n = 686) | |
| New | 351 (51.2) |
| Retreatment | 335 (48.8) |
| Relapse | 189 (27.6) |
| Treatment after loss to follow-up | 17 (2.5) |
| Treatment after treatment failure | 129 (18.8) |
Abbreviations: BMI, body mass index; TB, tuberculosis.
The total number and proportion of patients with the results of DST and the total number and proportion of patients with strains that were resistant to each drug among those who had test results are shown in Table 3. Among 680 patients with results of susceptibility testing of both FQs and SLIs, 520 (76.5%) had MDR-TB without additional resistance to FQs and/or SLIs (MDR-TB sensu stricto), 106 (15.6%) had FQ-resistant MDR-TB, 39 (5.7%) had SLI-resistant MDR-TB, and 15 (2.2%) had XDR-TB.
Table 3.
Results of Drug Susceptibility Testing (N = 686)
| Drug | No. Tested | % Tested | No. Resistant | % Resistant Among Tested |
|---|---|---|---|---|
| First-line drugs | ||||
| Isoniazid | 686 | 100.0 | 686 | 100.0 |
| Rifampicin | 686 | 100.0 | 686 | 100.0 |
| Ethambutol | 680 | 99.1 | 287 | 42.2 |
| Pyrazinamide | 652 | 95.0 | 198 | 30.4 |
| Streptomycin | 680 | 99.1 | 300 | 44.1 |
| Second-line injectable drugs | 683 | 99.6 | 54 | 7.9 |
| Kanamycin | 682 | 99.4 | 50 | 7.3 |
| Amikacin | 628 | 91.6 | 33 | 5.3 |
| Capreomycin | 674 | 98.3 | 22 | 3.3 |
| Fluoroquinolonesa | 680 | 99.1 | 121 | 17.8 |
| Ofloxacin | 673 | 98.1 | 116 | 17.2 |
| Levofloxacin | 175 | 25.5 | 42 | 24.0 |
| Moxifloxacin | 274 | 39.9 | 57 | 20.8 |
| Gatifloxacin | 387 | 56.4 | 27 | 7.0 |
| Others | ||||
| Prothionamide | 679 | 99.0 | 154 | 22.7 |
| Para-aminosalicylic acid | 677 | 98.7 | 62 | 9.2 |
| Rifabutin | 668 | 97.4 | 566 | 84.7 |
aOfloxacin was tested in 2007–2012; moxifloxacin and gatifloxacin were tested in 2010–2012; and levofloxacin was tested in 2012 [12].
Regarding drugs ever used, 599 (87.3%) were treated with moxifloxacin, 151 (22.0%) were treated with levofloxacin, 489 (71.3%) were treated with kanamycin, 45 (6.6%) were treated with capreomycin, 193 (28.1%) were treated with streptomycin, 631 (92.0%) were treated with prothionamide, 555 (80.9%) were treated with cycloserine, 52 (7.6%) were treated with terizidone, 428 (62.4%) were treated with para-aminosalicylic acid, 542 (79.0%) were treated with pyrazinamide, 189 (27.6%) were treated with isoniazid, 491 (71.6%) were treated with ethambutol, 47 (6.9%) were treated with rifabutin, 61 (8.9%) were treated with clofazimine, 28 (4.1%) were treated with amoxicillin/clavulanate, 23 (3.4%) were treated with clarithromycin, 8 (1.2%) were treated with linezolid, and 1 (0.2%) was treated with imipenem. Thirty-three (4.8%) received surgical intervention.
The association between characteristics of the patients and the outcomes of treatment are shown in Table 4 and that between drug resistance and the outcome of treatment in Table 5. The proportion of patients with treatment success was 84.2% in patients with MDR-TB sensu stricto, 80.2% in FQ-resistant MDR-TB patients, 79.5% in SLI-resistant MDR-TB patients, and 53.3% in XDR-TB patients (P < .01).
Table 4.
Patient Characteristics by Treatment Outcome
| Characteristic | Total No. (Column %) |
Success | Died | Treatment Failed |
Lost to Follow-up | P Value |
|---|---|---|---|---|---|---|
| No. (Row %) | ||||||
| Total | 568 (82.8) | 84 (12.2) | 18 (2.6) | 16 (2.3) | … | |
| Age, y (n = 686) | <.01 | |||||
| <45 | 224 (32.7) | 206 (92.0) | 9 (4.0) | 6 (2.7) | 3 (1.3) | |
| 45–64 | 294 (42.9) | 254 (86.4) | 26 (8.8) | 7 (2.4) | 7 (2.4) | |
| ≥65 | 168 (24.5) | 108 (64.3) | 49 (29.2) | 5 (3.0) | 6 (3.6) | |
| Sex (n = 686) | .65 | |||||
| Female | 186 (27.1) | 153 (82.3) | 26 (14.0) | 3 (1.6) | 4 (2.2) | |
| Male | 500 (72.9) | 415 (83.0) | 58 (11.6) | 15 (3.0) | 12 (2.4) | |
| BMI, kg/m2 (n = 685) | .09 | |||||
| <18.5 | 143 (20.9) | 108 (75.5) | 28 (20.0) | 4 (2.8) | 3 (2.1) | |
| 18.5–23.9 | 394 (57.5) | 335 (85.0) | 38 (9.6) | 12 (3.1) | 9 (2.3) | |
| ≥24 | 148 (21.6) | 125 (84.5) | 17 (11.5) | 2 (1.4) | 4 (2.7) | |
| Smear (n = 686) | .04 | |||||
| Negative | 354 (51.6) | 287 (81.1) | 54 (15.3) | 7 (2.0) | 6 (1.7) | |
| Positive | 332 (48.4) | 281 (84.6) | 30 (9.0) | 11 (3.3) | 10 (3.0) | |
| Cavitary lesions (n = 686) | .29 | |||||
| No | 441 (64.3) | 361 (81.9) | 61 (13.8) | 10 (2.3) | 9 (2.0) | |
| Yes | 245 (35.7) | 207 (84.5) | 23 (9.4) | 8 (3.3) | 7 (2.9) | |
| Alcohol use (n = 686) | .14 | |||||
| No | 587 (85.6) | 479 (81.6) | 75 (12.8) | 18 (3.1) | 15 (2.6) | |
| Yes | 99 (14.4) | 89 (89.9) | 9 (9.1) | 0 (0) | 1 (1.0) | |
| Diabetes (n = 686) | .51 | |||||
| No | 450 (65.6) | 379 (84.2) | 50 (11.1) | 12 (2.7) | 9 (2.0) | |
| Yes | 236 (34.4) | 189 (80.1) | 34 (14.4) | 6 (2.5) | 7 (3.0) | |
| Cancer (n = 684) | <.01 | |||||
| No | 643 (94.0) | 550 (85.5) | 61 (9.5) | 17 (2.6) | 15 (2.3) | |
| Yes | 41 (6.0) | 17 (41.5) | 22 (53.7) | 1 (2.4) | 1 (2.4) | |
| Chronic kidney disease (n = 680) | <.01 | |||||
| No | 634 (93.2) | 538 (84.9) | 67 (10.6) | 15 (2.4) | 14 (2.2) | |
| Yes | 46 (6.7) | 27 (58.7) | 14 (30.4) | 3 (6.5) | 2 (4.4) | |
| Liver disease (n = 679) | .68 | |||||
| No | 582 (85.7) | 487 (83.7) | 69 (11.3) | 16 (2.8) | 13 (2.2) | |
| Yes | 97 (14.3) | 77 (79.4) | 15 (15.5) | 2 (2.1) | 3 (3.1) | |
| Hypertension (n = 686) | <.01 | |||||
| No | 524 (76.4) | 446 (85.1) | 50 (9.5) | 15 (2.9) | 13 (2.5) | |
| Yes | 162 (23.6) | 122 (75.3) | 34 (21.0) | 3 (1.9) | 3 (1.9) | |
| Cardiovascular disease (n = 680) | <.01 | |||||
| No | 622 (91.5) | 525 (84.4) | 66 (10.6) | 17 (2.7) | 14 (2.3) | |
| Yes | 58 (8.5) | 40 (69.0) | 15 (25.9) | 1 (1.7) | 2 (3.5) | |
| History of anti-TB treatment | .05 | |||||
| No | 309 (45.1) | 258 (83.5) | 44 (14.2) | 4 (1.3) | 3 (1.0) | |
| First-line drugs | 324 (47.2) | 269 (83.0) | 34 (10.5) | 11 (3.4) | 10 (3.1) | |
| Second-line drugs | 53 (7.7) | 41 (77.4) | 6 (11.3) | 3 (5.7) | 3 (5.7) | |
| Type of case registration | .03 | |||||
| New | 351 (51.2) | 293 (83.5) | 48 (13.7) | 7 (2.0) | 3 (0.9) | |
| Retreatment | 335 (48.8) | 275 (82.1) | 36 (10.8) | 11 (3.3) | 13 (3.9) | |
| Relapse | 189 (27.6) | 157 (83.1) | 21 (11.1) | 5 (2.7) | 6 (3.2) | |
| Treatment after loss to follow-up | 17 (2.5) | 14 (82.4) | 1 (5.9) | 0 (0) | 2 (11.8) | |
| Treatment after treatment failure | 129 (18.8) | 104 (80.6) | 14 (10.9) | 6 (4.7) | 5 (3.9) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; TB, tuberculosis.
Table 5.
Susceptibility of Antituberculosis Drugs and the Treatment Outcomes of Multidrug-Resistant Tuberculosis in Taiwan, May 2007–April 2012 (N = 686)
| Drug | Total No. (Column %) |
Success | Died | Treatment Failed | Lost to Follow-up | P Value |
|---|---|---|---|---|---|---|
| No. (Row %) | ||||||
| Ethambutol (n = 680) | .09 | |||||
| Resistant | 287 (42.2) | 240 (83.6) | 33 (11.5) | 11 (3.8) | 3 (1.1) | |
| Susceptible | 393 (57.8) | 322 (81.9) | 51 (13.0) | 7 (1.8) | 13 (3.3) | |
| Streptomycin (n = 680) | .07 | |||||
| Resistant | 300 (44.1) | 245 (81.7) | 41 (13.7) | 11 (3.7) | 3 (1.0) | |
| Susceptible | 380 (55.9) | 317 (83.4) | 43 (11.3) | 7 (1.8) | 13 (3.4) | |
| Pyrazinamide (n = 652) | .56 | |||||
| Resistant | 198 (30.4) | 166 (83.8) | 20 (10.1) | 7 (3.5) | 5 (2.5) | |
| Susceptible | 454 (69.6) | 373 (82.2) | 60 (13.2) | 10 (2.2) | 11 (2.4) | |
| Injectable drugs (n = 683) | .03 | |||||
| Resistant | 54 (7.9) | 39 (72.2) | 13 (24.1) | 2 (3.7) | 0 (0) | |
| Susceptible | 629 (92.1) | 526 (83.6) | 71 (11.3) | 16 (2.5) | 16 (2.5) | |
| Fluoroquinolones (n = 680) | <.01 | |||||
| Resistant | 121 (17.8) | 93 (76.9) | 14 (11.6) | 12 (9.9) | 2 (1.7) | |
| Susceptible | 559 (82.2) | 469 (83.9) | 70 (12.5) | 6 (1.1) | 14 (2.5) | |
| Prothionamide (n = 679) | .11 | |||||
| Resistant | 154 (22.7) | 127 (82.5) | 17 (11.0) | 8 (5.2) | 2 (1.3) | |
| Susceptible | 525 (77.3) | 434 (82.7) | 67 (12.8) | 10 (1.9) | 14 (2.7) | |
| Para-aminosalicylic acid (n = 677) | .15 | |||||
| Resistant | 62 (9.2) | 50 (80.7) | 8 (12.9) | 4 (6.5) | 0 (0) | |
| Susceptible | 615 (90.8) | 511 (83.1) | 74 (12.0) | 14 (2.3) | 16 (2.6) | |
| Rifabutin (n = 668) | .08 | |||||
| Resistant | 566 (84.7) | 473 (83.6) | 64 (11.3) | 14 (2.5) | 15 (2.7) | |
| Susceptible | 102 (17.3) | 80 (78.4) | 19 (18.6) | 3 (2.9) | 0 (0.0) | |
| Type of MDR-TB (n = 680) | <.01 | |||||
| MDR-TB | 520 (76.5) | 438 (84.2) | 63 (12.1) | 5 (1.0) | 14 (2.7) | |
| FQ-resistant MDR-TB | 106 (15.6) | 85 (80.2) | 8 (7.6) | 11 (10.4) | 2 (1.9) | |
| SLI-resistant MDR-TB | 39 (5.7) | 31 (79.5) | 7 (18.0) | 1 (2.6) | 0 (0) | |
| XDR-TB | 15 (2.2) | 8 (53.3) | 6 (40.0) | 1 (6.7) | 0 (0) | |
Abbreviations: FQ, fluoroquinolone; MDR, multidrug resistant; SLI, second-line injectable; TB, tuberculosis; XDR, extensively drug resistant.
Patients with pre-XDR and XDR-TB were significantly more likely to have surgical intervention compared with MDR-TB patients (11.9% vs 2.7%; P < .01; Supplementary Table 1). Surgical intervention was not significantly associated with the outcome (P = .99).
Table 6 shows the factors associated with treatment success. Patients who were aged ≥65 years (adjusted odds ratio [aOR], 0.19 [95% confidence interval {CI}, .10–.35]) were significantly less likely to have treatment success compared with patients who were <45 years old. Patients with resistance to FQs (aOR, 0.49 [95% CI, .29–.85]) were significantly less likely to have treatment success compared with those susceptible to FQs. Patients with cancer (aOR, 0.11 [95% CI, .05–.24]) or chronic kidney disease (aOR, 0.28 [95% CI, .14–.55]) were significantly less likely to have treatment success compared with those without these diseases.
Table 6.
Univariate and Multivariate Predictors of Multidrug-Resistant Tuberculosis Treatment Success
| Predictor | Total No. |
Success, No. (%) |
Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| OR | (95% CI) | aOR | (95% CI) | |||
| Age, y | ||||||
| <45 | 224 | 206 (92.0) | Reference | Reference | ||
| 45–64 | 294 | 254 (86.4) | 0.55 | (.31–.99) | 0.71 | (.37–1.35) |
| ≥65 | 168 | 108 (64.3) | 0.16 | (.09–.28) | 0.19 | (.10–.35) |
| SLI resistance | ||||||
| No | 629 | 526 (83.6) | Reference | |||
| Yes | 54 | 39 (72.2) | 0.51 | (.27–.96) | ||
| FQ resistance | ||||||
| No | 559 | 469 (83.9) | Reference | Reference | ||
| Yes | 121 | 93 (76.9) | 0.64 | (.40–1.03) | 0.49 | (.29–.85) |
| Smear positive | ||||||
| No | 354 | 287 (81.1) | Reference | |||
| Yes | 332 | 281 (84.6) | 1.29 | (.86–1.92) | ||
| Cancer | ||||||
| No | 643 | 550 (85.5) | Reference | Reference | ||
| Yes | 41 | 17 (41.5) | 0.12 | (.06–.23) | 0.11 | (.05–.24) |
| Chronic kidney disease | ||||||
| No | 634 | 538 (84.9) | Reference | Reference | ||
| Yes | 46 | 27 (58.7) | 0.25 | (.14–.47) | 0.28 | (.14–.55) |
| Hypertension | ||||||
| No | 524 | 446 (85.1) | Reference | |||
| Yes | 162 | 122 (75.3) | 0.41 | (.23–.75) | ||
| Cardiovascular disease | ||||||
| No | 622 | 525 (84.4) | Reference | |||
| Yes | 58 | 40 (69.0) | 0.42 | (.23–.76) | ||
| Type of case registration | ||||||
| New | 351 | 293 (83.5) | Reference | |||
| Retreatment | 335 | 275 (82.1) | 0.91 | (.61–1.35) | ||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; FQ, fluoroquinolone; OR, odds ratio; SLI, second-line injectable agent.
Patients who were aged ≥65 years (aOR, 8.35 [95% CI, 3.59–19.45]) were significantly more likely to die during treatment compared with patients who were <45 years old. Patients with cancer (aOR, 10.74 [95% CI, 5.01–23.04]) or chronic kidney disease (aOR, 3.65 [95% CI, 1.71–7.76]) were significantly more likely to die compared with those without these diseases (Table 7).
Table 7.
Factors Associated With Death
| Factor | Total No. |
Death, No. (%) |
Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| OR | (95% CI) | aOR | (95% CI) | |||
| Age, y | ||||||
| <45 | 224 | 9 (4.0) | Reference | Reference | ||
| 45–64 | 294 | 26 (8.8) | 2.32 | (1.06–5.05) | 1.80 | (.74–4.42) |
| ≥65 | 168 | 49 (29.2) | 9.84 | (4.67–20.73) | 8.35 | (3.59–19.45) |
| SLI resistance | ||||||
| No | 629 | 71 (11.3) | Reference | |||
| Yes | 54 | 13 (24.1) | 2.49 | (1.27–4.88) | ||
| FQ resistance | ||||||
| No | 565 | 70 (12.5) | Reference | |||
| Yes | 121 | 14 (11.6) | 0.91 | (.50–1.68) | ||
| Smear positive | ||||||
| No | 355 | 54 (15.3) | Reference | |||
| Yes | 332 | 30 (9.0) | 0.55 | (.34–.89) | ||
| Cancer | ||||||
| No | 643 | 61 (9.5) | Reference | Reference | ||
| Yes | 41 | 22 (53.7) | 11.05 | (5.66–21.55) | 10.74 | (5.01–23.04) |
| Chronic kidney disease | ||||||
| No | 634 | 67 (10.6) | Reference | Reference | ||
| Yes | 46 | 14 (30.4) | 3.70 | (1.88–7.29) | 3.65 | (1.71–7.76) |
| Hypertension | ||||||
| No | 524 | 50 (9.5) | Reference | |||
| Yes | 162 | 14 (30.4) | 2.52 | (1.56–4.06) | ||
| Cardiovascular disease | ||||||
| No | 622 | 66 (10.6) | Reference | |||
| Yes | 58 | 15 (25.9) | 2.94 | (1.55–5.58) | ||
| Type of case registration | ||||||
| New | 351 | 48 (13.7) | Reference | |||
| Retreatment | 335 | 36 (10.8) | 0.76 | (.48–1.21) | ||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; FQ, fluoroquinolone; OR, odds ratio; SLI, second-line injectable agent.
Resistance to FQ was significantly associated with treatment failure (P < .01). In a multivariate analysis adjusted for age and sex, patients who were infected with strains that were resistant to FQs (aOR, 10.77 [95% CI, 3.93–29.55]) were significantly more likely to fail treatment than those who were not resistant to FQs (Supplementary Table 2).
Retreatment cases were significantly more likely to be lost to follow-up compared with new cases (3.9% vs 0.9%; aOR, 4.68 [95% CI, 1.32–16.59]); the proportion of patients who were lost to follow-up was particularly high among patients who received treatment after loss to follow-up (11.8%; Supplementary Table 3).
DISCUSSION
Our assessment revealed that the TMTC, which operates under a strong collaboration between the public health authority and clinical teams, has been a highly effective model of care in the management of MDR-TB. The proportion of MDR-TB patients with treatment success was 82.8%, which was much higher than that of earlier cohorts of MDR-TB patients in Taiwan [2, 3].
The most striking finding is the drastic reduction of loss to follow-up. The proportion of patients who were lost to follow-up in an earlier cohort in Taiwan was 29% [2], which was reduced to 2.9% under the care of the TMTC. The proportion of MDR-TB patients who were lost to follow-up was relatively high in several settings [15–28]: 38% in the Philippines [16], 21% in South Africa [17], 20% in Russia [18], 20% in Peru [20], 17% in Norway [21], and 13% in Vietnam [22]. A study from the Philippines reported that adverse drug reactions and use of alcohol are significantly associated with loss to follow-up; assistance from the TB program provided to patients, patients’ knowledge of TB, trust in health care workers, and support from physicians and nurses are factors protective against loss to follow-up [16]. Our study found that the TMTC has been able to tackle most of the barriers in adherence to treatment.
A substantial proportion of our patients died, which was mainly due to aging and comorbidities. Among patients who were ≥65 years old, 29% died during treatment. A better strategy to address comorbidities will be important in reducing the mortality of MDR-TB patients in Taiwan. The association between resistance to FQ and treatment failure has been previously reported [29]. These patients may benefit from the use of new drugs such as bedaquiline [30] and delamanid [31]. These new drugs have been recently procured by TCDC to be used, together with repurposed drugs (such as clofazimine, linezolid, and meropenem), in the management of difficult MDR-TB cases in TMTC. The proportion of XDR-TB patients with treatment success was relatively low in our cohort, which was mainly due to a high proportion of death that was confounded by age, as demonstrated in the multivariate analysis. Surgical intervention was not associated with a better outcome, likely because difficult cases (pre-XDR and XDR-TB) were more likely to have a surgical intervention in our study.
Our study has several strengths. This is a population-based study that covers >80% of MDR-TB cases detected in Taiwan during the study period. Hence, the findings of this study are highly representative. The sample size was relatively large, which enabled us to analyze relevant covariates that were potentially associated with the outcome of treatment. We identified factors associated with treatment failure, death, and loss to follow-up, all of which will be helpful in developing specific interventions to further improve the outcomes of patients with MDR-TB. The weakness of the study is that the DST results of second-line drugs of some patients in the early stage of TMTC were not available. This has been changed; current practice is that all MDR-TB patients should have DST of second-line drugs.
Some of the elements used in our program have been introduced by other groups. Mitnick et al reported that community-based DOT of individualized regimens and careful management of adverse drug effects has achieved a high cure rate [32]. A systemic review and meta-analysis reported that DOT was not associated with better treatment outcomes of TB as compared with self-administration of treatment [33]. Our experience shows that supportive DOT is crucial. The TMTC was led by senior clinicians who had substantial experience in TB control and clinical management of MDR-TB, thus ensuring that the regimens used were consistent with international recommendations and adverse reactions were managed in a timely and effective manner. A unique aspect of TMTC is that the operation of the TMTC was mainly funded by the government. Using the sufficient financial resources provided to the TMTC, outreach teams were organized to provide patient-centered care and supportive DOT was provided at the location (in the community or at the home) that was most convenient to the patients. Additionally, financial hardship and psychosocial problems of patients during the whole treatment course were addressed and managed effectively. Our experience supports a previous report that monetary incentives may help enhance adherence to treatment of MDR-TB patients [34].
The preliminary results of the “Evaluation of a standardized treatment regimen of anti-tuberculosis drugs for patients with multidrug-resistant tuberculosis” (STREAM) clinical trial reported that the control regimen performed better than expected in selected population under trial condition [35]. Our study clearly demonstrated that when a program is strongly supported by political commitment and has sufficient financial resources, it is feasible to achieve a very low proportion of loss to follow-up and a high proportion of treatment success among patients with MDR-TB under program condition. The findings of our study should encourage health authorities in other countries to invest in the fight against MDR-TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. Funding for the operation of the Taiwan MDR-TB Consortium came from the Taiwan Centers for Disease Control.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016. WHO/HTM/TB/2016.13:1–201. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Chiang CY, Enarson DA, Yu MC et al. . Outcome of pulmonary multidrug-resistant tuberculosis: a 6-yr follow-up study. Eur Respir J 2006; 28:980–5. [DOI] [PubMed] [Google Scholar]
- 3. Chan PC, Huang SH, Yu MC et al. . Taiwan Multidrug-Resistant Tuberculosis Consortium-TMTC Effectiveness of a government-organized and hospital-initiated treatment for multidrug-resistant tuberculosis patients—a retrospective cohort study. PLoS One 2013; 8:e57719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu MC, Chen HY, Chien SH, Jou R. An integrated MDR-TB management programme results in favourable outcomes in northern Taiwan. Eur Respir J 2015; 45:272–5. [DOI] [PubMed] [Google Scholar]
- 5. Huang SH, Wang KF, Chan PC, Yang CH, Chen CH. Evolution of MDR-TB control strategy in Taiwan. Taiwan Epidemiol Bull 2012; 28:269–78. [Google Scholar]
- 6. Jou R, Chiang CY, Yu CY, Wu MH. Proficiency of drug susceptibility testing for Mycobacterium tuberculosis in Taiwan. Int J Tuberc Lung Dis 2009; 13:1142–7. [PubMed] [Google Scholar]
- 7. Wu MH, Chiang CY, Deng YM, Wang TF, Jou R. Proficiency of drug susceptibility testing for Mycobacterium tuberculosis in Taiwan, 2007–2011. Int J Tuberc Lung Dis 2013; 17:113–9. [DOI] [PubMed] [Google Scholar]
- 8. Chiang CY, Enarson DA, Yang SL, Suo J, Lin TP. The impact of national health insurance on the notification of tuberculosis in Taiwan. Int J Tuberc Lung Dis 2002; 6:974–9. [PubMed] [Google Scholar]
- 9. Lo HY, Yang SL, Chou P, Chuang JH, Chiang CY. Completeness and timeliness of tuberculosis notification in Taiwan. BMC Public Health 2011; 11:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang WL, Chen HY, Kuo YM, Jou R. Performance assessment of the GenoType MTBDRplus test and DNA sequencing in detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 2009; 47:2520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang WL, Chi TL, Wu MH, Jou R. Performance assessment of the GenoType MTBDRsl test and DNA sequencing for detection of second-line and ethambutol drug resistance among patients infected with multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 2011; 49:2502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuang PH, Wu MH, Fan SY, Lin KY, Jou R. Population-based drug resistance surveillance of multidrug-resistant tuberculosis in Taiwan, 2007–2014. PLoS One 2016; 11:e0165222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. WHO/HTM/TB/2011.6:1–33. Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 14. Laserson KF, Thorpe LE, Leimane V et al. . Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9:640–5. [PubMed] [Google Scholar]
- 15. Kim HJ, Hong YP, Kim SJ, Lew WJ, Lee EG. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis 2001; 5:1129–36. [PubMed] [Google Scholar]
- 16. Tupasi TE, Garfin AMCG, Kurbatova EV et al. . Factors associated with loss to follow-up during treatment for multidrug-resistant tuberculosis, the Philippines, 2012–2014. Emerg Infect Dis 2016; 22:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brust JCM, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis 2010; 14:413–9. [PMC free article] [PubMed] [Google Scholar]
- 18. Keshavjee S, Gelmanova IY, Farmer PE et al. . Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet 2008; 372:1403–9. [DOI] [PubMed] [Google Scholar]
- 19. Palmero DJ, Ambroggi M, Brea A et al. . Treatment and follow-up of HIV-negative multidrug-resistant tuberculosis patients in an infectious diseases reference hospital, Buenos Aires, Argentina. Int J Tuberc Lung Dis 2004; 8:778–84. [PubMed] [Google Scholar]
- 20. Velásquez GE, Becerra MC, Gelmanova IY et al. . Improving outcomes for multidrug-resistant tuberculosis: aggressive regimens prevent treatment failure and death. Clin Infect Dis 2014; 59:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jensenius M, Winje BA, Blomberg B et al. . Multidrug-resistant tuberculosis in Norway: a nationwide study, 1995–2014. Int J Tuberc Lung Dis 2016; 20:786–92. [DOI] [PubMed] [Google Scholar]
- 22. Phuong NT, Nhung NV, Hoa NB et al. . Management and treatment outcomes of patients enrolled in MDR-TB treatment in Viet Nam. Public Health Action 2016; 6:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olaru ID, Lange C, Indra A, Meidlinger L, Huhulescu S, Rumetshofer R. High rates of treatment success in pulmonary multidrug-resistant tuberculosis by individually tailored treatment regimens. Ann Am Thorac Soc 2016; 13:1271–8. [DOI] [PubMed] [Google Scholar]
- 24. Mibei DJ, Kiarie JW, Wairia A, Kamene M, Okumu ME. Treatment outcomes of drug-resistant tuberculosis patients in Kenya. Int J Tuberc Lung Dis 2016; 20:1477–82. [DOI] [PubMed] [Google Scholar]
- 25. van Altena R, de Vries G, Haar CH et al. . Highly successful treatment outcome of multidrug-resistant tuberculosis in the Netherlands, 2000–2009. Int J Tuberc Lung Dis 2015; 19:406–12. [DOI] [PubMed] [Google Scholar]
- 26. Gurbanova E, Mehdiyev R, Blondal K, Altraja A. Predictors of cure in rifampicin-resistant tuberculosis in prison settings with low loss to follow-up. Int J Tuberc Lung Dis 2016; 20:645–51. [DOI] [PubMed] [Google Scholar]
- 27. Kwak N, Kim HR, Yoo CG, Kim YW, Han SK, Yim JJ. Changes in treatment outcomes of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2015; 19:525–30. [DOI] [PubMed] [Google Scholar]
- 28. Shin SS, Modongo C, Boyd R et al. . High treatment success rates among HIV-infected multidrug-resistant tuberculosis patients after expansion of antiretroviral therapy in Botswana, 2006–2013. J Acquir Immune Defic Syndr 2017; 74:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falzon D, Gandhi N, Migliori GB et al. . Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013; 42:156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pym AS, Diacon AH, Tang SJ et al. . TMC207-C209 Study Group Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2016; 47:564–74. [DOI] [PubMed] [Google Scholar]
- 31. Skripconoka V, Danilovits M, Pehme L et al. . Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 2013; 41:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitnick C, Bayona J, Palacios E et al. . Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med 2003; 348:119–28. [DOI] [PubMed] [Google Scholar]
- 33. Volmink J, Garner P. Directly observed therapy for treating tuberculosis (review). Cochrane Database Syst Rev 2007; CD003343. [DOI] [PubMed] [Google Scholar]
- 34. Sripad A, Castedo J, Danford N, Zaha R, Freile C. Effects of Ecuador’s national monetary incentive program on adherence to treatment for drug-resistant tuberculosis. Int J Tuberc Lung Dis 2014; 18:44–8. [DOI] [PubMed] [Google Scholar]
- 35. MRC Clinical Trials Unit. Preliminary results from STREAM trial provide insight into shorter treatment for multidrug-resistant tuberculosis Available at: http://www.ctu.mrc.ac.uk/news/2017/preliminary_results_from_stream_trial_provide_insight_into_shorter_treatment_for_multidrug_resistant_tuberculosis. Accessed 10 January 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.