This study showed that chimney sweeps and creosote workers had lower DNA methylation of F2RL3 and AHRR, which is a risk factor for lung cancer. PAH exposure from soot and creosote oil likely play a role in this epigenetic modification.
Abstract
Some polycyclic aromatic hydrocarbons (PAH) are known carcinogens and workplace PAH exposure may increase the risk of cancer. Monitoring early cancer-related changes can indicate whether the exposure is carcinogenic. Here, we enrolled 151 chimney sweeps, 152 controls and 19 creosote-exposed male workers from Sweden. We measured urinary PAH metabolites using LC/MS/MS, the cancer-related markers telomere length (TL) and mitochondrial DNA copy number (mtDNAcn) using qPCR, and DNA methylation of lung cancer-related genes F2RL3 and AHRR using pyrosequencing. The median 1-hydroxypyrene (PAH metabolite) concentrations were highest in creosote-exposed workers (8.0 μg/g creatinine) followed by chimney sweeps (0.34 μg/g creatinine) and controls (0.05 μg/g creatinine). TL and mtDNAcn did not differ between study groups. Chimney sweeps and creosote-exposed workers had significantly lower methylation of AHRR CpG site cg05575921 (88.1 and 84.9%, respectively) than controls (90%). Creosote-exposed workers (73.3%), but not chimney sweeps (76.6%) had lower methylation of F2RL3 cg03636183 than controls (76.7%). Linear regression analyses showed that chimney sweeps had lower AHRR cg05575921 methylation (B = –2.04; P < 0.057, adjusted for smoking and age) and lower average AHRR methylation (B = –2.05; P < 0.035), and non-smoking chimney sweeps had lower average F2RL3 methylation (B = –0.81; P < 0.042, adjusted for age) compared with controls. These cancer-related markers were not associated with urinary concentrations of PAH metabolites. In conclusion, although we found no associations with PAH metabolites in urine (short-term exposure), our results suggest dose–response relationship between PAH exposure and DNA hypomethylation of lung cancer-related loci. These findings indicate that further protective measures should be taken to reduce PAH exposure.
Introduction
Polycyclic aromatic hydrocarbons (PAH), a large group of chemicals with two or more fused aromatic rings, are produced from incomplete combustion of organic matter (1). Occupational exposure to PAH is common; in the 90s ~900 000 workers in the European Union, including 18 000 in Sweden, were estimated to be exposed to PAH at concentrations higher than background levels (2). Workers in some industries and workplaces, such as during chimney sweeping, creosote impregnation, coke production and asphalt paving, are consistently exposed to high levels of PAH (3).
Chimney sweeps are predominantly exposed to soot, which contains high amounts of PAH (3,4). Several PAH in soot are human carcinogens, e.g. benzo[a]pyrene (BaP), or suspected carcinogens; indeed, the International Agency for Research on Cancer (IARC) has classified ‘soot, as found in occupational exposure of chimney sweeps’ in group I, carcinogenic to humans (5). Chimney sweeps have increased incidence of lung, bladder and oesophageal cancer; moreover, working years as chimney sweep showed an exposure–response relationship with mortality of these cancer forms (6,7). Creosote-exposed workers (e.g. workers at creosote impregnation plants or railway switch assembly workers) are exposed to high levels of PAH from creosote oil which, among other uses, is used to preserve wooden railroad ties (8). Creosote oil is classified by IARC as group 2A, probably carcinogenic to humans (5) and increased incidences of lymphoma and skin cancer have been reported among workers (9,10). However, the risk of cancer among chimney sweeps and creosote-exposed workers in relation to their current exposure to PAH remains unknown. Therefore, characterization of the exposure to PAH and potential risk of developing cancer, by linking individual data on PAH exposure to individual data on early markers of cancer-related DNA changes will provide important insights into the effects of PAH on the risk of cancer among workers currently exposed to PAH.
Two important cancer-related DNA changes are telomere length (TL) and mitochondrial DNA copy number (mtDNAcn). Telomeres, DNA–protein structures at the ends of chromosomes of eukaryotic cells, play an essential role in maintaining DNA integrity (11). Shorter TL has been associated with chromosomal aberrations (12,13); however, both shorter and longer telomeres seem to predict cancer risk (14,15). The mitochondrial DNA (mtDNA) lacks histones and has limited DNA repair capacity (16); therefore, effects on the mtDNA provide a marker of oxidative DNA damage. Studies have found that alteration of mtDNAcn in peripheral blood cells can be related to toxic exposures, such as PAH (17).
DNA methylation of cancer-related genes provides another key cancer-related marker. Recently, several epidemiological studies showed that tobacco smoking, a major source of exposure to PAH, was consistently associated with DNA hypomethylation of CpG sites in specific genes in peripheral blood cells, particularly F2RL3 (encoding F2R like thrombin/trypsin receptor 3; also known as PAR-4) and AHRR (encoding aryl-hydrocarbon receptor repressor) (18). DNA hypomethylation of CpG cg03636183 in F2RL3 was shown to predict the risk of lung cancer (19–21) and DNA hypomethylation of cg05575921 in AHRR was suggested as a strong predictor of lung cancer and was linked to lymphoblastic leukemia (20,22).
The aim of this study was to explore early markers of cancer-related DNA changes in relation to current occupational exposure to PAH among chimney sweeps and creosote-exposed workers. We hypothesize that occupational exposure to PAH causes epigenetic changes, such as hypomethylation of F2RL3 and AHRR, as well as increased oxidative stress, measured as alterations in TL and mtDNAcn.
Materials and methods
Study population and recruitment
This study included two groups of workers with occupational exposure to PAH (chimney sweeps and creosote-exposed workers) and a group of controls who were not considered to be occupationally exposed to PAH. Detailed information about the chimney sweeps and controls has previously been published (4). Briefly, we recruited 151 chimney sweeps, and 152 controls, all males, from southern Sweden. Chimney sweeps’ work involves removing soot from chimneys in different types of buildings (private houses and industrial facilities) and performing other tasks that do not involve removal of soot, such as cleaning ventilation ducts. The control group included participants with no known occupational exposure to PAH, e.g. employees at food storage companies and municipalities. The recruitment of both groups took place during 2010–15. A trained occupational nurse collected questionnaires, blood and urine samples and recorded height and weight for the participants. The recruitment of both chimney sweeps and controls started by contacting the managers of the companies, not individual sweeps or controls. The company response rates for the chimney sweeps and controls were 87 and 58%, respectively (4). There was no pattern across the nonparticipating companies in terms of company location, size or kind of service provided to clients.
The second group of PAH-exposed workers included workers at a plant that produces railway switches; these switches are anchored to wooden ties, which are impregnated with creosote for preservation purposes prior to installation on the railway track. In total, we enrolled 19 male workers during 2013–16 from the plant, which is located in the middle of Sweden. The workers routinely handle wet, creosote-impregnated ties and dripping creosote oil, if necessary, during the manufacturing process. The workers come in contact with the impregnated ties with both their hands and legs. The manufacturing process takes place indoors, after which the finished railway switch, connected to the newly impregnated ties, is disassembled for transportation. A trained occupational nurse collected questionnaires, blood and urine samples from the participants. All workers were recruited from one company with full participation (100%).
Written informed consent was obtained from all participants. This study was approved by the Regional Ethics Committee in Lund, Sweden and the Regional Ethics Committee in Uppsala, Sweden.
Questionnaire
All participants answered a questionnaire containing questions about, age, education, chronic diseases, personal and family history of disease (cancer and cardiovascular disease), prescribed and non-prescribed medicines, dietary habits (frequency of consumption of vegetables, fruits and fish), physical activity, tobacco smoking, passive smoking, use of snus, employment history, residential history and exposure to PAH from hobbies. The questionnaire for the chimney sweeps further had questions about their work tasks (soot/non-soot sweeping tasks). The questions covered six different time periods (i.e. 1963–72, 1973–82, 1983–92, 1993–2002, 2003–12 and the past 12 months. Chimney sweeps were also asked to estimate the extent to which they used personal protective equipment (e.g. gloves and masks) during soot/non-soot sweeping over three time periods (1975–2002, 2003–12 and the past 12 months). The questionnaire for the creosote-exposed workers also explored work tasks (e.g. processing and handling the ties) and the use of gloves over the same six time periods as well as over the past working week and the past working day.
Sampling of blood and urine
We collected venous blood and post–shift spot urine samples from the participants on Wednesdays or Thursdays. Nine participants (five chimney sweeps and four controls) refrained from giving blood samples and four participants (three chimney sweeps and one control) could not urinate at the time of sampling. In total, we had 313 EDTA blood samples (146 from chimney sweeps, 148 from controls and 19 from creosote-exposed workers) and 318 urine samples (148 from chimney sweeps, 151 from the controls and 19 from creosote-exposed workers). Blood and urine samples of chimney sweeps, creosote-exposed workers and controls were kept at –20°C until DNA extraction and analysis of PAH metabolites.
Analysis of urinary PAH metabolites
We measured mono-hydroxylated metabolites of PAH in urine analysed as 1-hydroxypyrene (1-OH-PYR), 3-hydroxybenzo[a]pyrene (3-OH-BaP), 2–hydroxyphenanthrene (2-OH-PH) and 3-hydroxybenzo[a]anthracene (3-OH-BaA) to assess individual exposure to PAH; details of the measurements are described previously (4). In brief, internal standards of PAH metabolites were prepared and added to urine samples. Liquid chromatography tandem mass spectrometry (LC-MS/MS; QTRAP 5500, AB Sciex, Foster City, CA) was employed for measurements. Urine samples were hydrolysed with glucuronidase b and 5 µl of sample was injected onto a C18 column for analysis of 1-OH-PYR (four aromatic rings) and 2-OH-PH (three aromatic rings). The 3-OH-BaP (five aromatic rings) and 3-OH-BaA (four aromatic rings) were quantified using a two-dimensional chromatography system, with 20 µl of the sample on the column. All samples were run in duplicate and the average values for each sample were calculated. PAH metabolite concentrations in urine were adjusted to urinary creatinine (µg/g creatinine). Creatinine in urine was measured by an enzymatic colorimetric assay (23).
DNA extraction and measurement of TL
Peripheral blood samples were collected in EDTA tubes and stored at –20°C. A QIAamp DNA Blood Midi kit (Qiagen GmbH, Hilden, Germany; cat# 51185) was used for extracting genomic DNA from blood samples of chimney sweeps and controls, and the E.Z.N.A. Blood DNA Mini Kit (Omega Bio-tek, Norcross, GA; cat# D3392-02) for blood samples of creosote-exposed workers. NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE) was used to evaluate the quantity and purity of DNA and the 260/280 ratio was ≥1.8 for all samples except for five samples that had 260/280 = 1.7).
Relative TL was measured using a qPCR- and SYBR Green-based assay developed by Cawthon (24) and described previously (25) with minor adjustments. Relative TL was measured by calculating the ratio of the copy number of the telomere repeats (T) to that of a single-copy gene, HBB, encoding hemoglobin beta (S). The values of T and S were obtained from two independent qPCR runs, i.e. telomere run and HBB run, based on a standard curve. Sample preparation for standard curves, PCR conditions and sequences of primers for both assays are summarized in the supplementary material. All samples, including the standard curve and negative controls were conducted in triplicate. For quality control, 10% of randomly selected samples were re-analyzed with coefficient of variation of 7.3%. The coefficient of determination (R2) for all telomere and HBB runs was >0.99. Standard deviation (SD) for triplicate samples was accepted for Ct values at SD < 0.2; extreme values for some triplicates were excluded to obtain acceptable SD.
Measurement of mtDNA copy number (mtDNAcn)
The principle of the TL assay was applied to measure relative mtDNAcn using the same real-time PCR platform (LightCycler 480). Relative mtDNAcn was determined by calculating the ratio (M/S); mtDNAcn (M) to a single copy gene (HBB) copy number (S) from two separate PCR runs. For the mtDNAcn assay, 7.5 µl of KAPA master mix (KAPA SYBR FAST Master Mix 2×, Optimized for LightCycler 480; Kapa Biosystems) was used together with a 2.5-µl DNA sample. Details of the HBB assay and standard curve DNA preparation were the same as for the TL assay. All samples, standard curve points and negative controls were run in triplicate and 10% of the samples were re-run to ensure quality of the analysis (coefficient of variation = 14.4%). Accepted variation for Ct values between triplicates was set at SD < 0.2. The coefficient of determination (R2) for all runs was >0.99. Information about primer sequence and PCR conditions for mtDNAcn assay is found in Supplementary Table 1, available at Carcinogenesis Online.
Measurement of DNA methylation
Two previously reported (26,27) loci specific methylation assays for F2RL3 and AHRR were adopted. F2RL3 is a protein-coding gene with two exons located in chromosome 19 (19p13.11). Two CpG sites in exon 2 of F2RL3, i.e. cg03636183 [according to Illumina beadchips 450K; Illumina Inc., San Diego, CA; referred to as CpG2 in this study and in Hossain et al. (26)] that was linked to smoking and lung cancer and another CpG site downstream (CpG1), were analysed (Supplementary Table 2, Supplementary Figure 1a, available at Carcinogenesis Online). AHRR is a protein-coding gene with 11 exons located on chromosome 5 (5p15.33). The primary CpG site of interest in AHRR was cg05575921 (Illumina 450K) due to its associations with smoking and lung cancer (18,20). Two further CpG sites upstream of cg05575921 (within 25 bp proximity) were included in the analysis (Supplementary Table 2; Supplementary Figure 1b, available at Carcinogenesis Online).
DNA samples of the different study groups were randomized in 96-well plates prior to bisulfite conversion. Half a microgram of DNA from each sample was bisulfite-treated using EZ-96 DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, cat# D5008) and eluted in 20 µl following the manufacturer’s protocol. Unmethylated human control DNA (Qiagen) and methylated human control DNA (Zymo Research) were included in every randomized 96-well plate and bisulfite treated. SimpliAmp Thermal Cycler (Thermo Fisher Scientific, Carlsbad, CA) was used to amplify the sequence of interest for each assay. PCR and sequencing primers as well as PCR reagents (PyroMark PCR Kit; cat# 978703) were purchased from Qiagen. Representative samples taken from each plate after PCR were run on agarose gels to ensure amplification specificity. The pyrosequencing was performed using PyroMark Q96 ID platform (Qiagen) and all reagents were purchased from Qiagen, except Streptavidin Sepharose High Performance (GE Healthcare, Uppsala, Sweden). All pyrosequencing runs were carried out on the same day for each assay. Bisulfite-treated methylated and unmethylated DNA controls as well as negative controls were included in each pyrosequencing run. For quality control purposes, 10% of the samples were randomly selected and repeated. The coefficient of variation for each CpG site ranged between 2.5 and 4.1%.
Statistical analysis
Age was calculated based on birth and enrollment dates. Body mass index data were available for chimney sweeps and controls, but not for creosote-exposed workers, and calculated as weight (kg) divided by height (m) squared. Cigarette smoking was categorized into current smoker, party/ex-smoker and non-smoker. Average DNA methylation per gene was calculated based on individual CpG sites.
Frequencies were calculated for categorical variables (e.g. cigarette smoking and use of snus) and median, maximum and minimum values for continuous variables (e.g. PAH metabolites and DNA methylation). Differences between PAH-exposed groups and controls were examined by Fisher’s Exact test for categorical variables and Mann–Whitney U test for continuous variables. Spearman’s correlation was used to examine correlations for (i) age and cancer-related markers, (ii) PAH metabolites and cancer-related markers and (iii) the intercorrelations of cancer-related markers.
General linear models were fit to analyse the associations between outcome variables (i.e. TL, mtDNAcn and DNA methylation) and exposure (chimney sweeps versus controls; and creosote-exposed workers versus controls): Model 1 adjusted for age and cigarette smoking; Model 2 adjusted for age (only non-smokers); Model 3 adjusted for age, cigarette smoking, use of snus, exposure to PAH from hobby and passive smoking (fully adjusted). Only Model 1 was fit for comparison between controls and creosote-exposed workers, as the latter group was small (n = 19). Because smoking is a strong confounder, Model 2 was fit to assess the associations between outcome variables and PAH metabolites among chimney sweeps and controls separately. PAH metabolites were non-normally distributed and therefore they were natural log-transformed. The effect of smoking on the cancer-related markers was evaluated by a general linear model adjusted for age where smoking was divided into two categories; smokers versus party-/ex-/non-smokers.
The adjustment variables age and cigarette smoking were selected a priori based on published literature (19,28). Use of snus (yes/no), exposure to PAH from hobby (yes/no) and passive smoking (yes/no), were considered in the fully adjusted model due to correlations with either PAH exposure (PAH-exposed group and PAH metabolites) or outcome variables.
As a crude estimate of long-term exposure to PAH, working years of soot sweeping were calculated and analyses were made comparing models of working years versus cancer-related markers, with models of age versus cancer-related markers among non-smoking sweeps. The residuals of the linear regression models were reasonably normally distributed. Statistical analyses for creosote-exposed workers had low power due to the small sample size.
All statistical analyses were carried out with SPSS 23.0 (IBM SPSS Statistics, NY). Statistical significance (two-tailed) was denoted at P value <0.05.
Results
Characteristics of study groups
The demographic and lifestyle characteristics of chimney sweeps, creosote-exposed workers and controls are summarized in Table 1. The median ages of chimney sweeps and controls were similar (43 years), but lower for creosote-exposed workers (32 years). The three groups did not differ in cigarette smoking, passive smoking, exposure to PAH from hobbies, intake of vegetables, fruits and fish, physical activity, education and personal history of disease (P > 0.05). Use of snus was more common among chimney sweeps, and to a lesser degree among creosote-exposed workers, compared with controls (P = 0.007). Current place of residence differed between groups as more creosote-exposed workers resided in a big city compared with controls (P = 0.006).
Table 1.
Characteristics of controls, chimney sweeps and creosote-exposed workers
| Variables | Controls n = 152a | Chimney sweeps n = 151a | Creosote-exposed workers n = 19a | P* |
|---|---|---|---|---|
| Ageb (years) | 43 (20–63) | 43 (19–66) | 32 (22–58) | 0.492 |
| Body mass indexb (kg/m2) | 27 (20–45) | 26 (19–37) | –c | 0.019 |
| Smoking (non-smoker/party- and ex-smoker/current smoker) % | 55/29/16 | 49/33/18 | 50/33/17 | 0.915 |
| Snus (yes) % | 19 | 35 | 33 | 0.007 |
| Passive smoking (yes) % | 16 | 19 | 28 | 0.384 |
| Exposure to PAH from hobby (yes) % | 4 | 6 | 11 | 0.268 |
| Vegetablesd (≥5 times a week/<5 times a week) % | 58/42 | 64/36 | 61/39 | 0.609 |
| Fruitse (≥5 times a week/<5 times a week) % | 58/42 | 50/50 | 55/45 | 0.351 |
| Fishf (≥ once a week/< once a week) % | 46/54 | 49/51 | 50/50 | 0.884 |
| Physical activityg (high/low) % | 41/59 | 49/51 | 39/61 | 0.370 |
| Education (university or higher/high school or lower) % | 21/79 | 16/84 | 0/100h | 0.100 |
| Residency (big city/small city) % | 46/54 | 35/65 | 73/27h | 0.006 |
aUp to two missing cases for some of the variables.
bPresented as median (min–max).
cData not available.
dIntake of all kinds of vegetables, legumes and root vegetables (fresh, frozen, canned, stewed, juice, soup, etc.).
eIntake of all kinds of fruits and berries (fresh, frozen, canned, juice, jam, etc.).
fIntake of all kinds of fish.
gAverage physical activity during leisure time.
hMissing data for four participants.
*P-value of Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables.
PAH-exposed groups had higher concentrations of PAH metabolites in urine compared with controls
As previously reported (4), chimney sweeps had up to seven times higher concentrations of PAH metabolites in urine (1-OH-PYR, 2-OH-PH, 3-OH-BaP and 3-OH-BaA) than controls (P < 0.001; Table 2). Creosote-exposed workers had very high 1-OH-PYR, 2-OH-PH and 3-OH-BaA concentrations, up to 160 times higher than the controls (Table 2), but the concentrations of 3-OH-BaP for all creosote-exposed workers were very low. Differences in 1-OH-PYR, 2-OH-PH and 3-OH-BaA for chimney sweeps versus controls, creosote-exposed workers versus controls and creosote-exposed workers versus chimney sweeps were all statistically significant (P < 0.001).
Table 2.
PAH metabolites in urine presented for all participants and non-smokers in each study group
| Controlsa | Chimney sweepsa | Creosote-exposed workers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Min–Max | n | Median | Min–Max | P* | n | Median | Min–Max | P** | |
| 1-OH-PYRb | |||||||||||
| All participants | 151 | 0.06 | 0.00–0.73 | 148 | 0.39 | 0.02–8.77 | <0.001 | 19 | 10.7 | 3.2–41.3 | <0.001 |
| Non-smokers | 83 | 0.05 | 0.01–0.44 | 71 | 0.34 | 0.02–5.84 | <0.001 | 9 | 8.0 | 3.2–23.0 | <0.001 |
| 2-OH-PHc | |||||||||||
| All participants | 151 | 0.14 | 0.04–3.09 | 148 | 0.57 | 0.08–7.10 | <0.001 | 19 | 29.6 | 12.7–128.5 | <0.001 |
| Non-smokers | 83 | 0.12 | 0.05–3.09 | 71 | 0.48 | 0.08–3.64 | <0.001 | 9 | 32.4 | 13.8–128.5 | <0.001 |
| 3-OH-BaPd | |||||||||||
| All participants | 130 | 1.03 | 0.00–17.28 | 132 | 3.35 | 0.00–50.15 | <0.001 | 19 | 0.75 | 0.15–2.45 | 0.703 |
| Non-smokers | 73 | 0.92 | 0.00–9.01 | 66 | 3.09 | 0.00–16.23 | <0.001 | 9 | 0.75 | 0.15–2.45 | 0.683 |
| 3-OH-BaAe | |||||||||||
| All participants | 144 | 1.66 | 0.04–21.02 | 142 | 4.78 | 0.39–43.35 | <0.001 | 19 | 13.2 | 1.5–32.6 | <0.001 |
| Non-smokers | 80 | 1.49 | 0.04–6.84 | 68 | 4.08 | 0.84–30.64 | <0.001 | 9 | 13.2 | 1.5–30.3 | <0.001 |
aCreatinine–adjusted PAH metabolites in urine for controls and chimney sweeps have been previously reported in (4).
b1-hydroxypyrene (μg/g creatinine).
c2-hydroxyphenanthrene (μg/g creatinine).
d3-hydroxybenzo[a]pyrene (ng/g creatinine).
e3-hydroxybenzo[a]anthracene (ng/g creatinine).
*Mann–Whitney U test for differences between chimney sweeps and controls.
**Mann–Whitney U test for differences between creosote-exposed workers and controls.
Determinants of the cancer-related markers among the controls
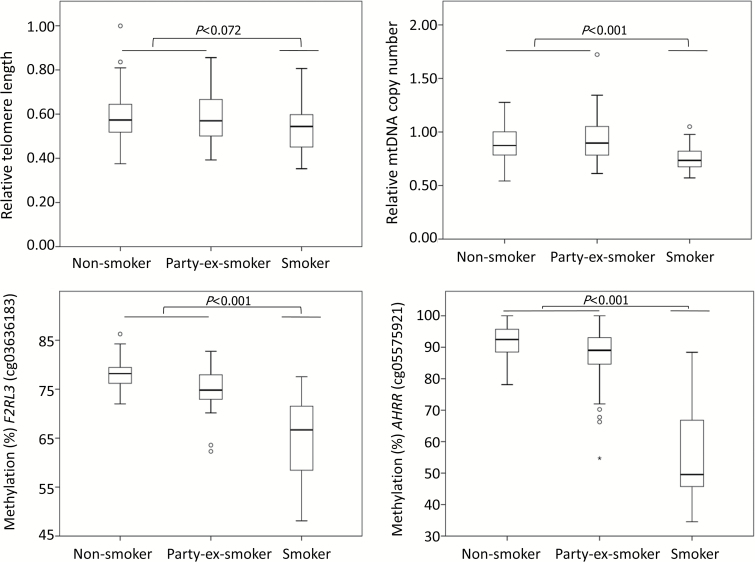
We first evaluated the effect of smoking on cancer-related markers among the controls. Current smokers had shorter telomeres compared with non-current smokers (non- and party/ex-smokers) but the difference was not significant (P = 0.072). The other markers were significantly associated with smoking, as current smokers had lower mtDNAcn and lower DNA methylation of F2RL3 and AHRR compared with non-current smokers (P ≤ 0.001; Figure 1). For all CpG sites, a dose–response relationship was found; median DNA methylation for smokers, party/ex-smokers and non-smokers was 67, 75 and 78% (F2RL3_cg03636183) and 50, 89 and 92% (AHRR_ cg05575921), respectively. Based on the correlations identified between smoking and the cancer-related markers, we performed further analyses evaluating occupational exposure to PAH and cancer-related markers using data from non-smokers.
Figure 1.
Variation of cancer-related markers across three different smoking categories among the controls. P values were derived from linear regression analysis adjusted for age where smoking status was allocated into two categories; smokers and party-/non-/ex-smokers).
Age has been associated with cancer-related markers (29) and we found that age was correlated inversely with TL (rS = −0.21) and positively with DNA methylation of AHRR (rS = 0.26 for average methylation of AHRR; Supplementary Table 3, available at Carcinogenesis Online).
We also investigated the intercorrelations between cancer-related markers in the control group. MtDNAcn and TL were positively correlated (rS = 0.3; Supplementary Table 4, available at Carcinogenesis Online), but showed either no correlation or weak positive correlations with DNA methylation (rS = 0.01–0.19; Supplementary Table 4, available at Carcinogenesis Online). DNA methylations of CpG sites were all positively intercorrelated (rS = 0.34–0.93; Supplementary Table 4, available at Carcinogenesis Online).
DNA methylation differed between PAH-exposed groups and controls, but TL and mtDNAcn did not
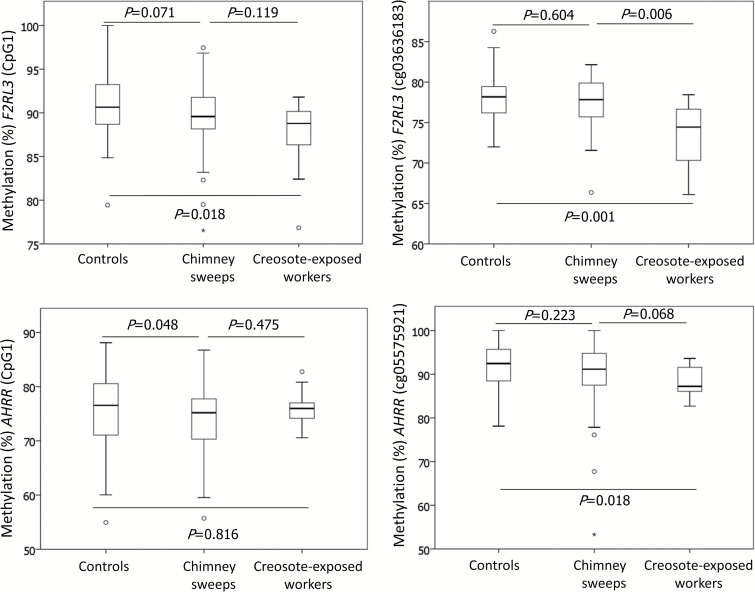
TL and mtDNAcn did not differ between the PAH-exposed groups and the controls (P > 0.05 Mann–Whitney U test; Table 3). Chimney sweeps had, compared with controls, lower median DNA methylation at all CpG sites and the differences were significant for AHRR (CpG1, CpG2 and average AHRR; P ≤ 0.048, Table 3). Creosote-exposed workers had, in comparison with controls, lower median DNA methylation at all CpGs except AHRR_CpG2, and significance was reached for F2RL3 (cg03636183, CpG1 and average F2RL3) and AHRR (cg05575921) (P ≤ 0.028; Table 3). When comparing chimney sweeps with creosote-exposed workers, we found that creosote-exposed workers had lower median DNA methylation at all CpG sites, apart from AHRR_CpG2 and the differences were significant for F2RL3 (cg03636183 and average F2RL3 methylation) and AHRR (cg05575921) (P ≤ 0.004; for non-smokers). This indicates a dose–response relationship between PAH exposure and methylation of cg03636183 and cg05575921 as DNA methylation decreased with higher level of PAH exposure (Table 3; Figure 2).
Table 3.
Telomere length, mtDNA copy number and DNA methylation of F2RL3 and AHRR in peripheral blood presented for all participants and non-smokers in each study group
| Controls | Chimney sweeps | Creosote-exposed workers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Min–Max | n | Median | Min–Max | P* | n | Median | Min–Max | P** | |
| Telomere length | |||||||||||
| All participants | 148 | 0.568 | 0.353–0.999 | 146 | 0.562 | 0.358–0.970 | 0.641 | 19 | 0.569 | 0.334–0.779 | 0.778 |
| Non-smokers | 83 | 0.573 | 0.375–0.999 | 73 | 0.555 | 0.358–0.970 | 0.578 | 9 | 0.571 | 0.429–0.779 | 0.708 |
| MtDNA copy number | |||||||||||
| All participants | 148 | 0.867 | 0.542–1.723 | 144 | 0.827 | 0.506–1.409 | 0.115 | 19 | 0.951 | 0.430–1.341 | 0.409 |
| Non-smokers | 83 | 0.874 | 0.542–1.277 | 71 | 0.851 | 0.532–1.382 | 0.140 | 9 | 0.940 | 0.430–1.228 | 0.498 |
| F2RL3_CpG1 | |||||||||||
| All participants | 147 | 89.5 | 58.1–100 | 146 | 88.8 | 54.7–98.6 | 0.063 | 19 | 87.2 | 64.9–96.4 | 0.014 |
| Non-smokers | 82 | 90.7 | 79.4–100 | 73 | 89.6 | 76.5–97.5 | 0.071 | 9 | 88.8 | 76.8–91.8 | 0.018 |
| F2RL3_CpG2 (cg03636183) | |||||||||||
| All participants | 147 | 76.7 | 48.1–86.3 | 146 | 76.6a | 44.8–82.5 | 0.786 | 19 | 73.3a | 49.5–78.4 | 0.003 |
| Non-smokers | 82 | 78.2 | 72.0–86.3 | 73 | 77.8a | 66.4–82.2 | 0.604 | 9 | 74.4a | 66.1–78.4 | 0.001 |
| F2RL3_average | |||||||||||
| All participants | 147 | 83.6 | 53.8–89.9 | 146 | 83.0a | 50.8–88.2 | 0.173 | 19 | 80.1a | 57.2–87.1 | 0.002 |
| Non-smokers | 82 | 84.5 | 77.1–89.9 | 73 | 84.2a | 71.5–88.2 | 0.094 | 9 | 81.1a | 71.5–85.0 | 0.001 |
| AHRR_CpG1 | |||||||||||
| All participants | 147 | 75.0 | 24.7–88.1 | 145 | 73.0 | 20.8–89.7 | 0.030 | 9 | 74.1 | 30.9–82.8 | 0.345 |
| Non-smokers | 82 | 76.5 | 55.0–88.1 | 72 | 75.2 | 55.7–86.7 | 0.048 | 9 | 76.0 | 70.6–82.8 | 0.816 |
| AHRR_CpG2 | |||||||||||
| All participants | 147 | 69.1 | 22.7–80.4 | 145 | 66.5 | 20.3–76.6 | 0.002 | 191 | 68.7 | 30.9–81.7 | 0.482 |
| Non-smokers | 82 | 71.3 | 56.9–80.4 | 72 | 69.4a | 46.6–76.6 | 0.005 | 9 | 72.7a | 68.1–81.7 | 0.111 |
| AHRR_CpG3 (cg05575921) | |||||||||||
| All participants | 147 | 90.0 | 34.5–100 | 145 | 88.1 | 29.3–100 | 0.038 | 19 | 84.9 | 37.3–93.6 | 0.003 |
| Non-smokers | 82 | 92.5 | 78.1–100 | 72 | 91.2 | 53.3–100 | 0.223 | 9 | 87.2 | 82.7–93.6 | 0.018 |
| AHRR_average | |||||||||||
| All participants | 147 | 78.6 | 27.3–87.2 | 145 | 76.2 | 23.5–87.5 | 0.007 | 19 | 77.0 | 33.7–82.9 | 0.262 |
| Non-smokers | 82 | 80.6 | 64.1–87.2 | 72 | 78.5 | 53.3–87.5 | 0.021 | 9 | 78.8 | 74.5–82.9 | 0.755 |
aSignificant differences in DNA methylation between chimney sweeps and creosote-exposed workers (Mann–Whitney U test).
*Mann–Whitney U test for differences between chimney sweeps and controls.
**Mann–Whitney U test for differences between creosote-exposed workers and controls.
Figure 2.
Variation of DNA methylation in the non-smoking control group, chimney sweeps and creosote-exposed workers. P values were derived from Mann–Whitney U test for differences between chimney sweeps and controls, creosote-exposed workers and controls and chimney sweeps and creosote-exposed workers.
In the linear regression analysis for chimney sweeps versus controls, TL and mtDNAcn showed no association with exposure group (Table 4(A)). DNA methylation of all CpG sites showed negative estimates for chimney sweeps in Models 1–3 (apart from cg03636183 in Model 1, Table 4(A)). The associations reached significance for AHRR_CpG2 and average AHRR methylation in all models (average AHRR, B = –1.94; 95% CI –3.55, –0.32; Model 2, Table 4(A)). Average F2RL3 methylation was significantly lower among chimney sweeps compared with controls (B = –0.81; –1.60, –0.03; Model 2, Table 4(A)).
Table 4.
Differences in cancer-related markers between PAH-exposed groups and control group (reference group); (A) chimney sweeps versus controls, and (B) creosote workers versus controls, explored by general linear models; Model 1, adjusted for age and smoking: Model 2, adjusted for age among non-smokers and Model 3, fully adjusted
| Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|
| P | B1 (95% CI) | P | B1 (95% CI) | P | B1 (95% CI) | |
| (A) Chimney sweeps versus controlsd | ||||||
| Telomere length | 0.698 | −0.005 (−0.028, 0.019) | 0.986 | 0.0003 (−0.034, 0.035) | 0.517 | −0.01 (−0.031, 0.016) |
| MtDNA copy number | 0.102 | −0.03 (−0.074, 0.007) | 0.126 | −0.04 (−0.089, 0.011) | 0.131 | −0.03 (−0.072, 0.009) |
| F2RL3_CpG1 | 0.110 | −0.98 (−2.18, 0.22) | 0.055 | −1.13 (−2.29, 0.02) | 0.125 | −0.96 (−2.19, 0.27) |
| F2RL3_CpG2 (cg03636183) | 0.962 | 0.03 (−1.02, 1.07) | 0.296 | −0.49 (−1.43, 0.44) | 0.952 | −0.03 (−1.10, 1.04) |
| F2RL3_average | 0.348 | −0.48 (−1.47, 0.52) | 0.042 | −0.81 (−1.60, −0.03) | 0.338 | −0.50 (−1.52, 0.52) |
| AHRR_CpG1 | 0.145 | −1.55 (−3.63, 0.54) | 0.063 | −1.87 (−3.83, 0.10) | 0.169 | −1.50 (−3.63, 0.64) |
| AHRR_CpG2 | 0.007 | −2.55 (−4.42, −0.69) | 0.007 | −2.30 (−3.96, −0.65) | 0.011 | −2.46 (−4.37, −0.56) |
| AHRR_CpG3 (cg05575921) | 0.057 | −2.04 (−4.14, 0.06) | 0.104 | −1.64 (−3.62, 0.34) | 0.078 | −1.93 (−4.08, 0.22) |
| AHRR_average | 0.035 | −2.05 (−3.95, −0.15) | 0.019 | −1.94 (−3.55, −0.32) | 0.048 | −1.96 (−3.91, −0.02) |
| (B) Creosote workers versus controlse | ||||||
| Telomere length | 0.893 | −0.004 (−0.055, 0.048) | — | — | — | — |
| MtDNA copy number | 0.532 | 0.030 (−0.065, 0.125) | — | — | — | — |
| F2RL3_CpG1 | <0.001 | −4.75 (−7.35, −2.15) | — | — | — | — |
| F2RL3_CpG2 (cg03636183) | <0.001 | −4.93 (−7.21, −2.64) | — | — | — | — |
| F2RL3_average | <0.001 | −4.84 (−7.00, −2.67) | — | — | — | — |
| AHRR_CpG1 | 0.075 | −4.25 (−8.95, 0.44) | — | — | — | — |
| AHRR_CpG2 | 0.501 | −1.46 (−5.72, 2.81) | — | — | — | — |
| AHRR_CpG3 (cg05575921) | <0.001 | −9.23 (−13.63, −4.94) | — | — | — | — |
| AHRR_average | 0.020 | −5.00 (−9.19, −0.81) | — | — | — | — |
Effect estimates are presented as B values and 95% confidence interval (95% CI).
a(cancer-related marker) = intercept + B1 × exposure group (2 categories) + B2 × age (continuous) + B3 × smoking status (3 categories) + e (residual error).
b(cancer-related marker) = intercept + B1 × exposure group (2 categories) + B2 × age (continuous) + e (residual error). Current and party-/ex-smokers were excluded from this analysis.
c(cancer-related marker) = intercept + B1 × exposure group (2 categories) + B2 × age (continuous) + B3 × smoking status (3 categories) + B4 × use of snus (2 categories) + B5 × passive smoking (2 categories) + B6 × exposure to PAH from hobby (2 categories) + e (residual error).
dNumber of cases included ranged between n = 143–145 for chimney sweeps and n = 147–148 for controls in Model 1; n = 71–73 for chimney sweeps and n = 82–83 for controls in Model 2 and n = 142–144 for chimney sweeps and n = 147–148 for controls in Model 3.
eNumber of cases included ranged between n = 17–18 for creosote-exposed workers and n = 147–148 for controls. Models 2 and 3 were not computed for this comparison due to small sample size of the group of creosote-exposed workers (only nine participants were non-smokers).
Creosote-exposed workers, compared with controls, showed lower DNA methylation for all CpG sites and the associations were significant for F2RL3 (cg03636183, CpG1 and average F2RL3) and AHRR (cg05575921 and average AHRR) (Table 4(B)). For instance, 9.2% lower methylation was estimated for creosote-exposed workers compared with controls (adjusted for age and smoking; P < 0.001; 95% CI, –13.6, –4.9; Model 1, Table 4(B)). Likewise, creosote-exposed workers had 4.9% lower methylation of cg03636183 compared with controls (adjusted for age and smoking; P < 0.001; 95% CI, –7.2, –2.6; Model 1, Table 4(B)).
PAH metabolites and working years with PAH exposure showed no clear relationship with cancer-related markers
There were no correlations between PAH metabolites in urine and cancer-related markers among non-smoking chimney sweeps. Age-adjusted linear regression analyses among non-smoking chimney sweeps and controls demonstrated no significant associations between PAH metabolites and cancer-related markers (Supplementary Table 5b, available at Carcinogenesis Online). Linear regression analysis for creosote-exposed workers was not performed due to small sample size (n = 9 non-smokers).
Working years of soot sweeping and age were highly correlated (rS = 0.89). Linear regression analyses among non-smoking chimney sweeps did not show bigger effect size of working years on cancer-related markers, compared with the effect size of age (Supplementary Table 6, available at Carcinogenesis Online).
Discussion
In this study, we showed that two groups of workers occupationally exposed to PAH had hypomethylation of F2RL3 and AHRR in blood, markers previously shown to indicate increased risk of lung cancer (19–21). Even though PAH metabolites in urine were not associated with DNA methylation, our data suggest a dose–response relationship between PAH exposure and DNA methylation as creosote-exposed workers, the group with higher PAH exposure (urinary concentrations of 1-OH-PYR were 23 times higher than those in chimney sweeps), showed even lower DNA methylation than chimney sweeps, compared with the controls. This should, however, be interpreted with some caution due to the small sample size of the creosote-exposed group. The lack of association between PAH metabolite concentrations in urine and DNA methylation may be explained by the fact that PAH metabolites are short-lived (6–36 h) (30) and thus reflect the very recent exposure, whereas alteration of DNA methylation takes place over a longer time period, days to weeks (31), and thus reflects longer-term exposure.
Our findings may have implications for cancer risk in these occupational groups. A recent population-based cohort study (N = 4987) with about 11 years follow-up suggested hypomethylation of F2RL3 (cg03636183) in blood as a powerful predictor of incidence and mortality of lung cancer; for every 10% lower methylation, the risk of developing lung cancer increased by 33% after adjustment for smoking, pack years and other confounders (32). In a longitudinal nested case-control study of 143 lung cancer cases and 457 age- and sex-matched controls, the same research group found that participants in the lowest quartile of F2RL3 (cg03636183) and AHRR (cg05575921) methylation had 10.5- and 15.9-fold higher odds of developing lung cancer, respectively, compared with participants in the highest quartile (adjusted for smoking and other confounders) (20). Likewise, a case–control study of 552 pairs found that hypomethylation of F2RL3 (cg03636183) and AHRR (cg05575921) in blood was associated with higher risk of lung cancer suggesting DNA methylation of these two CpG sites as a potential predictor of future lung cancer (33). F2RL3 plays an important role in blood coagulation, immune responses and signal regulation in endothelial cells (34). One can speculate that hypomethylation of F2RL3 results in higher F2RL3 expression, increased coagulation (35) and increased immune activity, that in turn may contribute to lung carcinogenesis. AHRR is expressed in a wide spectrum of tissues and acts as a negative feedback regulator at the aryl hydrocarbon receptor (AHR), which plays an important role in xenobiotic metabolism (e.g. PAH and dioxin) as well as in several biological functions including apoptosis, cell proliferation and differentiation (36,37). PAH act as agonists at the AHR in the cytosol resulting in upregulation of several genes, including PAH-metabolizing enzymes such as CYP1A1 and CYP1B1 that transform PAH to their carcinogenic derivatives (38,39). A study on biopsies from several human cancers demonstrated hypermethylation (excessive methylation) and low expression of AHRR suggesting a tumor suppressor role for AHRR (40). On the other hand, AHRR knockout mice exposed to BaP showed a delayed carcinogenesis in the skin contradicting the notion that AHRR has a tumor suppressor gene function (37). In light of this finding, hypomethylation of AHRR may result in AHHR upregulation, as seen in a study on human lung tissue (41), and thereby carcinogenesis. However, the regulatory role of AHRR and its methylation is more complex and pathways involved in AHR and AHRR signaling in relation to tumorigenesis are not fully understood and warrant further research (37).
In addition to predicting lung cancer, hypomethylation of F2RL3 (cg03636183) and AHRR (cg05575921) was associated with tobacco smoking as well as with exposure to welding fumes (19,26). Our data showed a profound effect of smoking on DNA methylation of cg03636183 and cg05575921 as well as other CpG sites in these genes and found a dose–response relationship between active smokers, former smokers and non-smokers. Consistent with previous studies, this indicates that the effect of smoking on DNA methylation of these genes can be reversible over time (42,43). Whether this is also the case for occupational exposure to PAH will need further research. However, it was apparent that active smoking had a more drastic effect on DNA methylation than occupational exposure to PAH, likely because the exposure is more complex and constant in active smokers than in chimney sweeps and creosote-exposed workers.
A dose–response relationship between PAH exposure groups and DNA methylation was found, despite the fact that chimney sweeps and creosote-exposed workers have different working routines and are exposed to different mixtures of PAH, as reflected in their PAH metabolite profiles. Most notably, the metabolite concentrations of the carcinogen BaP were low in creosote-exposed workers, but not in chimney sweeps. In addition to soot sweeping, chimney sweeps perform non-soot sweeping tasks (e.g. cleaning ventilation ducts and inspection of fire-safety systems). Therefore, intra- and inter-day exposures are highly variable. By contrast, the creosote-exposed workers have a uniform exposure profile since they carry out the same daily creosote-related work tasks at the same workplace (railway switch construction plant); hence they are constantly exposed to PAH and their PAH metabolites in urine better reflect their everyday exposure compared with chimney sweeps. Further, chimney sweeps are mainly exposed to particle-borne PAH in soot (not volatile PAH). The predominant PAH in soot produced from wood burning are pyrene, fluoranthene and phenanthrene, in addition to BaP (about 9–30% of the amount of pyrene) (44,45). By contrast, lower molecular weight PAH are predominating in the creosote oil, e.g. naphthalene and its methylated derivatives, acenaphthene, fluorene, phenanthrene, fluoranthene and pyrene, while BaP concentrations are rather low (when creosote production complies with the national and international regulations) (46,47). Chimney sweeps and creosote-exposed workers also have different exposure routes, which may explain the different sites of cancer linked to the occupations. Exposure to soot among chimney sweeps can occur through inhalation, skin contact and ingestion of soot particles (3). Epidemiological studies have shown increased incidence of lung and esophageal cancers, but not skin cancer (6,7). This may indicate that exposure through inhalation of particle-borne PAH is more pronounced than dermal exposure among today’s chimney sweeps. However, we cannot examine this hypothesis in our study, as we measured total PAH exposure, not route-specific exposure. In contrast to the sweeps, creosote-exposed workers are mainly exposed to lower molecular weight PAH through dermal contact with creosote oil or inhalation (8), which can be linked to the lip and skin cancer observed among these workers (48). Still, the evidence for creosote and cancer risk is so far ambiguous. No increase in cancer mortality was observed (49), but an increased risk for acute myeloid leukemia was recently reported in a population-based study (50), and previously a cohort study of exposed workers found an increased incidence of malignant lymphoma (48). Our findings of cancer-related DNA hypomethylation suggest that PAH-induced carcinogenicity is not solely attributable to one chemical (e.g. BaP), but rather to a mixture of PAH that are classified as possible and probable human carcinogens (e.g. BaA and naphthalene) as well as to other concurrent exposures such as particles, gases or other chemicals. This highlights the need for larger sample size and longitudinal design to further study cancer risk among creosote-exposed workers and chimney sweeps.
There were no associations found between occupational PAH exposure and TL or mtDNAcn in the present study. A few studies have found shorter TL among workers exposed to PAH, e.g. coke oven and coal tar pitch workers (51,52). A study among Chinese truck drivers exposed to particulate matter (2.5 and 10) from traffic air showed that longer TL was associated with short-term exposure to ambient particulate matter, and shorter TL with long-term exposure (average ambient particulate matter over 14 days prior sampling) (53), indicating a complex dynamics of TL in relation to exposure. Our results may, at least in part, be explained by the different levels of exposure (high PAH exposure among coke oven and coal tar pitch workers) as well as different exposure profile of PAH among truck drivers. In our study, cigarette smoking seemed to be associated with TL as smoking controls had shorter TL compared with non-smokers (P = 0.072). However, the effect of tobacco smoking on TL in the literature has been inconsistent (54).
The associations between PAH exposure and mtDNAcn have been inconsistent in the literature as well. Exposure to fluoranthene and pyrene in zebrafish and in vitro models was associated with higher mtDNAcn (55). This is consistent with another study that found increased mtDNAcn in relationship to PAH exposure among coke oven workers (17). In contrast, a study among male individuals environmentally exposed to PAH found decreased mtDNAcn in sperm in association with increased PAH exposure (56). The PAH-exposed groups in our study did not differ in their mtDNAcn from the controls, and no associations with PAH metabolites were observed. Similar to TL, smoking controls had decreased mtDNAcn compared with non-smokers.
A main strength of our study was that we included two PAH-exposed occupational groups exposed to different levels and mixtures of PAH, which enabled us to evaluate dose–response relationships between PAH exposure and DNA methylation. Another advantage was the use of gold-standard techniques for analyzing locus-specific DNA methylation (i.e. pyrosequencing) and for measuring PAH metabolites in urine (LC-MS/MS). On the other hand, some limitations should be considered. Our results might have been confounded by concurrent occupational exposures, which can potentially affect TL, mtDNAcn and DNA methylation. However, the effect of concurrent exposure is probably secondary as chimney sweeps and particularly creosote-exposed workers are known to be predominantly exposed to PAH (3,8). Another issue was the cross-sectional design of the study, which does not permit conclusions on causality between PAH exposure and cancer-related markers. Statistical analyses of DNA methylation should have preferably been adjusted for white blood cell count to account for heterogeneous methylation across different cell types. Also, the statistical power of creosote-exposed workers was low compared with chimney sweeps’ due to the small sample size. Yet, having a relatively large comparison control group has augmented the statistical power in the comparison analyses.
In conclusion, our study showed that chimney sweeps and creosote-exposed workers had lower DNA methylation of F2RL3 and AHRR, which is a risk factor for lung cancer. Exposure to PAH from soot and creosote oil is likely to play a role in this epigenetic modification. These findings stress the need to reduce current exposure to PAH at workplaces.
Supplementary material
Supplementary material can be found at Carcinogenesis online.
Funding
The study was financially supported by the Swedish Research Council for Health, Working Life and Welfare (FORTE) (grant no: 2012-00402), AFA Insurance (AFA Försäkring) (grant no: 120115), the Medical Training and Research Agreement (ALF grants; Region Örebro län) (grant no: OLL-550721), and Karolinska Institutet.
Conflict of Interest Statement
None declared.
Supplementary Material
Acknowledgements
The authors acknowledge the study participants as well as the nurses Pia Tallving, Patrice Milton, Eva Assarsson, Göte Mölleby and biomedical analyst Ina Lindell for recruiting the workers in the study. The authors also thank chimney sweeps’ trade union (Kommunal), and chimney sweeps’ employer organization (Sveriges Skorstensfejaremästares Riksförbund).
Abbreviations
- BaP
benzo[a]pyrene
- mtDNA
mitochondrial DNA
- PAH
polycyclic aromatic hydrocarbons
- TL
telomere length
References
- 1. ATSDR(1995)Toxicological Profile of Polycyclic Aromatic Hydrocarbons. Agency for Toxic Substances and Disease Registry. US Department of Health and Human Services, Atlanta, GA. [PubMed] [Google Scholar]
- 2. Kauppinen T., et al. (2000) Occupational exposure to carcinogens in the European Union. Occup. Environ. Med., 57, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. IARC(2012)Soot, as found in Occupational Exposure of Chimney Sweeps. Vol. 100F International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- 4. Alhamdow A., et al. (2017) Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci. Rep., 7, 9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. IARC(2017)Agents Classified by the IARC Monographs. Vol. 1–120 International Agency for Research on Cancer, Lyon France. [Google Scholar]
- 6. Evanoff B.A., et al. (1993) Mortality and incidence of cancer in a cohort of Swedish chimney sweeps: an extended follow up study. Br. J. Ind. Med., 50, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hogstedt C., et al. (2013) Cancer incidence in a cohort of Swedish chimney sweeps, 1958-2006. Am. J. Public Health, 103, 1708–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elovaara E., et al. (1995) Significance of dermal and respiratory uptake in creosote workers: exposure to polycyclic aromatic hydrocarbons and urinary excretion of 1-hydroxypyrene. Occup. Environ. Med., 52, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlsten C., et al. (2005) Squamous cell carcinoma of the skin and coal tar creosote exposure in a railroad worker. Environ. Health Perspect., 113, 96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Persson B., et al. (1989) Malignant lymphomas and occupational exposures. Br. J. Ind. Med., 46, 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackburn E.H. (1991) Structure and function of telomeres. Nature, 350, 569–573. [DOI] [PubMed] [Google Scholar]
- 12. Li H., et al. (2013) Telomere length and LINE1 methylation is associated with chromosomal aberrations in peripheral blood. Genes Chromosomes Cancer, 52, 1–10. [DOI] [PubMed] [Google Scholar]
- 13. Gisselsson D., et al. (2001) Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc. Natl Acad. Sci. USA, 98, 12683–12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anic G.M., et al. (2013) Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol., 37, 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanchez-Espiridion B., et al. (2014) Telomere length in peripheral blood leukocytes and lung cancer risk: a large case-control study in Caucasians. Cancer Res., 74, 2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H.C., et al. (2000) Mitochondrial role in life and death of the cell. J. Biomed. Sci., 7, 2–15. [DOI] [PubMed] [Google Scholar]
- 17. Pavanello S., et al. (2013) Mitochondrial DNA copy number and exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomarkers Prev., 22, 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao X., et al. (2015) DNA methylation changes of whole blood cells in response to active smoking exposure in adults: a systematic review of DNA methylation studies. Clin. Epigenet., 7, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fasanelli F., et al. (2015) Hypomethylation of smoking-related genes is associated with future lung cancer in four prospective cohorts. Nat. Commun., 6, 10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y., et al. (2016) Smoking-associated DNA methylation markers predict lung cancer incidence. Clin. Epigenet., 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y., et al. (2014) F2RL3 methylation in blood DNA is a strong predictor of mortality. Int. J. Epidemiol., 43, 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Smith A.J., et al. (2017) Correlates of prenatal and early-life tobacco smoke exposure and frequency of common gene deletions in childhood acute lymphoblastic leukemia. Cancer Res., 77, 1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzachi B.C., et al. (2000) Reference range and method comparison studies for enzymatic and Jaffé creatinine assays in plasma and serum and early morning urine. Clin. Lab., 46, 53–55. [PubMed] [Google Scholar]
- 24. Cawthon R.M. (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res., 30, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H., et al. (2012) Arsenic exposure through drinking water is associated with longer telomeres in peripheral blood. Chem. Res. Toxicol., 25, 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hossain M.B., et al. (2015) Exposure to welding fumes is associated with hypomethylation of the F2RL3 gene: a cardiovascular disease marker. Occup. Environ. Med., 72, 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu H.C., et al. (2014) Correlation of DNA methylation levels in blood and saliva DNA in young girls of the LEGACY Girls study. Epigenetics, 9, 929–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. López-Otín C., et al. (2013) The hallmarks of aging. Cell, 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goel N., et al. (2017) Role of DNA methylation in human age prediction. Mech. Ageing Dev., 166, 33–41. [DOI] [PubMed] [Google Scholar]
- 30. Jongeneelen F.J., et al. (1990) Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br. J. Ind. Med., 47, 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shenker N.S., et al. (2013) DNA methylation as a long-term biomarker of exposure to tobacco smoke. Epidemiology, 24, 712–716. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y., et al. (2015) F2RL3 methylation, lung cancer incidence and mortality. Int. J. Cancer, 137, 1739–1748. [DOI] [PubMed] [Google Scholar]
- 33. Baglietto L., et al. (2017) DNA methylation changes measured in pre-diagnostic peripheral blood samples are associated with smoking and lung cancer risk. Int. J. Cancer, 140, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gomides L.F., et al. (2012) Proteinase-activated receptor-4 plays a major role in the recruitment of neutrophils induced by trypsin or carrageenan during pleurisy in mice. Pharmacology, 89, 275–282. [DOI] [PubMed] [Google Scholar]
- 35. Ferrigno D., et al. (2001) Prognostic significance of blood coagulation tests in lung cancer. Eur. Respir. J., 17, 667–673. [DOI] [PubMed] [Google Scholar]
- 36. Köhle C., et al. (2007) Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem. Pharmacol., 73, 1853–1862. [DOI] [PubMed] [Google Scholar]
- 37. Vogel C.F.A., et al. (2017) The aryl hydrocarbon receptor repressor - more than a simple feedback inhibitor of AhR signaling: clues for its role in inflammation and cancer. Curr. Opin. Toxicol., 2, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hankinson O. (1995) The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol., 35, 307–340. [DOI] [PubMed] [Google Scholar]
- 39. Abel J., et al. (2010) An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem., 391, 1235–1248. [DOI] [PubMed] [Google Scholar]
- 40. Zudaire E., et al. (2008) The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J. Clin. Invest., 118, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shenker N.S., et al. (2013) Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum. Mol. Genet., 22, 843–851. [DOI] [PubMed] [Google Scholar]
- 42. Tsaprouni L.G., et al. (2014) Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics, 9, 1382–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guida F., et al. (2015) Dynamics of smoking-induced genome-wide methylation changes with time since smoking cessation. Hum. Mol. Genet., 24, 2349–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gauggel-Lewandowski S., et al. (2013) Bioavailability and potential carcinogenicity of polycyclic aromatic hydrocarbons from wood combustion particulate matter in vitro. Chem. Biol. Interact., 206, 411–422. [DOI] [PubMed] [Google Scholar]
- 45. Dilger M., et al. (2016) Toxicity of wood smoke particles in human A549 lung epithelial cells: the role of PAHs, soot and zinc. Arch. Toxicol., 90, 3029–3044. [DOI] [PubMed] [Google Scholar]
- 46. Heikkilä P. (2001)Respiratory and Dermal Exposure to Creosote. Doctoral Dissertation, Kuopio University Printing Office. Kuopio University, Kuopio, Finland. [Google Scholar]
- 47. EC(2001)Adapting to technical progress for the seventh time Annex I to Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (creosote). The European Communities Brussels, Belgium, vol. Commission Directive 2001/90/EC. [Google Scholar]
- 48. Karlehagen S., et al. (1992) Cancer incidence among creosote-exposed workers. Scand. J. Work. Environ. Health, 18, 26–29. [DOI] [PubMed] [Google Scholar]
- 49. Wong O., et al. (2005) Retrospective cohort mortality study and nested case-control study of workers exposed to creosote at 11 wood-treating plants in the United States. J. Occup. Environ. Med., 47, 683–697. [DOI] [PubMed] [Google Scholar]
- 50. Poynter J.N., et al. (2017) Chemical exposures and risk of acute myeloid leukemia and myelodysplastic syndromes in a population-based study. Int. J. Cancer, 140, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pavanello S., et al. (2010) Shorter telomere length in peripheral blood lymphocytes of workers exposed to polycyclic aromatic hydrocarbons. Carcinogenesis, 31, 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y., et al. (2015) [DNA methylation and telomere damage in occupational people exposed to coal tar pitch]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 33, 507–511. [PubMed] [Google Scholar]
- 53. Hou L., et al. (2012) Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ. Int., 48, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Astuti Y., et al. ; PILAR Research Network. (2017) Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ. Res., 158, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim H.Y., et al. (2014) Profiling of biomarkers for the exposure of polycyclic aromatic hydrocarbons: lamin-A/C isoform 3, poly[ADP-ribose] polymerase 1, and mitochondria copy number are identified as universal biomarkers. Biomed. Res. Int., 2014, 605135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ling X., et al. (2017) Polycyclic aromatic hydrocarbons exposure decreased sperm mitochondrial DNA copy number: a cross-sectional study (MARHCS) in Chongqing, China. Environ. Pollut., 220, 680–687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.