Abstract
Aims
The gut microbiome influences metabolic syndrome (MetS) and inflammation and is therapeutically modifiable. Arterial stiffness is poorly correlated with most traditional risk factors. Our aim was to examine whether gut microbial composition is associated with arterial stiffness.
Methods and results
We assessed the correlation between carotid-femoral pulse wave velocity (PWV), a measure of arterial stiffness, and gut microbiome composition in 617 middle-aged women from the TwinsUK cohort with concurrent serum metabolomics data. Pulse wave velocity was negatively correlated with gut microbiome alpha diversity (Shannon index, Beta(SE)= −0.25(0.07), P = 1 × 10−4) after adjustment for covariates. We identified seven operational taxonomic units associated with PWV after adjusting for covariates and multiple testing—two belonging to the Ruminococcaceae family. Associations between microbe abundances, microbe diversity, and PWV remained significant after adjustment for levels of gut-derived metabolites (indolepropionate, trimethylamine oxide, and phenylacetylglutamine). We linearly combined the PWV-associated gut microbiome-derived variables and found that microbiome factors explained 8.3% (95% confidence interval 4.3–12.4%) of the variance in PWV. A formal mediation analysis revealed that only a small proportion (5.51%) of the total effect of the gut microbiome on PWV was mediated by insulin resistance and visceral fat, c-reactive protein, and cardiovascular risk factors after adjusting for age, body mass index, and mean arterial pressure.
Conclusions
Gut microbiome diversity is inversely associated with arterial stiffness in women. The effect of gut microbiome composition on PWV is only minimally mediated by MetS. This first human observation linking the gut microbiome to arterial stiffness suggests that targeting the microbiome may be a way to treat arterial ageing.
Keywords: Gut microbiome diversity, Arterial stiffness, Microbial metabolites, Indolepropionate, Metabolic syndrome, Inflammation
Introduction
A substantial proportion of major adverse cardiovascular events (MACE) within the population are not explained by traditional cardiovascular risk factors. The gut microbiome has been implicated in a variety of potential disease mechanisms including oxidative stress and inflammation that could influence vascular disease.1,2 We therefore examined the relationship between the gut microbiome composition and arterial stiffness.
Arterial stiffness is an independent predictor of cardiovascular risk, especially in individuals with metabolic syndrome (MetS).3 Vascular stiffness is a consequence of pathophysiological alterations involving various functional elements of the vessel wall.4,5 It is a measure of vascular ageing predictive of MACE but (when adjusted for blood pressure) is weakly or unrelated to conventional risk factors.6–8
Several parameters are informative about arterial stiffness. Among these, pulse wave velocity (PWV) is currently considered the gold-standard measure of arterial stiffness and is predictive of future cardiovascular events.9 Both chronic hyperglycaemia and hyperinsulinaemia have been demonstrated to lead to hypertrophy of vascular smooth cells and fibrosis.10 In addition, levels of adipokines are significantly correlated with arterial stiffness.11 These associations suggest that factors contributing to insulin resistance and MetS may be involved in the development of arterial stiffness. Another important factor contributing to arterial stiffness is systemic inflammation.12 Evidence from this derives from the associations repeatedly reported between levels of c-reactive protein (CRP) and PWV in healthy individuals13,14 and from the higher levels of arterial stiffness seen in patients with primary inflammatory diseases after adjusting for cardiovascular risk factors.15,16
The gut microbiome, i.e. the community of microbes in the gastrointestinal tract,17,18 has recently emerged as an important regulator of systemic inflammation,19 glucose tolerance, and insulin sensitivity.13,14 We therefore hypothesized a relationship between gut microbiome composition and PWV. To test this, we first investigated the association between arterial stiffness, measured by PWV, and gut microbiome composition. We looked at the relationship between (i) arterial stiffness and loss of microbiome ‘diversity’ (loss in the number of species in the gut microbiome), a common finding in several disease states1,20 and (ii) between arterial stiffness and specific operational taxonomic units (OTUs). Secondly, we investigated whether any association between arterial stiffness and the microbiome might be accounted for by specific circulating metabolites known to be generated by the gut microbiome: phenylacetylglutamine and trimethylamine oxide (TMAO), previously linked to cardiovascular disease,2 and indoleproprionate (IPA),21 an antioxidant associated with MetS,22 and which might also influence arterial stiffness.
Methods
Study subjects were female twins enrolled in the TwinsUK registry, a national register of adult twins recruited as volunteers without selecting for any particular disease or traits.23 Here, we analysed data from 617 female twins with PWV, serum metabolites, and gut microbiome composition determined by 16S rRNA gene sequencing.24 The study was approved by NRES Committee London–Westminster, and all twins provided written informed consent to take part in the study.
Phenotype measurements
Brachial blood pressure was measured with the participants in a supine position according to British and Irish Hypertension Society Guidelines using a validated automated oscillometric device (Omron, 705 IT, Omron Health Care, Japan). Measurements were taken after at least 5 min of rest supine and an average of three measurements were used. Carotid-femoral PWV was calculated from sequential recordings of carotid and femoral artery pressure waveforms using the SphygmoCor system (AtCor medical, Australia). Difference in time of pulse arrival from the R-wave of the electrocardiogram between the two sites was taken as the transit time, and difference in path length was estimated using surface measurements as previously described.25 Measurements were made in triplicate, and mean values were used for analysis. The same system was used to obtain central and mean arterial blood pressure (MAP) by tonometric recording of radial artery pressure calibrated by brachial blood pressure. Estimates of visceral fat mass were derived from Dual-energy X-ray absorptiometry (DXA) measurements of whole body composition as previously described.26
Fasting insulin levels were measured for the twin cohort using the same methods as previously described.26 The homeostasis model assessment-estimated insulin resistance (HOMA-IR) was calculated multiplying overnight fasting plasma insulin (FPI) by overnight fasting plasma glucose (FPG), then dividing by the constant 22.5, i.e. HOMA-IR = (FPI × FPG)/22.515.
We calculated the 10-year atherosclerotic cardiovascular disease (ASCVD)27 to estimate the 10-year cardiovascular risk of an individual. The score is based on the individual age, sex, total and HDL cholesterol, systolic blood pressure, smoking status, use of blood pressure lowering medications, and the presence of type 2 diabetes28 and is calculated for women aged 40–79.
Environmental risk factors
Dietary intakes were estimated from a validated 131-item food frequency questionnaire (FFQ).29 Fibre and omega 3 intakes (grams per day) were derived from the UK Nutrient Database,30 which provided food content of non-starch polysaccharides (NSP) determined by the Englyst method.31 Alcohol intake was measured by questionnaire and coded based on the average units consumption per week (0 = never, 1 =1-5 units per week, 2 = 6–10 units per week, 3 = 11–15 units per week, 4 = 16–20 units per week, 5 = 21–40 units per week, 6= >41+ units per week). Adherence to a Mediterranean diet was calculated using the modified Mediterranean diet score (MDS) method, as outlined by Trichopoulou et al..32 Physical activity was measured by questionnaire asking their level of activity in a Likert scale (none, light, moderate, intense). In order to account for socioeconomic status, education, and access to health care an individual’s Index of Multiple Deprivation (IMD) score (2015) from the UK Office of National Statistics was used. This is based on the participants’ post code of residence (<seurld>https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015</seurld>) (details in the Supplementary material online).
Indoleproprionate, phenylacetylglutamine, and trimethylamine oxide measurement
Circulating serum levels of IPA, phenylacetylglutamine and TMAO were measured using ultra-high performance liquid chromatography-tandem mass spectrometry by the metabolomics provider Metabolon, Inc. (Research Triangle Park, USA) on fasting serum samples as described previously.33 We inverse normalized the metabolite data as it was not normally distributed. We imputed the missing values using the minimum run day measures.
C-reactive protein measurement
C-reactive protein was measured by a highly sensitive automated microparticle capture enzyme immunoassay, standardized on the World Health Organization International Reference Standard for CRP immunoassay 85/506,34 as previously reported.35
Microbiota analysis
A diagram summarizing the pipeline used in the analysis of stool samples for microbiome composition presented in Supplementary material online, Figure S1. A diagram of metabolomic profiling is presented in Supplementary material online, Figure S2.
The composition of the gut microbiome in faecal samples was determined by 16S rRNA gene sequencing carried out as previously described.24 Briefly, the V4 region of the 16S rRNA gene was amplified and sequenced on Illumina MiSeq. Reads were then summarized to operational taxonomic units (OTUs). Quality control was carried out on a per sample basis, discarding paired-ends with an overlap of less than 200 nt and removing chimeric sequences using de novo chimaera detection in USEARCH.36De novo OTU clustering was then carried across all reads using Sumaclust within Quantitative Insights Into Microbial Ecology (QIIME) 1.9.0, grouping reads with a 97% identity threshold.37,38 Quantitative Insights Into Microbial Ecology is a bioinformatic pipeline designated for the task of analysing microbial communities that were sampled through marker gene (e.g. 16S or 18S rRNA genes) amplicon sequencing. Sumaclust is a programme that clusters sequences and detects the ‘erroneous’ sequences created during amplification and sequencing protocols, deriving from ‘true’ sequences. OTU counts were converted to log transformed relative abundances, with zero counts handled by the addition of an arbitrary value (10−6). The residuals of the OTU abundances were taken from linear models, accounting for technical covariates including sequencing depth, sequencing run, sequencing technician, and sample collection method. These residuals were inverse normalized, as they were not normally distributed, and used in downstream analyses. In order to calculate alpha diversity, the complete OTU count table was rarefied to 10 000 sequences per sample 50 times. Alpha diversity metrics were calculated for each sample in each of the rarefied tables and final diversity measures taken as the mean score across all 50. Alpha diversities were quantified as observed OTU counts and Shannon and Simpson diversity indices (see the Supplementary material online for details). Alpha diversity indexes were standardized to have mean 0 and SD 1.
Statistical analysis
Statistical analysis was carried out using Stata version 11. Random intercept logistic regressions were undertaken to evaluate the association between PWV and gut microbial diversity (Shannon and Simpson indexes and number of observed OTUs) adjusting for age, body mass index (BMI), mean arterial pressure (MAP, the component of blood pressure that is thought to influence PWV, rather than systolic blood pressure, which is in large part determined by PWV), and family relatedness.
Linear regression was also employed to investigate the association between PWV and OTUs adjusting for covariates, family relatedness, and multiple testing using false discovery rate (FDR < 0.1).
We accounted for familial relatedness using random intercept linear regression:
| (1) |
where Yi and Xij are respectively PWV (Y) and the microbiome abundance of twin j from pair i. ζj is the family-specific error component, which represents the omitted family characteristics or unobserved heterogeneity. The comparison between PWV and variables is performed between each twin pair. We reran the analyses additionally adjusting for (i) diet (omega 3 and fibre intake, adherence to a Mediterranean diet), (ii) environmental factors including smoking, alcohol drinking habits, physical activity, socioeconomic status, PPI, and antibiotics use, that have been associated with microbiota changes, (iii) insulin resistance (assessed by HOMA-IR), and visceral fat mass that are considered important factors affecting arterial stiffness and were associated with PWV in our data, (iv) the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score27 to account for traditional CVD risk factors and serum levels of CRP to account for systemic inflammation, (v) uric acid, previously found to correlate with vascular stiffness.39 As faecal samples were taken 1.9 (SD = 1.5) years apart from the PWV measurement, we ensured that the associations between faecal microbiome composition and PWV were not influenced by the number of years between date of PWV measure and microbiome measure. We therefore (i) reran the analyses for the significant associations including samples taken more than 1 year apart and found no difference in the regression coefficients; (ii) further adjusted for the time elapsed between PWV measure and faecal sample collection and results were consistent.
We investigated for the association of three specific gut microbiome-derived metabolites: IPA, involved in insulin resistance, TMAO and phenylacetylglutamine and also adjusted for their levels.
Using standard multiple linear regressions, we computed the proportion of the variance (R2) and the corresponding 95% confidence intervals (95% CI)40 in PWV not explained by age, BMI, and MAP that was explained by microbiome diversity, microbiome OTUs, and microbiome-derived metabolites. We further employed partial least squares structural equation modelling (PLS-SEM)41 to test the mediation effects of ASCVD, HOMA + VFmass and CRP (indirect effect) on the total effect of microbiome factors on PWV which was adjusted for age, BMI, and MAP (Supplementary material online, Figure S3). We constructed a mediation model to quantify both the direct effect of microbiome factors on PWV and the indirect (mediated) effects mentioned above. The model goodness of fit was assessed by standardized path coefficient and effect size (f2)42–44 yield the lower and upper bound of the 95% CI. The variance accounted for (VAF) score, which represents the ratio of indirect-to-total effect and determines the proportion of the variance explained by the mediation process, was further used to determine the significance of mediation effect.41 A separate model was built to assess the association between Shannon index and PWV. All PLS-SEM analysis was conducted using the Smart-PLS 3 software. We also used IBM SPSS AMOS software to conduct covariance-based (CB) SEM analysis. See Supplementary material online for more details.
Results
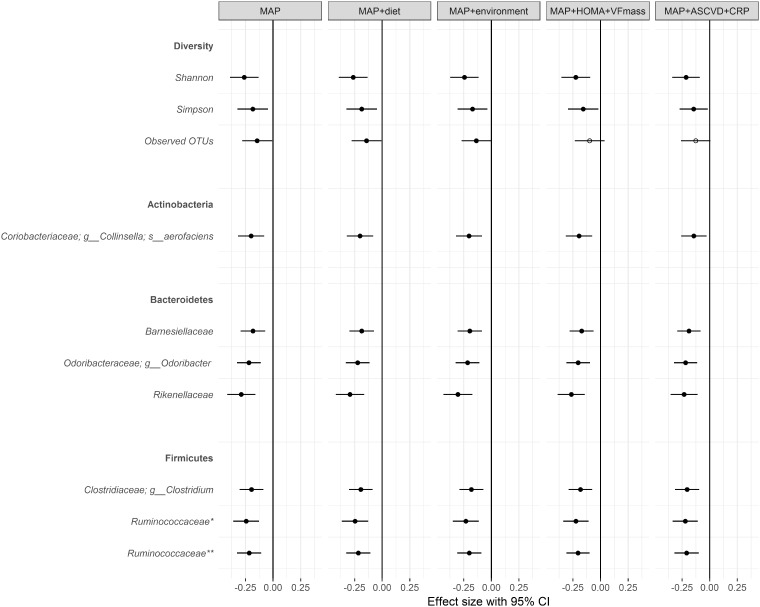
The characteristics of the study participants are presented in Table 1. 617 females with both PWV and microbiome data were included in the analysis. Both Shannon and Simpson indices of gut microbiome diversity were significantly associated with PWV after adjusting for age, BMI, MAP, and family relatedness (Figure 1). Examining the association between PWV and bacterial lineages or OTUs identified 7 OTUs that were significantly and negatively associated with PWV after adjusting for covariates and multiple testing, FDR < 0.05 (Figure 1 and Supplementary material online, Table S1). Two OTUs from the Ruminococcaceae family, one from the Rikenellaceae and one from the Clostridiaceae, a member of the Actinobateria Collinsella aerofaciens,45 a member of the Barnesiellaceae family, a member of the Clostridiaceae family, and the genus Odoribacter were negatively correlated with PWV.
Table 1.
Descriptive characteristics of the study population
| Phenotype | ||
|---|---|---|
| N | % | |
| N | 617 | |
| Females (%) | 617 | 100 |
| Physical activity | ||
| Low, n (%) | 89 | 14.47 |
| Medium or high, n (%) | 528 | 85.53 |
| T2D | ||
| Yes, n (%) | 25 | 4 |
| No, n (%) | 592 | 96 |
| Use of antibiotics | ||
| Yes, n (%) | 10 | 1.57 |
| No, n (%) | 207 | 98.43 |
| Use of PPIs | ||
| Yes, n (%) | 91 | 14.78 |
| No, n (%) | 526 | 85.22 |
| Mean | SD | |
| Age (years) | 61.42 | 7.34 |
| 10-years ASCVD risk score | 7.37 | 6.63 |
| BMI (kg/m2) | 26.33 | 4.67 |
| CRP (mmol/L) | 2.71 | 6.45 |
| DBP (mmHg) | 78.02 | 8.72 |
| Fibre intake (g/day) | 20.85 | 6.09 |
| HOMA-IR | 0.96 | 0.68 |
| IMD | 6.96 | 2.38 |
| MAP (mmHg) | 94.62 | 10.02 |
| MDS | 4.58 | 1.70 |
| Omega 3 intake (g) | 1.62 | 0.62 |
| PWV (m/s) | 9.39 | 1.86 |
| SBP (mmHg) | 127.79 | 15.77 |
| VFAT mass (g) | 585.79 | 275.66 |
| Indices of microbiome diversity | ||
| Number of observed OTUs | 309.27 | 87.83 |
| Shannon diversity | 5.19 | 0.71 |
| Simpson diversity | 0.93 | 0.066 |
DBP, diastolic blood pressure; PPI, proton pump inhibitors; SBP, systolic blood pressure; T2D, type 2 diabetes; VFAT, visceral fat.
Figure 1.
Microbes associated between pulse wave velocity and gut bacterial operational taxonomic units (false discovery rate < 0.1) adjusting for (i) age, body mass index, mean arterial blood pressure and family relatedness, (ii) age, body mass index, mean arterial blood pressure, fibre intake, omega 3 intake, adherence to a Mediterranean diet, and family relatedness, (iii) age, body mass index, mean arterial blood pressure, smoking, alcohol drinking habits, physical activity, PPI, antibiotics use, social deprivation status, and family relatedness, (iv) age, body mass index, mean arterial blood pressure, homeostasis model assessment-estimated insulin resistance, visceral fat mass, and family relatedness, (v) age, body mass index, mean arterial blood pressure, 10-years atherosclerotic cardiovascular disease risk score, c-reactive protein, and family relatedness.
Smoking/alcohol drinking habits, physical activity, fibre and omega 3 intake, adherence to a Mediterranean diet, socioeconomic status, PPI, and antibiotics use, have been associated with either microbiota changes46–49 or with arterial stiffness.50–53 Therefore, we additionally adjusted for these risk factors as potential confounders and found that the results remain consistent. Because levels of uric acid have been associated with arterial stiffness39 we also adjusted for this factor (Figure 1 and Supplementary material online, Table S1). In addition to lifestyle factors, we also adjusted for traditional cardiovascular risk factors and for systemic inflammation (Figure 1 and Supplementary material online, Table S1). To do this, we used the 10-year ASCVD risk score computed for each individual and CRP levels. The results remain unchanged by these adjustments (Figure 1).
Because both higher gut microbiome diversity and the relative abundance of microbes are associated with lower visceral fat mass54,55 and insulin resistance,56 we assessed whether these associations were simply caused by higher visceral fat or higher insulin resistance. Pulse wave velocity remained significantly associated with both microbiome diversity and the identified microbial lineages (Figure 1) after adjusting for these measures. Thus, in our cohort, PWV is significantly associated with gut microbiome composition after adjusting for the known likely covariates (insulin resistance, visceral fat) with each standard deviation of the microbial abundances contributing an effect size of -0.12 to -0.27 on PWV and achieving p-values ranging from 0.002 to 3 × 10−5 (Supplementary material online, Table S1).
We also explored whether specific compounds known to be generated by the gut microbiome that could be implicated in arterial ageing, like phenylacetylglutamine,57 TMAO,2 and the antioxidant IPA21 were related to arterial stiffness. The associations between PWV and gut microbiome diversity and specific OTUs remained significant after adjustment for these three compounds, although some of the associations were slightly attenuated suggesting that in part some of the effects of the gut microbiome on arterial stiffness are mediated by levels of phenylacetylglutamine and IPA.
Next, we quantified how much of arterial stiffness could be explained by gut microbiome composition, and two of the three compounds generated by the gut microbiome linked to this cardiovascular trait. We linearly combined the PWV-associated gut microbiome-derived variables—diversity measures (Shannon, Simpson, and number of OTUs), bacterial OTUs, circulating levels of the microbiome-derived metabolites phenylacetylglutamine, and IPA. After adjusting for age, BMI, and mean arterial pressure the overall proportion of variance explained by microbiome factors is 8.3% (95% CI 4.32–12.4%).
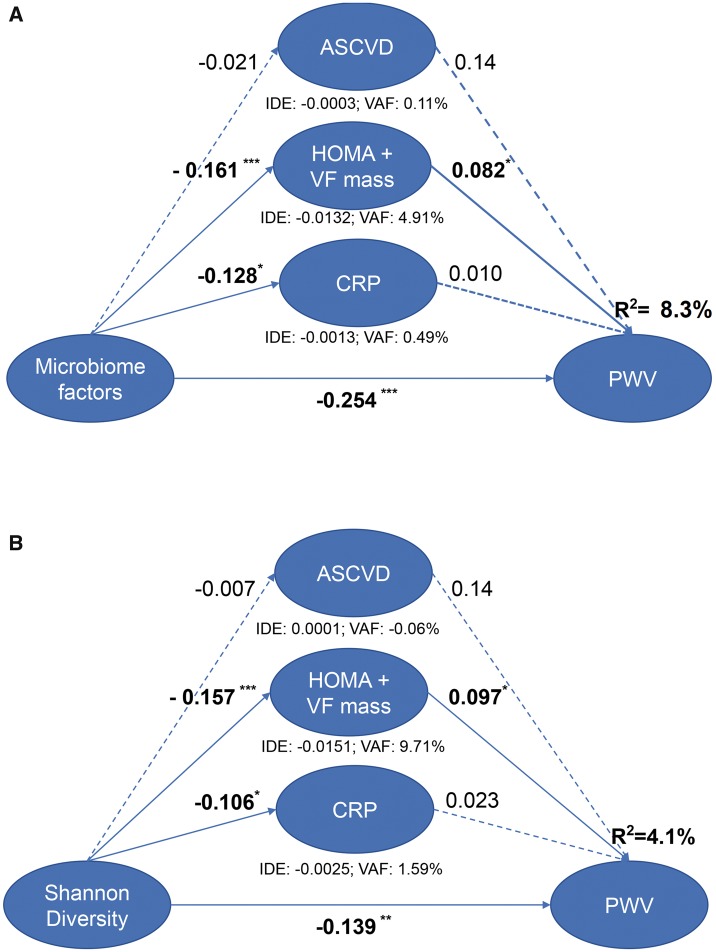
We finally conducted PLS-SEM analysis to determine the indirect effect of ASCVD, HOMA + VFmass, and CRP on the effect between microbiome factors and PWV. The mediation model (Figure 2A), which intends to evaluate the strength of the indirect effects, found that the direct relationship between microbiome factors and PWV was statistically significant (path coefficient = −0.254, P < 0.001) and the overall R2 was 8.4%. For indirect effect, only the effect of HOMA + VFmass was statistically significant. The VAF score for HOMA + VFmass was 4.91% while the combined VAF score of ASCVD, HOMA + VFmass, and CRP was 5.51%. The model to assess the relationship between Shannon diversity and PWV also found a significant direct effect (R2 4.1%; path coefficient −0.139; P < 0.001). The composite indirect effect was 11.24% (Figure 2B). We also conducted CB-SEM analysis and found similar results (see Supplementary material online, Figure S4). Thus, both PLS-SEM and CB-SEM analyses found that the indirect effect of the gut microbiome mediated by CRP, HOMA + VFmass, and ASCVD risk to be statistically significant but of a small magnitude, with the majority of the effect of gut microbiome composition on PWV not being mediated by these factors.
Figure 2.
Mediation analysis of the association between (A) microbiome factors and (B) Shannon diversity and pulse wave velocity using partial least squares structural equation modelling. Path coefficients are denoted beside each path and indirect effect and variance accounted for (variance accounted for) score is denoted below each mediator (*P < 0.05; **P < 0.01; ***P < 0.001).
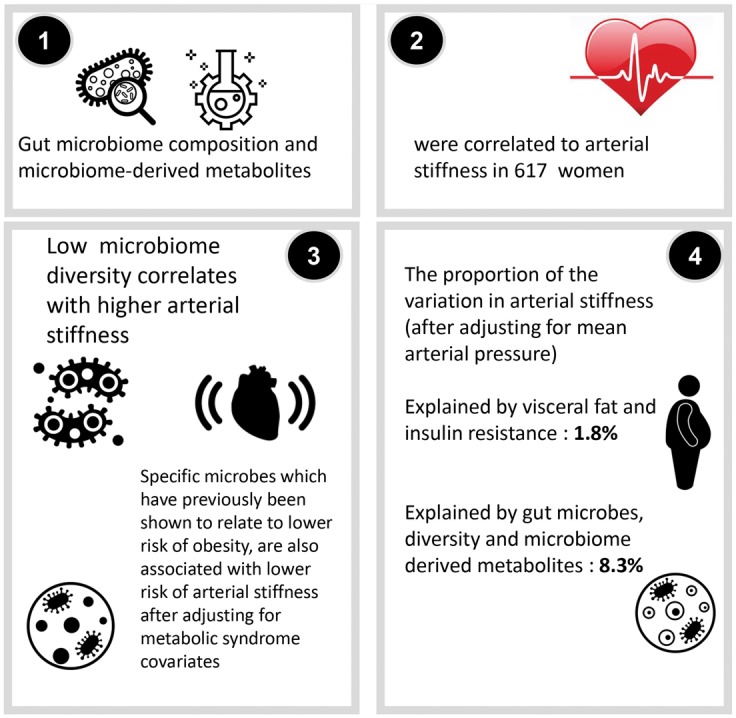
Take home figure.

The gut microbiome is related to metabolic syndrome and inflammation, is modifiable via diet, medication and probiotics. Arterial stiffness (measured by pulse wave velocity) is a predictor of major cardiovascular events, which is related to metabolic syndrome and inflammation but poorly correlated with most traditional risk factors other than mean arterial pressure. The hypothesis of this study was that the gut microbiome composition could be related to arterial stiffness. This was measured in 617 women and both specific microbes and gut microbiome diversity, a measure of gut dysbiosis, along with metabolites generated by the gut microbiome were found to be associated with arterial stiffness. In fact, the microbiome related factors explain 8.3% of the variance in pulse wave velocity compared with only 1.8% of insulin resistance combined with visceral fat. These data indicate a strong contribution of the gut microbiome to risk of arterial stiffness and suggest targeting the gut microbiome composition as a therapeutic strategy.
Discussion
The major novel finding of this study is that arterial stiffness as measured by carotid-femoral PWV is inversely correlated with gut microbiome diversity and with the abundance of specific microbes in the gut. These associations appear to be only in a small proportion mediated by an effect on MetS-related traits such as insulin resistance or visceral fat, but appear to be stronger than the associations of MetS and CRP in with PWV.
Specifically, we find that arterial stiffness correlates negatively with the abundance of Ruminococcaceae family bacteria. These are butyrate-producing bacteria whose abundance has been shown in mice to be linked to lower endotoxemia.58 Experimentally induced acute endotoxaemia is known to increase inflammatory cytokines and to cause endothelial dysfunction in humans, and chronic endotoxaemia is associated with MetS.59 It is well known that obesity, higher visceral fat, and insulin resistance all correlate with lower microbiome diversity.56 These factors contribute to arterial stiffness, but microbiome diversity remains negatively correlated with PWV after adjustment for all these factors. Similarly, microbiome-derived metabolites, such as phenylacetyl glutamine, are associated with lower PWV, but the association between OTUs and diversity with PWV remains significant even after adjusting for these microbiome-derive metabolites. We also find that all the microbiome factors that we identified, namely diversity, seven OTUs, and two microbiome-derived metabolites, make a contribution to explaining variation in PWV (Figure 2) and only a small proportion of that effect can be explained (after adjusting for MAP, age, and BMI) by traditional risk factors, MetS and CRP levels. However, it is known that inflammatory markers are very strongly associated with arterial stiffness60 and, although we find that only a small proportion of the effect of microbiome composition on PWV, CRP is not the only known biomarker for systemic inflammation predictive of CVD. Other markers, such as interleukin (IL)-661 are associated with increased risk in addition to the inflammation measured by CRP. Moreover, a meta-analysis of up to 29 population-based prospective studies, IL-6, IL-18, and TNF-α were all found to result in significantly higher relative risks for non-fatal myocardial infarction or CHD death after adjusting for traditional risk factors.62
The causal role of inflammation in the pathogenesis of cardiovascular disease63 has been clearly demonstrated recently by the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study).64 This trial has demonstrated beyond doubt that inflammation plays a role in the development of atherothrombosis by providing robust evidence that inhibiting IL-1β reduces the incidence of repetitive atherothrombotic events.
The fact that the gut microbiome composition plays a key role in inflammatory and autoimmune disease is now well characterized.65 A common theme in inflammatory diseases, ranging from rheumatoid arthritis, psoriasis, inflammatory bowel disease, and multiple sclerosis, is a reduced microbiome diversity.65 Although, clearly, functional characterization of the role of the various microbial lineages and microbial-derived compounds on arterial stiffness is needed, we hypothesize that the effect of gut microbiome composition on PWV is likely to be due caused by its role in modulating systemic inflammation and that only a part of this effect is captured by measuring CRP, insulin resistance, and traditional risk factors part of the 10-years ASCVD risk score.
This is relevant because the gut microbiome’s diversity and composition is modifiable. Gut microbial richness and composition are, to a large extent, modulated by diet.66 There is increasing evidence that insufficient consumption of dietary fibre leads to a loss of bacterial species in the human gut.67 Probiotics (live micro-organisms that influence the gut microbiome, mostly Bifidobacterium and Lactobacillus species) are another method to target the gut microbiome. A recent systematic review has shown that probiotic supplementation has a beneficial effect on blood pressure.68
We note several strengths and limitations to the current study. The study was based on middle-aged white female twins and hence may not be generalizable to other ethnic groups or to men. Although the characteristics of these women are representative of the general UK female population,23 clearly studies in men and in other ethnic groups are needed.
The faecal samples collected were not necessarily taken at the time of the PWV assessment. However, after adjustment for the time elapsed between the two measures we find no difference in the observed associations. For this reason, we suggest that our data are most likely valid, which would be consistent with data showing that the taxonomic composition and diversity of the gut microbiome remain constant over time69 in the absence of gross perturbation.70 It is postulated in the literature that long-term stability of the human indigenous microbial communities is maintained not by inertia but by the action of restorative forces within a dynamic system.70 Hence, the reported associations between gut microbiome composition and arterial stiffness unlikely to change majorly over time in a given individual.
Another limitation is the cross-sectional nature of the data. While there is biological plausibility via endotoxaemia for the association between arterial stiffness and microbiome composition being causal, this cannot be concluded from a cross-sectional study. On the other hand, we note several strengths, including the sample size of the study and the detailed clinical and molecular phenotyping of the study subjects, which has allowed us to test the relative contributions of different factors to arterial stiffness.
Here, we show that cardiovascular risk that is not explained by classical risk factors is likely to be in part captured by characterization of the microbiome and may in the future help stratify CVD risk, particularly in younger individuals and in women. Although it is unlikely to contribute to cardiovascular prevention guidelines at present, the findings presented here fit within the context of current European guidelines71 as follows: (i) The key outcome analysed was PWV. The Sixth Joint Task Force of the European Society of Cardiology concluded that PWV may serve as a useful biomarker to improve CVD risk prediction for patients close to decisional thresholds, although its systematic use in the general population to improve risk assessment is not recommended. (ii) One of the gaps in evidence identified by the European Task Force is that women continue to be under-represented in clinical trials. This study focuses specifically on cardiovascular risk in women. (iii) Gut microbiome composition is a modifiable factor influenced by dietary fibre intake. Fibre intake is part of the current recommendations for a healthy diet in the 2016 Task Force recommendation. In fact, the gut microbiome composition may contribute to the mechanism whereby dietary fibre intake influences cardiovascular risk, which is yet to be fully elucidated.
In conclusion, in this study we show for the first time that the composition of the gut microbiome is strongly correlated with levels of arterial stiffness in women independently of visceral fat and other obesity-related traits. Given the possibility of modifying the gut microbiome composition via diet and probiotic supplementation, this opens therapeutic avenues for reducing arterial stiffness targeting the gut microbiome.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank Dr Julia K. Goodrich, Dr Ruth E. Ley, and the Cornell technical team for generating the microbial data. We wish to express our appreciation to all study participants of the TwinsUK cohort.
Funding
The BHF special project grant SP/12/4/29573, the MRC AIM HY (MR/M016560/1) stratified medicines grant and by the FP7 project HEALS (Health and Environment-wide Associations based on Large population Surveys) Project No 603946 of the European Union’s Seventh Framework Programme; the TwinsUK microbiota project was funded by the National Institute of Health (NIH) RO1 DK093595, DP2 OD007444. Twins UK receives funding from the Wellcome Trust European Community’s Seventh Framework Programme (FP7/2007-2013 to TwinsUK); the National Institute for Health Research (NIHR) Clinical Research Facility at Guy’s & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. HLI collaborated with KCL to produce the metabolomics data from Metabolon Inc. T.D.S. is an NIHR Senior Investigator. A.M.V. is funded by the NIHR Nottingham Biomedical Research Centre. This work was also funded by the Chronic Disease Research and the Denise Coates Foundation.
Conflict of interest: R.P.M. is an employee of Metabolon, Inc. T.D.S. is co-founder of MapMygut Ltd and a consultant for Zoe Global Ltd. AMV is a consultant for Zoe Global Ltd. All other authors declare no competing financial interests.
Footnotes
See page 2398 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy300)
References
- 1. Weiss GA, Hennet T.. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci 2017;74:2959–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li DY, Tang WHW.. Gut microbiota and atherosclerosis. Curr Atheroscler Rep 2017;19:39.. [DOI] [PubMed] [Google Scholar]
- 3. Jia G, Aroor AR, Sowers JR.. Arterial stiffness: a nexus between cardiac and renal disease. Cardiorenal Med 2014;4:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jia G, Aroor AR, DeMarco VG, Martinez-Lemus LA, Meininger GA, Sowers JR.. Vascular stiffness in insulin resistance and obesity. Front Physiol 2015;6:231.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villacorta L, Chang L.. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig 2015;21:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cecelja M, Chowienczyk P.. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis 2012;1:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakagawa K, Morishima N, Shibata T.. A maturase-like subunit of the sequence-specific endonuclease endo.SceI from yeast mitochondria. J Biol Chem 1991;266:1977–1984. [PubMed] [Google Scholar]
- 8. McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, Gallacher J, Ben-Shlomo Y, Cockcroft JR, Wilkinson IB.. An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension 2010;56:36–43. [DOI] [PubMed] [Google Scholar]
- 9. Townsend RR. Arterial stiffness: recommendations and standardization. Pulse (Basel) 2016;4:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA.. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation 2001;103:1238–1244. [DOI] [PubMed] [Google Scholar]
- 11. Omelchenko E, Gavish D, Shargorodsky M.. Adiponectin is better predictor of subclinical atherosclerosis than liver function tests in patients with nonalcoholic fatty liver disease. J Am Soc Hypertens 2014;8:376–380. [DOI] [PubMed] [Google Scholar]
- 12. Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA.. Inflammation and arterial stiffness in humans. Atherosclerosis 2014;237:381–390. [DOI] [PubMed] [Google Scholar]
- 13. Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB.. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol 2004;24:969–974. [DOI] [PubMed] [Google Scholar]
- 14. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, Schalekamp MA, Asmar R, Hofman A, Witteman JC.. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis 2004;176:111–116. [DOI] [PubMed] [Google Scholar]
- 15. Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J, Wilkinson IB.. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006;114:1185–1192. [DOI] [PubMed] [Google Scholar]
- 16. Shang Q, Tam LS, Li EK, Yip GW, Yu CM.. Increased arterial stiffness correlated with disease activity in systemic lupus erythematosus. Lupus 2008;17:1096–1102. [DOI] [PubMed] [Google Scholar]
- 17. Everard A, Cani PD.. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013;27:73–83. [DOI] [PubMed] [Google Scholar]
- 18. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Dore J, Mattila I, Plichta DR, Poho P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jorgensen T, Holm JB, Trost K, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O.. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016;535:376–381. [DOI] [PubMed] [Google Scholar]
- 19. Grigg JB, Sonnenberg GF.. Host-microbiota interactions shape local and systemic inflammatory diseases. J Immunol 2017;198:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heiman ML, Greenway FL.. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab 2016;5:317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G.. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009;106:3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Mello VD, Paananen J, Lindstrom J, Lankinen MA, Shi L, Kuusisto J, Pihlajamaki J, Auriola S, Lehtonen M, Rolandsson O, Bergdahl IA, Nordin E, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Landberg R, Eriksson JG, Tuomilehto J, Hanhineva K, Uusitupa M.. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 2017;7:46337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moayyeri A, Hammond CJ, Valdes AM, Spector TD.. Cohort profile: twins UK and healthy ageing twin study. Int J Epidemiol 2013;42:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE.. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 2016;19:731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cecelja M, Jiang B, McNeill K, Kato B, Ritter J, Spector T, Chowienczyk P.. Increased wave reflection rather than central arterial stiffness is the main determinant of raised pulse pressure in women and relates to mismatch in arterial dimensions: a twin study. J Am Coll Cardiol 2009;54:695–703. [DOI] [PubMed] [Google Scholar]
- 26. Menni C, Migaud M, Glastonbury CA, Beaumont M, Nikolau A, Small K, Brosnan MJ, Mohney R, Spector TD, Valdes AM.. Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obesity (Silver Spring) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF.. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force. On practice guidelines . Circulation 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 28. Redon J. Global cardiovascular risk assessment: strengths and limitations. High Blood Press Cardiovasc Prev 2016;23:87–90. [DOI] [PubMed] [Google Scholar]
- 29. Bingham SA, Welch AA, McTaggart A, Mulligan AA, Runswick SA, Luben R, Oakes S, Khaw KT, Wareham N, Day NE.. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr 2001;4:847–858. [DOI] [PubMed] [Google Scholar]
- 30. McCance RA, Widdowson EM, Holland B, Welch A, Buss DH.. McCance and Widdowson’s the Composition of Foods. 5th edition, Cambridge: The Royal Society of Chemistry; 1991. [Google Scholar]
- 31. Englyst HN, Cummings JH.. Improved method for measurement of dietary fiber as non-starch polysaccharides in plant foods. J Assoc Off Anal Chem 1988;71:808–814. [PubMed] [Google Scholar]
- 32. Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocke MC, Peeters PH, van der Schouw YT, Boeing H, Hoffmann K, Boffetta P, Nagel G, Masala G, Krogh V, Panico S, Tumino R, Vineis P, Bamia C, Naska A, Benetou V, Ferrari P, Slimani N, Pera G, Martinez-Garcia C, Navarro C, Rodriguez-Barranco M, Dorronsoro M, Spencer EA, Key TJ, Bingham S, Khaw KT, Kesse E, Clavel-Chapelon F, Boutron-Ruault MC, Berglund G, Wirfalt E, Hallmans G, Johansson I, Tjonneland A, Olsen A, Overvad K, Hundborg HH, Riboli E, Trichopoulos D.. Modified Mediterranean diet and survival: ePIC-elderly prospective cohort study. BMJ 2005;330:991.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, Brewerton S, Turpaz Y, Perkins BA, Evans AM, Miller LA, Guo L, Caskey CT, Schork NJ, Garner C, Spector TD, Venter JC, Telenti A.. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet 2017;49:568–578. [DOI] [PubMed] [Google Scholar]
- 34. WHO Expert Committee on Biological Standardization. WHO Technical Report Series 760. Geneva, Switzerland: World Health Organization; 1987. p. 21–22. [PubMed] [Google Scholar]
- 35. Wilkins J, Gallimore JR, Moore EG, Pepys MB.. Rapid automated high sensitivity enzyme immunoassay of C-reactive protein. Clin Chem 1998;44(6 Pt 1):1358–1361. [PubMed] [Google Scholar]
- 36. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R.. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011;27:2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jackson MA, Bell JT, Spector T, Steves C.. A heritability-based comparison of methods used to cluster 16S rRNA gene sequences into operational taxonomic units. Peer J Preprints 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R.. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi HY, Kim SH, Choi AR, Kim SG, Kim H, Lee JE, Kim HJ, Park HC.. Hyperuricemia and risk of increased arterial stiffness in healthy women based on health screening in Korean population. PLoS One 2017;12:e0180406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen J, Cohen P, West SG, Aiken LS, Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Mahwah, NJ: Lawrence Earlbaum Associates; 2003. [Google Scholar]
- 41. Nitzl C, Roldan JL, Cepeda G.. Mediation analysis in partial least squares path modeling helping researchers discuss more sophisticated models. Ind Manag Data Syst 2016;116:1849–1864. [Google Scholar]
- 42. Hair JF, Sarstedt M, Ringle CM, Mena JA.. An assessment of the use of partial least squares structural equation modeling in marketing research. J Acad Mark Sci 2012;40:414–433. [Google Scholar]
- 43. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 44. Pavlou PA, Fygenson M.. Understanding and predicting electronic commerce adoption: an extension of the theory of planned behavior. Mis Q 2006;30:115–143. [Google Scholar]
- 45. Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A.. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007;133:24–33. [DOI] [PubMed] [Google Scholar]
- 46. Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, Steves CJ, Spector TD, Valdes AM.. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep 2017;7:11079.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Modi SR, Collins JJ, Relman DA.. Antibiotics and the gut microbiota. J Clin Invest 2014;124:4212–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ.. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Menni C, Jackson MA, Pallister T, Steves C, Spector T, Valdes A.. Gut microbiome diversity and high fibre intake are related to lower long term weight gain. Int J Obes (Lond) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tanaka H, Tomoto T, Kosaki K, Sugawara J.. Arterial stiffness of lifelong Japanese female pearl divers. Am J Physiol Regul Integr Comp Physiol 2016;310:R975–R978. [DOI] [PubMed] [Google Scholar]
- 51. Uemura H, Katsuura-Kamano S, Yamaguchi M, Arisawa K.. Relationships of elevated levels of serum hepatic enzymes and alcohol intake with arterial stiffness in men. Atherosclerosis 2015;238:83–88. [DOI] [PubMed] [Google Scholar]
- 52. Trudel X, Shipley MJ, McEniery CM, Wilkinson IB, Brunner EJ.. Socioeconomic status, education, and aortic stiffness progression over 5 years: the Whitehall II prospective cohort study. J Hypertens 2016;34:2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van de Laar RJ, Stehouwer CD, van Bussel BC, Prins MH, Twisk JW, Ferreira I.. Adherence to a Mediterranean dietary pattern in early life is associated with lower arterial stiffness in adulthood: the Amsterdam Growth and Health Longitudinal Study. J Intern Med 2013;273:79–93. [DOI] [PubMed] [Google Scholar]
- 54. Pallister T, Jackson MA, Martin TC, Glastonbury CA, Jennings A, Beaumont M, Mohney RP, Small KS, MacGregor A, Steves CJ, Cassidy A, Spector TD, Menni C, Valdes AM.. Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling. Int J Obes (Lond) 2017;41:1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beaumont M, Goodrich JK, Jackson MA, Yet I, Davenport ER, Vieira-Silva S, Debelius J, Pallister T, Mangino M, Raes J, Knight R, Clark AG, Ley RE, Spector TD, Bell JT.. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol 2016;17:189.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caricilli AM, Saad MJ.. Gut microbiota composition and its effects on obesity and insulin resistance. Curr Opin Clin Nutr Metab Care 2014;17:312–318. [DOI] [PubMed] [Google Scholar]
- 57. Menni C, Mangino M, Cecelja M, Psatha M, Brosnan MJ, Trimmer J, Mohney RP, Chowienczyk P, Padmanabhan S, Spector TD, Valdes AM.. Metabolomic study of carotid-femoral pulse-wave velocity in women. J Hypertens 2015;33:791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kang C, Wang B, Kaliannan K, Wang X, Lang H, Hui S, Huang L, Zhang Y, Zhou M, Chen M, Mi M.. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 2017;8:e00900-17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boutagy NE, McMillan RP, Frisard MI, Hulver MW.. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie 2016;124:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dregan A. Arterial stiffness association with chronic inflammatory disorders in the UK Biobank study. Heart 2018; doi:10.1136/heartjnl-2017-312610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tehrani DM, Gardin JM, Yanez D, Hirsch CH, Lloyd-Jones DM, Stein PK, Wong ND.. Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: cardiovascular Health Study. Atherosclerosis 2013;231:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J.. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D’Acierno L, Giordano R, Di Palma G, Conte M, Golino P, Russo MG, Calabrò R, Calabrò P.. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 2014;16:435.. [DOI] [PubMed] [Google Scholar]
- 64. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 65. Clemente JC, Manasson J, Scher JU.. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018;360:j5145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD.. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brunkwall L, Orho-Melander M.. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia 2017;60:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robles-Vera I, Toral M, Romero M, Jiménez R, Sánchez M, Pérez-Vizcaíno F, Duarte J.. Antihypertensive effects of probiotics. Curr Hypertens Rep 2017;19:26.. [DOI] [PubMed] [Google Scholar]
- 69. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ, Shaw G, Stevenson DK, Holmes SP, Relman DA.. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci USA 2015;112:11060–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Relman DA. The human microbiome: ecosystem resilience and health. Nutr Rev 2012;70:S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.