Abstract
Aims
While coronary artery calcium (CAC) has been extensively validated for predicting clinical events, most outcome studies of CAC have evaluated coronary heart disease (CHD) rather than atherosclerotic cardiovascular disease (ASCVD) events (including stroke). Also, virtually all CAC studies are of short- or intermediate-term follow-up, so studies across multi-ethnic cohorts with long-term follow-up are warranted prior to widespread clinical use. We sought to evaluate the contribution of CAC using the population-based MESA cohort with over 10 years of follow-up for ASCVD events, and whether the association of CAC with events varied by sex, race/ethnicity, or age category.
Methods and results
We utilized MESA, a prospective multi-ethnic cohort study of 6814 participants (51% women), aged 45–84 years, free of clinical CVD at baseline. We evaluated the relationship between CAC and incident ASCVD using Cox regression models adjusted for age, race/ethnicity, sex, education, income, cigarette smoking status, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, diabetes, lipid-lowering medication, systolic blood pressure, antihypertensive medication, intentional physical exercise, and body mass index. Only the first event for each individual was used in the analysis. Overall, 500 incident ASCVD (7.4%) events were observed in the total study population over a median of 11.1 years. Hard ASCVD included 217 myocardial infarction, 188 strokes (not transient ischaemic attack), 13 resuscitated cardiac arrest, and 82 CHD deaths. Event rates in those with CAC = 0 Agatston units ranged from 1.3% to 5.6%, while for those with CAC > 300, the 10-year event rates ranged from 13.1% to 25.6% across different age, gender, and racial subgroups. At 10 years of follow-up, all participants with CAC > 100 were estimated to have >7.5% risk regardless of demographic subset. Ten-year ASCVD event rates increased steadily across CAC categories regardless of age, sex, or race/ethnicity. For each doubling of CAC, we estimated a 14% relative increment in ASCVD risk, holding all other risk factors constant. This association was not significantly modified by age, sex, race/ethnicity, or baseline lipid-lowering use.
Conclusions
Coronary artery calcium is associated strongly and in a graded fashion with 10-year risk of incident ASCVD as it is for CHD, independent of standard risk factors, and similarly by age, gender, and ethnicity. While 10-year event rates in those with CAC = 0 were almost exclusively below 5%, those with CAC ≥ 100 were consistently above 7.5%, making these potentially valuable cutpoints for the consideration of preventive therapies. Coronary artery calcium strongly predicts risk with the same magnitude of effect in all races, age groups, and both sexes, which makes it among the most useful markers for predicting ASCVD risk.
Keywords: Risk prediction, Coronary artery calcium, Outcomes, Atherosclerotic Cardiovascular Disease, Population based study
Background
Coronary artery calcium (CAC) has undergone extensive validation as a predictor of cardiovascular risk; however, the main focus has been predicting coronary heart disease (CHD) events rather than atherosclerotic cardiovascular disease (ASCVD). Furthermore, the vast amount of outcome data with CAC has been limited to largely Caucasian cohorts with limited follow-up duration. The new American College of Cardiology/American Heart Association (ACC/AHA) Cholesterol and Prevention guidelines both focus on hard ASCVD [CHD death, non-fatal myocardial infarction (MI), and fatal or non-fatal stroke],1,2 consistent with statements from the European and Stroke Societies calling for the inclusion of stroke in the outcome of interest for CVD risk assessment.3,4 Questions raised by this working group called for more data on the ability of CAC to predict ASCVD, especially in population-based samples. Using MESA long-term outcome data following CAC scoring, in a population sample of asymptomatic apparently healthy adults ages 45–84 years, provided follow-up exceeding 10-years for ASCVD outcomes, rather than relying on extrapolating from shorter follow-up. This has been a limitation of almost every other CAC study to date.5,6 Thus, large scale 10-year risk data for CAC as a predictor of CVD are lacking. We therefore evaluated the long-term ASCVD outcome data from Multi-Ethnic Study of Atherosclerosis (MESA) cohort, a population-based study of Asians, blacks, Hispanics, and whites, with equal sex representation. Additionally, we sought to evaluate whether the association of CAC with events varied by age, sex, or race/ethnicity.
Methods
Recruitment and baseline examination
The MESA cohort7 is a longitudinal, population-based study of 6814 men and women, free of clinical cardiovascular disease, aged 45–84 at baseline recruited from six Field Centres: Baltimore, MD, USA; Chicago, IL, USA; Forsyth County, NC, USA; Los Angeles, CA, USA; New York, NY, USA; and St. Paul, MN, USA. Racial/ethnic groups enrolled included White, Black, Hispanic, and Chinese. Approximately 50% of the participants enrolled were female. The baseline visit took place between July 2000 and September 2002. The study was approved by Institutional Review Boards at each site and all participants gave written informed consent. Medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002) and explained in detail in Bild et al.7 Information about age, sex, ethnicity, and medical history were obtained by questionnaires. Physical activity was measured by using a detailed, semi-quantitative questionnaire adapted from the Cross-Cultural Activity Participation Study.4 Current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes was defined as a fasting glucose ≥126 mg/dL or on hypoglycaemic medication. Use of antihypertensive and other medications was based on clinic staff entry of prescribed medications. Resting blood pressure was measured three times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL, USA), and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of medication together with a self-reported diagnosis of high blood pressure. Total and high-density lipoprotein cholesterol (HDL-C) and triglyceride levels were measured from blood samples obtained after a 12-h fast. Low-density lipoprotein cholesterol (LDL-C) was calculated with the Friedewald equation.8 Analytical intra-assay CVs ranged from 2.3% to 4.4%, and inter-assay CVs ranged from 2.1% to 5.7%.
Computed tomographic scanning
Details on the MESA study’s methods for computed tomography (CT) scanning and interpretation have been published.9 Scanning centres assessed coronary calcium by chest CT using either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles, and New York Field Centres) or 4-16 detector CT systems (Baltimore, Forsyth County, and St. Paul Field Centers). Certified technologists scanned all participants twice over phantoms of known physical calcium concentration. Images were interpreted at the MESA CT reading centre (Los Angeles Biomedical Research Institute at Harbor–University of California Los Angeles Medical Center, Torrance, CA, USA). We used the average Agatston score10 for the two scans in all analyses. Intra-observer and inter-observer agreements were high (kappa 0.93 and 0.90, respectively).11,12 Participants were told either that they had no coronary calcification or that the amount was less than average, average, or greater than average for their age, and that they should discuss the results with their physicians.
Events surveillance
At time of analysis, the cohort had been followed for incident cardiovascular events for a median of 11.1 years. At intervals of 9–12 months, a telephone interviewer contacted each participant to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. In order to verify self-reported diagnoses, we requested copies of all death certificates and medical records for hospitalizations and outpatient cardiovascular diagnoses and conducted next-of-kin interviews for out-of-hospital cardiovascular deaths. We obtained records on 98% of reported hospitalized cardiovascular events. Some information was available on 95% of reported outpatient diagnostic encounters. Trained personnel abstracted medical records suggesting possible cardiovascular events. Two physicians independently classified and assigned incidence dates. Differences between reviewers were resolved between the reviewers via discussion. For purposes of this study, we used incident ASCVD events (total events), including definite or probable MI, resuscitated cardiac arrest, fatal CHD, fatal and non-fatal stroke (not transient ischaemic attack), other atherosclerotic death, and other cardiovascular death. Hard ASCVD included MI, fatal or non-fatal strokes (not transient ischaemic attack (TIA)), resuscitated cardiac arrest, and fatal CHD. Additional details on the MESA study’s follow-up methods and event adjudication are available on the MESA web site at http://www.mesa-nhlbi.org.
Reviewers classified MI as definite, probable, or absent, based primarily on combinations of symptoms, electrocardiogram (ECG), and cardiac biomarker levels. In most cases, definite or probable MI required either abnormal cardiac biomarkers (two times upper limits of normal) regardless of pain or ECG findings; evolving Q waves regardless of pain or biomarker findings; or a combination of chest pain, and ST-T evolution or new left bundle branch block (LBBB), and biomarker levels 1–2 times upper limits of normal.
Reviewers classified resuscitated cardiac arrest when a patient successfully recovered from a full cardiac arrest through cardiopulmonary resuscitation (including cardioversion).
Fatal CHD required a documented MI within the previous 28 days, chest pain within the 72 h before death, or a history of CHD, and required the absence of a known non-atherosclerotic or non-cardiac cause of death.
Neurologists reviewed and classified stroke as present if there was a focal neurologic deficit lasting 24 h or until death, with a clinically relevant lesion on brain imaging, and no non-vascular cause.
Statistical methods
We evaluated the relationship between CAC and incident ASCVD. Our analytic sample (n = 6783) excluded five participants who were discovered to be ineligible for the study after baseline and 26 participants who had no follow-up data. Multiple imputation by chained equations, with 10 imputed datasets and using Rubin’s Rules to combine the standard errors, was used to handle small amounts (<4%) of missing risk factor data. Only the first ASCVD event for each participant was included. Event rates were estimated by dividing the number of events by the number of person-years at risk. These are expressed as expected events per 100 participants followed for 10-years (e.g. 1000 person-years) and can thus be interpreted as 10-year event rates. Cox proportional hazards regression models was used to estimate the hazard ratio (HR) for CAC after adjustment for age, race/ethnicity, sex, education, income, cigarette smoking status, LDL-C, high-density lipoprotein cholesterol, diabetes, lipid-lowering medication, systolic blood pressure, antihypertensive medication, intentional physical exercise minutes per day, and body mass index. To determine if the relationship of CAC to the risk of ASCVD was the same in in age, sex, and race or ethnic groups, we modelled the interaction of these variables with CAC. In these models we used CAC continuously, with a log-base 2 transformation of CAC score plus 1 to be consistent with prior literature and to show how a doubling of CAC is related to events.8 Unadjusted Kaplan–Meier cumulative incidence curves were performed as described by Kaplan and Meier.13 All statistical analyses were performed using Stata version 12.1.
Results
Baseline demographic characteristics and risk factors by race/ethnic group are shown in Table 1. Overall, 500 incident hard CVD (7.4%), 709 total CVD (10.5%), 321 incident hard CHD (4.7%), and 498 total CHD (7.3%) events were observed in the total study population over a median of 11.1 years follow-up. Hard ASCVD included 217 MI, 188 strokes (not TIA), 13 resuscitated cardiac arrest, and 82 CHD deaths.
Table 1.
Baseline demographic characteristics and risk factors by race or ethnic group
| Variable | Black (n = 1880) | Chinese (n = 801) | Hispanic (n = 1488) | White (n = 2614) |
|---|---|---|---|---|
| Coronary artery calcium present (%) | 43.5 | 50.2 | 45.2 | 57.0 |
| Coronary artery calcium, if non-zero | 282.6 (576.9) | 188.8 (354.1) | 274.6 (540.7) | 331.4 (568.9) |
| Age (years) | 62.1 (10.1) | 62.4 (10.3) | 61.3 (10.3) | 62.6 (10.3) |
| Male (%) | 44.6 | 48.6 | 48.3 | 48.0 |
| Education (%) | ||||
| Less than high school | 12.2 | 24.5 | 44.6 | 5.0 |
| High school diploma | 53.8 | 36.5 | 45.5 | 45.4 |
| Bachelor’s degree | 17.4 | 22.8 | 5.6 | 22.2 |
| Graduate degree | 16.7 | 16.3 | 4.4 | 27.4 |
| Income, median category | $35 000–$39 999 | $25 000–$29 999 | $25 000–$29 999 | $50 000–$74 999 |
| Cigarette use | ||||
| Former (%) | 36.9 | 19.0 | 32.5 | 44.2 |
| Current (%) | 18.0 | 5.6 | 13.6 | 11.6 |
| High-density lipoprotein | 52.4 (15.3) | 49.6 (12.7) | 47.6 (13.1) | 52.3 (15.7) |
| Low-density lipoprotein | 116.5 (33.1) | 115.1 (29.0) | 119.5 (32.7) | 117.0 (30.2) |
| Blood pressure (mmHg) | ||||
| Systolic | 131.7 (21.6) | 124.6 (21.7) | 126.7 (21.9) | 123.5 (20.4) |
| Diastolic | 74.5 (10.2) | 72.0 (10.4) | 71.6 (10.1) | 70.2 (10.0) |
| Diabetes (%) | 16.6 | 11.1 | 16.8 | 5.3 |
| Lipid-lowering medication (%) | 16.6 | 14.5 | 13.1 | 18.4 |
| Hypertension medication (%) | 50.3 | 28.7 | 32.6 | 33.1 |
| Intentional exercise (met-min/week), median (IQR) | 810 (105–2130) | 735 (0–1470) | 630 (0–1620) | 1050 (315–2205) |
| Body mass index (kg/m2) | 30.2 (5.9) | 24.0 (3.3) | 29.4 (5.1) | 27.7 (5.1) |
| ACC/AHA ASCVD 10-year risk (%) | 14.6 (11.9) | 13.2 (14.1) | 13.9 (14.5) | 12.7 (12.8) |
Cells show mean (standard deviation) except where noted.
IQR, interquartile range.
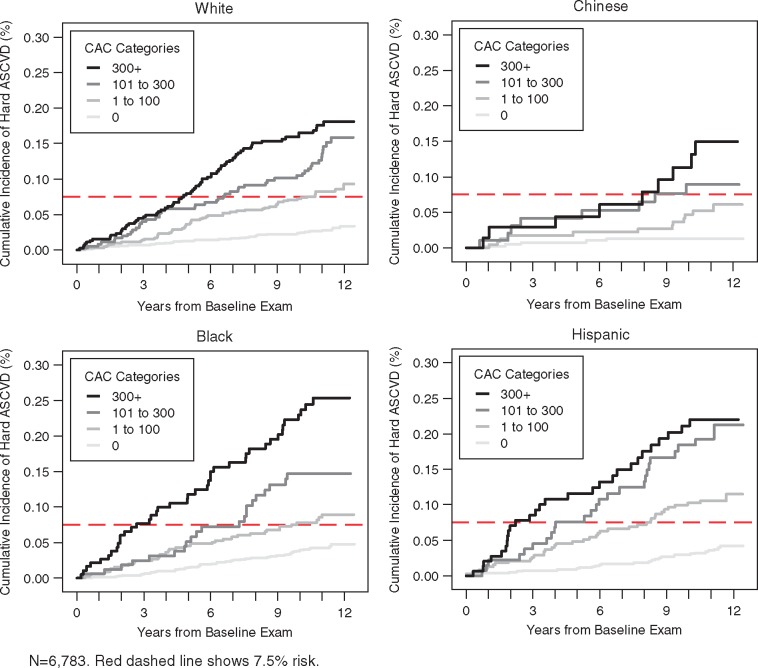
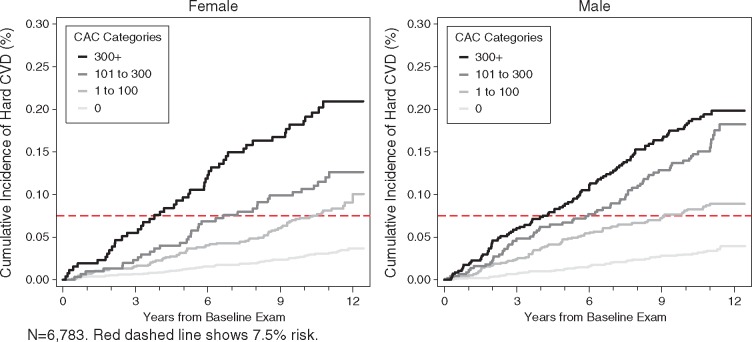
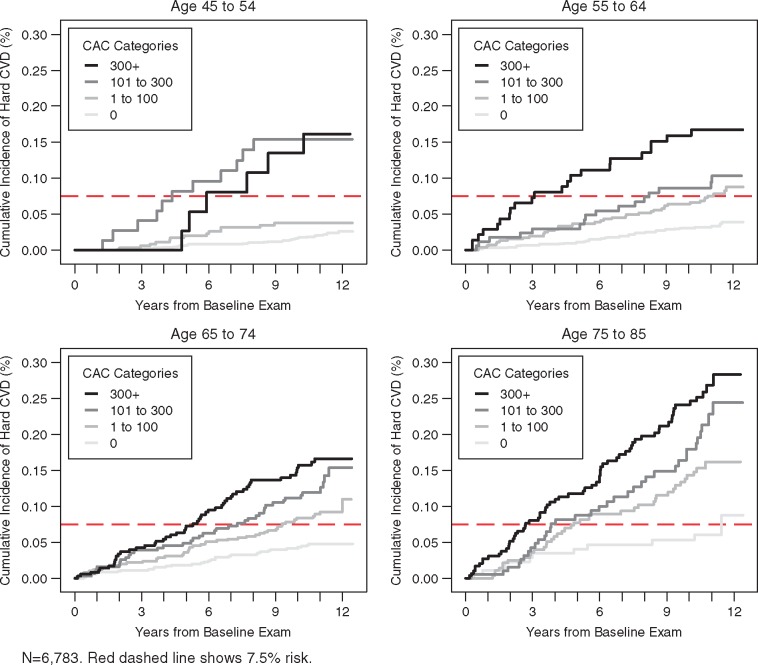
Figures 1–3 illustrate the unadjusted relationship between CAC categories and ASCVD event rates over time, stratified by categories of race/ethnicity, age, and sex, respectively. A dashed horizontal line indicates the risk threshold of 7.5%. At 10 year follow-up, all participants with CAC over 100 had more than 7.5% risk regardless of demographic subset.
Figure 1.
Unadjusted Kaplan–Meier cumulative incidence curves for hard atherosclerotic cardiovascular disease events by categories of coronary artery calcium and race or ethnicity. n = 6783. Red dashed line shows 7.5% risk.
Figure 3.
Unadjusted Kaplan–Meier cumulative incidence curves for hard atherosclerotic cardiovascular disease events by categories of coronary artery calcium and sex. n = 6783. Red dashed line shows 7.5% risk.
Figure 2.
Unadjusted Kaplan–Meier cumulative incidence curves for hard atherosclerotic cardiovascular disease events by categories of coronary artery calcium and age. n = 6783. Red dashed line shows 7.5% risk.
Ten-year ASCVD event rates increase steadily across CAC categories (Table 2) and this holds true regardless of race/ethnicity, age, sex, or education. Event rates in the CAC = 0 group ranged from 1.3% to 5.6%, while for the CAC > 300 group rates ranged from 13.1% to 25.6%. Table 3 shows the hazards ratios for age, sex, race, and age with and without adjustment for CAC. Overall, the associations were significantly attenuated with CAC adjustment, though significant associations still existed for age, sex, and race. For each doubling of CAC, we estimated a 14% relative increment in ASCVD risk, holding all other risk factors constant.
Table 2.
Event rates examining the likelihood of hard atherosclerotic cardiovascular disease events by categories of coronary artery calcium
| CAC categories |
||||
|---|---|---|---|---|
| 0 | 1 to 100 | 101 to 300 | 300+ | |
| 10-year event rate [No. of events/N] | ||||
| Race or ethnicity | ||||
| Black | 3.9 [43/1062] | 7.7 [36/461] | 14.7 [21/168] | 24.5 [41/189] |
| Chinese | 1.3 [5/399] | 4.7 [12/235] | 8.3 [8/98] | 13.1 [9/69] |
| Hispanic | 3.1 [29/815] | 10.3 [38/391] | 19.1 [25/135] | 21.7 [28/147] |
| White | 2.4 [33/1124] | 7.3 [56/700] | 10.8 [47/354] | 16.2 [69/436] |
| Age | ||||
| 45 to 54 | 1.7 [32/1464] | 3.8 [13/361] | 15.4 [11/74] | 16.2 [6/42] |
| 55 to 64 | 3.1 [35/1035] | 6.5 [40/529] | 8.6 [16/169] | 16.7 [22/140] |
| 65 to 74 | 4.2 [32/721] | 8.3 [50/604] | 11.8 [39/317] | 15.0 [53/363] |
| 75 to 85 | 5.6 [11/180] | 14.3 [39/293] | 18.1 [35/195] | 24.7 [66/296] |
| Sex | ||||
| Male | 3.0 [42/1245] | 8.0 [75/930] | 14.4 [67/446] | 18.2 [99/578] |
| Female | 2.8 [68/2155] | 7.3 [67/857] | 10.6 [34/309] | 19.0 [48/263] |
| Lipid-lowering medication at baseline | ||||
| No | 2.7 [96/3029] | 7.7 [114/1437] | 13.4 [76/588] | 19.9 [111/613] |
| Yes | 2.8 [13/361] | 7.4 [27/348] | 12.9 [24/164] | 16.5 [36/228] |
ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium.
Table 3.
Hazard ratio examining the likelihood of hard atherosclerotic cardiovascular disease events
| Model without CAC |
Model with CAC |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Race or ethnicity | ||||
| White | 1.0 | 1.0 | ||
| Black | 0.73 (0.58–0.92) | 0.008 | 0.89 (0.71–1.13) | 0.333 |
| Chinese | 0.44 (0.30–0.64) | <0.001 | 0.48 (0.33–0.71) | <0.001 |
| Hispanic | 0.82 (0.63–1.06) | 0.132 | 0.94 (0.73–1.22) | 0.666 |
| Age | ||||
| 45–54 | 1.0 | 1.0 | ||
| 55–64 | 1.65 (1.21–2.26) | 0.002 | 1.37 (1.00–1.88) | 0.054 |
| 65–74 | 2.27 (1.66–3.10) | <0.001 | 1.53 (1.10–2.11) | 0.011 |
| 75–85 | 4.34 (3.12–6.06) | <0.001 | 2.47 (1.73–3.52) | <0.001 |
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 1.63 (1.32–2.00) | <0.001 | 1.31 (1.06–1.62) | 0.012 |
| Lipid-lowering medication at baseline | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.02 (0.81–1.28) | 0.896 | 0.92 (1.02–1.51) | 0.033 |
| CAC (log2[CAC+1]) | 1.14 (1.11–1.17) | <0.001 | ||
Proportional hazard Cox regression models adjusted for variables shown, and, education, income, cigarette smoking status (never, former, current), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), diabetes status (yes/no), systolic blood pressure (SBP), antihypertensive medication use, intentional physical exercise minutes per day, and body mass index.
ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CI, confidence interval.
Table 4 explored whether the association of CAC with risk differs across important demographic subsets. The association was highly consistent across all categories. Tests for interaction with age, sex, or race/ethnicity were all non-significant. This demonstrates that CAC is independently associated with events, independent of race and ethnicity (Table 4), despite different prevalence of CAC in these subgroups (CAC was present in 43.5% of blacks, 50.2% of Chinese-Americans, 45.2% of Hispanics, and 57% of non-Hispanic Whites). Similarly, CAC is associated with ASCVD events independent of gender and age. Each doubling of CAC was association with approximately 14–20% (relative) increase in risk regardless of subset.
Table 4.
Risk of atherosclerotic cardiovascular disease associated with coronary artery calcium by age categories, sex, and race or ethnicity
| Subset | HR (95% CI) | P-value |
|---|---|---|
| Age category | ||
| 45–54 | 1.20 (1.11–1.29) | <0.001 |
| 55–64 | 1.16 (1.10–1.22) | <0.001 |
| 65–74 | 1.13 (1.08–1.18) | <0.001 |
| 75–84 | 1.12 (1.06–1.17) | <0.001 |
| Sex | ||
| Male | 1.14 (1.10–1.18) | <0.001 |
| Female | 1.13 (1.08–1.17) | <0.001 |
| Race or ethnicity | ||
| White | 1.13 (1.08–1.18) | <0.001 |
| Chinese | 1.16 (1.05–1.28) | 0.003 |
| Black | 1.13 (1.08–1.18) | <0.001 |
| Hispanic | 1.14 (1.09–1.20) | <0.001 |
| Lipid-lowering medication at baseline | ||
| No | 1.14 (1.10–1.17) | <0.001 |
| Yes | 1.14 (1.10–1.17) | <0.001 |
| Overall | 1.14 (1.10–1.17) | <0.001 |
All models adjust for age, sex, race/ethnicity, education, income, cigarette smoking status (never, former, current), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL-C), diabetes status (yes/no), lipid-lowering medication use, systolic blood pressure (SBP), antihypertensive medication use, intentional physical exercise minutes per day, and body mass index. Hazard ratios (HRs) represent the multiplicative increase in risk for a doubling of CAC and were calculated for baseline levels of log2(CAC + 1) after adjustment for risk factors and interaction between age group, sex, and race or ethnic group, lipid-lowering medication use at baseline, and CAC score.
ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CI, confidence interval.
Discussion
This is the first study to evaluate true 10-year predictive ability of varying CAC score cutpoints specifically for ASCVD in different ages, genders, and race-ethnicities. This is also, to our knowledge, the first paper demonstrating that CAC is similarly predictive of ASCVD in patients on or off lipid-lowering therapy (Table 2). There were previous concerns that since statins cause micro-calcification, CAC would not be predictive in this population. We also found that CAC strongly predicts risk with the same magnitude of effect in all races, age groups, and both sexes.
This study examined ASCVD events as the primary endpoint as they are the focus of the 2013 ACC/AHA Prevention Guidelines and ESC guidelines, and because statin therapy reduces the risk of both coronary and cerebrovascular events.1,2,14 Prior publications from MESA reported the ability of CAC to predict CVD events and that CAC was superior to carotid intimal media thickness (IMT); however, follow-up was limited to mean of 5.3 years, and the number of strokes in the study was limited (n = 59).15 Blaha et al.16 showed that CAC predicted ASCVD events in the subset of the MESA population who met entry criteria for Jupiter (≥50 years for men and ≥60 years for women, LDL-C <3.37 mmol/L, not on lipid-lowering therapy, free of diabetes, triglycerides <5.65 mmol/L, and creatinine <176.8 μmol/L and hsCRP ≥ 2 mg/L), but mean follow-up was only 5.8 years and the subcohort was limited to 950 individuals. A strength of the current study was inclusion of all patients in MESA, including statin-treated patients, as MESA involved a population-based cohort free of CVD at baseline.
We demonstrated that CAC is strongly associated with major adverse cardiovascular events (i.e. stroke, cardiovascular death or non-fatal MI) regardless of sex, race/ethnicity, or age group. Yeboah et al.17 recently reported an improvement in discrimination using CAC to predict 10-year ASCVD risk over the pooled cohort equation in a subgroup of non-statin users in MESA using CAC as a continuous variable; however, these investigators did not report differences in race–ethnicity, gender, or cutpoints of CAC that can be used in the clinical setting, and excluded those on statin therapy at baseline. Our analysis included the entire baseline MESA cohort and provides further validation for CAC cutpoints used to predict CHD risk in a general population.
The Cardiovascular Risk Assessment and Blood Cholesterol Management Work Groups (2013) stated that ‘assessing CAC is likely to be the most useful of the current approaches to improving risk assessment among individuals found to be at intermediate risk after formal risk assessment’, concordant with the 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults.18 The work group noted three limitations of using CAC for CVD risk assessment prior to expansion of its current role. The first was that the vast majority of existing outcome data was related to CHD, not hard ASCVD events that included stroke; hence, uncertainty remained regarding the additional contribution of CAC to estimating 10-year risk of first hard ASCVD events. Here, we demonstrated that CAC is robustly associated with hard ASCVD events over a >10 year period. The second concern related to radiation; however, the median CAC radiation exposure in MESA (using multiple contemporary CT scanners) was only 0.95 mSv, a little more than an average mammogram, and less than annual background radiation.19,20 The final concern of the work group was related to cost, but a recent poll at the 2016 Society of Cardiovascular Computed Tomography Annual Scientific Sessions demonstrated average costs of <$100, and as low as $49 per test.
Research to date has found a very low-cardiovascular event rate among those with zero CAC.21 Approximately 50% of the MESA sample had CAC scores of zero and were largely <5% over the 10 year follow-up and, demonstrate an excellent opportunity for this negative risk factor to accurately ‘de-risk’ certain subjects.22,23 This is concordant with other studies from MESA and elsewhere, demonstrating that CAC score of 0 results in the most accurate downward risk reclassification.24,25 As seen in Figures 1–3, the 10+ year average event rates for those participants with zero CAC remains well under 6%, signifying a low risk state. This is similar to Valenti et al.,26 who recently demonstrated that persons with zero CAC had lower risk of mortality than persons characterized with zero CV risk factors. Of the available options, a CAC score of zero may be used as a rationale to downgrade risk and reconsider lifelong statin therapy when the patient or clinician is otherwise uncertain.24 Since the pooled cohort equation has demonstrated marked over-estimation in some contemporary cohorts,27 a zero CAC score can be very reassuring as to the true low risk of the patient, and potentially avoid both statin and aspirin use in that individual, at least for 5 years.20,28 Obviating use of statin and aspirin has been shown to be cost-effective.29 Conversely, we show that regardless of sociodemographic group, a CAC score ≥ 100 signifies at least a 7.5% 10-year risk of ASCVD for which statin therapy could be warranted (after a careful patient–clinician discussion as recommended by the guidelines1,2), even if the calculated 10-year risk does not reach this threshold. Currently the guidelines use a CAC score of ≥300 or ≥75th percentile for age, gender, and ethnicity as a criterion for further informing the treatment decision following global risk assessment.
Coronary artery calcium measurement has emerged as a non-invasive test that can risk stratify asymptomatic individuals into low-, intermediate-, and high-risk groups.30–32 McClelland et al. recently concluded that ‘Inclusion of CAC in the MESA risk score offered significant improvements in risk prediction (C-statistic 0.80 vs. 0.75; P < 0.0001)’. External validation in both the Recall and Dallas Heart studies ‘provides evidence for very good to excellent discrimination and calibration for the model including CAC’.33 Recent guidelines have incorporated CAC testing in the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) risk estimator, suggesting that CAC measurement may be appropriate both in patients with 10-year risk <7.5% and when treatment decisions are uncertain in higher risk patients.
The multi-ethnic and population-based nature of MESA allows for some generalizability; however, participants were restricted to 45–84 years at entry. Most importantly, because the MESA cohort was asymptomatic at baseline, our findings cannot be extrapolated to individuals with symptoms or known coronary artery disease. Our data suggest that CAC is similarly associated with ASCVD events independent of sex, race/ethnicity, age group, and education studied in MESA. This is important when one considers that the pooled cohort equation tends to perform poorly when applied to recent populations with contemporary risk factor exposures, often with concomitant preventive therapy, and greater racial and ethnic diversity.14,34–37 In this study, blacks and Hispanics had virtually the same event HRs for CAC as whites, despite generally lower CAC scores and lower prevalence of CAC in non-whites.
It has been previously shown that CAC adds value to both the ACC/AHA Pooled Risk Cohort as well as the ESC SCORE algorithm. In a German cohort, Mahabadi demonstrated when CAC score leads to significant improvement of risk prediction, especially in addition to European recommendations.38 Importantly, this effect of higher CAC being associated with higher ASCVD rates is not attenuated by baseline lipid lower therapy (Tables 2–4). While statins may have a very small effect on raising CAC scans, CAC remains highly predictive in all subgroups. It must be noted that the overall population studied is large, certain subgroups have few patients (Chinese with >300+ calcium score).
Coronary artery calcium has been associated with higher compliance, in both MESA and other studies.39–41 In MESA, there was a significant relationship between baseline CAC score and medication continuation rates for both statins and aspirin.42
One limitation was the use of electron beam tomography (EBT) and 4- and 16-detector CT systems, and there has been robust change of technology. We do not believe this should affect the interpretation of their results in current practice, as we have demonstrated that there was similar reproducibility among the different scanners (EBT, 4, 16, and 64 detector systems) and similarity in scores between EBT and 64-MDCT.43,44
In conclusion, we demonstrated four important findings related to contemporary use of risk equations and CAC. First, CAC is independently and strongly associated with ASCVD, including stroke, in all models. Second, a zero score carries a low 10-year risk and could be used to down-stratify a patient’s risk based on global risk assessment for possible reconsideration of certain preventive therapies. Third, a score of ≥100 signifies at least a 7.5% 10-year risk of ASCVD regardless of age group, gender, or ethnicity, where based on current guidelines, individuals may be eligible for preventive therapies such as statins. Finally, unlike other CHD risk factors, CAC strongly predicts risk with the same magnitude of effect in all races, age groups, and both sexes, and recently been demonstrated to be predictive of ASCVD in symptomatic patients as well.45 This property makes it among the most useful markers for predicting ASCVD risk.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
R01 HL071739 and MESA; HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute; and by grants UL1-TR-000040, UL1 TR 001079, and UL1-RR-025005.
Conflict of interest: M.B. has grants from the National Institutes of Health and General Electric. No other author reports any conflict of interest.
Footnotes
See page 2409 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy343)
References
- 1. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 3. Lackland DT, Elkind MS, D’Agostino R Sr, Dhamoon MS, Goff DC Jr, Higashida RT, McClure LA, Mitchell PH, Sacco RL, Sila CA, Smith SC Jr, Tanne D, Tirschwell DL, Touzé E, Wechsler LR.. Inclusion of stroke in cardiovascular risk prediction instruments: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:1998–2027. [DOI] [PubMed] [Google Scholar]
- 4. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Budoff MJ, Möhlenkamp S, McClelland R, Delaney JA, Bauer M, Jöckel HK, Kälsch H, Kronmal R, Nasir K, Lehmann N, Moebus S, Mukamal K, Erbel R.. A comparison of outcomes with coronary artery calcium scanning in unselected populations: the Multi-Ethnic Study of Atherosclerosis (MESA) and Heinz Nixdorf RECALL study (HNR). J Cardiovasc Comput Tomogr 2013;7:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS.. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–1870. [DOI] [PubMed] [Google Scholar]
- 7. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP.. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 8. Friedewald WT, Levy RI, Fredrickson DS.. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 9. Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC.. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 10. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R.. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 11. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA.. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 12. Budoff MJ, McClelland RL, Chung H, Wong ND, Carr JJ, Gray MM, Blumenthal RS, Detrano RC.. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: the Multi-Ethnic Study of Atherosclerosis. Am J Roentgenol 2009;192:613–617. [DOI] [PubMed] [Google Scholar]
- 13. Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 14. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL.. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2999–3058.27567407 [Google Scholar]
- 15. Folsom AR, Kronmal R, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL.. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O'Leary DH, Lima J, Blumenthal RS, Nasir K.. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC Jr, Psaty BM, Greenland P, Herrington DM.. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol 2016;67:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC Jr, Taylor AJ, Weintraub WS, Wenger NK.. American College of Cardiology F and American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–103. [DOI] [PubMed] [Google Scholar]
- 19. Messenger B, Li D, Nasir K, Carr JJ, Blankstein R, Budoff MJ.. Coronary calcium scans and radiation exposure in the multi-ethnic study of atherosclerosis. Int J Cardiovasc Imaging 2016;32:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. deGoma EM, Karlsberg RP, Judelson DR, Budoff MJ.. The underappreciated impact of heart disease. Womens Health Issues 2010;20:299–303. [DOI] [PubMed] [Google Scholar]
- 21. Shareghi S, Ahmadi N, Young E, Liu ST, Gopal A, Budoff MJ.. The prognostic significance of zero coronary calcium scores on cardiac computed tomography. J Cardiovasc Comput Tomogr 2007;1:155–159. [DOI] [PubMed] [Google Scholar]
- 22. Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatston AS, Rivera JJ, Miemdema MD, Sibley CT, Shaw LJ, Blumenthal RS, Budoff MJ, Krumholz HM.. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 23. Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS.. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2009;158:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K.. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study Of Atherosclerosis (MESA). Circulation 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joshi PH, Blaha MJ, Budoff MJ, Miedema MD, McClelland RL, Lima JAC, Agatston AS, Blankstein R, Blumenthal RS, Nasir K.. The 10-year prognostic value of zero and minimal CAC. JACC Cardiovasc Imaging 2017;10:957–958. [DOI] [PubMed] [Google Scholar]
- 26. Valenti V, Hartaigh BÓ, Heo R, Cho I, Schulman-Marcus J, Gransar H, Truong QA, Shaw LJ, Knapper J, Kelkar AA, Sandesara P, Lin FY, Sciarretta S, Chang HJ, Callister TQ, Min JK.. A 15-year warranty period for asymptomatic individuals without coronary artery calcium. J Am Coll Cardiol Cardiovasc Imaging 2015;8:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Sung SH, Ballantyne CM, Go AS.. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol 2016;67:2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR.. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes 2014;7:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts ET, Horne A, Martin SS, Blaha MJ, Blankstein R, Budoff MJ, Sibley C, Polak JF, Frick KD, Blumenthal RS, Nasir K.. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 2015;doi:0.1371/journal.pone.0116377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JAC, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE.. Assessment of coronary artery disease by cardiac computed tomography, a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114:1761–1791. [DOI] [PubMed] [Google Scholar]
- 31. Budoff MJ, Raggi P, Beller GA, Berman DS, Druz RS, Malik S, Rigolin VH, Weigold WG, Soman P; Imaging Council of the American College of Cardiology . Noninvasive cardiovascular risk assessment of the asymptomatic diabetic patient: the Imaging Council of the American College of Cardiology. JACC Cardiovasc Imaging 2016;9:176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, Kondos GT, Kronmal RA.. Coronary calcium predicts events better with absolute calcium scores than age-gender-race percentiles—the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2009;53:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, Folsom AR, Khera A, Ayers C, Mahabadi AA, Lehmann N, Jöckel KH, Moebus S, Carr JJ, Erbel R, Burke GL.. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, Ikram MA, Stricker BH, Hofman A, Franco OH.. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA 2014;311:1416–1423. [DOI] [PubMed] [Google Scholar]
- 35. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ.. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pursnani A, Massaro JM, D’Agostino RB, O’Donnell CJ, Hoffmann U.. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA 2015;314:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeFilippis AP, Blaha MJ.. Predicted vs observed clinical event risk for cardiovascular disease. JAMA 2015;314:2082. [DOI] [PubMed] [Google Scholar]
- 38. Mahabadi AA, Möhlenkamp S, Lehmann N, Kälsch H, Dykun I, Pundt N, Moebus S, Jöckel KH;. Erbel R on behalf of the Heinz Nixdorf Recall Study Investigators. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. J Am Coll Cardiol Cardiovasc Imaging 2017;10:143–153. [DOI] [PubMed] [Google Scholar]
- 39. Kalia NK, Cespedes L, Youssef G, Li D, Budoff MJ.. Motivational effects of coronary artery calcium scores on statin adherence and weight loss. Coron Artery Dis 2015;26:225–230. [DOI] [PubMed] [Google Scholar]
- 40. Mamudu HM, Paul TK, Veeranki SP, Budoff M.. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: a systematic review. Atherosclerosis 2014;236:338–350. [DOI] [PubMed] [Google Scholar]
- 41. Ladapo JA, Hoffmann U, Lee KL, Coles A, Huang M, Mark DB, Dolor RJ, Pelberg RA, Budoff M, Sigurdsson G, Severance HW, Douglas PS.. Changes in medical therapy and lifestyle after anatomical or functional testing for coronary artery disease. J Am Heart Assoc 2016;5:e003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasir K, McClelland RL, Blumenthal RS, Goff DC Jr, Hoffmann U, Psaty BM, Greenland P, Kronmal RA, Budoff MJ.. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: the Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Qual Outcomes 2010;3:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Budoff MJ, Katz R, Wong ND, Nasir K, Mao S, Takasu J, Kronmal R, Detrano RC, Carr JJ.. Effect of scanner type on the reproducibility of extra-coronary measures of calcification: the multi-ethnic study of atherosclerosis. Acad Radiol 2007;14:1043–1049. [DOI] [PubMed] [Google Scholar]
- 44. Mao SS, Pal RS, McKay CS, Gao YG, Gopal A, Ahmadi N, Child J, Carson S, Takasu J, Sarlak B, Bechmann D, Budoff MJ.. Comparison of coronary artery calcium scores between electron beam computed tomography and 64-multidetector computed tomographic scanner. J Comput Assist Tomogr 2009;33:175–178. [DOI] [PubMed] [Google Scholar]
- 45. Budoff MJ, Mayrhofer T, Ferencik M, Bittner DO, Lee KL, Lu MT, Coles A, Jang JJ, Krishnam MS, Douglas PS, Hoffmann U;. PROMISE Investigators. The prognostic value of coronary artery calcium in the PROMISE study. Circulation 2017;135:2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.