Abstract
The loss of flower-rich habitats and agricultural intensification have resulted in significant losses of wild bee diversity from agricultural landscapes that is increasingly threatening the pollination of zoochorous agricultural crops and agricultural sustainability. However, the links of different wild bee functional trait groups with habitat types and plant resources in agricultural landscapes remain poorly understood, thus impeding the formulation of effective policies for bee conservation. We therefore analyzed how bees representing different functional groups responded to variations in habitat type, vegetation composition and plant diversity. Natural shrubland sustained the highest diversity in bees overall, in large-sized bees, solitary bees and belowground-nesting bees, while each habitat harbored unique species. In half of the functional bee groups, species were negatively linked to tree coverage and herb coverage, respectively, while plant diversity was positively related to all functional groups except large-sized bees and aboveground-nesting bees. Overall bee abundance was positively related to abundance of plants in the Sympetalae, and negatively related to abundance of plants in the Archichlamydeae. Different bee functional groups showed distinct preferences for different plant communities. In order to conserve the diversity of wild bees across functional groups to optimize associated pollination services, a diverse habitat mosaic, and particularly plant species in Sympetalae need to be promoted in agricultural landscapes. Future studies should aim to enhance our understanding of plant-pollinator associations and specific food requirement of different wild bee species for their effective conservation.
Keywords: pollinator, body size, sociality, nest location, floral resource
Pollination is a crucial ecosystem service for many agricultural crops and wild plants alike (Biesmeijer et al. 2006), with an estimated 87.5% of all flowering plants (Ollerton et al. 2011) and 75% of the world’s leading food crops (Klein et al. 2007) depending on pollinators to various degrees. Bees (Hymenoptera: Apoidea), including managed honey bees and wild bees, are believed to be the most effective pollinators because of their specific pollen-carrying body structures, with wild bees representing the main pollinators for a large number of agricultural crops (Biesmeijer et al. 2006). However, with the acute declines in managed honey bee and wild bee populations over recent decades, there are great concerns about the loss of pollination services from these key providers (Biesmeijer et al. 2006, Stokstad 2007, Hayes et al. 2008, Potts et al. 2010, Meeus et al. 2018).
In comparison to honey bees, wild bees are considered to be more reliable pollinators (Kremen et al. 2004, Garibaldi et al. 2013) with a great adaptability and species diversity (Kremen et al. 2002) associated with a large variation in a number of their traits like nest location (Cane et al. 2006, Jha and Vandermeer 2010), social behavior (Jha and Vandermeer 2009, 2010), dispersal ability (Zurbuchen et al. 2010), tongue length (Miller-Struttmann et al. 2015) or their body size. This influences their use of resources like nectar and pollen, and their response to disease and environment changes such as changes to their habitats and climatic conditions (Henle et al. 2004, Miller-Struttmann et al. 2015). For example, belowground-nesting bees often have higher thermal requirements for flight activity and commonly require bare ground (Osborne et al. 1991, Cane and Tepedino 2001). Large-sized bees, with a higher requirement for energy and a high pollination efficiency, may be more sensitive to habitat loss than smaller species (Cane et al. 2006). If these functional traits that are sensitive to environment changes are also those that affect ecosystem services, then tailored conservation policies will be required that rely on a thorough understanding of response patterns in the different functional groups to environmental change (Lavorel and Garnier 2002, Forrest et al. 2015) to effectively tackle the decline in pollination services for many agricultural crops and wild plants that is linked to the decline of wild bee diversity (Biesmeijer et al. 2006, Potts et al. 2010).
In fragmented agricultural landscapes, habitat diversity is often considered as a basis for bee species diversity, because different habitats often provide different floral resources, nest sites and nest materials for wild bee species (Westrich 1996, Steffan-Dewenter et al. 2002), with habitat diversity additionally linked to a more continuous provision of floral resources throughout the seasons when bees are active (Wojcik et al. 2008, Potts et al. 2010). Habitat quality, linked, e.g., to the complexity of the vegetation structure and composition, is another factor impacting wild bee diversity (Klein et al. 2003, Potts et al. 2003, Müller et al. 2006). Alongside species richness, functional group diversity represents a second important component of bee diversity (Tilman et al. 1997, Scherber et al. 2006, Hoehn et al. 2008), and it has been shown to better predict pollination effectiveness than species diversity (Hoehn et al. 2008, Fründ et al. 2013). Nonetheless, knowledge gaps persist in relation to the distribution of wild bees and bee trait groups across different habitats in fragmented agricultural landscapes (Mendenhall et al. 2011), as well as to the relationship between wild bee diversity and the composition and diversity of the vegetation. With modern, intensive agricultural practices often resulting in a dramatic loss of flower-rich habitats and a decrease in habitat quality (Newbold et al. 2015), these knowledge gaps need to be urgently addressed to allow for a better conservation of wild bees and the important ecosystem services they provide.
China harbors rich wild bee resources, with more than 1,000 species known to science (Xu et al. 2009), and the estimated total species richness exceeding 3,000 species of Apoidea (Wu 1965). With the country’s rapid economic development and greatly improved living standards, the demand for crops heavily relying on pollinators like many fruits is increasing (Aizen and Harder 2009). To meet the associated increased demand on pollination service, a sound knowledge on wild bee diversity, their distribution patterns among different habitats in the agricultural landscape and their associations with the vegetation are essential—especially for functional groups with a high pollination efficiency.
In this study, we therefore aim to investigate the distribution pattern of different functional trait groups in wild bees between different habitat types to establish how habitat type, vegetation composition, and plant diversity influence the abundance and diversity of species within these functional groups. We hypothesize that 1) wild bees are more diverse in remaining pristine habitat types than in other semi-natural and in heavily managed habitats; 2) overall wild bee diversity is strongly impacted by the composition and diversity of the vegetation, and particularly by the diversity of pollen- and nectar-providing flowering plants; 3) different wild bee functional groups show diverging response patterns to habitat type, vegetation composition and diversity in agricultural landscapes due to their traits being linked to different requirements of food sources, nest sites, and different dispersal abilities.
Materials and Methods
Study Area and Site Selection
The study was conducted in Changping district (40°2′–40°23′ N, 115°50′–116°29′ E, 1,352 km2) in the northwestern suburbs of Beijing City, China. The local climate is continental with an average annual temperature of ~12°C and an average annual rainfall of ~550 mm. The area is located in the transitional zone between the Yan and Taihang Mountains and the north China Plain, with an altitudinal range from 30 to 1,400 m. The mountains, located in the western and northern parts of the study region, are dominated by natural forest and shrubland habitats and account for about 48% of the total area in the region. The plain area located in the southeastern parts of the region is dominated by urban spaces (29%), planted woodland (12%), and fruit orchards (7%), with abandoned fields and other land-use types accounting for about 4%.
Six distinct habitat types were selected for bee sampling in 2016. They consisted of 1) natural shrubland in the mountainous areas, 2) planted woodland, 3) abandoned fields representing previously cultivated, naturally regenerating grassland habitats, 4) apple orchards, 5) cherry orchards, and 6) peach orchards in the plain area (Fig. 1). In each habitat, five study sites were established at distances of at least 1 km from each other.
Fig. 1.
The distribution of sampling sites in Changping district, Beijing.
Bee Sampling and Classification of Functional Groups
At each study site, three parallel 50 m transects were set at distances of 10 m. Along each transect, wild bees were sampled using sweep nets at monthly intervals from April to September. We defined ‘wild bees’ as all bees not belonging to Apis spp. (Hymenoptera: Apidae), excluding the latter from the analysis. All sites were sampled during three to four consecutive days during each sampling run. Bee sampling took place between 0830 and 1730 hours during sunny, partially cloudy, or bright, overcast days with a wind velocity <2.5 m/s and an air temperature > 15°C. In each sampling run, bees were netted for total of 10 min along each transect, excluding the time to transfer specimens from the net into a polypropylene centrifugal tube.
All wild bees were identified to species level and divided into small (≤7.5 mm), medium (>7.5 and ≤11.5 mm) and large (>11.5 mm) species according to their averaged body size (Wu 1965, Tscheulin et al. 2011), measured using a vernier caliper or based on the literature. Bees were also categorized into social (primitively social to eusocial), solitary or parasitic (cleptoparasitic) bees according to social behavior (Wu 1965, Kremen and M’Gonigle 2015). We furthermore classified bees according to their nest location as aboveground- or belowground-nesting (Wu 1965, Kremen and M’Gonigle 2015).
Plant Sampling
The vegetation was surveyed separately in May and September. At each sampling site, a 20 × 40 m block was set up for vegetation investigation. Five 5 × 5 m plots were then established in the four corners and the center of each block for tree and shrub layer sampling, while one 1 m2 plot was set up within each of the 5 × 5 m plots for herb species sampling. The percentage coverage of each plant species, separated into tree layer, shrub layer, and herb layer, was recorded for each sampling plot. Vascular plant data from the two records of the same plot were pooled, and the maximum coverage recorded for each species on the plot was used for analysis.
Statistical Analyses
Wild bee data from the six sweep netting samples of the same plot were pooled for analysis. The subsequent analysis strongly focuses on abundances because these are considered to be crucial for the delivery of ecosystem services (Naeem and Wright 2003).
Total species richness was estimated based on the Chao 1 species richness estimator calculated in Past3 (Hammer et al. 2001) to account for the differences in sample sizes that result from the varied shapes of investigated habitats, localized weather patterns and arrangements of pan traps. To determine habitat-specific differences in wild bee abundances and their estimated species richness, a one-way ANOVA was calculated using Duncan’ test as post-hoc test in the base package in R 3.2.5 (R Core Team 2016).
Four parameters: coverage of herbaceous, coverage of flowering herbs, coverage of trees, and overall plant species richness, were used as independent parameters to reflect the vegetation composition. To investigate the effects of vegetation composition on wild bee functional groups, we used linear mixed effect models with fixed variance (gls, nlme package version 3.1–128) (Pinheiro et al. 2017). The coverage of herbs (or of zoochorous herbs), trees and plant species richness were not significantly correlated (Spearman correlation, P > 0.36 in all cases, Table 1) with each other, and were hence used as fixed variables, with wild bee abundance included as a dependent variable. The selected gls model was optimized using the step-AIC function in the MASS package (Venables and Ripley 2002). Transformations were applied for response variables to meet normal distribution requirements where necessary. To account for spatial autocorrelation, we fitted gls models to response variables with Gauss–Krüger coordinates treated as spatial covariates, assuming a spherical spatial correlation structure (Pinheiro and Bates 2000). All analysis was performed in R 3.2.5 (R Core Team 2016).
Table 1.
Average species richness and coverage (±SD) of the vegetation at the different habitats
| Habitats | Species richness | % Coverage of the respective layer | ||||||
|---|---|---|---|---|---|---|---|---|
| Herbs | Shrubs | Trees | Overall | Herbs | Shrubs | Trees | Overall | |
| Natural shrubland | 9.2 ± 3.0 | 11.2 ± 3.0 | 0.4 ± 0.9 | 20.8 ± 4.4 | 18.0 ± 11.5 | 86.0 ± 12.5 | 1.4 ± 3.1 | 92.0 ± 4.5 |
| Planted woodland | 21.8 ± 3.1 | 0 | 3.8 ± 1.3 | 25.6 ± 2.4 | 74.2 ± 15.5 | 0 | 57.0 ± 17.9 | 83.0 ± 9.1 |
| Abandoned field | 17.0 ± 5.2 | 0 | 0 | 17.0 ± 5.2 | 82.0 ± 9.1 | 0 | 0 | 85.0 ± 6.1 |
| Apple orchard | 16.4 ± 2.7 | 0 | 1.0 ± 0 | 17.4 ± 2.7 | 62.0 ± 19.2 | 0 | 63.0 ± 11.0 | 80.0 ± 9.4 |
| Cherry orchard | 16.0 ± 1.9 | 0 | 1.0 ± 0 | 17.0 ± 1.9 | 54.2 ± 31.2 | 0 | 71.0 ± 6.5 | 80.0 ± 13.2 |
| Peach orchard | 21.2 ± 5.5 | 0 | 1.0 ± 0 | 22.2 ± 5.5 | 77.0 ± 20.8 | 0 | 64.8 ± 18.5 | 91.0 ± 6.5 |
In order to investigate the effects of plant species composition on different wild bee functional groups, vegetation data based on the coverage of each plant species at each site was firstly condensed by means of principal components analysis (PCA) using PAST version 3 (Hammer et al. 2001). Then, multiple linear regression (MLR) models were used to investigate how the abundance of different wild bee functional groups was linked to the vegetation composition, with the principal components (PCs) used as independent variables. We applied the dredge function in the MuMIn package (Bartoń et al. 2016) to compute models with all possible combinations with no more than four predictors (k ≤ n/3), and then ranked the models using the corrected version of Akaike’s information criterion (AICc) to account for the small sample size shared by all plots (n = 30). For each functional group, the model with the lowest AICc was retained as the best-fitted model.
Results
Bee Diversity and Functional Group Composition
In total, 561 wild bee specimens representing 76 species, 25 genera and six families were collected, and 130 plant species were recorded. Overall bee abundance was highest in shrubland habitats followed by abandoned fields, but overall differences between these and the other habitat types were not significant. The estimated species richness again peaked in natural shrubland (F(5,24) = 2.76, P = 0.042) and was significantly higher there than in all other habitat types except the abandoned fields (Fig. 2). Similarly, the abundance of medium-sized bees (F(5,24) = 4.34, P = 0.006) and solitary bees (F(5,24) = 3.17, P = 0.024) and the diversity of large bees (F(5,24) = 5.22, P = 0.002), belowground-nesting bees (F(5,24) = 2.68, P = 0.046) and solitary bees (F(5,24) = 2.80, P = 0.040) all peaked in natural shrubland habitats, while small-sized bees showed the higher abundance in planted woodland (F(5,24) = 2.89, P = 0.035). The differences between habitats for the other trait groups and measures were nonsignificant, although a trend for highly diverse assemblages encountered at shrubland habitats was visible across all groups. In contrast, social bees were particularly abundant at woodland habitats and abandoned fields, with parasitic bees also showing a high abundance at woodland habitats.
Fig. 2.
Individuals and diversity (±SE) of each bee functional group in habitats. Chao 1 is the species richness estimator.
The number of species uniquely encountered in plots of a single habitat type again peaked in natural shrubland (10 species), followed by abandoned fields (eight species), apple orchards (three species) and peach orchards (two species), while only one species was uniquely encountered in planted woodland and cherry orchard habitats, respectively.
Effects of Vegetation Composition on the Abundance Patterns of Different Bee Functional Groups
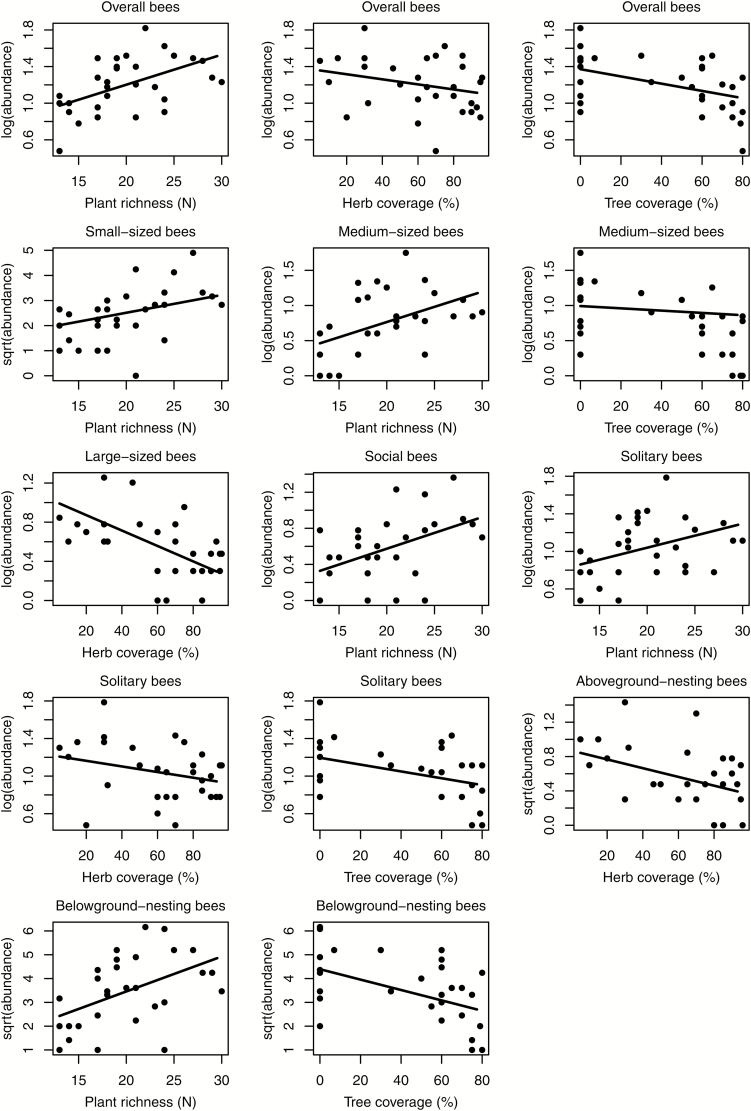
The models revealed that the abundance of bees overall (F(4,26) = 11.48, P = 0.002), of medium-sized bees (F(3,27) = 18.11, P = 0.0002), solitary bees (F(4,26) = 6.53, P = 0.02) and belowground-nesting bees (F(3,27) = 11.01, P = 0.003) was negatively correlated with overall tree coverage (Fig. 3). Abundance of overall bees (F(4,26) = 132.84, P < 0.0001), large-sized bees (F(2,28) = 178.53, P < 0.0001), solitary bees (F(4,26) = 76.54, P < 0.0001) and aboveground-nesting bees (F(2,28) = 5.51, P = 0.03) in contrast was negatively linked to herb coverage. When overall herb coverage was replaced by the coverage of potentially nectar-producing herbs, chiefly excluding the grasses, as explanatory variable, there was no significant correlation with the abundance of any of the functional groups (P > 0.085 in all cases). In contrast, overall plant species richness showed positive links to the abundance of bees overall (F(4,26) = 20.57, P = 0.0001), and to all functional groups except for large-sized bees and aboveground-nesting bees.
Fig. 3.
Effects of vegetation composition on the abundance of each bee functional group.
Effects of Plant Species Composition on Abundance of Bee Functional Groups
In the PCA, the first nine PCs jointly explained 80.5% of the total variance in the plant dataset (Supp. Table S1) and were hence selected as predictors in the subsequent analysis, with the 10th PC only adding a further 2.7% toward the explained variance. In the context of this analysis, the PCs indicate the main trends in the distribution of the different plant species, and hence describe the main shifts in the community composition of the vegetation.
The MLR models comprising the nine PCs as independent parameters showed that only the PCs 1 and 3–7 were significantly linked to the abundance of the different wild bee functional groups (Table 2). Abundance of overall bees was associated with PCs 1, 3, 6, and 7, representing plant communities with a high coverage of Vitexnegundo L. (Tubiflorae: Verbenaceae), Artemisia argyi Lévl. et Van. (Campanulales: Compositae), Digitaria sanguinalis (L.) Scop. (Graminales: Gramineae), Cirsium setosum (Willd.) MB. (Campanulales: Compositae), Trigonotis peduncularis (Trev.) Benth. ex Baker et Moore (Tubiflorae: Boraginaceae) and Artemisia capillaris Thunb. (Campanulales: Compositae).
Table 2.
Effects of plant species on the different bee functional groups
| Functional groups | Explanatory variables | P-value | Estimate of PCs | Effects of plant species with high loading in PCs (estimate) |
|---|---|---|---|---|
| Overall bees | PC1 | 0.005 | − | Convolvulus arvensis (−), Amygdalus persica (−), Malus pumila (−) |
| PC3 | 0.009 | + | Vitex negundo (+), Artemisia argyi (+), Cerasus pseudocerasus (−) | |
| PC6 | 0.035 | + | Digitaria sanguinalis (+), Cirsium setosum (+), Trigonotis peduncularis (+) | |
| PC7 | 0.014 | + | Artemisia argyi (+), Artemisia capillaries (+), Portulaca oleracea (−) | |
| Small-sized bees | PC4 | 0.038 | − | Humulus scandens (+), Vitex negundo (−), Amygdalus persica (−) |
| PC6 | 0.008 | + | Digitaria sanguinalis (+), Cirsium setosum (+), Trigonotis peduncularis (+) | |
| Large-sized bees | PC4 | 0.036 | + | Vitex negundo (+), Amygdalus persica (+), Humulus scandens (−) |
| Social bees | PC6 | 0.017 | + | Digitaria sanguinalis (+), Cirsium setosum (+), Trigonotis peduncularis (+) |
| Solitary bees | PC3 | 0.003 | + | Vitex negundo (+), Artemisia argyi (+), Cerasus pseudocerasus (−) |
| Aboveground-nesting bees | PC4 | 0.012 | + | Vitex negundo (+), Amygdalus persica (+), Humulus scandens (−) |
| PC5 | 0.018 | + | Cerasus pseudocerasus (+), Humulus scandens (+), Malus pumila (−) | |
| Belowground-nesting bees | PC3 | 0.010 | + | Vitex negundo (+), Artemisia argyi (+), Cerasus pseudocerasus (−) |
| PC6 | 0.010 | + | Digitaria sanguinalis (+), Cirsium setosum (+), Trigonotis peduncularis (+) |
−, negative effect; +, positive effect.
Small-sized bee abundance was negatively linked to PC 4 and positively associated with PC 6, representing plant communities with a high coverage of Humulus scandens (Lour.) Merr. (Urticales: Moraceae), D. sanguinalis, C. setosum, and T. peduncularis. Large-sized bees showed the opposite trend in response to PC 4, hence occurring at sites with a high coverage of V. negundo and A. persica.
Abundance of social bees positively correlated with PC 6, representing plant communities with a high coverage of D. sanguinalis, C. setosum, and T. peduncularis. Solitary bees abundance showed a positive association with PC 3, representing plant communities with a high coverage of V. negundo and A. argyi.
Aboveground-nesting bee abundance was positively linked to PCs 4 and 5, representing plant communities with a high coverage of V. negundo, A. persica, and C. pseudocerasus. Abundance of belowground-nesting bees was positively linked to PCs 3 and 6, representing plant communities with a high coverage of V. negundo, A. argyi, D. sanguinalis, C. setosum, and T. peduncularis.
Discussion
Effects of Habitat Types and Vegetation Composition on Overall Wild Bee Diversity
An ideal wild bee habitat needs to provide foraging resources throughout the seasonal activity period of bees, sufficient nest sites and also specific nest materials (Osborne et al. 1991, Grundel et al. 2010). Our results indicate that natural shrubland, the dominant natural habitat type in the study region, represents such a good wild bee habitat, providing sufficient quantities of nectar and pollen, suitable nest sites, and experiencing low levels of human interference to satisfy the resource requirement of wild bees. Hence, in support of our first hypothesis, the highest wild bee diversity was indeed found in this natural habitat.
Although planted woodlands and abandoned fields also harbored diverse plant communities, these plants appeared to provide lower quantities of suitable nectar and pollen sources, because 21.9 and 25.7% of the herb coverage was linked to anemochorous plants in these habitats, respectively. This potentially explains the relatively low diversity of wild bees in these habitat types in comparison to natural shrubland. In orchards, fruit trees, such as apple, cherry and peach, mainly bloomed in April, thus providing a highly ‘pulsed’, short-term nectar resources during their respective short flowering periods (Mandelik et al. 2012). Otherwise, they undergo intensive management such as plowing for soil aeration, weeding and application of pesticides and herbicides, that are likely to all contribute to the low wild bee diversity in these habitats. In accordance with our second hypothesis, overall plant species richness was strongly positively correlated with the abundance of overall bees, with diverse plant species potentially providing a greater diversity and continuity in nectar and pollen resources throughout the seasons of bee activity (Wojcik et al. 2008, Potts et al. 2010).
The negative correlation between tree coverage and the abundance of bees overall could have a number of explanations. Firstly, bees often prefer to forage in open habitats with high irradiance, which is particularly true for solitary and belowground-nesting bees (McKinney and Goodell 2010) that have commonly higher thermal requirements for flight activity and larval development chiefly linked to open or lightly-shaded sites (Osborne et al. 1991, Cane and Tepedino 2001). High tree coverage, in contrast, is generally associated with heavy shading causing cooler, more humid microclimatic conditions. Furthermore, a dense tree coverage also leads to less dense undergrowth and a distinctly lower provision of flowers and florets in the herb and shrub layers while trees are in foliage, rendering these sites less attractive to wild bees (Peet 1978, Wulf and Naaf 2009, McKinney and Goodell 2010). It could be argued that the low abundance of wild bees in habitats with a dense tree coverage could also relate to the sampling technique, where bees were chiefly sampled close to the ground, but not within the tree canopies. Nonetheless, most trees, apart from the fruit trees in the orchards with their aforementioned flowering pulse, were anemochorous, and may be less attractive to bees. In orchards, although the fruit trees were entomophilous, the large areas covered by orchards will have led to a dilutive effect on the density of the limited number of wild bees present in the area.
There are also several potential explanations for the observed negative relationship between herb coverage and the overall abundance of bees. Firstly, plant composition of the herb layer and particularly the balance between the area covered by anemochorous versus zoochorous species could impact the correlation between the herb layer coverage overall and wild bees. Overall, 62.4, 25.7, 21.9, 12.6, 11.8, and 8.9% of herb coverage were anemochorous and therefore potentially less attractive for bees in natural shrubland, abandoned fields, planted woodland, cherry orchards, peach orchards, and apple orchards, respectively. If the coverage of zoochorous herbs only was used as a predictor, there were indeed no significant links between their coverage and the abundance of overall bees and each functional group. A dense undergrowth is also often linked to fertile soils where individual, fast-growing plant species dominate, in turn suppressing the growth of other species and therefore also the plant diversity and nectar resources available throughout the season.
Considering the negative links in at least some bee groups with tree and herbaceous ground cover and the contrasting positive links between wild bee populations and the plant species richness observed in this study, vegetation management aimed at reducing the dominant and anemochorous plant species and at increasing overall plant species richness might benefit bees. For example, reducing fertilizer applications that favor the aforementioned small set of fast-growing, dominating plant species might be an effective way to increase ground cover diversity and enhance the local bee populations (Curry 1994, Steffan-Dewenter and Leschke 2003, Boch et al. 2013).
Effects of Habitat Types and Vegetation Composition on Different Wild Bee Functional Groups
The strong differences in the distribution patterns of wild bees representing different functional groups between the varied habitat types and in response to vegetation composition and plant diversity can potentially be explained by their different resource requirements and dispersal ability.
A large body size is commonly associated with a high dispersal ability, and with a high requirement for energy and hence amount of foraging (Cane et al. 2006, Westphal et al. 2006, Zurbuchen et al. 2010). Therefore, bees with different body size showed varied distribution pattern among habitat types with different levels of available resources. Unlike small- and medium-sized bees, the lack of significant correlations between large-sized bees and overall plant species richness is likely linked to their greater foraging radii and high dispersal rates across the study area, enabling them to flexibly use available resources across the fragmented agricultural landscape (Greenleaf et al. 2007, Bommarco et al. 2010, Woodcock et al. 2014)—with variables acting at larger spatial scale like overall landscape structure potentially being more important predictors for the abundance and diversity of large-sized bees (Chapman et al. 2003, Osborne et al. 2008, Kovács-Hostyánszki et al. 2011).
Social behavior also affected the distribution of wild bees and their response to vegetation variables. Living in colonies (Michener 2000) allows social bees to have a prolonged foraging season and larger dispersal ability in comparison to their solitary cousins (Osborne et al. 1991, Westphal et al. 2008). They can also gain warmth from other individuals in their hives or nests (Heinrich and Esch 1994), and hence allow them the foraging of resources from across the entire landscape. In contrast, solitary bees have a higher general thermal requirement that is chiefly provided by habitats with low tree coverage (Osborne et al. 1991, Klein et al. 2002).
Nest location is shown to be another important factor that is shown to affect the wild bee distribution and their relationship with vegetation variables. The diversity of belowground-nesting bees in our study region peaked in natural shrubland, and their abundance was negatively related to tree coverage, which is likely due to their requirement for warm, bare ground and low disturbance levels at their nesting sites (Williams et al. 2010), while aboveground-nesting bees did not have these requirements. Another difference from belowground-nesting bees is that aboveground-nesting bees often possess a high nesting and floral specialization (Kremen and M’Gonigle 2015, Le Féon et al. 2016), which possibly results in the lack of correlation between aboveground-nesting bee abundance and overall plant richness.
Effects of Plant Species Composition on Wild Bee Diversity
The observed negative influence of the presence of fruit trees on overall bee abundances is likely related to the short flowering time of these trees, the competition of managed honey bees and the intensive management of the orchards throughout the year, including the application of pesticides, and the dilution effect due to large areas of orchards flowering simultaneously in the study region. In contrast, the positive relationship between plant communities containing V. negundo and overall bees and most of the investigated functional groups with the exception of small-sized bees could be contributed to that plant species’ long flowering time and the high quality of both pollen and nectar it provides (Zhang et al. 2010). On the other hand, a differential preference for different flowering plants is likely associated with the observed overall variation in associations between plant communities and the abundance of the different functional groups of wild bees. These associations are nonetheless to date poorly known and understood, and further research into this topic is hence urgently required.
Generally, herb communities which benefited wild bee abundance contained a large number of species belonging to the Sympetalae, while herb communities with a high coverage of Archichlamydeae were negatively associated with bee abundance. The reason could be that Sympetalae at least partly co-evolved with wild bees, and hence are a preferred source of nectar and pollen in comparison to members of the Archichlamydeae (Wernham 1911). Nonetheless, few studies have to date investigated the potential links between bee species as pollinators and these two subclasses within the Dicotyledoneae. However, a sound understanding of these plant-pollinator associations is crucial for both the conservation of pollinator assemblages and the strengthening associated ecosystem services, with the mutual dependencies between pollinators and plant species potentially accelerating their parallel declines in response to agricultural intensification (Biesmeijer et al. 2006, Weiner et al. 2014), especially for those functional groups within the bees that show traits sensitive to other environment change-related factors.
Conclusions
The different resource requirements and foraging radii of different wild bee functional groups result in diverging responses to their environment, including responses to habitat type, vegetation composition, and plant diversity. In our study area, protecting natural shrubland, reducing vegetation coverage to increase overall plant species richness, particularly of Sympetalae and coverage of V. negundo, combined with low intensity of habitat management are likely to be beneficial in supporting a diversity of wild bees that can effectively provide pollination services for zoochorous agricultural crops.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
The authors are very grateful to the farmers for allowing access to their orchards and supporting our experiment. We are also greatly indebted to the Natural Science Foundation of Beijing Municipality (5162017) and the National Natural Science Foundation of China (31470514).
References Cited
- Aizen M. A. and Harder L. D.. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19: 915–918. [DOI] [PubMed] [Google Scholar]
- Bartoń K. 2016. MuMIN: Multi-Model Inference. R package version 1.15.6 https://cran.r-project.org/web/packages/MuMIn/index.html (accessed 29 July 2017).
- Biesmeijer J. C., Roberts S. P., Reemer M., Ohlemüller R., Edwards M., Peeters T., Schaffers A. P., Potts S. G., Kleukers R., Thomas C. D., et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 313: 351–354. [DOI] [PubMed] [Google Scholar]
- Boch S., Prati D., Mueller J., Socher S., Baumbach H., Buscot F., Gockel S., Hemp A., Hessenmoeller D., and Kalko E. K. V.,. et al. 2013. High plant species richness indicates management-related disturbances rather than the conservation status of forests. Basic Appl. Ecol. 14: 496–505. [Google Scholar]
- Bommarco R., Biesmeijer J. C., Meyer B., Potts S. G., Pöyry J., Roberts S. P., Steffan-Dewenter I., and Ockinger E.. 2010. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. Biol. Sci. 277: 2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane J. H., and Tepedino V. J.. 2001. Causes and extent of declines among native North American invertebrate pollinators: detection, evidence, and consequences. Conserv. Ecol. 5: 1. [Google Scholar]
- Cane J. H., Minckley R. L., Kervin L. J., Roulston T. H., and Williams N. M.. 2006. Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 16: 632–644. [DOI] [PubMed] [Google Scholar]
- Chapman R. E., Wang J., and Bourke A. F.. 2003. Genetic analysis of spatial foraging patterns and resource sharing in bumble bee pollinators. Mol. Ecol. 12: 2801–2808. [DOI] [PubMed] [Google Scholar]
- Curry J. P. 1994. Grassland invertebrates: ecology, influence on soil fertility and effects of plant growth. Chapman & Hall, London, United Kingdom. [Google Scholar]
- Forrest J. R. K., Thorp R. W., Kremen C., and Williams N. M.. 2015. Contrasting patterns in species and functional-trait diversity of bees in an agricultural landscape. J. Appl. Ecol. 52: 706–715. [Google Scholar]
- Fründ J., Dormann C. F., Holzschuh A., and Tscharntke T.. 2013. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology. 94: 2042–2054. [DOI] [PubMed] [Google Scholar]
- Garibaldi L. A., Steffan-Dewenter I., Winfree R., Aizen M. A., Bommarco R., Cunningham S. A., Kremen C., Carvalheiro L. G., Harder L. D., Afik O., et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 339: 1608–1611. [DOI] [PubMed] [Google Scholar]
- Greenleaf S. S., Williams N. M., Winfree R., and Kremen C.. 2007. Bee foraging ranges and their relationship to body size. Oecologia. 153: 589–596. [DOI] [PubMed] [Google Scholar]
- Grundel R., Jean R. P., Frohnapple K. J., Glowacki G. A., Scott P. E., and Pavlovic N. B.. 2010. Floral and nesting resources, habitat structure, and fire influence bee distribution across an open-forest gradient. Ecol. Appl. 20: 1678–1692. [DOI] [PubMed] [Google Scholar]
- Hammer Ø., Harper D. A. T., and Ryan P. D.. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4: 1–9. [Google Scholar]
- Hayes J. Jr, Underwood R. M., and Pettis J.. 2008. A survey of honey bee colony losses in the US, fall 2007 to spring 2008. PloS One 3: e4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B., Esch H. 1994. AThermoregulation in bees. Am. Sci. 82: 164–170. [Google Scholar]
- Henle K., Davies K. F., Kleyer M., Margules C., and Settele J.. 2004. Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 13: 207–251. [Google Scholar]
- Hoehn P., Tscharntke T., Tylianakis J. M., and Steffan-Dewenter I.. 2008. Functional group diversity of bee pollinators increases crop yield. Proc. Biol. Sci. 275: 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S., and Vandermeer J. H.. 2009. Contrasting bee foraging in response to resource scale and local habitat management. Oikos. 118: 1174–1180. [Google Scholar]
- Jha S., and Vandermeer J. H.. 2010. Impacts of coffee agroforestry management on tropical bee communities. Biol. Conserv. 143: 1423–1431. [Google Scholar]
- Klein A. M., Steffan-Dewenter I., Buchori D., and Tscharntke T.. 2002. Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv. Biol. 16: 1003–1014. [Google Scholar]
- Klein A. M., Steffan-Dewenter I., and Tscharntke T.. 2003. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. Biol. Sci. 270: 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. M., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., and Tscharntke T.. 2007. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács-Hostyánszki A., Batáry P., and Báldi A.. 2011. Local and landscape effects on bee communities of Hungarian winter cereal fields. Agr. Forest Entomol. 13: 59–66. [Google Scholar]
- Kremen C., and M’Gonigle L. K.. 2015. Small-scale restoration in intensive agricultural landscapes supports more specialized and less mobile pollinator species. J. Appl. Ecol. 52: 602–610. [Google Scholar]
- Kremen C., Williams N. M., and Thorp R. W.. 2002. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. U. S. A. 99: 16812–16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C., Williams N. M., Bugg R. L., Fay J. P., and Thorp R. W.. 2004. The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecol. Lett. 7: 1109–1119. [Google Scholar]
- Lavorel S., and Garnier E.. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 16: 545–556. [Google Scholar]
- Le Féon V., Poggio S. L., Torretta J. P., Bertrand C., Molina G. A. R., Burel F., Baudry J., and Ghersa C. M.. 2016. Diversity and life-history traits of wild bees (Insecta: Hymenoptera) in intensive agricultural landscapes in the Rolling Pampa, Argentina. J. Nat. Hist. 50: 1175–1196. [Google Scholar]
- Mandelik Y., Winfree R., Neeson T., and Kremen C.. 2012. Complementary habitat use by wild bees in agro-natural landscapes. Ecol. Appl. 22: 1535–1546. [DOI] [PubMed] [Google Scholar]
- McKinney A. M., and Goodell K.. 2010. Shading by invasive shrub reduces seed production and pollinator services in a native herb. Biol. Invasions. 12: 2751–2763. [Google Scholar]
- Meeus I., Pisman M., Smagghe G., and Piot N.. 2018. Interaction effects of different drivers of wild bee decline and their influence on host-pathogen dynamics. Curr. Opin. Insect Sci. 26: 136–141. [DOI] [PubMed] [Google Scholar]
- Mendenhall C. D., Sekercioglu C. H., Brenes F. O., Ehrlich P. R., and Daily G. C.. 2011. Predictive model for sustaining biodiversity in tropical countryside. Proc. Natl. Acad. Sci. U. S. A. 108: 16313–16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C. D. 2000. The bees of the world. The Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Miller-Struttmann N. E., Geib J. C., Franklin J. D., Kevan P. G., Holdo R. M., Ebert-May D., Lynn A. M., Kettenbach J. A., Hedrick E., and Galen C.. 2015. Functional mismatch in a bumble bee pollination mutualism under climate change. Science. 349: 1541–1544. [DOI] [PubMed] [Google Scholar]
- Müller A., Diener S., Schnyder S., Stutz K., Sedivy C., and Dorn S.. 2006. Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee-flower relationships. Biol. Conserv. 130: 604–615. [Google Scholar]
- Naeem S., and Wright J. P.. 2003. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 6: 567–579. [Google Scholar]
- Newbold T., Hudson L. N., Hill S. L., Contu S., Lysenko I., Senior R. A., Börger L., Bennett D. J., Choimes A., Collen B., et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature. 520: 45–50. [DOI] [PubMed] [Google Scholar]
- Ollerton J., Winfree R., and Tarrant S.. 2011. How many flowering plants are pollinated by animals?Oikos 120: 321–326. [Google Scholar]
- Osborne J. L., Williams I. H., and Corbet S. A.. 1991. Bees, pollination and habitat change in the European community. Bee World 72: 99–116. [Google Scholar]
- Osborne J. L., Martin A. P., Carreck N. L., Swain J. L., Knight M. E., Goulson D., Hale R. J., and Sanderson R. A.. 2008. Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol. 77: 406–415. [DOI] [PubMed] [Google Scholar]
- Peet R. K. 1978. Forest vegetation of the Colorado Front Range: patterns of species diversity. Plant Ecol. 37: 65–78. [Google Scholar]
- Pinheiro J. C., and Bates D. M.. 2000. Mixed-effects models in S and S-PLUS. Springer Verlag, New York, NY. [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D.,and R Core Team 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1–131 http://cran.r-project.org/web/packages/nlme/index.html (accessed 15 May 2017).
- Potts S. G., Vulliamy B., Dafni A., Ne’eman G., and Willmer P.. 2003. Linking bees and flowers: how do floral communities structure pollinator communities?Ecology. 84: 2628–2642. [Google Scholar]
- Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., and Kunin W. E.. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25: 345–353. [DOI] [PubMed] [Google Scholar]
- R Core Team.. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Scherber C., Milcu A., Partsch S., Scheu S., and Weisser W. W.. 2006. The effects of plant diversity and insect herbivory on performance of individual plant species in experimental grassland. J. Ecol. 94: 922–931. [Google Scholar]
- Steffan-Dewenter I., and Leschke K.. 2003. Effects of habitat management on vegetation and above-ground nesting bees and wasps of orchard meadows in Central Europe. Biodivers. Conserv. 12: 1953–1968. [Google Scholar]
- Steffan-Dewenter I., Münzenberg U., Bürger C., Thies C., and Tscharntke T.. 2002. Scale-dependent effects of landscape context on three pollinator guilds. Ecology. 83: 1421–1432. [Google Scholar]
- Stokstad E. 2007. The case of the empty hives. Science. 316: 970–972. [DOI] [PubMed] [Google Scholar]
- Tilman D., Knops J., Wedin D., Reich P., Ritchie M., and Siemann E.. 1997. The influence of functional diversity and composition on ecosystem processes. Science. 277: 1300–1302. [Google Scholar]
- Tscheulin T., Neokosmidis L., Petanidou T., and Settele J.. 2011. Influence of landscape context on the abundance and diversity of bees in Mediterranean olive groves. Bull. Entomol. Res. 101: 557–564. [DOI] [PubMed] [Google Scholar]
- Venables W. N., and Ripley B. D.. 2002. Modern applied statistics with S-PLUS. Springer, New York, NY. [Google Scholar]
- Weiner C. N., Werner M., Linsenmair K. E., and Blüthgen N.. 2014. Land-use impacts on plant-pollinator networks: interaction strength and specialization predict pollinator declines. Ecology. 95: 466–474. [DOI] [PubMed] [Google Scholar]
- Wernham H. F. 1911. Floral evolution: with particular reference to the sympetalous Dicotyledons II. The Archichlamydeae, and their phylogenetic relations to the Sympetalae. New Phytol. 10: 109–120. [Google Scholar]
- Westphal C., Steffan-Dewenter I., and Tscharntke T.. 2006. Bumblebees experience landscapes at different spatial scales: possible implications for coexistence. Oecologia. 149: 289–300. [DOI] [PubMed] [Google Scholar]
- Westphal C., Bommarco R., Carré G., Lamborn E., Morison N., Petanidou T., Potts S. G., Roberts S. P. M., Szentgyorgyi H., and Tscheulin T.,. et al. 2008. Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 78: 653–671. [Google Scholar]
- Westrich P. 1996. Habitat requirements of Central European bees and the problems of partial habitats, pp. 1–16. InMatheson A., Buchmann S. L., O’Toole C., Westrich P., Williams I. H. (eds.), The conservation of bees. Academic Press, London, United Kingdom. [Google Scholar]
- Williams N. M., Crone E. E., T’ai H. R., Minckley R. L., Packer L., and Potts S. G.. 2010. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 143: 2280–2291. [Google Scholar]
- Wojcik V. A., Frankie G. W., Thorp R. W., and Hernandez J. L.. 2008. Seasonality in bees and their floral resource plants at a constructed urban bee habitat in Berkeley, California. J. Kansas Entomol. Soc. 81: 15–28. [Google Scholar]
- Woodcock B. A., Harrower C. A., Redhead J. W., Edwards M., Vanbergen A. J., Heard M. S., Roy D. B., and Pywell R. F.. 2014. National patterns of functional diversity and redundancy in predatory ground beetles and bees associated with key UK arable crops. J. Appl Ecol. 51: 142–151. [Google Scholar]
- Wu Y. R. 1965. Economic Insect Fauna of China. Fasc. 9. Hymenoptera: Apoidea. Science Press, Beijing, China. [Google Scholar]
- Wulf M., and Naaf T.. 2009. Herb layer response to broadleaf tree species with different leaf litter quality and canopy structure in temperate forests. J. Veg. Sci. 20: 517–526. [Google Scholar]
- Xu H. L., Yang J. W., and Sun J. R.. 2009. Current status on the study of wild bee-pollinators and conservation strategies in China. Acta Phytophylacica Sinica. 36: 371–376. [Google Scholar]
- Zhang B. W., Wang L. L., Zhao L. C., Chen B. Y., Kui Y. L., and Fu H. Y.. 2010. Investigation of wild nectar and pollen plant resources in Beijing. Journal of Beijing Forestry University. 32: 18–22. [Google Scholar]
- Zurbuchen A., Landert L., Klaiber J., Müller A., Hein S., and Dorn S.. 2010. Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol. Conserv. 143: 669–676. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.