Abstract
Risk factors can drive socioeconomic inequalities in cardiovascular disease (CVD) through differential exposure and differential vulnerability. In this paper, we show how econometric decomposition directly enables simultaneous, policy-oriented assessment of these 2 mechanisms. We specifically estimate contributions of neighborhood environment and proximal risk factors to socioeconomic inequality in CVD incidence via these mechanisms. We followed 5,608 participants in the Multi-Ethnic Study of Atherosclerosis (2000–2012) to their first CVD event (median length of follow-up, 12.2 years). We used a summary measure of baseline socioeconomic position (SEP). Covariates included baseline demographics, neighborhood characteristics, and psychosocial, behavioral, and biomedical risk factors. Using Poisson models, we decomposed the difference (inequality) in incidence rates between low- and high-SEP groups into contributions of 1) differences in covariate means (differential exposure) and 2) differences in CVD risk associated with covariates (differential vulnerability). Notwithstanding large uncertainty in neighborhood estimates, our analysis suggested that differential exposure to poorer neighborhood socioeconomic conditions, adverse social environment, diabetes, and hypertension accounted for most of the inequality. Psychosocial and behavioral contributions were negligible. Further, neighborhood SEP, female sex, and white race were more strongly associated with CVD among low-SEP (vs. high-SEP) participants. These differentials in vulnerability also accounted for nontrivial portions of the inequality and could have important implications for intervention.
Keywords: cardiovascular disease, decomposition, differential vulnerability, Multi-Ethnic Study of Atherosclerosis, neighborhood, residence characteristics, socioeconomic inequality, socioeconomic status
Etiological investigation of socioeconomic inequalities in cardiovascular disease (CVD) has been a long-standing pursuit since the early 1960s (1). Accumulating evidence suggests that social differentials in exposure to risk factors, such as smoking, physical inactivity, diabetes, and hypertension, account for 20% to over 50% of the higher CVD burden among socioeconomically disadvantaged groups (1–12). Although considerable evidence has linked neighborhood conditions and CVD (13–20), less attention has been given to quantifying how much of the socioeconomic inequality in CVD is driven by adverse neighborhood environment (21–24).
Risk factors may drive social inequalities in health through differential exposure and differential vulnerability (25–27): Low socioeconomic position (SEP) may increase exposure to CVD risk factors (e.g., adverse environments and associated psychobiological responses), and it can also exacerbate vulnerability to CVD (i.e., risk) given such exposures (26, 28). While differential exposure (a mediation hypothesis) has received the most attention, assessments of differential vulnerability (an interaction hypothesis) remain scarce (29–31).
Differential vulnerability—the notion that SEP could modify the effects of risk factors—is plausible for at least 2 reasons. First, low SEP constrains a person’s access to flexible material (e.g., health care) and social (e.g., social support) resources (32–34). A lack of such resources compromises the ability to manage risk factors or buffer their pathophysiological consequences (26, 28), potentially exacerbating their cardiovascular effects among the disadvantaged. Second, clustering of risk factors in low-SEP groups (9, 35–42) may trigger biological synergism. Depressive symptoms, for example, appear to magnify the associations of smoking and diabetes with coronary heart disease risk (43, 44). Low-SEP groups may disproportionately experience such stronger effects given co-occurrence of these risk factors.
The absence of systematic evidence on differential vulnerability (30, 31) might reflect the paucity of methods for quantifying mechanistic contributions of interaction, until the recent development of epidemiologic decompositions of mediation and interaction (45, 46). In this article, we show how econometric decomposition (47–49), which has become increasingly attractive in public health research (50–52), can also provide a unified empirical framework with which to simultaneously quantify differential exposure (mediation) and differential vulnerability (interaction) contributions of risk factors to health inequalities. Analytically, econometric decomposition assesses mediation while relaxing the requirement of no exposure-mediator interaction by isolating the contribution of such an interaction.
Econometric decomposition also enables estimation of more policy-relevant contributions than traditional epidemiologic approaches, in 2 ways. First, contributions reflect counterfactual intervention scenarios in which exposure levels and associated disease risk among the disadvantaged are brought up to those present among the most advantaged. Such scenarios correspond to more plausible policies than those entailed in calculating population attributable fractions (complete elimination of risk factors) (53) and the “proportion explained/mediated” in mediation analyses (invoking unrealistic “cross-worlds” natural indirect effects) (54, 55). Second, contributions are estimated directly on the absolute, additive scale, with arguably more immediate policy relevance than relative measures (53, 56).
Using rich, multiethnic cohort data, we estimated contributions of neighborhood, psychosocial, behavioral, and biomedical risk factors to socioeconomic inequality in CVD incidence. We hypothesized that while differential exposure to these factors could account for the majority of CVD inequality, differential vulnerability contributions would also be sizable. Contributions, through either mechanism, would be larger for more causally distal risk factors (e.g., neighborhoods) than for factors more proximal to CVD (e.g., biomarkers).
METHODS
Study sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of subclinical CVD in 6,814 adults aged 45–84 years who self-identified as white, black, Chinese, or Latino and were free of clinically overt CVD at baseline. Participants were recruited at 6 US sites: Forsyth County, North Carolina; New York, New York; Baltimore, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles County, California. The baseline examination was conducted in 2000–2002, with follow-up examinations being held every 1.5–2 years (57). Institutional review boards approved the study at each site. All participants gave written informed consent. Of the 6,191 participants who consented to participation in the MESA Neighborhood Study, 583 were excluded due to incomplete covariate data, leaving 5,608 participants in the study sample. Excluded participants had similar characteristics and follow-up time as those included, but they were more likely to be black and to live in poorer neighborhoods.
Measures
Incident CVD was defined as first definite angina, probable angina followed by revascularization, myocardial infarction, resuscitated cardiac arrest, coronary heart disease death, stroke, or stroke death on or before and adjudicated through December 31, 2012 (median duration of follow-up from baseline, 12.2 years; largely uniform across sample subgroups). Every 9–12 months, participants (or proxies) were asked about hospitalizations, CVD diagnoses, and death. Possible CVD events were extracted from available records and were reviewed and adjudicated by an independent committee (57).
Socioeconomic position
We measured SEP as a summary score (range, 0–10) of measures of participants’ baseline income, education, and wealth, following the method of Lemelin et al. (58) (see Web Appendix 1, available at https://academic.oup.com/aje). Larger values indicated higher SEP. Overall scores were then categorized into low-, middle-, and high-SEP tertiles.
Neighborhood characteristics
Participants’ baseline neighborhoods were proxied by Census 2000 census tracts (basic characteristics are given in Web Table 1). Neighborhood SEP was captured by a scale summarizing scores for 6 standardized variables (Web Appendix 2) representing tract-level wealth, income, education, and occupation (13). Higher values indicated better socioeconomic conditions. We also included percentage of foreign-born residents in each neighborhood (highly correlated with percent Latino) to capture neighborhoods’ ethnic makeup.
To characterize the quality of social and physical neighborhood environments, we used scales developed in the MESA Neighborhood Ancillary Study, detailed elsewhere (59). All neighborhood scales were constructed using conditional empirical Bayes estimation to improve accuracy for tracts with few respondents (59).
Social environment was measured by means of a score summarizing 3 composite scales of perceived neighborhood social cohesion, aesthetic quality, and safety (Cronbach’s α = 0.89). Physical environment was assessed as a summary score from scales of walkability and perceived availability of healthy food (Cronbach’s α = 0.75). Higher scores indicated a better physical environment. Perceived availability of healthy food captures constructs relevant to dietary decision-making (60, 61) and may be more consequential for dietary behavior than the spatial availability of food stores (62, 63). One-mile (1.6-km) densities of total physical activity resources were derived as in prior work (64, 65) using Walls & Associates’ National Establishment Time-Series database (66). We also used tract-level population density to account for systematic variability in neighborhood amenities by location (urban/suburban) and residential density across study sites.
Individual-level covariates
We used baseline measures of the participants’ demographic, psychosocial, behavioral, and biomedical characteristics. Distributions of the characteristics are shown in Table 1, overall and by tertile of SEP index.
Table 1.
Observed Baseline Characteristics of Sample Participants by Socioeconomic Position, Multi-Ethnic Study of Atherosclerosis, 2000–2012
| Characteristic | Tertile of Individual-Level SEP Index Score | P for Trend | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (Score 0–4) (n = 2,227) | Middle (Score 5–7) (n = 1,879) | High (Score 8–10) (n = 1,452) | Overall (n = 5,608) | ||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | ||
| Individual SEP measures | |||||||||
| Annual family income, thousands of dollars | 21.7 (13.5) | 53.2 (23.1) | 91.5 (23.6) | 50.3 (34.2) | <0.001 | ||||
| Education, years | 10.4 (4.0) | 14.0 (2.3) | 16.8 (1.6) | 13.3 (4.0) | <0.001 | ||||
| Wealth index, no. of assets (possible range, 0–4) | 1.4 (1.0) | 2.9 (0.8) | 3.5 (0.6) | 2.5 (1.2) | <0.001 | ||||
| Demographic characteristics | |||||||||
| Age ≥65 years | 52.8 | 38.2 | 31.0 | 42.3 | <0.001 | ||||
| Female sex | 60.0 | 49.7 | 42.6 | 52.0 | <0.001 | ||||
| Race | |||||||||
| White | 19.3 | 48.7 | 62.3 | 40.3 | <0.001 | ||||
| Chinese | 15.7 | 8.8 | 10.1 | 12.0 | |||||
| Black | 26.3 | 28.3 | 23.6 | 26.3 | |||||
| Latino | 38.7 | 14.2 | 4.1 | 21.5 | |||||
| Married/cohabiting | 48.5 | 65.7 | 79.9 | 62.4 | <0.001 | ||||
| Population density | |||||||||
| Bottom tertile | 21.0 | 42.4 | 41.1 | 33.4 | <0.001 | ||||
| Middle tertile | 38.2 | 32.0 | 27.6 | 33.4 | |||||
| Top tertile | 40.8 | 25.6 | 31.3 | 33.3 | |||||
| Neighborhood environmenta | |||||||||
| % foreign-born | 28.7 (20.6) | 17.1 (15.8) | 14.9 (12.4) | 21.2 (18.3) | <0.001 | ||||
| Neighborhood SEP score | −3.0 (5.9) | 0.8 (5.5) | 4.2 (5.4) | 0.2 (6.3) | <0.001 | ||||
| Perceived physical environment | −0.5 (1.5) | −0.4 (1.9) | 0.5 (2.3) | −0.2 (1.9) | <0.001 | ||||
| Physical activity resources | 4.0 (5.6) | 3.7 (6.4) | 5.3 (8.7) | 4.2 (6.8) | <0.001 | ||||
| Perceived social environment | −1.4 (2.5) | 0.3 (2.6) | 1.1 (2.5) | −0.2 (2.7) | <0.001 | ||||
| Psychosocial factors | |||||||||
| Perceived lifetime discrimination (possible range, 0–6) | 0.6 (1.0) | 0.8 (1.1) | 0.9 (1.1) | 0.7 (1.1) | <0.001 | ||||
| Chronic stress (Chronic Burden Scale score; possible range, 0–5) | 1.2 (1.2) | 1.1 (1.1) | 1.0 (1.1) | 1.1 (1.2) | 0.0149 | ||||
| Depressive symptoms (CES-D score; possible range, 0–60) | 9.1 (8.4) | 6.8 (6.7) | 5.4 (5.9) | 7.4 (7.4) | <0.001 | ||||
| Social support (ESSI score; possible range, 6–30) | 23.6 (5.7) | 24.1 (5.2) | 25.2 (4.5) | 24.2 (5.3) | <0.001 | ||||
| Behavioral factors | |||||||||
| Pack-years of smoking | 10.3 (19.3) | 13.1 (23.3) | 9.3 (17.9) | 11.0 (20.4) | 0.415 | ||||
| Moderate/vigorous exercise, thousands of MET-hours/week | 5.6 (6.0) | 6.5 (6.7) | 5.3 (4.6) | 5.8 (6.0) | 0.437 | ||||
| Body mass indexb | 28.6 (5.6) | 28.5 (5.4) | 27.4 (4.8) | 28.3 (5.4) | <0.001 | ||||
| Current alcohol consumption (yes) | 41.8 | 62.3 | 75.6 | 57.4 | <0.001 | ||||
| Uninsured (no health insurance) | 15.6 | 4.3 | 1.5 | 8.1 | <0.001 | ||||
| Biomedical factors | |||||||||
| Diabetesc | 16.6 | 10.0 | 6.2 | 11.7 | <0.001 | ||||
| Hypertensiond | 50.7 | 41.6 | 34.6 | 43.5 | <0.001 | ||||
| Total cholesterol, mg/dL | 194.6 (34.9) | 195.3 (35.4) | 191.2 (33.6) | 193.9 (34.8) | 0.009 | ||||
| Interleukin-6, pg/mL | 1.7 (1.2) | 1.5 (1.1) | 1.3 (1.2) | 1.5 (1.2) | <0.001 | ||||
| C-reactive protein, mg/dL | 3.9 (5.3) | 3.4 (4.3) | 3.0 (4.9) | 3.5 (4.9) | <0.001 | ||||
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; ESSI, Emotional Social Support Inventory; MET, metabolic equivalent of task; SD, standard deviation; SEP, socioeconomic position.
a See Methods section of text for details on individual measures. Conditions improve as values increase.
b Weight (kg)/height (m)2.
c Fasting glucose concentration ≥126 mg/dL and/or use of insulin/oral hypoglycemic medication (74).
d Systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication (73).
Among psychosocial factors, we measured perceived discrimination as a summary score (range, 0–6) of the 6-domain Lifetime Discrimination Scale (67). Chronic stress was measured using the Chronic Burden Scale (68) (range, 0–5), summarizing participants’ ratings of their ongoing material/social problems as moderately/very stressful. Depressive symptoms were measured using the 20-item Centers for Epidemiologic Studies Depression Scale (69) (range, 0–60). Social support was measured using the 7-item Emotional Social Support Inventory (70) (range, 6–30), which assesses perceived frequency of the availability of social/emotional support.
Behavioral measures included smoking (pack-years of cigarettes smoked), physical activity, body mass index (weight (kg)/height (m)2), current alcohol consumption (yes/no), and lack of health insurance. Typical-week physical activity was assessed using a detailed questionnaire (71), and then metabolic equivalent values were derived from durations of moderate/vigorous exercise (72). We included a diet index in preliminary analyses but eventually dropped it, as it made other covariate estimates imprecise while hardly contributing to the inequality.
As biomedical risk factors, we included whether participants had hypertension (systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication (73)) or diabetes (fasting glucose concentration ≥126 mg/dL and/or use of insulin/oral hypoglycemic medication (74)), as well as their blood levels of total cholesterol (mg/dL) and the inflammatory markers C-reactive protein (mg/dL) and interleukin-6 (pg/mL).
Statistical analysis
Econometric decomposition
While econometric decomposition has been popular in the social sciences (47, 75), its epidemiologic applications remain few (50–52). Here, we conceptually discuss decomposition quantities, particularly: 1) their correspondence with the mechanistic notions of differential exposure and differential vulnerability; 2) their underlying counterfactual intervention scenarios; and 3) their practical interpretation.
In simplified notation, socioeconomic inequality (difference) in incident CVD, ΔCVD, can be expressed as
| (1) |
where , the estimated Poisson incidence rate, is a function of mean values of characteristics and coefficients , for socioeconomic groups L (low-SEP) and H (high-SEP). Through further algebraic manipulation, ΔCVD can be decomposed into 2 components:
| (2) |
Equation 2 is the standard Oaxaca-Blinder linear decomposition expression, which approximately holds for nonlinear models (76), and clearly delineates the components. Derivation of equation 2, which involves key counterfactuals, is detailed in Web Appendix 3.
ΔE and ΔV are traditionally known as the “explained” and “unexplained” components of the decomposition (51, 77), respectively. That is because group differences in observed characteristics explain a portion (equal to ΔE) of the inequality (ΔCVD). The remaining portion, unexplained by observed characteristics, is captured in ΔV, the differences in coefficients . These coefficient differences are, fundamentally, tests of additive interactions between SEP and covariates, as they capture the excess CVD risk associated with covariates at different SEP levels and thus represent empirical assessments of the differential vulnerability hypothesis. Since measures the prevalence of exposure to risk factor X and measure CVD vulnerability (risk associated with exposure), ΔE and ΔV estimate differential exposure and differential vulnerability contributions to inequality, respectively.
ΔE and ΔV capture the total change in ΔCVD that would be expected if, respectively, exposure to and CVD vulnerability associated with considered risk factors in the low-SEP group were set, by means of some intervention(s), to the high-SEP levels. Such hypothetical “interventions” are ceteris paribus; that is, they assume that all other conditions remain unchanged (47) (though inherent in regression-based analysis, this assumption is untenable in reality, where conditions do change). Aggregate ΔE and ΔV can undergo “detailed” decomposition to retrieve the respective contributions of individual covariates, using a first-order Taylor series expansion (78, 79). Causal interpretation of aggregate contributions requires ignorability of unmeasured confounders with respect to SEP, whereas for covariate-specific contributions, no unmeasured confounding must strictly hold (47).
ΔE and ΔV values can be positive or negative. Given a positive ΔCVD (higher incidence at low SEP), a positive ΔE for covariate X suggests that differential exposure to X across SEP groups is the culprit; eliminating that differential would reduce ΔCVD. A negative ΔE for a covariate, on the other hand, would suggest that differential exposure to that covariate is “protective” against greater inequality; ΔCVD would have been larger than it actually is, absent that differential.
Modeling strategy
We used Stata’s (StataCorp LP, College Station, Texas) nonlinear decomposition routine—mvdcmp—by Powers et al. (79), with standard errors calculated using the delta method (80). In all analyses, we decomposed the observed socioeconomic difference in CVD incidence rates with reference to the high-SEP group, which had the lowest incidence.
Traditionally-reported multivariable decompositions, including all covariates in 1 model, assume additivity of covariate contributions and sum them to 100% merely as a mathematical artifact. This is problematic, particularly for more upstream factors such as neighborhood conditions, whose contributions are likely to be mediated through the proximal psychosocial, behavioral, and biomedical factors (13–20). To respect the plausible causal ordering of covariates, we incorporated covariate blocks sequentially, in 3 sets of decomposition models: 1) models adjusting for all demographic factors; 2) models further adjusting for all neighborhood characteristics; and 3) models additionally adjusting for all psychosocial, behavioral, and biomedical factors together. By adjusting for covariates presumed to be causally/temporally antecedent (confounders) to the covariate set of interest, our strategy allows the “total” contributions of demographic characteristics (model 1), neighborhood factors (model 2), and proximal risk factors (model 3) to more realistically sum to more than 100% (81). Stata code for all decompositions is available in Web Appendix 4.
Sensitivity analyses
To aid interpretation of decomposition estimates and to assess whether vulnerability differentials (ΔV) reflect substantive interactions, we performed 2 additional analyses. First, we separately fitted Poisson incidence models for each SEP group (sequentially adjusted as above) and compared coefficients across these models using the Chow test (82), accounting for error correlation across models (83). Second, in similar models but pooling socioeconomic groups together, we assessed effect modification by SEP for each covariate on both additive and multiplicative scales. We further assessed the sensitivity of our findings to alternative specification of covariates (e.g., categorical vs. continuous specifications of age, depressive symptoms, smoking, or cholesterol).
RESULTS
Differential exposure: socioeconomic differences in prevalence of risk factors
Among the 5,608 participants (62,846 person-years (PY)), 619 CVD events occurred, including 430 coronary heart disease events and 179 stroke events. Incidence rates were inversely patterned by SEP (Figure 1). Relative to high-SEP participants, lower-SEP participants were more likely to be older, female, and Latino; less likely to be white; and more likely to live in neighborhoods with poorer socioeconomic, physical, and social environments (Table 1). Lower-SEP participants also had more severe depressive symptoms, higher prevalences of diabetes and hypertension, elevated levels of inflammatory biomarkers, and less access to health insurance.
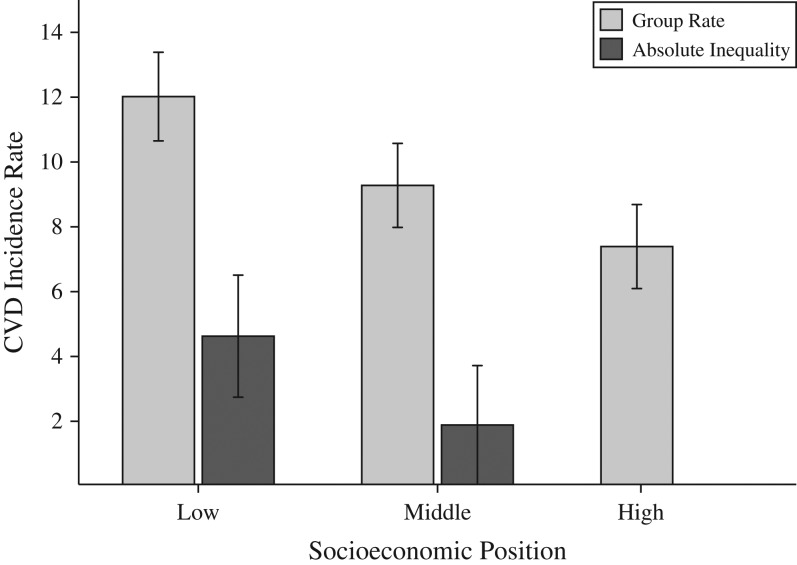
Figure 1.
Observed cardiovascular disease (CVD) incidence rates and absolute inequalities by socioeconomic position (SEP) (n = 5,608), Multi-Ethnic Study of Atherosclerosis, 2000–2012. The incidence rate is the observed number of incident CVD cases per 1,000 person-years. (P < 0.001 for linear trend in incidence rates across SEP groups.) The absolute inequality is the difference between the rate in the lower-SEP groups (low and middle SEP) and the rate in the high-SEP group. Decomposition is applied to these observed group differences. Low-, middle-, and high-SEP categories are tertiles of the baseline SEP index and correspond to SEP score ranges of 0–4, 5–7, and 8–10, respectively. Bars, 95% confidence intervals.
Differential vulnerability: socioeconomic differences in CVD risk associated with risk factors
We found little evidence that associations of risk factors with CVD incidence differed by SEP, with a few exceptions. In Poisson models stratified by SEP and sequentially adjusted for confounding (Table 2), being female was generally “protective” against CVD, but this protective association was weaker in lower-SEP groups (for female sex, incidence rate ratios (IRRs) were 0.5 (95% confidence interval (CI): 0.4, 0.6) and 0.3 (95% CI: 0.2, 0.4) in low- and high-SEP participants, respectively). Being white (vs. black) was more strongly associated with CVD among low-SEP participants than among high-SEP participants (IRRs were 1.6 (95% CI: 1.2, 2.2) and 0.9 (95% CI: 0.6, 1.4), respectively). However, being Latino (vs. white) was protective only among low-SEP participants (IRRs were 0.7 (95% CI: 0.5, 0.9) and 2.3 (95% CI: 1.2, 4.3) among low- and high-SEP participants, respectively).
Table 2.
Sequentially Adjusted Associations of Cardiovascular Disease Risk Factors With Incident Cardiovascular Disease by Baseline Socioeconomic Position, Multi-Ethnic Study of Atherosclerosis, 2000–2012
| Covariate | Tertile of SEP | |||||||
|---|---|---|---|---|---|---|---|---|
| Low SEP (n = 2,227) | Middle SEP (n = 1,879) | High SEP (n = 1,452) | Overall (n = 5,608) | |||||
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | |
| Model 1a | ||||||||
| Baseline SEP tertile | ||||||||
| Low | 1.6b | 1.3, 2.1 | ||||||
| Middle | 1.2c | 1.0, 1.5 | ||||||
| High | 1.0 | Referent | ||||||
| Demographic characteristics | ||||||||
| Age ≥65 years | 2.5b | 1.9, 3.2 | 2.6b | 2.0, 3.5 | 2.5b | 1.7, 3.5 | 2.6b | 2.2, 3.0 |
| Female sex | 0.5b,d | 0.4, 0.6 | 0.6b,d | 0.4, 0.8 | 0.3b | 0.2, 0.4 | 0.5b | 0.4, 0.6 |
| Race | ||||||||
| Whitee (referent) | 1.6b,d | 1.2, 2.2 | 1.3 | 0.9, 1.9 | 0.9 | 0.6, 1.4 | 1.3f | 1.1, 1.6 |
| Chinese | 0.4b | 0.3, 0.7 | 0.9 | 0.5, 1.5 | 0.6 | 0.3, 1.3 | 0.6b | 0.4, 0.8 |
| Black | 0.6b,d | 0.5, 0.9 | 0.7 | 0.5, 1.1 | 1.1 | 0.7, 1.7 | 0.8f | 0.6, 0.9 |
| Latino | 0.7d,f | 0.5, 0.9 | 1.1d | 0.7, 1.6 | 2.3f | 1.2, 4.3 | 0.9 | 0.7, 1.1 |
| Married/cohabiting | 0.8 | 0.7, 1.1 | 0.8 | 0.6, 1.2 | 0.9 | 0.6, 1.4 | 0.8f | 0.7, 1.0 |
| Population density | ||||||||
| Bottom tertile | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent |
| Middle tertile | 0.9 | 0.7, 1.2 | 1.2 | 0.9, 1.7 | 1.0 | 0.6, 1.5 | 1.0 | 0.8, 1.2 |
| Top tertile | 0.7c | 0.5, 1.0 | 1.0 | 0.7, 1.5 | 0.8 | 0.5, 1.2 | 0.8f | 0.7, 1.0 |
| Model 2g | ||||||||
| Neighborhood environmenth | ||||||||
| % foreign-born | 1.0 | 0.9, 1.2 | 1.0 | 0.8, 1.3 | 0.9 | 0.7, 1.3 | 1.0 | 0.9, 1.1 |
| Neighborhood SEP | 1.0d | 0.8, 1.2 | 0.8 | 0.6, 1.1 | 0.7f | 0.5, 0.9 | 0.9c | 0.8, 1.0 |
| Physical environment | 1.0 | 0.8, 1.3 | 1.2 | 0.9, 1.5 | 1.1 | 0.9, 1.4 | 1.1 | 1.0, 1.2 |
| Total PA resources | 1.0 | 0.8, 1.2 | 1.0 | 0.8, 1.2 | 1.0 | 0.8, 1.2 | 1.0 | 0.9, 1.1 |
| Social environment | 0.9 | 0.8, 1.1 | 1.2 | 0.9, 1.6 | 1.1 | 0.8, 1.5 | 1.0 | 0.9, 1.2 |
| Model 3i | ||||||||
| Psychosocial factorsh | ||||||||
| Lifetime discrimination | 1.0 | 0.9, 1.2 | 1.0 | 0.9, 1.2 | 1.0 | 0.8, 1.2 | 1.0 | 0.9, 1.1 |
| Chronic stress | 1.0 | 0.9, 1.2 | 0.9 | 0.8, 1.1 | 1.0 | 0.8, 1.2 | 1.0 | 0.9, 1.1 |
| Depressive symptoms | 1.0 | 0.9, 1.2 | 1.3b | 1.1, 1.5 | 1.0 | 0.8, 1.3 | 1.1c | 1.0, 1.2 |
| Social support | 1.0 | 0.9, 1.1 | 0.9 | 0.8, 1.1 | 0.9 | 0.7, 1.2 | 1.0 | 0.9, 1.1 |
| Behavioral factors | ||||||||
| Uninsured (no health insurance) | 1.0 | 0.7, 1.5 | 2.7b | 1.5, 4.8 | 2.2 | 0.7, 7.1 | 1.3 | 0.9, 1.8 |
| Pack-years of smokingh | 1.1f | 1.0, 1.2 | 1.0 | 0.9, 1.2 | 1.1 | 0.9, 1.2 | 1.1c | 1.0, 1.1 |
| Current alcohol consumption | 0.8 | 0.7, 1.1 | 0.9 | 0.7, 1.2 | 0.8 | 0.5, 1.2 | 0.9c | 0.7, 1.0 |
| Moderate/vigorous PAh | 0.9 | 0.8, 1.0 | 0.9 | 0.7, 1.0 | 0.9 | 0.7, 1.2 | 0.9f | 0.8, 1.0 |
| Body mass indexh,j | 1.0 | 0.8, 1.1 | 1.0 | 0.9, 1.2 | 1.1 | 0.9, 1.4 | 1.0 | 0.9, 1.1 |
| Biomedical factors | ||||||||
| Diabetesk (yes/no) | 1.6b | 1.2, 2.1 | 1.4c | 1.0, 2.1 | 1.6c | 1.0, 2.8 | 1.5b | 1.2, 1.8 |
| Hypertensionl (yes/no) | 1.8b | 1.4, 2.4 | 2.1b | 1.5, 2.8 | 2.1b | 1.5, 3.1 | 2.0b | 1.7, 2.4 |
| Total cholesterolh | 1.1 | 1.0, 1.2 | 1.0 | 0.9, 1.2 | 1.1 | 0.9, 1.4 | 1.1f | 1.0, 1.2 |
| Interleukin-6h | 1.1f | 1.0, 1.2 | 1.0 | 0.9, 1.2 | 1.2f | 1.0, 1.4 | 1.1b | 1.0, 1.2 |
| C-reactive proteinh | 1.0 | 0.9, 1.1 | 1.0 | 0.9, 1.2 | 1.0 | 0.8, 1.2 | 1.0 | 1.0, 1.1 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; PA, physical activity; SEP, socioeconomic position.
a IRRs for listed SEP and demographic variables adjusted for in model 1 (demographic factors; demographic factors + SEP in the “overall” model).
bP < 0.01.
cP < 0.10.
d IRR is different from its counterpart in the high-SEP model, based on the Chow test (82) following “seemingly unrelated estimation” (P < 0.1).
e IRRs in this row are for the reference group (white race), and they come from model 1 but with black race used as the referent. Additive- and multiplicative-scale interactions between white race and low SEP were also highly significant (P < 0.001) in models pooling SEP groups together (see Web Table 2).
fP < 0.05.
g IRRs for listed neighborhood variables adjusted for in model 2 (demographic factors + neighborhood variables).
h IRRs are for a 1–standard-deviation increase in the value of the variable (see Table 1 for units).
i IRRs for listed psychosocial, behavioral, and biomedical factors adjusted for in model 3 (demographic factors + neighborhood variables + psychosocial factors + behavioral factors + biomedical factors).
j Weight (kg)/height (m)2.
k Fasting glucose concentration ≥126 mg/dL and/or use of insulin/oral hypoglycemic medication (74).
l Systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication (73).
Overall, a 1–standard-deviation higher neighborhood SEP score (i.e., better socioeconomic conditions) was “protective” against CVD (Table 2). This protective association, however, was stronger in high-SEP individuals (IRRs for neighborhood SEP among participants with low and high individual SEP were 1.0 (95% CI: 0.8, 1.2) and 0.7 (95% CI: 0.5, 0.9), respectively). Interaction analyses pooling SEP groups together (Web Table 2) largely supported these observations. IRRs for psychosocial, behavioral, and biomedical factors did not materially differ across SEP groups.
Decomposing inequality and comparing contributions
Relative to high-SEP participants, CVD incidence was higher by 4.6 cases per 1,000 PY (95% CI: 2.7, 6.5) among low-SEP participants and by 1.9 cases per 1,000 PY (95% CI: 0.5, 3.7) among middle-SEP participants (Figure 1). We focus on the larger low-SEP versus high-SEP socioeconomic inequality.
Before discussing decomposition findings, we reiterate 3 key points: 1) The only quantity decomposed is the observed, unadjusted 4.6-case low-high SEP inequality —it is the denominator for all relative contributions. 2) Adding covariates to decomposition models only adjusts existing covariate contributions; remains unchanged. 3) While contributions from the same model sum to 100% (e.g., see Web Table 3), contributions across different models may sum to more than 100%. We note that whenever appropriate.
Demographic factors
Differentials in the demographic composition of SEP groups, particularly differentials in age, sex, and race distributions, appeared to account for substantial portions of CVD inequality (Table 3). Nonetheless, much of the contribution of these differential exposures was offset by the contributions of differential vulnerability in the opposite direction. For example, in Table 3 (model 1), the less protective association of female sex in the low-SEP group accounted for 2.3 cases per 1,000 PY (49.4% of the inequality). This almost fully offset the negative contribution (−2.2 cases per 1,000 PY; −47.9%) of the greater prevalence of females in the low-SEP group. After adjustment for neighborhood characteristics and proximal risk factors (Table 3, model 3), demographic factors accounted for only 16.9% of the inequality in total.
Table 3.
Adjusted Contributionsa of Demographic Factors to the Observed Socioeconomic Inequality (Low-High) in Incident Cardiovascular Disease (n = 3,729), Multi-Ethnic Study of Atherosclerosis, 2000–2012
| Covariate | Differential Exposureb (ΔE) Contributions | Differential Vulnerabilityc (ΔV) Contributions | ||||
|---|---|---|---|---|---|---|
| Absolute, Cases/1,000 | 95% CI | Relative, % | Absolute, Cases/1,000 | 95% CI | Relative, % | |
| Model 1d | ||||||
| Aggregate contributione | −2.0f | −4.3, 0.4 | −42.3 | 4.8f | −0.5, 10.1 | 103.2 |
| Age ≥65 years | 3.6g | 1.5, 5.7 | 77.7 | 0.1 | −1.2, 1.3 | 1.4 |
| Female sex | −2.2g | −3.3, −1.1 | −47.9 | 2.3g | 0.6, 4.0 | 49.4 |
| Race | ||||||
| White | −3.3g | −4.9, −1.7 | −71.0 | 2.9h | 0.4, 5.4 | 62.7 |
| Chinese | −0.4g | −0.7, −0.1 | −8.8 | 0.2 | −0.4, 0.8 | 3.9 |
| Black | 0.0 | −0.1, 0.1 | −0.5 | −0.1 | −1.0, 0.8 | −1.5 |
| Latino | 0.2 | −1.1, 1.4 | 3.5 | −0.3h | −0.4, −0.1 | −5.4 |
| Married/cohabiting | 0.9 | −0.6, 2.5 | 20.4 | −0.4 | −4.2, 3.4 | −8.3 |
| Population density | ||||||
| Bottom tertile | −0.5 | −1.1, 0.2 | −10.7 | 0.2 | −0.9, 1.2 | 3.3 |
| Middle tertile | 0.0 | −0.3, 0.3 | 0.5 | −0.1 | −0.9, 0.7 | −1.9 |
| Top tertile | −0.3f | −0.5, 0.0 | −5.5 | 0.0 | −0.9, 0.9 | −0.3 |
| Model 3i | ||||||
| Aggregate contributionj | −2.4g | −4.1, −0.7 | −51.5 | 3.2 | −1.2, 7.5 | 68.4 |
| Age ≥65 years | 1.4g | 0.4, 2.3 | 29.3 | −0.1 | −1.1, 0.9 | −2.2 |
| Female sex | −1.2g | −2.0, −0.5 | −26.5 | 1.3f | −0.1, 2.7 | 28.7 |
| Race | ||||||
| White | −2.1g | −3.3, −0.9 | −45.8 | 2.3h | 0.0, 4.6 | 49.3 |
| Chinese | −0.2f | −0.4, 0.0 | −3.8 | 0.2 | −0.4, 0.7 | 3.3 |
| Black | 0.0 | −0.1, 0.0 | −0.8 | 0.1 | −0.8, 0.9 | 1.1 |
| Latino | −0.2 | −0.8, 0.5 | −3.4 | −0.2h | −0.4, 0.0 | −4.7 |
| Married/cohabiting | 0.5 | −0.4, 1.3 | 9.8 | −0.4 | −3.3, 2.5 | −8.6 |
| Population density | ||||||
| Bottom tertile | −0.3 | −0.8, 0.2 | −7.1 | 0.3 | −1.0, 1.6 | 6.5 |
| Middle tertile | 0.0 | −0.1, 0.2 | 0.5 | 0.0 | −0.6, 0.6 | 0.3 |
| Top tertile | −0.2 | −0.4, 0.1 | −3.8 | −0.2 | −1.2, 0.8 | −5.3 |
Abbreviations: CI, confidence interval; SEP, socioeconomic position.
a “Absolute” columns list absolute contributions (number of cases per 1,000 person-years) to inequality by each demographic factor through differential exposure and differential vulnerability. “Relative” columns list those contributions as a percentage of the inequality. Estimates were generated in decompositions of the observed, 4.6-extra-case low-high SEP inequality.
b Difference in the prevalence of the risk factor between low- and high-SEP groups.
c Difference in the association of the risk factor with cardiovascular disease between low- and high-SEP groups.
d Contributions of demographic factors in model 1 represent “total” contributions, unadjusted for downstream factors.
e The overall aggregate contribution (ΔE + ΔV) of demographic factors in model 1 was 2.8 (95% CI: −2.2, 7.8) cases per 1,000 person-years (60.9% of the inequality). The SEP difference in intercepts accounted for the remaining 39.1% of the inequality.
fP < 0.10.
gP < 0.01.
hP < 0.05.
i Contributions of each demographic factor in model 3 were adjusted for all other demographic factors listed, as well as neighborhood, psychosocial, behavioral, and biomedical factors.
j The overall aggregate contribution (ΔE + ΔV) of demographic factors in model 3 was 0.8 (95% CI: −3.8, 5.4) cases per 1,000 person-years (16.9% of the inequality).
Neighborhood environment
There was substantial uncertainty in estimating the contributions of differentials in neighborhood environment (Table 4). However, 3 particularly large contributions merit highlighting. After adjustment for demographic factors (Table 4, model 2), differential exposure to lower neighborhood SEP and adverse social environment accounted for 35.3% (1.6 cases per 1,000 PY; 95% CI: −8.4, 11.6) and 41.8% (1.9 cases per 1,000 PY; 95% CI: −6.8, 10.7) of the inequality, respectively. The differential vulnerability associated with neighborhood SEP (being protective mainly in the high-SEP group) accounted for another 33.7% (1.6 cases per 1,000 PY; 95% CI: −0.3, 3.5). These 3 contributions, together with the contributions of other neighborhood factors and demographic factors (not shown), all from model 2, summed to 100%. Further adjustment for proximal risk factors (Table 4, model 3) reduced these neighborhood contributions by about 60%.
Table 4.
Adjusted Contributionsa of Neighborhood Conditions to the Observed Socioeconomic Inequality (Low-High) in Incident Cardiovascular Disease (n = 3,729), Multi-Ethnic Study of Atherosclerosis, 2000–2012
| Covariate | Differential Exposureb (ΔE) Contributions | Differential Vulnerabilityc (ΔV) Contributions | ||||
|---|---|---|---|---|---|---|
| Absolute, Cases/1,000 | 95% CI | Relative, % | Absolute, Cases/1,000 | 95% CI | Relative, % | |
| Model 2d | ||||||
| Aggregate contributione,f | 4.0 | −14.0, 21.9 | 85.4 | 0.3 | −1.4, 1.9 | 5.8 |
| % foreign-born | 0.8 | −3.9, 5.5 | 17.4 | −0.3 | −1.3, 0.8 | −6.1 |
| Neighborhood SEP | 1.6 | −8.4, 11.6 | 35.3 | 1.6 | −0.3, 3.5 | 33.7 |
| Physical environment | −0.5 | −3.7, 2.7 | −10.8 | −0.3 | −1.3, 0.7 | −6.5 |
| Total PA resources | 0.1 | −1.1, 1.2 | 1.6 | 0.0 | −0.4, 0.4 | −0.2 |
| Social environment | 1.9 | −6.8, 10.7 | 41.8 | −0.7 | −2.0, 0.6 | −15.1 |
| Model 3g | ||||||
| Aggregate contributionf,h | 1.1 | −0.9, 3.1 | 23.1 | 0.1 | −1.2, 1.4 | 2.3 |
| % foreign-born | 0.2 | −0.9, 1.4 | 5.1 | 0.0 | −0.8, 0.9 | 0.5 |
| Neighborhood SEP | 0.5 | −1.6, 2.6 | 10.0 | 0.7 | −0.8, 2.2 | 14.7 |
| Physical environment | −0.1 | −1.1, 0.8 | −3.0 | −0.2 | −1.0, 0.6 | −3.8 |
| Total PA resources | 0.0 | −0.3, 0.4 | 0.9 | 0.0 | −0.3, 0.3 | −0.1 |
| Social environment | 0.5 | −1.1, 2.0 | 10.0 | −0.4 | −1.5, 0.6 | −9.0 |
Abbreviations: CI, confidence interval; PA, physical activity; SEP, socioeconomic position.
a “Absolute” columns list absolute contributions (number of cases per 1,000 person-years) to inequality by each neighborhood variable through differential exposure and differential vulnerability. “Relative” columns list those contributions as a percentage of the inequality. Estimates were generated in decompositions of the observed, 4.6-extra-case low-high SEP inequality.
b Difference in the prevalence of the risk factor between low- and high-SEP groups.
c Difference in the association of the risk factor with cardiovascular disease between low- and high-SEP groups.
d Contributions of each neighborhood variable in model 2 represent “total” contributions, with adjustment only for demographic confounders and all listed neighborhood characteristics. All P values were greater than 0.1.
e The overall aggregate contribution (ΔE + ΔV) of neighborhood variables in model 2 was 4.2 (95% CI: −13.5, 21.9) cases per 1,000 person-years (90.2% of the inequality).
f Variables were specified in decomposition models as z scores (standard deviation units).
g Contributions of each neighborhood variable in model 3 were adjusted for demographic, psychosocial, behavioral, and biomedical factors, as well as for all other neighborhood covariates listed. All P values were greater than 0.1.
h The overall aggregate contribution (ΔE + ΔV) of neighborhood variables in model 3 was 1.2 (95% CI: −0.9, 3.3) cases per 1,000 person-years (25.4% of the inequality).
Proximal risk factors
After adjustment for demographic factors and neighborhood characteristics (model 3), differentials in all proximal risk factors accounted in total for 54.7% of the inequality (Table 5). Differential exposure was the primary mechanism driving these contributions (50.7%). All differential vulnerability contributions were negligible. Psychosocial risk factors and behaviors also accounted for little of the inequality. Differential exposure to biomedical risk factors accounted overall for 37.7% of the inequality (1.7 cases per 1,000 PY), chiefly reflecting the contributions of the higher prevalences of hypertension (19.3%; 0.9 cases per 1,000 PY, 95% CI: 0.3, 1.5), diabetes (9.8%; 0.5 cases per 1,000 PY, 95% CI: 0.1, 0.8), and elevated interleukin-6 levels (5.9%; 0.3 cases per 1,000 PY, 95% CI: 0.0, 0.6) in the low-SEP group (Table 5). These findings were robust to alternative covariate specifications.
Table 5.
Adjusted Contributionsa of Psychosocial, Behavioral, and Biomedical Risk Factors to the Observed Socioeconomic Inequality (Low-High) in Incident Cardiovascular Disease (n = 3,729), Multi-Ethnic Study of Atherosclerosis, 2000–2012
| Covariate | Differential Exposureb (ΔE) Contributions | Differential Vulnerabilityc (ΔV) Contributions | ||||
|---|---|---|---|---|---|---|
| Absolute, Cases/1,000 | 95% CI | Relative, % | Absolute, Cases/1,000 | 95% CI | Relative, % | |
| Aggregate contribution—all factorsd | 2.3e | 0.4, 4.3 | 50.7 | 0.2 | −2.6, 3.0 | 4.0 |
| Psychosocial factorsf | ||||||
| Aggregate contribution | 0.2 | −0.5, 0.8 | 3.4 | 0.0 | −0.5, 0.6 | 0.6 |
| Lifetime discriminationg | −0.1 | −0.3, 0.2 | −1.4 | 0.0 | −0.2, 0.2 | 0.6 |
| Chronic stressg | 0.0 | −0.1, 0.2 | 0.9 | 0.0 | −0.1, 0.1 | −0.5 |
| Depressive symptomsg | 0.2 | −0.4, 0.7 | 4.0 | −0.1 | −0.6, 0.4 | −1.5 |
| Social supportg | 0.0 | −0.3, 0.3 | −0.1 | 0.1 | −0.3, 0.4 | 1.9 |
| Behavioral factorsf | ||||||
| Aggregate contribution | 0.4 | −0.6, 1.4 | 9.5 | 0.5 | −2.0, 2.9 | 10.1 |
| Uninsured (no health insurance) | 0.0 | −0.5, 0.5 | 0.0 | −0.1 | −0.2, 0.0 | −1.6 |
| Pack-years of smokingg | 0.0 | 0.0, 0.1 | 1.0 | 0.0 | −0.1, 0.1 | −0.4 |
| Current alcohol consumption | 0.5 | −0.4, 1.4 | 11.1 | 0.4 | −2.1, 2.8 | 8.5 |
| Moderate/vigorous PAg | 0.0 | −0.1, 0.0 | −1.0 | 0.0 | −0.1, 0.2 | 0.4 |
| Body mass indexg,h | −0.1 | −0.3, 0.2 | −1.5 | 0.1 | −0.1, 0.4 | 3.1 |
| Biomedical factorsf | ||||||
| Aggregate contribution | 1.7i | 0.6, 2.9 | 37.7 | −0.3 | −1.4, 0.7 | −6.7 |
| Diabetesj | 0.5e | 0.1, 0.8 | 9.8 | 0.0 | −0.3, 0.2 | −0.1 |
| Hypertensionk | 0.9i | 0.3, 1.5 | 19.3 | −0.3 | −1.3, 0.7 | −7.0 |
| Total cholesterolg | 0.1 | 0.0, 0.2 | 1.7 | 0.0 | −0.1, 0.1 | 0.3 |
| Interleukin-6g | 0.3l | 0.0, 0.6 | 5.9 | 0.1 | −0.1, 0.2 | 1.2 |
| C-reactive proteing | 0.1 | −0.1, 0.2 | 1.2 | 0.0 | −0.2, 0.1 | −1.0 |
Abbreviations: CI, confidence interval; PA, physical activity; SEP, socioeconomic position.
a “Absolute” columns list absolute contributions (number of cases per 1,000 person-years) to inequality by each risk factor through differential exposure and differential vulnerability. “Relative” columns list those contributions as a percentage of the inequality. Estimates were generated in decompositions of the observed, 4.6-extra-case low-high SEP inequality.
b Difference in the prevalence of the risk factor between low- and high-SEP groups.
c Difference in the association of the risk factor with cardiovascular disease between low- and high-SEP groups.
d The overall aggregate contribution (ΔE + ΔV) of all risk factors in model 3 was 2.5 (95% CI: −0.6, 5.7) cases per 1,000 person-years (54.7% of the inequality).
eP < 0.05.
f Contributions of each risk factor in model 3 were adjusted for demographic factors and neighborhood variables, as well as for all other psychosocial, behavioral, and biomedical factors listed.
g Variable was specified in decomposition models as a z score (standard deviation units).
h Weight (kg)/height (m)2.
iP < 0.01.
j Fasting glucose concentration ≥126 mg/dL and/or use of insulin/oral hypoglycemic medication (74).
k Systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication (73).
lP < 0.1.
DISCUSSION
Three sets of findings emerged from our analyses. First, differential exposure to poor neighborhood socioeconomic conditions and adverse social environment among low-SEP individuals appeared to account for large portions of the inequality in CVD incidence. Although they were undermined by considerable statistical imprecision, the large point estimates resonate with existing evidence on the role of neighborhood deprivation and stressful environment in the development of risk factors for and incidence of CVD events (15, 16, 20). Absence of strong socioeconomic differentials in the measures of neighborhood physical environment (perceived walkability and healthy food availability) and physical activity resources in MESA was the probable reason why these factors accounted for little of the inequality.
Our analysis also suggested that the differential vulnerability associated with neighborhood socioeconomic conditions (being protective mainly at high SEP) had a nontrivial contribution. This could reflect high-SEP individuals’ ability to better harness improvements in the neighborhood socioeconomic landscape (e.g., business developments, gentrification) without feeling disenfranchised or fearing displacement—concerns that are common among low-SEP residents in response to such neighborhood changes (84–86).
Second, net of demographic factors and neighborhood characteristics, differential exposure to proximal risk factors accounted for 50.7% of the inequality, primarily reflecting contributions of hypertension and diabetes with magnitudes close to those in the Whitehall II Study, a British study of civil servants (10). Conversely, there was generally no evidence of differential vulnerability related to our measures of proximal risk factors. While our broad differential exposure finding is consistent with existing literature (7, 8, 10–12, 87), our estimated contributions of specific risk factors varied. For instance, while smoking accounted for very little of the inequality in our study, it had a much larger contribution in studies where it exhibited a steep inverse social gradient, such as the Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands (MORGEN) (12) and the Whitehall II Study (10). Likewise, the absence of strong socioeconomic differentials in the prevalence of psychosocial measures (e.g., discrimination and chronic stress) in our sample was probably why they accounted for little of the inequality.
Our third set of findings was incidental but compelling: Although demographic contributions nearly canceled out overall, differential vulnerability associated with race and sex accounted for significant shares of the inequality. Being Latino was protective only in the low-SEP group, potentially reflecting low-SEP Latinos’ lower degree of US acculturation, with likely healthier lifestyles (particularly diet) and greater social support (88, 89). On the other hand, the weaker protection against CVD among females in the low-SEP group might have been driven by the compound disadvantage that low-SEP women are likely to experience (e.g., single motherhood, economic hardship, and lack of adequate workplace protections) (90, 91). These experiences might erode protections against CVD (particularly coronary heart disease) related to female biology (e.g., hormones) (92).
The stronger association of white race (relative to other racial groups) with CVD incidence among low-SEP individuals is also intriguing. Documented clustering of multiple negative health behaviors among low-SEP whites (93–96), also visible in our sample, might be the culprit. Another possibility is that CVD incidence might have also been artificially lower among minorities due to selection bias in study participation by race. Blacks, for instance, generally have shorter life expectancies (97, 98) and are more likely to experience cardiovascular events earlier in life than whites (95, 99). It is therefore possible that black low-SEP participants in MESA were healthier than the low-SEP black population.
Our findings should be interpreted in light of the following limitations. While our broad set of covariates makes major confounding unlikely, causal interpretation of covariate contributions requires more precise confounding control, such as meeting the standard mediation assumptions (100–103). However, it remains unclear what specific assumptions of this kind (and relevant sensitivity analyses) apply in the econometric decomposition context. Further, we could only analyze baseline covariates; modeling of time-varying covariates in decomposition analyses like ours remains underdeveloped (75). Accordingly, time-dependent confounding cannot be ruled out, and it might have been partly underlying the attenuation of neighborhood contributions upon adjustment for contemporaneous risk factors.
With relatively few CVD events and the interactions decomposition involves, our analyses also wound up lacking the necessary statistical power to precisely estimate covariate contributions, despite our emphasis on covariate parsimony, broadness, and continuous specification. Neighborhood contributions were especially imprecise, which may also reflect the modest within-SEP-group variability and overlap across SEP groups in neighborhood characteristics. Our neighborhood findings are therefore only generally suggestive, and they should be cautiously interpreted.
This analysis is one of the first attempts to empirically disentangle differential exposure and differential vulnerability contributions of risk factors to socioeconomic inequality in incident CVD. While our neighborhood estimates merit further investigation, our findings corroborate existing wisdom that controlling disproportionate exposure to adverse environments and cardiovascular risk factors, particularly diabetes and hypertension, in low-SEP groups could help curb inequalities in CVD incidence. Further, better understanding of the sources of differential vulnerability by neighborhood SEP, sex, and race may inform more effective interventions for reducing such inequalities.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Joseph J. Zilber School of Public Health, University of Wisconsin–Milwaukee, Milwaukee, Wisconsin (Mustafa Hussein); Department of Epidemiology and Biostatistics, Dornsife School of Public Health, Drexel University, Philadelphia, Pennsylvania (Ana V. Diez Roux); Department of Epidemiology, School of Public Health, University of California, Berkeley, Berkeley, California (Mahasin S. Mujahid); Department of Oncology, School of Medicine and Karmanos Cancer Institute, Wayne State University, Detroit, Michigan (Theresa A. Hastert); Division of Epidemiology, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Kiarri N. Kershaw); Department of Epidemiology and Prevention, Wake Forest School of Medicine, Winston-Salem, North Carolina (Alain G. Bertoni); and Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Ana Baylin).
This work was supported by grant 2R01 HL071759 (Principal Investigator: A.V.D.R.), by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grant 2P60MD002249 (Center for Integrative Approaches to Health Disparities; Principal Investigator: A.V.D.R.) from the National Institute on Minority Health and Health Disparities.
We thank Dr. Tyler VanderWeele for thoughtful comments on an earlier version of this article.
A preliminary version of this research was presented as a contributed poster at the Epidemiology Congress of the Americas in Miami, Florida, June 21–24, 2016.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- CVD
cardiovascular disease
- IRR
incidence rate ratio
- MESA
Multi-Ethnic Study of Atherosclerosis
- PY
person-years
- SEP
socioeconomic position
REFERENCES
- 1. Pell S, D’Alonzo CA. Blood pressure, body weight, serum cholesterol, and smoking habits among executives and non-executives. J Occup Med. 1961;3(10):467–470. [PubMed] [Google Scholar]
- 2. Holme I, Helgeland A, Hjermann I, et al. Coronary risk factors and socioeconomic status. The Oslo Study. Lancet. 1976;308(8000):1396–1398. [DOI] [PubMed] [Google Scholar]
- 3. Salonen JT. Socioeconomic status and risk of cancer, cerebral stroke, and death due to coronary heart disease and any disease: a longitudinal study in eastern Finland. J Epidemiol Community Health. 1982;36(4):294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marmot MG, Shipley MJ, Rose G. Inequalities in death—specific explanations of a general pattern? Lancet. 1984;323(8384):1003–1006. [DOI] [PubMed] [Google Scholar]
- 5. Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4):1973–1998. [DOI] [PubMed] [Google Scholar]
- 6. Mulatu MS, Schooler C. Causal connections between socio-economic status and health: reciprocal effects and mediating mechanisms. J Health Soc Behav. 2002;43(1):22–41. [PubMed] [Google Scholar]
- 7. Albert MA, Glynn RJ, Buring J, et al. Impact of traditional and novel risk factors on the relationship between socioeconomic status and incident cardiovascular events. Circulation. 2006;114(24):2619–2626. [DOI] [PubMed] [Google Scholar]
- 8. Lynch J, Davey Smith G, Harper S, et al. Explaining the social gradient in coronary heart disease: comparing relative and absolute risk approaches. J Epidemiol Community Health. 2006;60(5):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kivimäki M, Lawlor DA, Davey Smith G, et al. Socioeconomic position, co-occurrence of behavior-related risk factors, and coronary heart disease: the Finnish Public Sector Study. Am J Public Health. 2007;97(5):874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh-Manoux A, Nabi H, Shipley M, et al. The role of conventional risk factors in explaining social inequalities in coronary heart disease: the relative and absolute approaches to risk. Epidemiology. 2008;19(4):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramsay SE, Morris RW, Whincup PH, et al. Socioeconomic inequalities in coronary heart disease risk in older age: contribution of established and novel coronary risk factors. J Thromb Haemost. 2009;7(11):1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kershaw KN, Droomers M, Robinson W, et al. Quantifying the contributions of behavioral and biological risk factors to socioeconomic disparities in coronary heart disease incidence: the MORGEN Study. Eur J Epidemiol. 2013;28(10):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 14. Diez Roux AV. Residential environments and cardiovascular risk. J Urban Health. 2003;80(4):569–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 16. Kershaw KN, Diez Roux AV, Bertoni A, et al. Associations of chronic individual-level and neighbourhood-level stressors with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. J Epidemiol Community Health. 2015;69(2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76. [DOI] [PubMed] [Google Scholar]
- 18. Dow WH, Schoeni RF, Adler NE, et al. Evaluating the evidence base: policies and interventions to address socioeconomic status gradients in health. Ann N Y Acad Sci. 2010;1186:240–251. [DOI] [PubMed] [Google Scholar]
- 19. Robert SA. Socioeconomic position and health: the independent contribution of community socioeconomic context. Annu Rev Sociol. 1999;25:489–516. [Google Scholar]
- 20. Pujades-Rodriguez M, Timmis A, Stogiannis D, et al. Socioeconomic deprivation and the incidence of 12 cardiovascular diseases in 1.9 million women and men: implications for risk prediction and prevention. PLoS One. 2014;9(8):e104671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King KE, Morenoff JD, House JS. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med. 2011;73(7):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morenoff JD, House JS, Hansen BB, et al. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007;65(9):1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schulz AJ, Mentz G, Lachance L, et al. Do observed or perceived characteristics of the neighborhood environment mediate associations between neighborhood poverty and cumulative biological risk? Health Place. 2013;24:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mujahid MS, Diez Roux AV, Cooper RC, et al. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diderichsen F, Hallqvist J. Social inequalities in health: some methodological considerations for the study of social position and social context In: Arve-Pares B, ed. Inequality in Health: A Swedish Perspective. Stockholm, Sweden: Swedish Council for Social Research; 1998:25–39. [Google Scholar]
- 26. Diderichsen F, Evans T, Whitehead M. The social basis of disparities in health In: Evans T, Whitehead M, Diderichsen F, et al., eds. Challenging Inequities in Health: From Ethics to Action. New York, NY: Oxford University Press, 2001:13–23. [Google Scholar]
- 27. Solar O, Irwin A. A Conceptual Framework for Action on the Social Determinants of Health (Social Determinants of Health discussion paper 2 (Policy and Practice).) Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 28. Hoven H, Siegrist J. Work characteristics, socioeconomic position and health: a systematic review of mediation and moderation effects in prospective studies. Occup Environ Med. 2013;70(9):663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nordahl H, Lange T, Osler M, et al. Education and cause-specific mortality: the mediating role of differential exposure and vulnerability to behavioral risk factors. Epidemiology. 2014;25(3):389–396. [DOI] [PubMed] [Google Scholar]
- 30. Eikemo TA, Hoffmann R, Kulik MC, et al. How can inequalities in mortality be reduced? A quantitative analysis of 6 risk factors in 21 European populations. PLoS One. 2014;9(11):e110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoffmann R, Eikemo TA, Kulhánová I, et al. The potential impact of a social redistribution of specific risk factors on socioeconomic inequalities in mortality: illustration of a method based on population attributable fractions. J Epidemiol Community Health. 2013;67(1):56–62. [DOI] [PubMed] [Google Scholar]
- 32. Link BG, Phelan JC. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;Spec No:80–94. [PubMed] [Google Scholar]
- 33. Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(suppl):S28–S40. [DOI] [PubMed] [Google Scholar]
- 34. Graham H. Social determinants and their unequal distribution: clarifying policy understandings. Milbank Q. 2004;82(1):101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma S, Malarcher AM, Giles WH, et al. Racial, ethnic and socioeconomic disparities in the clustering of cardiovascular disease risk factors. Ethn Dis. 2004;14(1):43–48. [PubMed] [Google Scholar]
- 36. Clark AM, DesMeules M, Luo W, et al. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol. 2009;6(11):712–722. [DOI] [PubMed] [Google Scholar]
- 37. Ljung R, Hallqvist J. Socioeconomic position, clustering of risk factors, and the risk of myocardial infarction. Am J Public Health. 2007;97(11):1927–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–897. [DOI] [PubMed] [Google Scholar]
- 39. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART Study): case-control study. Lancet. 2004;364(9438):937–952. [DOI] [PubMed] [Google Scholar]
- 40. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. [DOI] [PubMed] [Google Scholar]
- 41. Halonen JI, Kivimäki M, Pentti J, et al. Quantifying neighbourhood socioeconomic effects in clustering of behaviour-related risk factors: a multilevel analysis. PLoS One. 2012;7(3):e32937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feng X, Astell-Burt T. Neighborhood socioeconomic circumstances and the co-occurrence of unhealthy lifestyles: evidence from 206,457 Australians in the 45 and Up Study. PLoS One. 2013;8(8):e72643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carroll AJ, Carnethon MR, Liu K, et al. Interaction between smoking and depressive symptoms with subclinical heart disease in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Health Psychol. 2017;36(2):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28(6):1339–1345. [DOI] [PubMed] [Google Scholar]
- 45. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. 2014;25(5):749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. VanderWeele TJ. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology. 2013;24(2):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fortin N, Lemieux T, Firpo S. Decomposition methods in economics In: Ashenfelter O, Card D, eds. Handbook of Labor Economics, Volume 4A. 1st ed Oxford, United Kingdom: Elsevier/North-Holland Publishing Company; 2011:1–102. [Google Scholar]
- 48. Blinder A. Wage discrimination: reduced form and structural estimates. J Hum Resour. 1973;8(4):436–455. [Google Scholar]
- 49. Oaxaca RL. Male-female wage differentials in urban labor markets. Int Econ Rev. 1973;14(3):693–709. [Google Scholar]
- 50. Powell LM, Wada R, Krauss RC, et al. Ethnic disparities in adolescent body mass index in the United States: the role of parental socioeconomic status and economic contextual factors. Soc Sci Med. 2012;75(3):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sen B. Using the Oaxaca-Blinder decomposition as an empirical tool to analyze racial disparities in obesity. Obesity (Silver Spring). 2014;22(7):1750–1755. [DOI] [PubMed] [Google Scholar]
- 52. Basu S, Hong A, Siddiqi A. Using decomposition analysis to identify modifiable racial disparities in the distribution of blood pressure in the United States. Am J Epidemiol. 2015;182(4):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poole C. A history of the population attributable fraction and related measures. Ann Epidemiol. 2015;25(3):147–154. [DOI] [PubMed] [Google Scholar]
- 54. VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology. 2013;24(1):175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Naimi AI, Kaufman JS, MacLehose RF. Mediation misgivings: ambiguous clinical and public health interpretations of natural direct and indirect effects. Int J Epidemiol. 2014;43(5):1656–1661. [DOI] [PubMed] [Google Scholar]
- 56. VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3(1):33–72. [Google Scholar]
- 57. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 58. Lemelin ET, Diez Roux AV, Franklin TG, et al. Life-course socioeconomic positions and subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Soc Sci Med. 2009;68(3):444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mujahid MS, Diez Roux AV, Morenoff JD, et al. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. [DOI] [PubMed] [Google Scholar]
- 60. Williams LK, Thornton L, Crawford D, et al. Perceived quality and availability of fruit and vegetables are associated with perceptions of fruit and vegetable affordability among socio-economically disadvantaged women. Public Health Nutr. 2012;15(7):1262–1267. [DOI] [PubMed] [Google Scholar]
- 61. Williams L, Ball K, Crawford D. Why do some socioeconomically disadvantaged women eat better than others? An investigation of the personal, social and environmental correlates of fruit and vegetable consumption. Appetite. 2010;55(3):441–446. [DOI] [PubMed] [Google Scholar]
- 62. Caspi CE, Kawachi I, Subramanian SV, et al. The relationship between diet and perceived and objective access to supermarkets among low-income housing residents. Soc Sci Med. 2012;75(7):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Giskes K, Van Lenthe FJ, Brug J, et al. Socioeconomic inequalities in food purchasing: the contribution of respondent-perceived and actual (objectively measured) price and availability of foods. Prev Med. 2007;45(1):41–48. [DOI] [PubMed] [Google Scholar]
- 64. Powell LM, Chaloupka FJ, Slater SJ, et al. The availability of local-area commercial physical activity-related facilities and physical activity among adolescents. Am J Prev Med. 2007;33(4 suppl):S292–S300. [DOI] [PubMed] [Google Scholar]
- 65. Gordon-Larsen P, Nelson MC, Page P, et al. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117(2):417–424. [DOI] [PubMed] [Google Scholar]
- 66. Walls & Associates National Establishment Time-Series (NETS) Database: 2012 Database Description Denver, CO: Walls & Associates; 2013. http://exceptionalgrowth.org/downloads/NETSDatabaseDescription2013.pdf. Accessed December 30, 2016.
- 67. Williams DR, Spencer MS, Jackson JS. Race, stress, and physical health: the role of group identity In: Contrada RJ, Ashmore RD, eds. Self, Social Identity, and Physical Health: Interdisciplinary Explorations. New York, NY: Oxford University Press; 1999:71–100. [Google Scholar]
- 68. Bromberger JT, Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychol Aging. 1996;11(2):207–213. [DOI] [PubMed] [Google Scholar]
- 69. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 70. Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23(6):398–403. [DOI] [PubMed] [Google Scholar]
- 71. Bertoni AG, Whitt-Glover MC, Chung H, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of Physical Activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S516. [DOI] [PubMed] [Google Scholar]
- 73. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2571. [DOI] [PubMed] [Google Scholar]
- 74. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. [DOI] [PubMed] [Google Scholar]
- 75. Powers DA, Yun M-S. Multivariate decomposition for hazard rate models. Sociol Methodol. 2009;39(1):233–263. [Google Scholar]
- 76. Bazen S, Joutard X. The Taylor Decomposition: A Unified Generalization of the Oaxaca Method to Nonlinear Models Marseille, France: Aix-Marseille School of Economics; 2013. (AMSE working paper no. 1332). https://ideas.repec.org/p/aim/wpaimx/1332.html. Accessed September 20, 2016. [Google Scholar]
- 77. Jann B. The Blinder–Oaxaca decomposition for linear regression models. Stata J. 2008;8(4):453–479. [Google Scholar]
- 78. Yun MS. Decomposing differences in the first moment. Econ Lett. 2004;82(2):275–280. [Google Scholar]
- 79. Powers DA, Yoshioka H, Yun MS. mvdcmp: Multivariate decomposition for nonlinear response models. Stata J. 2011;11(4):556–576. [Google Scholar]
- 80. Yun MS. Hypothesis tests when decomposing differences in the first moment. J Econ Soc Meas. 2005;30(4):295–304. [Google Scholar]
- 81. Krieger N. Health equity and the fallacy of treating causes of population health as if they sum to 100. Am J Public Health. 2017;107(4):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chow GC. Tests of equality between sets of coefficients in two linear regressions. Econometrica. 1960;28(3):591–605. [Google Scholar]
- 83. Weesie J. sg121: seemingly unrelated estimation and the cluster-adjusted sandwich estimator. Stata Tech Bull. 1999;9:231–248. [Google Scholar]
- 84. Huyser M, Meerman JR. Resident perceptions of redevelopment and gentrification in the Heartside neighborhood: lessons for the social work profession. J Sociol Soc Welf. 2014;41(3):3–22. [Google Scholar]
- 85. Doucet B. Living through gentrification: subjective experiences of local, non-gentrifying residents in Leith, Edinburgh. J Hous Built Environ. 2009;24(3):299–315. [Google Scholar]
- 86. Freeman L. There Goes the ‘Hood: Views of Gentrification From the Ground Up. Philadelphia, PA: Temple University Press; 2006. [Google Scholar]
- 87. Marmot MG, Shipley MJ, Hemingway H, et al. Biological and behavioural explanations of social inequalities in coronary heart disease: the Whitehall II Study. Diabetologia. 2008;51(11):1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Diez Roux AV, Detrano R, Jackson S, et al. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation. 2005;112(11):1557–1565. [DOI] [PubMed] [Google Scholar]
- 89. Rodriguez CJ, Allison M, Daviglus ML, et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130(7):593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Borrell C, Muntaner C, Benach J, et al. Social class and self-reported health status among men and women: what is the role of work organisation, household material standards and household labour? Soc Sci Med. 2004;58(10):1869–1887. [DOI] [PubMed] [Google Scholar]
- 91. Malmusi D, Vives A, Benach J, et al. Gender inequalities in health: exploring the contribution of living conditions in the intersection of social class. Glob Health Action. 2014;7(1):23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Spence JD, Pilote L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis. 2015;241(1):208–210. [DOI] [PubMed] [Google Scholar]
- 93. LaVeist T, Pollack K, Thorpe R, et al. Place, not race: disparities dissipate in southwest Baltimore when blacks and whites live under similar conditions. Health Aff (Millwood). 2011;30(10):1880–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Karlamangla AS, Merkin SS, Crimmins EM, et al. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Ann Epidemiol. 2010;20(8):617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. [DOI] [PubMed] [Google Scholar]
- 96. Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci USA. 2015;112(49):15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Frieden TR; Centers for Disease Control and Prevention . CDC health disparities and inequalities report—United States, 2013. Foreword. MMWR Suppl. 2013;62(3):1–2. [PubMed] [Google Scholar]
- 98. National Center for Health Statistics Health, United States, 2011: With Special Feature on Socioeconomic Status and Health Hyattsville, MD: National Center for Health Statistics; 2012. (DHHS publication no. 2012-1232). https://www.cdc.gov/nchs/data/hus/hus11.pdf. Accessed January 7, 2016. [PubMed] [Google Scholar]
- 99. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. [DOI] [PubMed] [Google Scholar]
- 100. Jiang Z, VanderWeele TJ. When is the difference method conservative for assessing mediation? Am J Epidemiol. 2015;182(2):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. VanderWeele T. Mediation: introduction and regression-based approaches In: VanderWeele T, ed. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press; 2015:20–65. [Google Scholar]
- 103. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.