Abstract
Background
Mass drug administration (MDA) is a control and elimination tool for treating infectious diseases. For malaria, it is widely accepted that conducting MDA during the dry season results in the best outcomes. However, seasonal movement of populations into and out of MDA target areas is common in many places and could potentially fundamentally limit the ability of MDA campaigns to achieve elimination.
Methods
A mathematical model was used to simulate malaria transmission in two villages connected to a high-risk area into and out of which 10% of villagers traveled seasonally. MDA was given only in the villages. Prevalence reduction under various possible timings of MDA and seasonal travel was predicted.
Results
MDA is most successful when distributed outside the traveling season and during the village low-transmission season. MDA is least successful when distributed during the traveling season and when traveling overlaps with the peak transmission season in the high-risk area. Mistiming MDA relative to seasonal travel resulted in much poorer outcomes than mistiming MDA relative to the peak transmission season within the villages.
Conclusions
Seasonal movement patterns of high-risk groups should be taken into consideration when selecting the optimum timing of MDA campaigns.
Keywords: Malaria, Mass drug administration, Mathematical modelling, Seasonal human movement
Introduction
Mass drug administration (MDA), where drugs are presumptively distributed to a population, is a common tool for control of infectious diseases including trachoma, lymphatic filariasis, schistosomiasis and soil-transmitted helminths.1 For malaria, MDA is under consideration as a tool for elimination in several areas where other interventions such as case management and vector control have already decreased regional malaria transmission.2 Because MDA is a resource-intensive intervention, deploying MDA to maximal effect requires understanding and avoiding means by which it can fail.
Good coverage is acknowledged to be essential to MDA. In diseases with seasonal transmission, such as malaria, the low-transmission season is presumed to be the best season for conducting MDA, as interrupting transmission is most likely during this time and drug prophylactic effects will confer protection into the high-transmission season.3,4 However, in many settings there is frequent population turnover and migration, and seasonal movement for economic reasons, schooling, social activities or subsistence farming can result in systematically missing these high-risk individuals if MDA occurs while they are away.5–8 In Gambia, human movement was identified as a major barrier to MDA coverage, with as much as 20% of the population estimated to be unstable and one-third of non-participation in MDA attributed to mobility.9,10 In a study in Karen State, Myanmar, a majority of 15–35 year olds were present only intermittently during 24 months of MDA and follow-up, and their absence was attributed to seasonal work or schooling.11 While seasonal movement is presumed to be important in impacting MDA outcomes, its impact relative to other considerations, such as timing MDA to the dry season and the duration of drug prophylactic effects, has yet to be examined in detail.
Mathematical modeling has been used to describe how various aspects of MDA, including choice of drug, coverage, adherence and timing, affect MDA outcome.12–16 In this study, a simple model framework is developed of two villages connected to a high-risk area to which some villagers travel seasonally (Figure 1); this transmission system is characteristic of Southeast Asian settings where forests are often loci of malaria transmission,17,18 although seasonal movement and seasonal malaria are common in many endemic areas. The effect of MDA is tested under various possible timings of seasonal travel and MDA distribution.
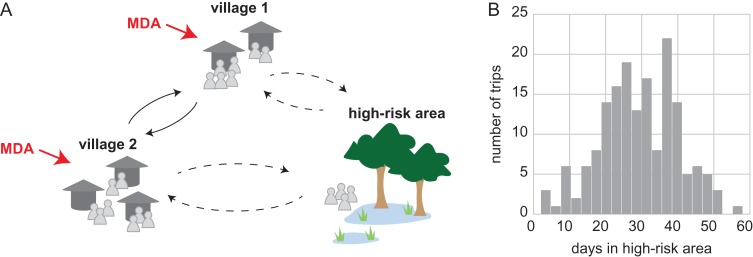
Figure 1.
A hypothetical malaria transmission system where villagers move seasonally to and from a high-risk area. (A) People from two neighboring villages, each with 300 villagers, can seasonally move to a high-risk area for agricultural, economic, social or other activities (dashed lines). Individuals also move between villages year-round (solid lines). MDA is given in the villages to reduce malaria transmission. (B) Distribution of durations of trips to the high-risk area observed in a representative simulation over the course of 1 y and the traveling season lasts 6 months. Most trips last several weeks. Since only 10% of villagers are travelers to the high-risk area, most travelers make more than one trip to the high-risk area during the traveling season.
Materials and methods
Malaria transmission model
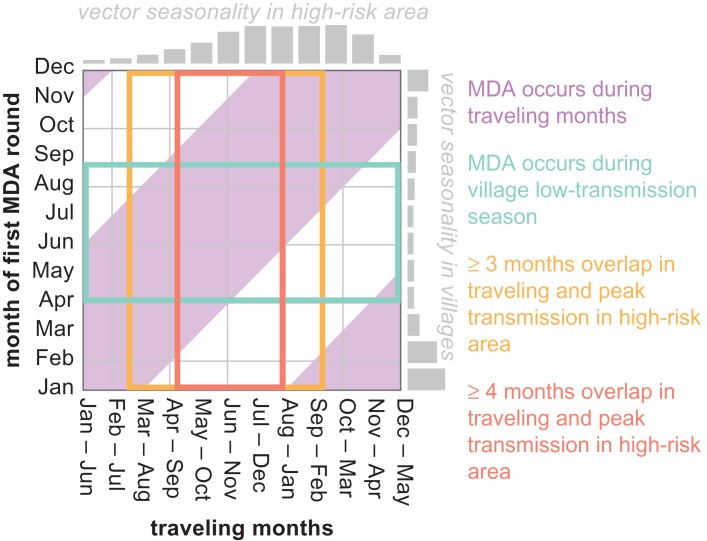
EMOD version 2.11,19 an agent-based model of Plasmodium falciparum transmission with exposure-dependent within-host immune effects20,21 and vector life cycle dynamics,22 was used for all simulations. The simulation framework included two villages, each with 300 individuals, and a high-risk area (Figure 1). Transmission within each village and the high-risk area was modeled as well mixed, where any infected individual can potentially infect any other individual. Demographic cycling was modeled with birth and death rates of 23 per 1000 per year. Vectors were modeled with peak biting between November and January in the villages and peak biting between May and November in the high-risk area (Figure 2). The true prevalence of infection over all areas varied seasonally between 25% and 50% prior to MDA.
Figure 2.
Outcomes of MDAs occurring in villages may depend on the interaction between relative timings of the MDA, travel between villages and the high-risk area, peak within-village transmission and peak transmission in the high-risk area.
Human movement
In each village, 35% of individuals between the ages of 15 and 35 y (10% of all individuals) were categorized as travelers. These individuals were eligible to make trips to the high-risk area at any time during the traveling season, which lasted 6 months. Each trip had a duration drawn from a Gaussian distribution with a mean of 30 d and a standard deviation of 10 d (Figure 1B). Travelers could make more than one trip to the high-risk area each season. All individuals were also eligible to move between the two villages.
An alternative travel pattern where travelers visited the high-risk area only at the beginning and end of the travel season, reflecting an agricultural high-risk area that is visited only for planting and harvesting, was also considered. Trip durations were drawn from the same Gaussian described above, resulting in a travel pattern where, for example, if travelers depart the villages in January, 26% return in January, 68% return in February and 6% return in March.
The average parasite prevalence across multiple stochastic realizations varied seasonally in travelers between 48% and 60% and in non-travelers between 26% and 47%.
Interventions
MDA was distributed in the two villages as two rounds separated by 60 d. Coverage was set at 70% and assumed to be independent between rounds. Individuals in the high-risk area during the MDA rounds were not eligible to receive MDA, nor were they included in the coverage denominator. The antimalarial drug given during MDA was the combination therapy dihydroartemisinin–piperaquine (DP) for the drug with a duration of prophylactic protection of 30 d (Figure 3) and artemether–lumefantrine (AL) for the drug with a shorter duration of prophylactic protection of 15 d (Figure 4). Drug pharmacokinetics and pharmacodynamics were explicitly modeled.9 No interventions other than MDA were modeled in the villages or high-risk area.
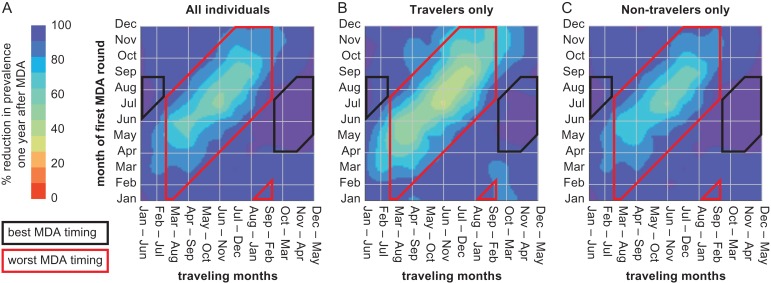
Figure 3.
The success of MDA is strongly dependent on the timing of travel and transmission in the high-risk area. (A) The percentage reduction in malaria prevalence 1 y after MDA compared with 1 y prior to MDA is greatest when MDA occurs during the low-transmission season in the villages and does not overlap with travel to the high-risk area (small left and right boxes) and is least when MDA occurs while travelers are away and traveling overlaps at least 3 months with peak transmission in the high-risk area (central box). The impact of mistimed MDA is (B) greatest in travelers but (C) is also felt in non-travelers. In all panels, MDA is with DP and consists of two rounds separated by 60 d.
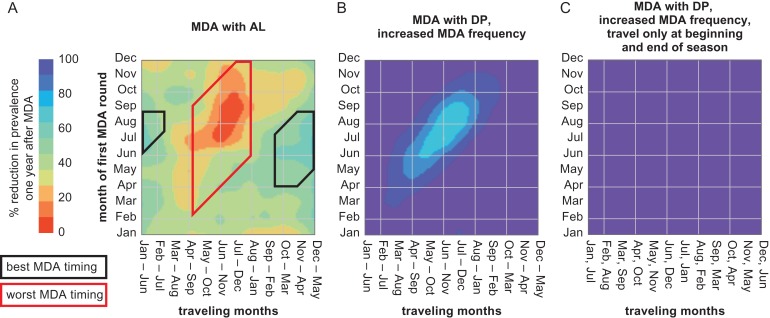
Figure 4.
Most and least successful timing of MDA depends on the duration of drug prophylaxis, frequency of MDA and specific patterns of seasonal travel. All panels show the percentage reduction in malaria prevalence in all individuals 1 y after MDA. (A) When MDA is with a drug with a shorter duration of prophylactic protection (AL), the least successful timing of MDA is limited to when MDA occurs while travelers are away and traveling overlaps at least 4 months with peak transmission in the high-risk area (central box). The most successful timing of MDA continues to be when MDA occurs during the low-transmission season in the villages and does not overlap with travel to the high-risk area (left and right boxes). MDA consists of two rounds separated by 60 d. (B) Increasing the frequency of MDA from two rounds with 60 d between rounds to three rounds with 30 d between rounds shrinks the zone of least successful MDA (contrast with Figure 3A). MDA is with DP. (C) A three-round MDA campaign with DP when travelers visit the high-risk area only at the beginning and end of the travel season is highly successful at any timing.
Twelve MDA timings and 12 travel timings were each simulated with 100 stochastic realizations, for a total of 14 400 simulations, and each realization was simulated for 3 y with MDA distributed during the second year. Prevalence reductions were calculated as the difference between mean true prevalence 1 y after the first MDA round and 1 y prior to the first MDA round divided by the mean true prevalence 1 y prior to the first MDA round. The areas shown in Figures 3 and 4 were calculated with the radial basis function and smoothing value of 1 in SciPy version 0.19.1.
In scenarios with increased MDA frequency, MDA was given as three rounds with 30 d between rounds, with coverage at 70% and independent between rounds.
Results
The success of MDA campaigns depends on the relative timings of MDA, travel between villages and the high-risk area, peak within-village transmission and peak transmission in the high-risk area (Figures 2–4). Depending on how these relative timings align, simulations show that MDA using DP with the same measured coverage can reduce prevalence by as much as 98% and as little as 32% in a setting where prevalence ranges seasonally from 25% to 50% (Figure 3).
Conditions for the best MDA outcomes are twofold: first, that MDA does not coincide with travel months and, second, that MDA occurs during the village low-transmission season (Figure 3A). Even with only 10% of individuals visiting the high-risk area, conducting MDA during traveling months is more detrimental to MDA outcome than conducting MDA during peak transmission season in the villages.
MDA campaigns are least successful when they occur while travelers are away and traveling overlaps at least 3 months with peak transmission season in the high-risk area. Not only is prevalence reduced less in travelers under these conditions (Figure 3B), but those who do not visit the high-risk area also see less successful outcomes from the MDA as infected travelers will return to the village to reseed transmission (Figure 3C).
Optimal MDA timing is affected by the duration of the MDA drug’s prophylactic effects. Figure 3 shows outcomes when MDA is given with DP, a drug combination with a 30 d prophylactic period. When MDA is instead given with a drug combination with a shorter period of prophylactic protection (AL), MDA is overall less effective, with prevalence reductions ranging from 0% to 70% (Figure 4A). However, the window of least effective MDA timing shrinks to when traveling overlaps at least 4 months with the peak transmission season in the high-risk area and MDA is distributed while travelers are away.
Adding another round of MDA with a shorter interval between rounds, reaching the recommended MDA configuration of three rounds separated by 1 month,23 increases the effectiveness of MDA at any timing, as the extra round offers an additional chance of contacting travelers (Figure 4B). The impact of seasonal travel is also lessened under specific travel patterns, such as a planting and harvesting pattern where travelers visit the high-risk area only at the beginning and end of the travel season, as under this pattern travelers are likely to be at home during at least one MDA round (Figure 4C).
Discussion
Coverage is known to be a strong determinant of MDA effectiveness. When transmission is heterogeneous, however, systematic missing of high-risk groups can have disproportionately detrimental effects on campaign outcomes. Repeating MDA campaigns or increasing their frequency will not result in better outcomes if the same high-risk group is missed each time, but increasing MDA frequency will have a benefit if additional rounds offer some chance of contacting high-risk individuals. This work highlights travel as a potentially high-risk behavior that is also systematically missed by intervention. However, other behaviors, such as non-compliance with recommended vector control measures coupled with consistent non-participation in MDA even when the individual is present in the village can also lead to similar patterns in MDA outcomes.
Three rounds with a 1-month separation between rounds is currently the recommended MDA structure23 but, depending on the scale of MDA, programs may be constrained to fewer rounds and longer intervals between rounds. In the model presented above, failure to reach the up to 10% of the population that experiences occupation-based exposure is the difference between a campaign that reduces prevalence by 98%, putting elimination within reach, and one that reduces prevalence by only 32%, which is unlikely to be cost effective.
Because coverage in this model is defined as within-village coverage and ignores individuals currently in the high-risk area, there is a small difference in overall population coverage when MDA campaigns are distributed during traveling months rather than non-traveling months. However, this difference is maximally one of 63% overall coverage vs 70% overall coverage, which is too small to account for the large variation in campaign outcome.
The importance of reaching seasonal travelers depends on transmission conditions at the travelers’ destination. When travel to the high-risk area occurs during the low-transmission season in the high-risk area, failure to treat these individuals during the MDA incurs a much lower cost than if travel occurs during the peak transmission season in the high-risk area. A survey of human movement in four sub-Saharan countries identified women traveling with children and youth workers, depending on the context, as populations likely to travel to high-risk areas.24 The degree to which MDA campaigns should be planned around seasonal movements such as forest-going or lakeside fishing depends on whether travel coincides with peak transmission at the destination.
Distributing MDA during the dry season is predicted to be superior to distribution during the wet season.3,4 However, the difference between optimal and suboptimal timing of MDA relative to the local transmission season is not large compared with the costs of mistiming relative to the travel season. Consistent with other modeling studies,3 this model shows that when MDA is given outside the travel season, there is a larger penalty for distributing MDA during the wet season when the MDA drug has a shorter period of prophylactic protection.
In this study, seasonality of transmission was modeled as offset between the villages and the high-risk area. While peak transmission times are usually similar in proximal areas, seasonal exposure can also vary across quite short distances due to the presence of standing water or differences in vector species composition. Whatever the relative seasonality, what is important is that MDA should not be conducted when travelers are away acquiring malaria. If the connected areas have the same seasonality, and high-transmission coincides with a high level of travel, then the optimal time for MDA is the dry season: the same as it would be when travel is not considered. If travel instead occurs during the dry season, optimal timing will depend on how much of the population is traveling and whether an increase in coverage from timing MDA to when travelers are at home will offset any loss of impact due to mistiming relative to seasonality of exposure.
While high levels of case management, such as can be achieved through village malaria workers, are critical to alter baseline transmission and sustain elimination, this work isolates the effect of MDA by including only that intervention. However, in realistic situations, MDA is but one component of an elimination strategy that includes other interventions such as case management and vector control. While this study has focused on factors affecting the impact of the MDA component, high-risk individuals such as seasonal travelers may also be less likely to access the health system, particularly if treatment is unavailable at their destination, or bring bed nets with them while traveling. Including these individuals in MDA campaigns is therefore even more critical.
Malaria transmission, particularly in low-transmission conditions, is heterogeneous, and there is an increasing focus on characterizing spatial heterogeneity in transmission and its implications.25–27 One consequence of spatial heterogeneity is the movement of parasites from one area to another, frustrating elimination or control efforts. When travel is seasonal and predictable, however, and when programs have the logistical flexibility to select from a range of possible timings, interventions can be scheduled such that they are implemented when travelers are most easily reached.
The findings of this study are generalizable to other infectious diseases, particularly those also with seasonal transmission, where MDA forms a critical component of control efforts. In addition to considering the seasonality of disease dynamics and seasonal patterns of physical accessibility to areas that are targeted for MDA, operations should also investigate and plan around seasonal human movement. MDA campaigns are resource intensive and should not be embarked upon without a local understanding of which people move, where they go, at what times of year and for how long.
Conclusions
Seasonal movement to and from high-risk regions outside of MDA target areas can have a strongly detrimental effect on MDA outcomes. Control and elimination programs should plan to time MDA campaigns to when high-risk individuals are most likely to be successfully reached.
Acknowledgments
Author contributions: JG, ABV, PAE and EAW conceived the study. JG implemented and analyzed the simulations. ABV designed the simulation framework. JG, ABV and EAW interpreted the data. JG drafted the manuscript. All authors revised the manuscript and read and approved the final manuscript.
Acknowledgments: We thank Daniel Parker for helpful discussion.
Funding: This work was supported by Bill and Melinda Gates through the Global Good Fund.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1. Smits HL. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev Anti Infect Ther 2014;7(1):37–56. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization Guidelines for the treatment of malaria, 3rd ed Geneva: World Health Organization, 2015. [Google Scholar]
- 3. Griffin JT. The interaction between seasonality and pulsed interventions against malaria in their effects on the reproduction number. PLoS Comput Biol 2015;11(1):e1004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gu W, Killeen GF, Mbogo CM et al. An individual-based model of Plasmodium falciparum malaria transmission on the coast of Kenya. Trans R Soc Trop Med Hyg 2003;97(1):43–50. [DOI] [PubMed] [Google Scholar]
- 5. Kajeechiwa L, Thwin MM, Shee PW et al. The acceptability of mass administrations of anti-malarial drugs as part of targeted malaria elimination in villages along the Thai–Myanmar border. Malar J 2016;15:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Seidlein L, Walraven G, Milligan PJ et al. The effect of mass administration of sulfadoxine-pyrimethamine combined with artesunate on malaria incidence: a double-blind, community-randomized, placebo-controlled trial in The Gambia. Trans R Soc Trop Med Hyg 2003;97(2):217–25. [DOI] [PubMed] [Google Scholar]
- 7. Peto T, Tripura R, von Seidlein L. Model citizen. Lancet Glob Health 2017;5(1):e973. [DOI] [PubMed] [Google Scholar]
- 8. Kajeechiwa L, Thwin MM, Nosten S et al. Community engagement for the rapid elimination of malaria: the case of Kayin State, Myanmar. Wellcome Open Res 2017;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dial NJ, Ceesay SJ, Gosling RD et al. A qualitative study to assess community barriers to malaria mass drug administration trials in the Gambia. Malar J 2014;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dierickx S, Gryseels C, Mwesigwa J et al. Factors associated with non-participation and non-adherence in directly observed mass drug administration for malaria in The Gambia. PLoS One 2016;11(2):e0148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landier J, Kajeechiwa L, Thwin MM et al. Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: a pilot trial in four villages of Eastern Myanmar. Wellcome Open Res 2017;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brady OJ, Slater HC, Pemberton-Ross P et al. Role of mass drug administration in elimination of Plasmodium falciparum malaria: a consensus modelling study. Lancet Glob Health 2017:5(7):e680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerardin J, Eckhoff P, Wenger EA. Mass campaigns with antimalarial drugs: a modelling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination. BMC Infect Dis 2015;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okell LC, Drakeley CJ, Bousema T et al. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med 2008;5(11):e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maude RJ, Socheat D, Nguon C et al. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One 2012;7(5):e37166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowell V, Briët OJT, Hardy D et al. Modelling the cost-effectiveness of mass screening and treatment for reducing Plasmodium falciparum malaria burden. Malar J 2013;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Somboon P, Aramrattana A, Lines J et al. Entomological and epidemiological investigations of malaria transmission in relation to population movements in forest areas of north-west Thailand. Southeast Asian J Trop Med Public Health 1998;29(1):3–9. [PubMed] [Google Scholar]
- 18. Parker DM, Landier J, von Seidlein L et al. Limitations of malaria reactive case detection in an area of low and unstable transmission on the Myanmar-Thailand border. Malar J 2016;15:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Institute for Disease Modeling Epidemiological modeling software. www.idmod.org/software [accessed 1 August 2017].
- 20. Eckhoff P. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg 2013;88(5):817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerardin J, Ouédraogo AL, McCarthy KA et al. Characterization of the infectious reservoir of malaria with an agent-based model calibrated to age-stratified parasite densities and infectiousness. Malar J 2015;14:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eckhoff PA. A malaria transmission-directed model of mosquito life cycle and ecology. Malar J 2011;10:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malaria Policy Advisory Committee Evidence Review Group on MDA, MSAT and FSAT. Geneva: World Health Organization, 2015. [Google Scholar]
- 24. Marshall JM, Touré M, Ouédraogo AL et al. Key traveller groups of relevance to spatial malaria transmission: a survey of movement patterns in four sub-Saharan African countries. Malar J 2016;15:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bousema T, Griffin JT, Sauerwein RW et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 2012;9(1):e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bousema T, Stresman G, Baidjoe AY et al. The impact of hotspot-targeted interventions on malaria transmission in Rachuonyo South District in the western Kenyan highlands: a cluster-randomized controlled trial. PLoS Med 2016;13(4):e1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerardin J, Bever CA, Bridenbecker D et al. Effectiveness of reactive case detection for malaria elimination in three archetypical transmission settings: a modelling study. Malar J 2017;16:248. [DOI] [PMC free article] [PubMed] [Google Scholar]