ABSTRACT
Background
There is mixed evidence regarding the nature of cognitive function in patients who have undergone renal transplantation. The aim of this meta-analysis was to examine which cognitive domains are impacted following kidney transplantation and how performance compares with non-transplanted patients or healthy controls/normative data.
Method
A systematic search was conducted using keywords within three databases (Embase, MEDLINE and PsychINFO), yielding 458 unique studies, 10 of which met the inclusion criteria. Neuropsychological tests were grouped into nine cognitive domains and three separate analyses were undertaken within each domain: (i) within subjects pre- versus post-transplant, (ii) transplanted versus non-transplanted patients and (iii) transplanted versus healthy matched controls and standardized normative data.
Results
Transplanted patients showed moderate to large improvements in the domains of general cognitive status (g = 0.526), information and motor speed (g = 0.558), spatial reasoning (g = 0.376), verbal memory (g = 0.759) and visual memory (g = 0.690) when compared with their pre-operative scores. Test scores in the same five domains were significantly better in post-transplanted patients when compared with dialysis-dependant or conservatively managed chronic kidney disease patients. However, post-transplanted patients’ performance was significantly low compared with that of healthy controls (and standardized normative data) in the domains of executive functioning (g = −0.283), verbal fluency (g = −0.657) and language (g = −0.573).
Conclusions
Two key issues arise from this review. First, domain-specific cognitive improvement occurs in patients after successful transplantation. Nevertheless, transplanted patients still performed significantly below healthy controls in some domains. Second, there are important shortcomings in existing studies; the length of follow-up is typically short and only limited neuropsychological test batteries are employed. These factors are important in order to support the recovery of cognitive function among patients following renal transplant.
Keywords: chronic kidney disease, cognition, dialysis, kidney transplant, systematic review
INTRODUCTION
Chronic kidney disease (CKD) can result from multiple factors, including hypertension, diabetes and genetic disorders [1–3]. End-stage kidney disease (ESKD) is the fifth and most severe category of CKD and is defined as the inability of the kidneys to metabolize and remove waste substances such as creatinine [4]. Patients suffering from ESKD depend on renal replacement therapy (RRT) for survival. RRT options include kidney transplantation, peritoneal dialysis (PD) and haemodialysis (HD) [5] and transplantation is currently the recommended gold standard [6]. The majority of transplanted patients spend a period of months or years dependent on dialysis before being transplanted, although a minority (22%) receive a transplant pre-emptively, bypassing the need for maintenance dialysis [7].
CKD is a debilitating illness with a broad impact on physical health and psychological function (both well-being and cognitive function) [8, 9]. CKD has been shown to be an independent risk factor for cognitive decline across multiple domains of functioning, including memory and spatial reasoning [10]. The onset and course of cognitive difficulties is poorly understood, but there is evidence that cognitive function may improve following kidney transplantation [11]. There are various potential mechanisms to account for the cognitive change experienced by CKD patients. For instance, the impact of CKD on cerebral vasculature may affect cognition [12]. Previous research has shown that reduced creatinine clearance (between 15 and 60 mL/min) is associated with a 43% increase in stroke and white matter deterioration, which are factors associated with dementia and cognitive impairments [13]. In addition, CKD patients often have elevated homocysteine levels [14], which in turn have been associated with subclinical cerebral infarcts [15], high carotid intima-media thickness [16] and cerebral white matter changes [17], as revealed by magnetic resonance imaging studies employing diffusion -weighted sequences. While these are subclinical associations, they can prompt cognitive impairment through multiple mechanisms such as cerebral systemic inflammation, oedema and atherosclerosis [18]. Although there are only limited data, another putative mechanism for cognitive decline in this group relates to a possible increased risk of vascular dementia in patients with CKD [19]. Vascular mechanisms may also underlie cognitive changes noted in patients receiving dialysis [20]. This may be caused by hypotension (a common result of rapid reduction of blood volume during HD), cerebral oedema due to excess water in the brain because of water retention or even cortical atrophy as a result of dialysis [21]. Anaemia could also contribute to cognitive difficulties experienced by those with CKD. The prevalence of anaemia has been found to be twice as high among CKD patients (15.4%) compared with the general population (7.6%) [22]. Furthermore, the prevalence of anaemia has been shown to increase with the severity of CKD, from 8.4% at Stage 1 to 53.4% at Stage 5 [22]. Unlike other mechanisms, which reflect permanent alterations of neural function, reversal of anaemia is associated with a concomitant improvement in cognitive function [23]. Thus there are several potential mechanisms that contribute to poor cognitive status in CKD patients, and the question remains open as to whether these impairments recover with effective treatment of CKD.
Some studies suggest that cognitive decline of CKD can to some extent be reversed following kidney transplantation [11, 24–26], while others indicate that cognitive decline may be exacerbated by the adverse effects of immunosuppressive medications that patients are required to take after transplantation [27–30] or due to mood disturbances. Cognitive impairments may impact on post-transplant daily living. For instance, decreased cognitive functioning (e.g. in memory and executive function [31]) may lead to lapses in the self-administration of and adherence to complex immunosuppressive medication regimes [32].
There is mixed evidence regarding the nature of cognitive changes following kidney transplantation. Identification of the precise nature of cognitive change is imperative for pre-transplant advice and post-transplant clinical support. The aim of this meta-analysis was to examine which cognitive domains are impacted following kidney transplantation and how performance compares with non-transplanted patients or healthy controls/normative data.
MATERIALS AND METHODS
The review complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33] (Supplementary data, Table S1).
Review strategy
Three databases were searched through the Ovid platform: Embase (1947–February 2016), MEDLINE (1946–February 2016) and PsychINFO (1806–February 2016). No limits were applied for publication dates. Search terms were categorized into three blocks: Block 1, renal or kidney; Block 2, immunosuppression or transplant and Block 3, cognitive function* or cogniti* or neuropsycho* or neuroimag* or memory or language or attention or executive or spatial. A search was then performed using the AND function combining the results of the three blocks. Duplicates were omitted. Reference lists were hand searched (backwards search) and a forward (citation) search was conducted to identify additional relevant publications. Where relevant, authors were contacted to request further information about the methodology or data.
Studies were conducted if they (i) reported cognitive performance data for kidney transplant patients; (ii) included relevant comparison data of either pre- or post-transplant data, dialysis patients, CKD patients, healthy controls or standardized norms; (iii) were written in English and (iv) age >18 years. Exclusion criteria included reviews of conference abstracts, case studies, dissertations or book chapters. The following information was systematically extracted from each identified article and entered into a pre-designed data acquisition sheet: authors (year of publication), aims, design, country of origin, sample size (age range), comparison groups, treatment type, stage of CKD, prospective follow-up period, length of immunosuppressive treatment, time tested after transplant surgery, cognitive domains tested and findings.
Methodological quality assessment
Selected articles underwent quality assessment using an 11-item quality assessment tool [34]. Two researchers undertook this process independently and issues were resolved through consensus. Scoring was dependant on the extent to which a study fulfilled each criterion: a score of two was assigned if a study fully met the criterion, one was assigned if it partially met the criterion and zero was assigned if a criterion was not met. Criteria were excluded if they were not applicable to a particular study. An average score was calculated and studies were categorized as high quality (score > 1.50), medium (score 1.00–1.50) or low quality (score < 1.00).
Meta-analysis procedure
Outcome measures were grouped into nine cognitive domains (Supplementary data, Table S2): attention, executive function, general cognitive status, information and motor speed, spatial reasoning, language, verbal fluency, verbal memory and visual memory. Three separate meta-analyses were performed: (i) within subjects pre- versus post-transplant, (ii) transplanted versus non-transplanted patients and (iii) transplanted versus healthy matched controls and standardized normative data. Data from each study could be included in one, two or all three analyses depending on study design. Random effects models were used to calculate the effect sizes (Hedges’ g) for changes in cognitive performance in each of the nine domains. The Hedges’ g effect size is a measure of the difference between groups reported on a common dimensionless scale. It is obtained by dividing the difference of each measure by the pooled and weighted standard deviation of the sample. An effect size (g) of 0.2 is regarded as being small, because 85% of the area from normally distributed values shared between two groups of equal size and variability overlap; 0.5 is regarded as medium, because 66% of normally distributed values overlap; and 0.8 is regarded as large, because only 20% of combined normally distributed values overlap [35].
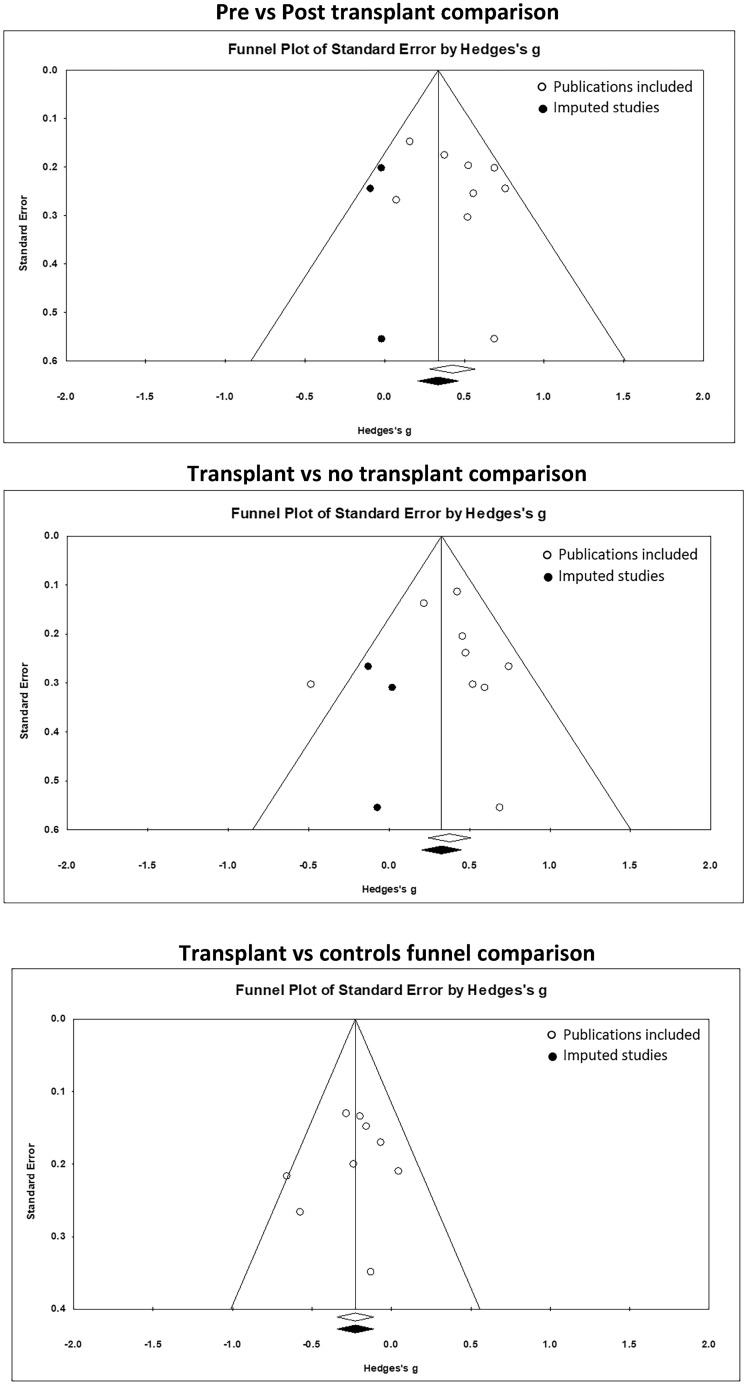
Heterogeneity between articles was assessed using I2, τ2 and Q tests. The I2 statistic is especially relevant to this review, since it allows researchers to approximate the proportion of total variation in the effect sizes across studies. Higher I2 values indicate higher levels of heterogeneity. These proportions have been categorized into low, moderate and high (25%, 50% and 75%, respectively) I2 values representing various levels of heterogeneity [36]. Publication bias was assessed using funnel plots with the trim and fill method (Figure 2). Meta-analysis calculations were undertaken with Comprehensive Meta-Analysis software (version 2; Biostat, Englewood, NJ, USA) [37].
FIGURE 2.
Funnel plots assessing publication bias by analyses conducted, cognitive domain effect sizes and significance level. Hollow symbols represent publication bias of studies included and solid symbols represent imputed studies required to avoid publication bias.
RESULTS
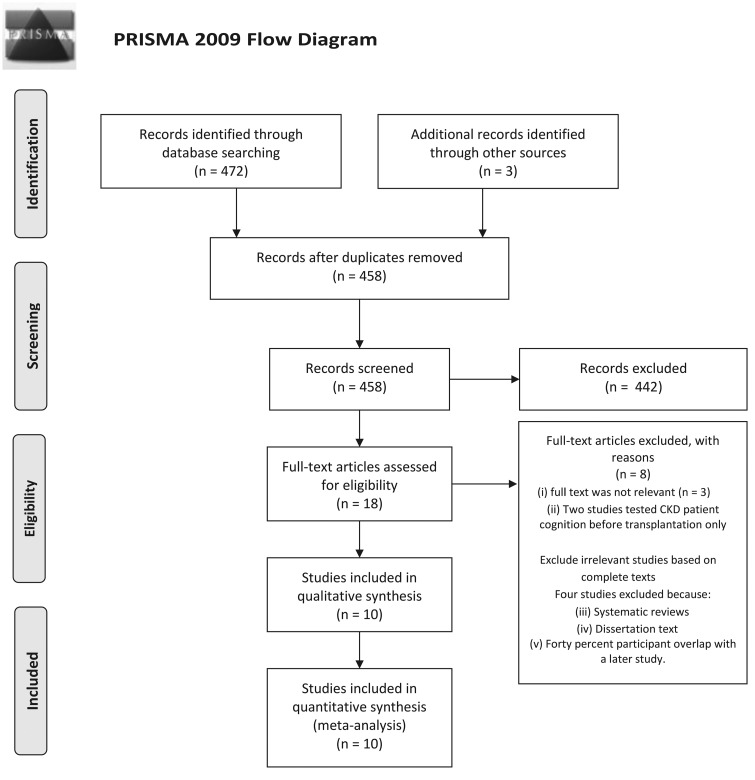
The search methodology identified 472 articles, which was reduced to 458 by eliminating duplicates (Figure 1). After screening titles and abstracts, 442 non-relevant articles were excluded. The remaining 16 articles were retrieved in full text for review against the inclusion and exclusion criteria. Three articles were excluded as they were not relevant to the review [38–40] and a further two studies were excluded because they reported no post-transplant comparative date [41, 42]. A backwards search identified one article and a forwards search revealed two further articles. Of these 14 articles, 3 were excluded because they were systematic reviews or a dissertation without empirical findings. In addition, one article had a 40% participant overlap with a previous study by the same authors and therefore the earlier article was excluded [45, 46]. Ten articles remained; six studies were categorized as high quality [11, 24, 26, 30, 46, 47] and four as moderate quality [14, 25, 48, 49]. Four studies used a longitudinal design in which follow-up assessment periods ranged from 6 to 28 months post-transplant.
FIGURE 1.
Overview of search methodology: (i) Koushik et al. [38], Olbrisch et al. [39] and Wolkowitz et al. [40]; (ii) Hailpern et al. [41] and Kurella et al. [42]; (iii) Carrasco et al. [43] and Gutierrez-Dalmau and Campistol [44] and (v) Harciarek et al. [45].
The most frequently assessed domain across all studies was verbal memory (Supplementary data, Table S3), which was assessed across seven studies. Performance scores from one assessment in an included study were omitted because distinct scores were not provided for the forward and backward parts of the digit span test [47]. Study sample sizes ranged from 15 to 262 (Table 1), with a total sample size of 1118. The age range across the studies was 42–59 years. Publication bias was assessed by visually inspecting funnel plots for asymmetry (Figure 2). Some bias was present, but when plots were re-analysed with the trim and fill method, no significant changes in effect size were found.
Table 1.
Summary of study key significant findings and sample size information
| Study/date | Sample and age (years) | Design | Comparative group, n (age ± SD) | Time tested post-transplant | Outcomes: domains assessed |
|---|---|---|---|---|---|
| Kramer et al. [14], Austria | 15 transplant patients (45 ± 13) previously on HD | Prospective longitudinal Within-subjects pre-transplant dialysis patients assessed after transplantation compared with healthy controls; 14 ± 5-month follow-up |
|
14 months |
|
| Harciarek et al. [46], Poland | 27 transplant patients (46.1 ± 10.9) previously on HD: 17, PD: 10 | Prospective longitudinal Within-subjects pre-transplant dialysis patients assessed after transplantation compared with dialysis and healthy controls; 8-month and 20-month follow-up |
|
None stated |
|
| Griva et al. [26], United Kingdom | 28 transplant patients (follow-up age not stated) previously on HD: 10, PD: 18 | Prospective longitudinal Within-subjects pre-transplant dialysis patients assessed after transplantation; 6-month follow up |
|
6 months | Executive function Information and motor speed Verbal memory Visual memory |
| Radic et al. [24], Croatia | 21 transplant patients (45.14 ± 7.86) previously on HD | Prospective longitudinal Within-subjects pre-transplant dialysis patients assessed after transplantation; 20 ± 8-month follow-up |
|
20.5 months |
|
| Griva et al. [25], United Kingdom | 117 transplant patients (50.26 ± 12.33) | Cross-sectional transplant patients compared with dialysis patients |
|
None stated |
|
| Troen et al. [11], Israel | 183 transplant patients (54 ± 9.5) | Cross-sectional transplant patients assessed only compared with norms | Standardized norms | None stated |
|
| Gelb et al. [48], Canada | 42 transplant patients (55.24 ± 10.96) | Cross-sectional transplant patients compared with healthy controls and CKD patients | CKD patients: 45 (59.67 ± 11.88) Healthy controls: 49 (57.00 ± 13.59) | None stated |
|
| Martinez-Sanchis et al. [30], Spain | 32 transplant patients (42.69 ± 8.28) | Cross-sectional transplant patients compared with healthy controls | Healthy controls: 10 (37.20 ± 9.90) | None stated |
|
| Anwar et al. [47], Egypt | 50 transplant patients | Cross-sectional transplant patients compared with dialysis and healthy controls |
|
None stated |
|
| Ozcan et al. [49], Turkey | 69 transplant patients (50.9 ± 16.5) | Cross-sectional transplant patients compared with dialysis patients |
|
None stated | General cognitive status |
Age ± SD refers to mean age ± standard deviation.
Cognitive findings by domain
All nine cognitive domains (Supplementary data, Table S2) were entered into the analyses.
Within subjects pre- versus post-transplant
Three studies in this analysis included pre-transplant patients on HD [14, 24, 46] and one study included pre-transplant patients on HD or PD [26].
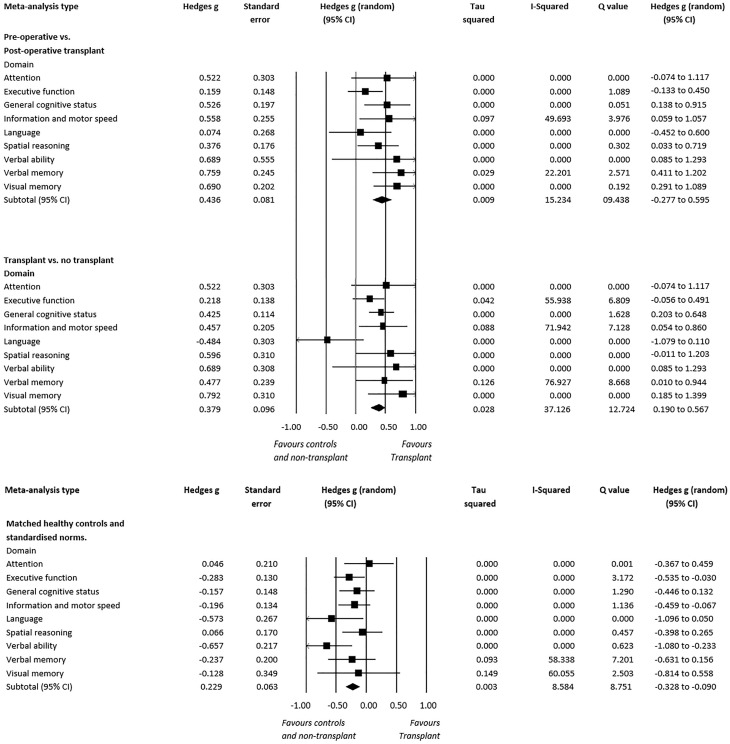
Post-transplantation performance showed significant improvement in general cognitive status {g = 0.526 [95% confidence interval (CI) 0.138–0.915], P = 0.008, I2 = 0.000}, information and motor speed [g = 0.558 (95% CI 0.059–1.057), P = 0.029, I2 = 49.693], spatial reasoning [g = 0.376 (95% CI 0.033–0.719), P = 0.053, I2 = 0.000], verbal memory [g = 0.759 (95% CI 0.411–1.202), P = 0.002, I2 = 22.201] and visual memory [g = 0.690 (95% CI 0.291–1.089), P < 0.001, I2 = 0.000] (Figure 3). No significant effect sizes were observed for the other domains.
FIGURE 3.
Cognitive performance by domains of transplant patients’ baseline performance compared with follow-up. Transplant patients compared with non-transplanted patients and transplant patients compared with matched healthy controls and standardized norms.
Transplanted versus non-transplanted patients
Kidney transplant patients’ scores were compared with non-transplanted dialysis-dependant patients in four studies, one of which measured cognition in patients who were on HD [47], while the remaining three assessed patients who were dialysing through HD or PD [25, 46, 49]. One study was also included in this analysis that compared kidney transplant patients with individuals with CKD [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] who had not received a transplant or dialysis [48]. Scores of patients measured after they had been transplanted and compared with patients who are dialysis-dependant or CKD managed showed significantly better general cognitive status performance [g = 0.425 (95% CI 0.203–0.648), P < 0.001, I2 = 0.000], information and motor speed [g = 0.457 (95% CI 0.054–0.860), P = 0.026, I2 = 71.942], spatial reasoning [g = 0.596 (95% CI −0.011–1.203), P = 0.054, I2 = 0.000], verbal memory [g = 0.477 (95% CI 0.010–0.944), P = 0.046, I2 = 76.927] and visual memory [g = 0.792 (95% CI 0.185–1.399), P = 0.011, I2 = 0.000]. However, no significant differences were found in domains of attention, executive functioning, language and verbal fluency (Figure 3).
Transplanted versus healthy matched controls and standardized norms
Transplanted patients’ scores were compared with healthy matched control participants or standardized norms. One study compared patients with healthy controls first while they were dependent on HD and then after they had been transplanted [46]. Another study made similar comparisons but did not re-test healthy controls after HD patients were transplanted [14]. Five further studies were cross-sectional and assessed cognition at one time point following transplantation and compared with healthy controls or standardized norms [11, 30, 47, 48]. Healthy controls were matched to transplanted patients according to age, mean years of education, gender, baseline intelligence (Mini Mental State Examination, vocabulary), physical activity, occupation, marital status, comorbid conditions and biological factors (haemoglobin, serum creatinine, blood urea nitrogen, eGFR) across studies.
Significant differences were found in executive functioning [g = −0.283 (95% CI −0.535 to −0.030), P = 0.030, I2 = 0.000], language [g = −0.573 (95% CI −1.095 to −0.051), P = 0.032, I2 = 0.000] and verbal fluency [g = −0.657 (95% CI −1.080 to −0.233), P < 0.01, I2 = 0.000], in which post-operative transplant patients scored below healthy matched controls and standardized norms (Figure 3). No significant differences were found in domains of attention, spatial reasoning, information and motor speed, general cognitive status, verbal memory and visual memory across studies (Figure 3).
DISCUSSION
The aim of this meta-analysis was to examine which cognitive domains were impacted following kidney transplantation and how performance compares with non-transplanted patients or healthy controls/normative data. The meta-analysis identified that cognitive performance is better in patients who have received a transplant compared with those who are dialysis dependant or CKD managed in the domains of verbal and visual memory, spatial reasoning, processing speed and general cognitive status. While reviewing changes within people who received a transplant, significant improvements were apparent in the domains of general cognitive status, information and motor speed, spatial reasoning, verbal memory and visual memory. These changes appear to be stable, as cognitive improvement remained measurable at the 1-year [46] and even the 2-year follow-up [24]. However, these findings highlight that transplant patients are unlikely to recover fully after transplant surgery, as patients still performed significantly below healthy matched controls/normative data in three cognitive domains (executive function, verbal fluency and language). Moreover, cognition in some domains (attention, executive function, verbal fluency and language) did not improve after transplantation (even with long-term follow-up) compared with pre-transplant levels and was not superior compared with dialysis patients.
Improved understanding of the aetiology of cognitive impairment after kidney transplantation may enable development of interventions to improve or maintain cognition [50, 51]. Determining whether there are effects upon cognition of specific immunosuppressive medication has important clinical implications. Impairments in attention and executive functioning in kidney transplant patients treated with either tacrolimus or sirolimus have been reported [30]. In contrast, no such cognitive side effects were reported among cyclosporine-treated patients, who performed similarly to healthy controls after transplantation [30]. Unfortunately, any discrete cognitive outcomes related to medication treatments in the study had to be averaged across immunosuppressive groups to be included in the review, losing the effects of individual immunosuppressive therapies on cognition. However, it is well established that immunosuppressive medication can impact neural function. In normal central nervous system function, the cytokine interleukin-2 (IL-2) has been demonstrated to be essential for neurogenesis and cognition [52]; its production and action is inhibited by a number of immunosuppressive drugs, including tacrolimus, cyclosporine and sirolimus [53–55]. In addition, dopamine and acetylcholine have been found to be innervated to the prefrontal and parietal regions [56], which may be involved in executive function tasks requiring working memory, visuospatial working memory, planning and inhibition [29]. Innervation of these neurotransmitters has been shown to be mediated through IL-2 [57]. Thus interference from immunosuppression in the midbrain area (substantia nigra), where IL-2 is abundant, could lead to a blockade in neurotransmitter processes essential for executive functions [58]. The severity of immunosuppressive-induced cognitive changes appears to be reversible and related to the length of immunosuppressive exposure rather than to drug dosages [59–61]. Since tacrolimus and sirolimus have been associated with cognitive impairment in past research [30], it is important to assess the individual effects of specific immunosuppressant medications on cognition. Moreover, this is due to the neural pathways in which these drugs inhibit IL-2. Tacrolimus inhibits IL-2 through calcineurin inhibition [62], whereas sirolimus inhibits T-cell and B-cell activation through action on mammalian target of rapamycin, which regulates cell growth, proliferation and motility [63]. These underlying mechanisms could have pleiotropic effects influencing various phenotypic traits [64, 65]. The possibility of medication-specific cognitive impairment should be one of the issues discussed to fully prepare and inform potential transplant recipients.
There are several alternative mechanisms that might underlie sustained cognitive impairment in kidney transplant patients. There is a well-established link between mood disturbance and cognitive changes, particularly in the domains of attention, executive function and working memory [66–68]. Prevalence rates of depression in kidney transplant patients range between 13 and 46% [69–71]. While some studies of depression in dialysed patients who subsequently received a transplant typically report lower levels of depression after transplantation [26], others found no significant differences or interactions in depression levels between dialysed patients pre- and post-transplantation [45, 46]. The literature illustrates that depression is prevalent in transplant patients. However, uncertainty exists about the carry-over effects of depression from dialysis to transplantation due to mixed findings.
Posterior reversible encephalopathy syndrome (PRES) is an identified mechanism of cognitive impairment and may affect brain function in kidney transplant patients in the short term [72]. In most cases, irregularities are confined to bilateral frontal lobes, right occipital cortices, and parietal regions [73], which are regions important to executive functions [74, 75]. However, longitudinal follow-up studies included in this review suggest that symptoms of PRES are resolved in the long term compared with pre-transplant levels [14, 24, 46]. Investigations of PRES have been explored, but only on a case study [76] or small sample size basis [77]. Therefore, the role and identification of PRES warrants further investigation in longitudinal designs. Other variables including cerebrovascular disease, subclinical infarcts and carotid intima-media thickness could merge with more acute risk factors to influence cognitive changes after transplantation [16, 17]. Delineating between these chronic and acute risk factors should be explored through further research for effective management. Also possible is that improved toxin clearance following transplantation results in improved cognitive performance [46]. Poorer toxin clearance (indicated by a lower eGFR) has been shown to correlate with poorer performance on tests of memory and executive function [11]. Studies that assess the trajectory of cognitive function and how that correlates with toxin clearance would help test this hypothesis.
There are several limitations in the meta-analysis presented here. Only four studies assessed cognition longitudinally (6 months to 2 years follow-up periods). Moreover, it should be noted that only English-language studies were included and that non-English-language studies may influence the overall findings reported here. Additionally, discrepancies exist between global approaches to the management of renal transplant patients and practices change over time. Therefore, the meta-analysis and review conducted should be interpreted with caution, as location and time are relevant to outcomes. Another concern is that studies lack large enough sample sizes required to match for multiple comparisons across dialysis, transplant and healthy patients. Therefore, study designs lacked the power to compare for multiple confounds across samples and findings may be biased. Publication bias was apparent in the within-subjects comparisons and non-transplant versus transplant patient comparisons. However, when the trim and fill method was used to adjust for the suspected bias, only marginal effect size shifts were apparent, indicating no significant changes even with imputed studies. In addition, studies employed study-specific neuropsychological measures to assess cognition, which makes it challenging to compare and group tests into domains because sensitivities and specificities of tests may differ according to the population being studied [78]. Furthermore, cut-off scores defined as being abnormal or normal may vary for each incorporated test [78].
Organization of assessments was grouped into cognitive domains with specific distinctions made between certain cognitive domains. Categorization of tests primarily dependent on language and executive control abilities were classified as verbal fluency. However, those tasks involving predominantly executive functions such as inhibition and categorization [79] with a working memory retrieval component were grouped in the executive function domain. Arguably, the validity of both verbal fluency and executive function assessments have been found to overlap [80]. Due to the hybrid nature of both the domains, the number of possible variations that tests could be classified as could vary overall outcomes. The development of a cognitive taxonomy for the classification of assessments is required and recommended to limit this shortcoming.
The analysis included a pre- versus post- within-subjects design. One weakness of the studies in this analysis (pre- versus post-) is that a control group was not used to control for changes in cognition of patients over time [81]. Therefore, a control group of ESRD patients actively waiting for a transplant from the deceased donor list is recommended in further research. This group represents the closest match to transplant patients postoperatively and may assist in controlling for confounding factors among both groups over time.
We know of no interventions that have been developed specifically to mitigate the effects of cognitive changes among transplant patients. However, cognitive interventions have been shown to be effective in improving cognitive functioning in other groups, including executive functioning after brain injury and mild cognitive impairment in other clinical populations, and it would be useful to test their benefit for kidney transplant recipients [82, 83]. Our findings provide evidence for restoration of cognitive function in transplanted patients to pre-end-stage but not normal levels in the majority of cognitive domains. There is a need for research that identifies the basis for this improvement. The recovery of cognitive abilities may not extend to the important domain of executive functions and may be inhibited by some groups of immunosuppressive medication. Thus, there is a strong case for additional research aimed at supporting optimal cognitive outcomes among patients receiving kidney transplants.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Ligtenberg G. Regulation of blood pressure in chronic renal failure: determinants of hypertension and dialysis-related hypotension. Neth J Med 1999; 55: 13–18 [DOI] [PubMed] [Google Scholar]
- 2. Pyram R, Kansara A, Banerji MA. et al. Chronic kidney disease and diabetes. Maturitas 2012; 71: 94–103 [DOI] [PubMed] [Google Scholar]
- 3. Satko SG, Freedman BI, Moossavi S.. Genetic factors in end-stage renal disease. Kidney Int 2005; 67(Suppl 94): S46–S49 [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care. http://www.nice.org.uk/guidance/CG182. [PubMed]
- 5. Ramesh Prasad GV, Ruzicka M, Burns KD. et al. Hypertension in dialysis and kidney transplant patients. Can J Cardiol 2009; 25: 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tonelli M, Wiebe N, Knoll G. et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- 7. NHS Blood and Transplant. Summary of Donor and Transplant Activity. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/4492/section_1_summary_of_transplant_activity.pdf
- 8. Perlman RL, Finkelstein FO, Liu L. et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis 2005; 45: 658–666 [DOI] [PubMed] [Google Scholar]
- 9. Schiffrin EL, Lipman ML, Mann JF.. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007; 116: 85–97 [DOI] [PubMed] [Google Scholar]
- 10. Khatri M, Nickolas T, Moon YP. et al. CKD associates with cognitive decline. J Am Soc Nephrol 2009; 20: 2427–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Troen AM, Scott TM, D'Anci KE. et al. Cognitive dysfunction and depression in adult kidney transplant recipients: baseline findings from the FAVORIT Ancillary Cognitive Trial (FACT). J Ren Nutr 2012; 22: 268–276 e261-263.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drew DA, Weiner DE.. Cognitive impairment in chronic kidney disease: keep vascular disease in mind. Kidney Int 2014; 85: 505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khatri M, Wright CB, Nickolas TL. et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke 2007; 38: 3121–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kramer L, Madl C, Stockenhuber F. et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int 1996; 49: 833–838 [DOI] [PubMed] [Google Scholar]
- 15. Seshadri S, Wolf PA, Beiser AS. et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol 2008; 65: 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mykkanen L, Zaccaro DJ, O'Leary DH. et al. Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS). Stroke 1997; 28: 1710–1716 [DOI] [PubMed] [Google Scholar]
- 17. Feng L, Isaac V, Sim S. et al. Associations between elevated homocysteine, cognitive impairment, and reduced white matter volume in healthy old adults. Am J Geriatr Psychiatry 2013; 21: 164–172 [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Rosenberg GA.. Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011; 42: 3323–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helmer C, Stengel B, Metzger M. et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 2011; 77: 2043–2051 [DOI] [PubMed] [Google Scholar]
- 20. Weiner DE, Scott TM, Giang LM. et al. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 2011; 58: 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madero M, Gul A, Sarnak MJ.. Cognitive function in chronic kidney disease. Semin Dial 2008; 21: 29–37 [DOI] [PubMed] [Google Scholar]
- 22. Stauffer ME, Fan T.. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014; 9: e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray-Kolb LE, Beard JL.. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007; 85: 778–787 [DOI] [PubMed] [Google Scholar]
- 24. Radic J, Ljutic D, Radic M. et al. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol 2011; 34: 399–406 [DOI] [PubMed] [Google Scholar]
- 25. Griva K, Hansraj S, Thompson D. et al. Neuropsychological performance after kidney transplantation: a comparison between transplant types and in relation to dialysis and normative data. Nephrol Dial Transplant 2004; 19: 1866–1874 [DOI] [PubMed] [Google Scholar]
- 26. Griva K, Thompson D, Jayasena D. et al. Cognitive functioning pre- to post-kidney transplantation—a prospective study. Nephrol Dial Transplant 2006; 21: 3275–3282 [DOI] [PubMed] [Google Scholar]
- 27. Alparslan M, Bora U, Hüseyin K. et al. Posterior reversible encephalopathy syndrome in a renal transplanted patient. Am J Case Rep 2013; 14: 241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DiMartini A, Crone C, Fireman M. et al. Psychiatric aspects of organ transplantation in critical care. Crit Care Clin 2008; 24: 949–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koenigs M, Barbey AK, Postle BR. et al. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 2009; 29: 14980–14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez-Sanchis S, Bernal MC, Montagud JV. et al. Effects of immunosuppressive drugs on the cognitive functioning of renal transplant recipients: a pilot study. J Clin Exp Neuropsychol 2011; 33: 1016–1024 [DOI] [PubMed] [Google Scholar]
- 31. Griva K, Davenport A, Harrison M. et al. Non-adherence to immunosuppressive medications in kidney transplantation: intent vs. forgetfulness and clinical markers of medication intake. Ann Behav Med 2012; 44: 85–93 [DOI] [PubMed] [Google Scholar]
- 32. Stoehr GP, Lu SY, Lavery L. et al. Factors associated with adherence to medication regimens in older primary care patients: the Steel Valley Seniors Survey. Am J Geriatr Pharmacother 2008; 6: 255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kmet LM, Lee RC, Cook LS. et al. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Edmonton, AL, Canada: Alberta Heritage Foundation for Medical Research, 2004 [Google Scholar]
- 35. Zakzanis KK. Statistics to tell the truth, the whole truth, and nothing but the truth: formulae, illustrative numerical examples, and heuristic interpretation of effect size analyses for neuropsychological researchers. Arch Clin Neuropsychol 2001; 16: 653–667 [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ. et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borenstein M, Hedges LV, Higgins JPT. et al. Comprehensive Meta-Analysis Version 2 www.Meta-Analysis.com (12 April 2017, date last accessed)
- 38. Koushik NS, McArthur SF, Baird AD.. Adult chronic kidney disease: neurocognition in chronic renal failure. Neuropsychol Rev 2010; 20: 33–51 [DOI] [PubMed] [Google Scholar]
- 39. Olbrisch ME, Benedict SM, Ashe K. et al. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol 2002; 70: 771–783 [DOI] [PubMed] [Google Scholar]
- 40. Wolkowitz OM, Reus VI, Weingartner H. et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990; 147: 1297–1303 [DOI] [PubMed] [Google Scholar]
- 41. Hailpern SM, Melamed ML, Cohen HW. et al. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 2007; 18: 2205–2213 [DOI] [PubMed] [Google Scholar]
- 42. Kurella M, Chertow GM, Luan J. et al. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 2004; 52: 1863–1869 [DOI] [PubMed] [Google Scholar]
- 43. Carrasco FR, Moreno A, Ridao N. et al. Kidney transplantation complications related to psychiatric or neurological disorders. Transplant Proc 2009; 41: 2430–2432 [DOI] [PubMed] [Google Scholar]
- 44. Gutierrez-Dalmau A, Campistol JM.. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs 2007; 67: 1167–1198 [DOI] [PubMed] [Google Scholar]
- 45. Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M. et al. Cognitive performance before and after kidney transplantation: a prospective controlled study of adequately dialyzed patients with end-stage renal disease. J Int Neuropsychol Soc 2009; 15: 684–694 [DOI] [PubMed] [Google Scholar]
- 46. Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M. et al. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int 2011; 79: 1353–1360 [DOI] [PubMed] [Google Scholar]
- 47. Anwar W, Ezzat H, Mohab A.. Comparative study of impact of hemodialysis and renal transplantation on cognitive functions in ESRD patients. Nefrologia 2015; 35: 567–571 [DOI] [PubMed] [Google Scholar]
- 48. Gelb S, Shapiro RJ, Hill A. et al. Cognitive outcome following kidney transplantation. Nephrol Dial Transplant 2008; 23: 1032–1038 [DOI] [PubMed] [Google Scholar]
- 49. Ozcan H, Yucel A, Avsar UZ. et al. Kidney transplantation is superior to hemodialysis and peritoneal dialysis in terms of cognitive function, anxiety, and depression symptoms in chronic kidney disease. Transplant Proc 2015; 47: 1348–1351 [DOI] [PubMed] [Google Scholar]
- 50. Smith GB, Schwebel AI, Dunn RL. et al. The role of psychologists in the treatment, management, and prevention of chronic mental illness. Am Psychol 1993; 48: 966–971 [DOI] [PubMed] [Google Scholar]
- 51. Katon W, Unutzer J, Russo J.. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety 2010; 27: 19–26 [DOI] [PubMed] [Google Scholar]
- 52. Snyder SH, Lai MM, Burnett PE.. Immunophilins in the nervous system. Neuron 1998; 21: 283–294 [DOI] [PubMed] [Google Scholar]
- 53. Yoon KH. Efficacy and cytokine modulating effects of tacrolimus in systemic lupus erythematosus: a review. J Biomed Biotechnol 2010; 2010: 686480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuga K, Nishifuji K, Iwasaki T.. Cyclosporine A inhibits transcription of cytokine genes and decreases the frequencies of IL-2 producing cells in feline mononuclear cells. J Vet Med Sci 2008; 70: 1011–1016 [DOI] [PubMed] [Google Scholar]
- 55. Rabinovitch A, Suarez-Pinzon WL, Shapiro AMJ. et al. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 2002; 51: 638–645 [DOI] [PubMed] [Google Scholar]
- 56. Katsuki F, Constantinidis C.. Time course of functional connectivity in primate dorsolateral prefrontal and posterior parietal cortex during working memory. PLoS One 2013; 8: e81601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alonso A, Klink R.. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol 1993; 70: 128–143 [DOI] [PubMed] [Google Scholar]
- 58. McAfoose J, Baune BT.. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 2009; 33: 355–366 [DOI] [PubMed] [Google Scholar]
- 59. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008; 29: 1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lamy C, Oppenheim C, Meder JF. et al. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging 2004; 14: 89–96 [PubMed] [Google Scholar]
- 61. Ayas M, Al-Jefri A, Al-Seraihi A.. In cyclosporine induced neurotoxicity, is tacrolimus an appropriate substitute or is it out of the frying pan and into the fire? Pediatr Blood Cancer 2008; 50: 426–426 [DOI] [PubMed] [Google Scholar]
- 62. Naesens M, Kuypers DRJ, Sarwal M.. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009; 4: 481–508 [DOI] [PubMed] [Google Scholar]
- 63. Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003; 35(Suppl): S7–S14 [DOI] [PubMed] [Google Scholar]
- 64. Wagner GP, Zhang J.. The pleiotropic structure of the genotype–phenotype map: the evolvability of complex organisms. Nat Rev Genet 2011; 12: 204–213 [DOI] [PubMed] [Google Scholar]
- 65. Martinet W, De Loof H, De Meyer GR.. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 2014; 233: 601–607 [DOI] [PubMed] [Google Scholar]
- 66. Airaksinen E, Larsson M, Lundberg I. et al. Cognitive functions in depressive disorders: evidence from a population-based study. Psychol Med 2004; 34: 83–91 [DOI] [PubMed] [Google Scholar]
- 67. Andrew James I, Reichelt FK, Carlsonn P. et al. Cognitive behavior therapy and executive functioning in depression. J Cogn Psychother 2008; 22: 210–218 [Google Scholar]
- 68. Cella M, Dymond S, Cooper A.. Impaired flexible decision-making in major depressive disorder. J Affect Disord 2010; 124: 207–210 [DOI] [PubMed] [Google Scholar]
- 69. Zelle DM, Dorland HF, Rosmalen JG. et al. Impact of depression on long-term outcome after renal transplantation: a prospective cohort study. Transplantation 2012; 94: 1033–1040 [DOI] [PubMed] [Google Scholar]
- 70. Griva K, Davenport A, Harrison M. et al. The impact of treatment transitions between dialysis and transplantation on illness cognitions and quality of life - a prospective study. Br J Health Psychol 2012; 17: 812–827 [DOI] [PubMed] [Google Scholar]
- 71. Spencer BW, Chilcot J, Farrington K.. Still sad after successful renal transplantation: are we failing to recognise depression? An audit of depression screening in renal graft recipients. Nephron Clin Pract 2011; 117: c106–c112 [DOI] [PubMed] [Google Scholar]
- 72. Agildere AM, Basaran C, Cakir B. et al. Evaluation of neurologic complications by brain MRI in kidney and liver transplant recipients. Transplant Proc 2006; 38: 611–618 [DOI] [PubMed] [Google Scholar]
- 73. Postma IR, Slager S, Kremer HP. et al. Long-term consequences of the posterior reversible encephalopathy syndrome in eclampsia and preeclampsia: a review of the obstetric and nonobstetric literature. Obstet Gynecol Surv 2014; 69: 287–300 [DOI] [PubMed] [Google Scholar]
- 74. Casey BJ, Forman SD, Franzen P. et al. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp 2001; 13: 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Halpern JH, Pope HG Jr, Sherwood AR. et al. Residual neuropsychological effects of illicit 3,4-methylenedioxymethamphetamine (MDMA) in individuals with minimal exposure to other drugs. Drug Alcohol Depend 2004; 75: 135–147 [DOI] [PubMed] [Google Scholar]
- 76. Haughey D, Narsipur SS.. Posterior reversible encephalopathy syndrome after renal transplant: a simple solution for a complicated patient. Case Rep Nephrol Dial 2015; 5: 20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Giussani A, Ardissino G, Belingheri M. et al. Posterior reversible encephalopathy syndrome after kidney transplantation in pediatric recipients: two cases. Pediatr Transplant 2016; 20: 68–71 [DOI] [PubMed] [Google Scholar]
- 78. Tombaugh TN, McIntyre NJ.. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992; 40: 922–935 [DOI] [PubMed] [Google Scholar]
- 79. Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist 2007; 13: 214–228 [DOI] [PubMed] [Google Scholar]
- 80. Shao Z, Janse E, Visser K. et al. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 2014; 5: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leong RLF, Lo JC, Sim SKY. et al. Longitudinal brain structure and cognitive changes over 8 years in an East Asian cohort. NeuroImage 2017; 147: 852–860 [DOI] [PubMed] [Google Scholar]
- 82. Manly T, Murphy FC.. Rehabilitation of executive function and social cognition impairments after brain injury. Curr Opin Neurol 2012; 25: 656–661 [DOI] [PubMed] [Google Scholar]
- 83. Reijnders J, van Heugten C, van Boxtel M.. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev 2013; 12: 263–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.