Abstract
Aims
There is a paucity of data on the influence of diabetes on long-term outcomes after ischaemic stroke (IS). We assessed whether outcomes after IS differ between patients with and without diabetes.
Methods and results
Patients aged ≥65 years (n = 409 060) in Get With The Guidelines-Stroke (nationwide registry of stroke patients from 1690 sites in the USA) were followed for 3 years post-discharge. The outcomes of interest were mortality, cardiovascular and non-cardiovascular hospitalizations, heart failure (HF), and recurrence of IS/transient ischaemic attack (TIA). Patients with diabetes (29.6%) were younger and had more comorbidities. At 3 years post-discharge after IS, diabetes was associated with higher risks of adverse outcomes: all-cause mortality [cumulative incidence 46.0% vs. 44.2%, absolute difference (AD) 1.8%; adjusted hazard ratio (aHR) 1.24, 95% confidence interval 1.23–1.25], all-cause readmission (71.3% vs. 63.7%, AD 7.6%; aHR 1.22, 1.21–1.23), composite of mortality and all-cause readmission (84.1% vs. 79.3%, AD 4.8%; aHR 1.21, 1.20–1.22), composite of mortality and cardiovascular readmission (69.5% vs. 64.3%, AD 5.2%; aHR 1.19, 1.18–1.20), IS/TIA readmission (15.9% vs. 13.3%, AD 2.6%; aHR 1.18, 1.16–1.20), HF readmission (10.3% vs. 6.4%, AD 3.9%; aHR 1.60, 1.56–1.64), non-cardiovascular readmission (58.3% vs. 50.3%, AD 8.0%; aHR 1.28, 1.26–1.29), and non-IS/TIA readmission (67.6% vs. 59.7%, AD 7.9%; aHR 1.23, 1.22–1.25). Accounting for the initial severity of stroke using the National Institute of Health Stroke Scale as well as using propensity score matching method as a sensitivity analysis, did not modify the results.
Conclusion
Among older IS patients diabetes was associated with increased risks of death, cardiovascular and non-cardiovascular hospitalizations, HF, and IS/TIA recurrence.
Keywords: Diabetes , Glycated haemoglobin , Stroke , Ischaemic stroke , Transient ischaemic attack
Introduction
Diabetes and stroke are each highly prevalent in the USA.1,2 Patients with diabetes display an increased the risk of ischaemic stroke (IS) by at least twice that of those without the condition.3 Diabetes and IS tend to coexist, with at least one in four patients with IS having diabetes.4 Diabetes may promote adverse outcomes of IS through an acceleration of the atherosclerosis process.5 However, it is unclear to what extent diabetes influences long-term IS outcomes other than mortality, especially cardiovascular events and hospitalizations. There have been a limited number of investigations on this topic. Such investigations may clarify our understanding of the natural history and clinical outcomes (including stroke recurrence) of patients with both IS and diabetes, including the magnitude of possible differences within clinically important subgroups defined by age, comorbidities, previous IS/transient ischaemic attack (TIA), initial severity of stroke, chronic kidney disease (CKD), or racial/ethnic background.3
Population-based studies have shown that two-thirds of all strokes occur in patients aged 65 years and older.2 Older patients with IS have worse functional outcomes and higher recurrent event risk than younger patients.6 Using Get With The Guidelines (GWTG)-Stroke registry data linked to Centres for Medicare & Medicaid Services claims, we assessed the association of diabetes and long-term outcomes of patients age 65 years of age and older with IS, including mortality, all-cause readmission, hospitalization for cardiovascular reasons, hospitalization for non-cardiovascular causes, heart failure (HF), and readmission for IS/TIA. We hypothesized that diabetes will be associated worse outcomes in older patients with IS, even after accounting for demographics and other comorbid conditions.
Methods
Data source
The study cohort was formed from the Get With The Guidelines (GWTG)-Stroke, a national prospective stroke registry and quality improvement program sponsored by the American Heart Association (AHA)/American Stroke Association (ASA). Details of the design and conduct of the GWTG-Stroke registry have been previously described.7 In GWTG-Stroke, participating hospitals use an internet-based patient management tool (IQVIA, Inc, Durham NC) to add data to a central database on consecutive acute IS patients. The methods for data extraction have been previously described,8 as well as the validity and reliability of data collection.9 As the primary objective of data collection is quality improvement, each participating hospital received either human research approval to enrol cases without individual patient consent under the common rule or a waiver of authorization and exemption from subsequent review by their Institutional Review Board. The Duke Clinical Research Institute serves as the data analysis centre.
The patients enrolled in the GWTG-Stroke registry were those hospitalized with new IS or TIA, or patients who developed significant stroke/TIA symptoms such that stroke was the primary discharge diagnosis. The in-hospital data from the GWTG-Stroke registry were linked to the Centres for Medicare & Medicaid Services (CMS) claims data. The linked clinical and claims data set (GWTG-Stroke/CMS) was created by matching on a series of indirect identifiers, including admission date, discharge date, patient age or date of birth, and sex. This probabilistic linkage methodology has previously been successfully completed and validated using the 100% Medicare inpatient claims and GWTG-Stroke.10 The Medicare claims for outpatient and skilled nursing facilities data were limited to fee-for-service Medicare patients aged ≥65 years at the time of first stroke diagnosis. Prior work has shown that patients in the linked GWTG-Stroke/CMS database are representative of the national Medicare IS population.8 In case multiple stroke admission records were linked for a single patient, we used the earliest record for this analysis.
Study population (inclusion and exclusion criteria)
The study cohort was restricted to patients age 65 years and above with a final diagnosis of IS. Only patients aged ≥65 years were included in the study because this is the group of patients for whom data post-discharge follow-up data on outcomes can be obtained through linkage to the CMS data. Case ascertainment of admissions for IS was conducted using the International Classification of Diseases 9th revision (ICD-9) discharge codes for IS, with chart review for eligibility confirmation. Patients were further excluded if: (i) they were transferred in from another facility; (ii) transferred out to another acute care facility; (iii) left against medical advice or if the discharge status was unknown; and (iv) had an unknown diabetes status or (v) they died during hospitalization.
Diabetes ascertainment
Diabetes mellitus was defined by prior medical history of diabetes mellitus or new clinical diagnosis of diabetes mellitus during index hospitalization. We used an alternative definition of diabetes to perform a sensitivity analysis, which was based on a combination of criteria including a past history of diabetes, the use of diabetes medications prior to admission, the use of diabetes medications at discharge, and a new diagnosis of diabetes during index admission.
Outcomes
The primary outcome was all-cause mortality. The secondary outcomes included a composite of mortality and all-cause readmission, a composite of death and cardiovascular readmission, all-cause admission, admission for recurrent IS (IS/TIA) (stroke specific event), admission for recurrent IS only (stroke specific event), admission for acute myocardial infarction (AMI), admission for HF, cardiovascular readmission, non-cardiovascular admission, and non IS/TIA readmission. The outcomes were assessed at 1 year and at 3 years post-discharge. Mortality was ascertained on the basis of death dates from the CMS vital status files,10,11 and admission on the basis of subsequent Medicare inpatient claims. The ICD-9 discharge codes were used for defining outcomes and are shown in the Supplementary material online, Table S1.
Statistical analysis
We compared patient (demographic and clinical) and hospital characteristics between patients with and without diabetes. Categorical variables were presented as proportions, and differences tested using the Pearson’s χ2 test. Continuous variables were presented as median (interquartile range), and differences between groups were tested using the Wilcoxon rank sum test. Percent standardized differences were also provided for all variables between the diabetes and no-diabetes groups. The cumulative incidence of each outcome was estimated by diabetes status. The differences in mortality were tested using the log-rank test. For the hospitalization outcomes, we accounted for the competing risk of death, and assessed the differences between those with and without diabetes using the Gray’s test. We used Cox proportional hazards models to test the association between diabetes and each clinical outcome. The models used a robust variance estimation to account for correlation within sites. The covariates included in the multivariable models were patients characteristics {age, sex, race [white, black, other], medical history [hypertension, dyslipidaemia, smoking, atrial fibrillation/flutter, previous stroke/TIA, coronary artery disease (CAD)/prior myocardial infarction, HF, carotid stenosis, peripheral vascular disease (PVD)], arrival via Emergency Medical Services (EMS), on vs. off hours admission, discharge year} and hospital characteristics (geographic region, teaching status, rural location, bed size, annual admissions for IS, primary stroke centre).
We performed a series of additional analyses, to assess the robustness of our findings and explore potential factors that may influence the relation of diabetes status and stroke outcomes. These analyses are as follows: (i) using an alternative diabetes definition, (ii) adjusting for NIHSS score in patients with recorded NIHSS, (iii) assessing the outcomes by diabetes status among patients, who received thrombolysis with tissue plasminogen activator (tPA), and (iv) prespecified stratified analyses in clinically relevant subgroups by age (<80 and ≥80 years), sex (female and male), race (non-Hispanic black and other), baseline pre-existing cardiovascular disease and CKD [estimated glomerular filtration rate (eGFR) <60 and ≥60 ml/min/1.73m2]. We also assessed the association between glycated haemoglobin (HbA1C ≥7% vs. <7%) and outcomes in diabetes patients who had HbA1C data recorded. To account for the association of diabetes treatment with outcomes, we assessed the outcomes among patients with diabetes discharged on insulin therapy compared to those not on insulin therapy at discharge. Missing covariates were handled by imputation based on the extent of missingness, as detailed in Supplementary material online, Table S2.
Analyses were performed using the SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All P-values are 2-sided tests and were considered statistically significant at <0.05.
Results
A total of 1 124 670 stroke patients were admitted between 1 April 2003 and 31 December 2011, to GWTG-Stroke fully-participating hospitals (1755 sites). Of these, we excluded a number of patients for following reasons: age <65 years (n = 365 286), transferred-out hospitalizations (n = 12 220), left against medical advice/discharge disposition not determined or missing (n = 10 210), unknown diabetes status (n = 2202), no link to CMS claims data (n = 243 393), non-index admission (n = 30 692), fee-for-service ineligible at index discharge (n = 23 967), or in-hospital death (n = 27 640). The final study sample contained 409 060 patients aged ≥65 years (from 1690 sites) followed for up to 3 years post-discharge (until 31 December 2014).
Baseline characteristics
The baseline patients (demographic and clinical) and hospitals characteristics between the diabetes and no-diabetes groups are shown in Table 1. Patients with diabetes (29.6%, n = 120 989) were more likely to be younger, from an ethnic minority (Hispanic or Black), to have a higher body mass index, as well as to have a history of comorbidities including hypertension, dyslipidaemia, CKD, prior IS/TIA, carotid stenosis, CAD, PVD, and HF. Accordingly, diabetic patients were also more likely to be on cholesterol-lowering medication, antihypertensives and antiplatelets.
Table 1.
Baseline characteristics of ischaemic stroke patients by diabetes status in the GWTG-Stroke registry
| Variables | Overall | Diabetes | No diabetes | P-value | Percent standardized differences |
|---|---|---|---|---|---|
| n = 409 060 | n = 120 989 | n = 288 071 | |||
| Demographics | |||||
| Agea | 80 (73–86) | 77 (71–83) | 81 (74–87) | <0.001 | 35.8 |
| Age ≥80 | 51.85 | 41.22 | 56.32 | <0.001 | 30.5 |
| Female | 58.11 | 54.82 | 59.49 | <0.001 | 9.5 |
| Race/ethnicity | <0.001 | ||||
| White | 81.16 | 74.03 | 84.15 | 25.1 | |
| Black | 9.98 | 14.82 | 7.94 | 21.8 | |
| Hispanic | 3.66 | 5.50 | 2.89 | 13.1 | |
| Asian | 1.67 | 2.10 | 1.48 | 4.7 | |
| Other | 3.54 | 3.55 | 3.54 | 0.1 | |
| Medical history | |||||
| Atrial fibrillation/flutter | 23.94 | 21.52 | 24.96 | <0.001 | 8.2 |
| CAD/prior MI | 31.10 | 38.65 | 27.92 | <0.001 | 22.9 |
| Carotid stenosis | 4.88 | 5.55 | 4.60 | <0.001 | 4.3 |
| Peripheral vascular disease | 5.63 | 7.71 | 4.75 | <0.001 | 12.3 |
| Hypertension | 78.54 | 85.62 | 75.56 | <0.001 | 25.7 |
| Smoker | 9.81 | 8.68 | 10.28 | <0.001 | 5.5 |
| Dyslipidaemia | 41.26 | 49.78 | 37.67 | <0.001 | 24.6 |
| Heart failure | 7.29 | 9.63 | 6.30 | <0.001 | 12.3 |
| Previous stroke/TIA | 29.97 | 33.51 | 28.48 | <0.001 | 10.9 |
| Prosthetic heart valve | 1.57 | 1.55 | 1.58 | 0.409 | 0.3 |
| Measurements | |||||
| BMIa | 25.9 (22.8–29.8) | 27.9 (24.4–32.1) | 25.2 (22.2–28.7) | <0.001 | 40.9 |
| SBP (50–250) (mmHg)a | 155 (137–177) | 155 (137–178) | 155 (137–176) | <0.001 | 2.6 |
| Blood glucose (20–800) (mg/dL)a | 116 (100–145) | 148 (115–197) | 110 (97–128) | <0.001 | 86.5 |
| Serum creatinine (0–150) (mg/dL)a | 1.0 (0.8–1.3) | 1.1 (0.9–1.5) | 1.0 (0.8–1.3) | <0.001 | 4.3 |
| eGFRa | 59.7 (45.1–77.1) | 57.5 (40.9–73) | 60.2 (47.1–78.8) | <0.001 | 14.2 |
| CKD (eGFR <60 mL/min/1.73 m2)b | 51.06 | 55.43 | 49.07 | <0.001 | 12.8 |
| Arrival information | |||||
| Arrival mode: EMS | 60.07 | 58.82 | 60.60 | <0.001 | 3.6 |
| Ambulatory status at admission | <0.001 | ||||
| Able to ambulate independently | 33.93 | 33.08 | 34.32 | 2.6 | |
| With assistance from person | 29.59 | 30.00 | 29.40 | 1.3 | |
| Unable to ambulate | 36.48 | 36.92 | 36.28 | 1.3 | |
| On-time arrival (non-holiday, weekday, 7 a.m.–6 p.m.) | 48.72 | 48.52 | 48.80 | 0.100 | 0.6 |
| Initial NIHSS score (0–42)a | 5 (2–11) | 5 (2–11) | 5 (2–11) | <0.001 | 3.4 |
| Initial NIHSS score missing | 48.51 | 49.19 | 48.22 | <0.001 | 1.9 |
| Discharge year | <0.001 | ||||
| 2011 | 20.19 | 21.31 | 19.72 | 3.9 | |
| 2010 | 18.43 | 19.36 | 18.04 | 3.4 | |
| 2009 | 16.52 | 16.96 | 16.33 | 1.7 | |
| 2008 | 13.96 | 13.65 | 14.09 | 1.3 | |
| 2007 | 11.95 | 11.18 | 12.27 | 3.4 | |
| 2006 | 9.47 | 8.76 | 9.77 | 3.5 | |
| 2005 | 5.79 | 5.41 | 5.95 | 2.3 | |
| 2004 | 2.51 | 2.31 | 2.60 | 1.8 | |
| 2003 | 1.17 | 1.06 | 1.22 | 1.5 | |
| Medications prior to admission | |||||
| Antiplatelets | 50.72 | 56.20 | 48.25 | <0.001 | 16.0 |
| Anticoagulants | 13.40 | 13.94 | 13.16 | <0.001 | 2.3 |
| Hypertension medications | 76.35 | 84.18 | 73.05 | <0.001 | 27.4 |
| Cholesterol-lowering medications | 41.69 | 53.90 | 36.55 | <0.001 | 35.4 |
| Hospital characteristics | |||||
| Rural location | 4.53 | 4.69 | 4.46 | 0.001 | 1.1 |
| Region | <0.001 | ||||
| West | 14.53 | 13.13 | 15.11 | 5.7 | |
| South | 36.86 | 38.41 | 36.21 | 4.5 | |
| Midwest | 22.31 | 22.69 | 22.15 | 1.3 | |
| Northeast | 26.30 | 25.77 | 26.52 | 1.7 | |
| Primary stroke centre | 46.74 | 46.48 | 46.85 | 0.032 | 0.7 |
| Academic hospital | 58.91 | 58.54 | 59.06 | 0.002 | 1.1 |
| Number of bedsa | 356 (248–530) | 358 (249–531) | 355 (248–527) | 0.004 | 1.1 |
| Annual volume of IS admissionsa | 226 (154–345) | 228 (153–343) | 226 (154–345) | 0.221 | 0.4 |
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; EMS, Emergency Medical Services; IS, ischaemic stroke; MI, myocardial infarction; NIHSS, NIH stroke scale; PVD, peripheral vascular disease; SBP, systolic blood pressure; TIA, transient ischaemic attack.
Continuous variables are present as median (interquartile range), categorical variables are presented n (%).
Chronic kidney disease defined using the modification of diet in renal disease equation as eGFR < 60 mL/min/1.73 m2.
Clinical outcomes
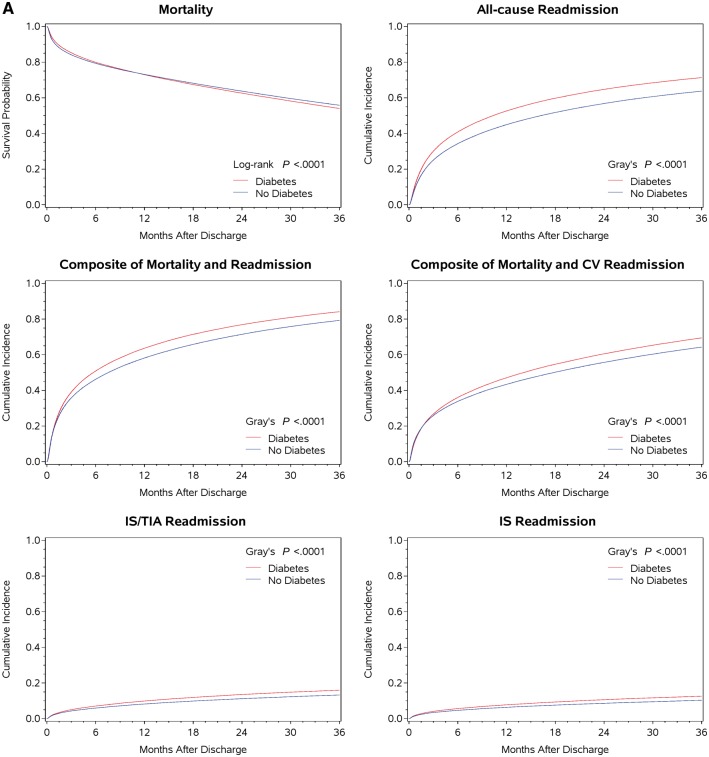
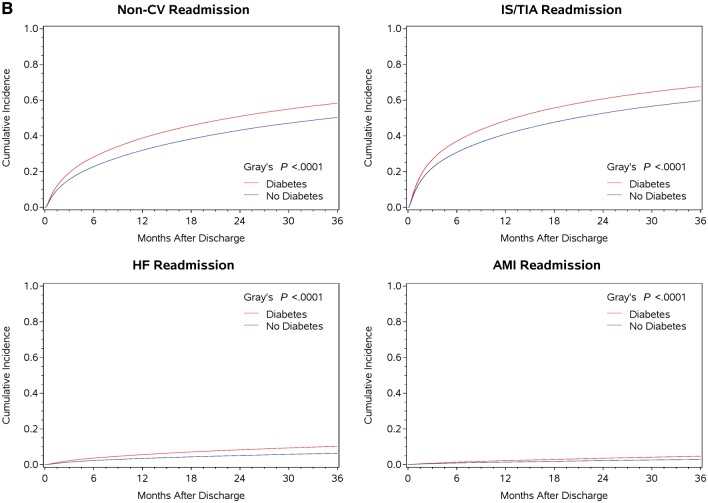
The cumulative incidence rates of mortality and other outcomes were significantly higher in those with diabetes compared with those without diabetes, over the 3-year follow-up period (Tables 1 and2). At 1 year and 3 years post-discharge, the cumulative incidence rates of outcomes were different between the diabetes and non-diabetes groups (Table 2 and Figure 1): all-cause mortality (absolute differences (ADs) at 1 year and 3 years 0.1% and 1.8%, respectively), all-cause readmission (ADs 7.7% and 7.6%), composite of mortality and readmission (ADs 5.4% and 4.8%), composite of death and cardiovascular readmission (ADs 3.7% and 5.2%), IS/TIA readmission (ADs 1.7% and 2.6%), IS readmission (ADs 1.4% and 2.3%), AMI readmission (ADs 0.8% and 1.7%), HF admission (ADs 2.1% and 3.9%), non-cardiovascular admission (ADs 6.8% and 8%), and non IS/TIA admission (ADs 7.5% and 7.9%). These differences were significant for all outcomes (P < 0.0001), except for all-cause mortality at 1 year (P = 0.54).
Table 2.
Unadjusted and adjusted hazard ratios for outcomes at 1 year and 3 years post-discharge among patients with ischaemic stroke
| Cumulative incidence |
Unadjusted analysis |
Adjusted analysis |
||||
|---|---|---|---|---|---|---|
| N (%) | P-valuea | HR (95% CI) | P-valueb | HR (95% CI) | P-valueb | |
| At 1 year | ||||||
| All-cause mortality | ||||||
| Diabetes | 32 670 (27.00) | 0.54 | 1.00 (0.98–1.01) | 0.57 | 1.20 (1.18–1.22) | <0.0001 |
| No diabetes | 77 402 (26.87) | Reference | Reference | |||
| All-cause readmission | ||||||
| Diabetes | 62 749 (52.61) | <0.0001 | 1.23 (1.22–1.24) | <0.0001 | 1.21 (1.20–1.23) | <0.0001 |
| No diabetes | 127 971 (44.94) | Reference | Reference | |||
| Composite of mortality and all-cause readmission | ||||||
| Diabetes | 75 995 (63.63) | <0.0001 | 1.14 (1.13–1.15) | <0.0001 | 1.19 (1.18–1.20) | <0.0001 |
| No diabetes | 165 843 (58.16) | Reference | Reference | |||
| Composite of mortality and CV readmission | ||||||
| Diabetes | 56 137 (47.05) | <0.0001 | 1.10 (1.09–1.11) | <0.0001 | 1.16 (1.15–1.18) | <0.0001 |
| No diabetes | 123 675 (43.38) | Reference | Reference | |||
| IS/TIA readmission | ||||||
| Diabetes | 11 759 (9.87) | <0.0001 | 1.20 (1.17–1.23) | <0.0001 | 1.15 (1.12–1.17) | <0.0001 |
| No diabetes | 23 364 (8.21) | Reference | Reference | |||
| HF readmission | ||||||
| Diabetes | 6653 (5.60) | <0.0001 | 1.61 (1.56–1.67) | <0.0001 | 1.55 (1.50–1.61) | <0.0001 |
| No diabetes | 9868 (3.47) | Reference | Reference | |||
| Non-CV readmission | ||||||
| Diabetes | 46 156 (38.77) | <0.0001 | 1.26 (1.24–1.27) | <0.0001 | 1.27 (1.26–1.29) | <0.0001 |
| No diabetes | 90 851 (31.95) | Reference | Reference | |||
| Non-IS/TIA readmission | ||||||
| Diabetes | 57 783 (48.47) | <0.0001 | 1.24 (1.22–1.25) | <0.0001 | 1.22 (1.21–1.24) | <0.0001 |
| No diabetes | 116 608 (40.97) | Reference | Reference | |||
| IS readmission | ||||||
| Diabetes | 9228 (7.74) | <0.0001 | 1.22 (1.19–1.25) | <0.0001 | 1.17 (1.13–1.20) | <0.0001 |
| No diabetes | 18 070 (6.34) | Reference | Reference | |||
| AMI readmission | ||||||
| Diabetes | 2669 (2.24) | <0.0001 | 1.56 (1.49–1.64) | <0.0001 | 1.52 (1.45–1.60) | <0.0001 |
| No diabetes | 4060 (1.43) | Reference | Reference | |||
| At 3 year | ||||||
| All-cause mortality | ||||||
| Diabetes | 55 627 (45.98) | <0.0001 | 1.04 (1.03–1.05) | <0.0001 | 1.24 (1.23–1.25) | <0.0001 |
| No diabetes | 127 320 (44.20) | Reference | Reference | |||
| All-cause readmission | ||||||
| Diabetes | 83 474 (71.30) | <0.0001 | 1.22 (1.21–1.24) | <0.0001 | 1.22 (1.21–1.23) | <0.0001 |
| No diabetes | 178 385 (63.74) | Reference | Reference | |||
| Composite of mortality and all-cause readmission | ||||||
| Diabetes | 98 713 (84.12) | <0.0001 | 1.15 (1.14–1.16) | <0.0001 | 1.21 (1.20–1.22) | <0.0001 |
| No diabetes | 222 467 (79.28) | Reference | Reference | |||
| Composite of mortality and CV readmission | ||||||
| Diabetes | 80 991 (69.45) | <0.0001 | 1.13 (1.12–1.14) | <0.0001 | 1.19 (1.18–1.20) | <0.0001 |
| No diabetes | 179 792 (64.30) | Reference | Reference | |||
| IS/TIA readmission | ||||||
| Diabetes | 18 499 (15.94) | <0.0001 | 1.22 (1.20–1.24) | <0.0001 | 1.18 (1.16–1.20) | <0.0001 |
| No diabetes | 36 944 (13.27) | Reference | Reference | |||
| HF readmission | ||||||
| Diabetes | 11 820 (10.27) | <0.0001 | 1.64 (1.60–1.68) | <0.0001 | 1.60 (1.56–1.64) | <0.0001 |
| No diabetes | 17 676 (6.39) | Reference | Reference | |||
| Non-CV readmission | ||||||
| Diabetes | 67 775 (58.30) | <0.0001 | 1.25 (1.24–1.26) | <0.0001 | 1.28 (1.26–1.29) | <0.0001 |
| No diabetes | 140 119 (50.33) | Reference | Reference | |||
| Non-IS/TIA readmission | ||||||
| Diabetes | 79 000 (67.62) | <0.0001 | 1.23 (1.22–1.24) | <0.0001 | 1.23 (1.22–1.25) | <0.0001 |
| No diabetes | 166 898 (59.72) | Reference | Reference | |||
| IS readmission | ||||||
| Diabetes | 14 589 (12.57) | <0.0001 | 1.24 (1.21–1.26) | <0.0001 | 1.20 (1.18–1.23) | <0.0001 |
| No diabetes | 28 644 (10.29) | Reference | Reference | |||
| AMI readmission | ||||||
| Diabetes | 5398 (4.72) | <0.0001 | 1.62 (1.56–1.67) | <0.0001 | 1.55 (1.49–1.60) | <0.0001 |
| No diabetes | 8136 (2.95) | Reference | Reference | |||
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; IS, ischaemic stroke; TIA, transient ischaemic attack.
Log-rank P-values were presented for mortality outcomes, Gray’s P-values were presented for other outcomes.
P < 0.05 indicates that the care differs significantly between those with and without diabetes.
Figure 1.
(A and B) The Kaplan–Meier curve for mortality outcome and cumulative incidence function plots for admission and composite outcomes at 3 years post-discharge.
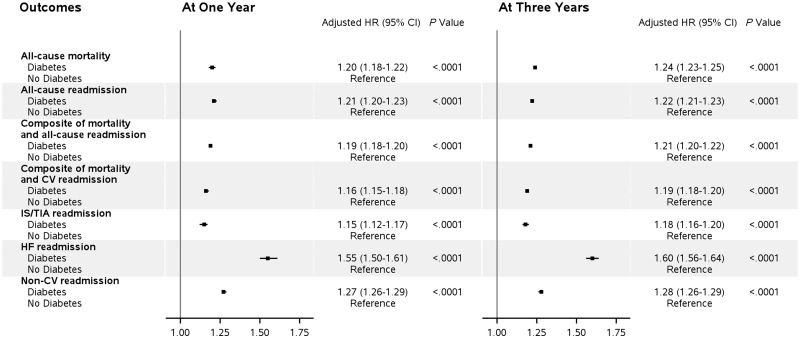
After multivariable adjustment for relevant patient characteristics (Table 2 and Figure 2), compared with the no-diabetes group, diabetes was associated with higher risks of outcomes at 1 year, including all-cause mortality [adjusted hazard ratio (aHR) 1.20, 95% confidence interval (CI) 1.18–1.22], composite of mortality and all-cause readmission (aHR 1.19, 95% CI 1.18–1.20), composite of death and cardiovascular readmission (aHR 1.16, 1.15–1.18), all-cause readmission (aHR 1.21, 1.20–1.23), IS/TIA readmission (aHR 1.15, 1.12–1.17), IS readmission (aHR 1.17, 1.13–1.20), AMI readmission (aHR 1.52, 1.45–1.60), HF readmission (aHR 1.55, 1.50–1.61), non-cardiovascular readmission (aHR 1.27, 1.26–1.29), and non-IS/TIA readmission (aHR 1.22, 1.21–1.24). Similar differences in diabetes-related multivariable adjusted risk estimates were observed at 3-years for all outcomes: all-cause mortality (aHR 1.24, 1.23–1.25), all-cause readmission (aHR 1.22, 1.21–1.23), composite of mortality and all-cause readmission (aHR 1.21, 1.20–1.22), composite of death and cardiovascular readmission (aHR 1.19, 1.18–1.20), IS/TIA readmission (aHR 1.18, 1.16–1.20), IS readmission (aHR 1.20, 1.18–1.23), AMI readmission (aHR 1.55, 1.49–1.60), HF readmission (aHR 1.60, 1.56–1.64), non-cardiovascular readmission (aHR 1.28, 1.26–1.29), and non-IS/TIA readmission (aHR 1.23, 1.22–1.25).
Figure 2.
Comparative outcomes of ischaemic stroke between patients with and without diabetes.
In sensitivity analysis conducted on a one-on-one propensity score matched sample (N = 216 562), we found higher risks of adverse 1-year and 3-year IS outcomes among patients with diabetes compared with those without diabetes (see Supplementary material online, Table S3), which is consistent with the findings (both in terms of the magnitude and significance of associations) obtained in the primary analysis using traditional multivariable models.
Alternative definition of diabetes and outcomes
Using an alternative definition of diabetes, a higher proportion of participants (N = 132 220, 32.3%) were identified as having diabetes. In this newly defined group, similar results were observed both in terms of the magnitude and significance of the estimates of association of diabetes with outcomes. This included significantly higher cumulative incidence rates of all outcomes in those with diabetes (see Supplementary material online, Table S4 and Figure S1), as well as significantly higher risk of all outcomes (all-cause mortality, all-cause admission, composite outcome of mortality and admission, composite of death and cardiovascular readmission, IS/TIA admission, IS admission, AMI admission, HF admission, non-cardiovascular readmission, and non-IS/TIA readmission) over the 1-year and 3-year follow-up periods (see Supplementary material online, Table S4).
Influences of stroke severity and use of tissue plasminogen activator on the association of diabetes and outcomes
Given that the National Institutes of Health Stroke Scale (NIHSS), a validated tool for assessing the initial stroke severity, has been shown to predict mortality in acute IS, we conducted an analysis restricted to participants with available data on the NIHSS (N = 210 631—51.5% of the initial sample of patients). The multivariable aHRs for all outcomes at 1 year and 3 years among those with data on NIHSS were significantly higher among those with diabetes compared with those without diabetes (see Supplementary material online, Table S5), to an extent that was similar to estimates observed in the main analyses. Among IS stroke patients who received tPA, the multivariable aHRs for IS outcomes at 1 year and 3 years were significantly worse among those with diabetes compared with those without diabetes, excepted for estimates of the associations of diabetes with IS readmission that were smaller in magnitude (see Supplementary material online, Table S6).
Glycated haemoglobin and insulin therapy associations with outcomes in patients with diabetes
Among patients with diabetes and available data on HbA1C (N = 58 455, corresponding to 48.3% of patients with diabetes), we examined the association of HbA1C with IS outcomes, by subdividing patients into two groups: (i) HbA1C<7% and (ii) HbA1C ≥7% (see Supplementary material online, Table S7). There were no significant differences in outcomes between the two groups, except for higher risks of all-cause readmission (aHR 1.03, 1.01–1.05), composite of death and cardiovascular readmission (aHR 1.03, 1.01–1.05), IS/TIA readmission (aHR 1.15, 1.11–1.20), HF admission (aHR 1.14, 1.08–1.20), IS readmission (aHR 1.20, 1.15–1.26), and AMI readmission (aHR 1.26, 1.16–1.37) among those with an HbA1C ≥7% at 3-year follow-up. In the data subset consisting of patients who received diabetes treatment at discharge (N = 108 969), those treated with insulin at discharge had similar mortality outcomes but higher hazards of readmissions at 1-year, and worse 3-year IS outcomes, compared with diabetes patients discharged on non-insulin therapies (see Supplementary material online, Table S8).
Subgroups analyses
We conducted stratified analyses to assess the effect of age, sex, CKD (defined on the basis of eGFR), and race (non-Hispanic Black vs. non-Black) and history of previous cardiovascular disease. Across all subgroups, the hazard ratios for all outcomes at 1 year and 3 years were significantly higher among those with diabetes compared with those without diabetes (see Supplementary material online, Table S9A and B). The difference in subgroups pertained to age, with the risks of all outcomes being significantly higher among those aged <80 years vs. patients aged ≥80 years. Chronic kidney disease also appeared to have an influence on outcomes; CKD patients had worse outcomes than those without CKD for all outcomes except for the IS/TIA and HF readmissions. Of note, there were no differences in the risk of 1-year or 3-year IS outcomes between those with a history of cardiovascular disease and those without baseline cardiovascular disease.
Discussion
We examined long-term outcomes of IS by diabetes status in a large, real-world population of 409 060 older patients in the GWTG-Stroke Registry. Overall 29.6% of patients had diabetes. Those with diabetes had a higher burden of comorbidities (cardiovascular risk factors or prior cardiovascular events), as well as significantly higher long-term risks of death, all-cause hospitalization, recurrent IS, HF hospitalization, cardiovascular hospitalization, and non-cardiovascular hospitalizations over a 3-year period. The association of diabetes with worse outcomes persisted, with few exceptions, after accounting for demographics, comorbid conditions, and the IS severity. These findings highlight that there may be an important opportunity to apply a more systematic and structured approach to management of IS patients with pre-existing or newly defined diabetes.
While the association of diabetes and incident stroke has been reported in population-based studies,3 a limited number of studies have evaluated the association between diabetes and long-term outcomes clinical outcomes among patients after acute IS. With a few exceptions,12 prior studies of the impact of diabetes on long-term IS outcomes have generally found that diabetes is related to worse outcomes in the aftermath of an IS, mostly overall mortality.13–21 However, these studies have a number of limitations, which include the limited examination of outcomes other than mortality except in one study (that included vascular outcomes),21 relatively small sample sizes13 and a shorter follow-up period (<2 years).12 Furthermore, these studies included limited adjustment for confounding factors, lacked the diversity of our population with respect to race/ethnicity as these have mostly been conducted in the European countries,12,14–20 conflated ischaemic and haemorrhagic stroke types,14,16–19 and did not account for the degree of blood glucose control. Our finding of an increased risk of IS recurrence among those with diabetes is consistent with prior data.21–23 Indeed, the extant studies suggest an increased risk of recurrent IS among patients with previous stroke of up to 44%.23 However, these studies have been small in size, and taken together these have included fewer patients than in the current investigation.23 In addition to the study size, the observed differences in estimates of IS recurrence in relation to diabetes may be also related (at least in part) to variations in diabetes ascertainment or IS identification methods.
Our study provides significant complementary information on the associations of diabetes with outcomes of IS. In addition to mortality risk among IS patients, we examined long-term admission and cardiovascular hospitalization, as well as IS recurrence. Our findings highlight the importance of achieving more effective treatment of diabetes (in addition to using antihypertensives and statins where relevant) among IS patients, as this may potentially contribute to reducing cardiovascular events, HF, and mortality. However, whether blood glucose control would lead to reduction of first stroke, stroke recurrence rates, or other cardiovascular outcomes is still to be unequivocally demonstrated in clinical trials. The majority of trials of diabetes treatment, both old24,25 and recent [using sodium-glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP1) receptor agonists],26–28 have not demonstrated clear benefits in terms of reduction of the incidence of stroke, except one trial of the GLP1 agonist semaglutide that showed a significant 29% reduction in non-fatal stroke events.29 It is however important to point out that none of these trials was powered for the stroke outcomes and if tested in appropriately powered trials, there may be benefit. It is also notable that the use of pioglitazone was shown to achieve a significant 24% reduction in the risk of stroke recurrence among insulin resistant patients.30
In terms of excess risk, HF hospitalizations had the highest aHRs at 1.6 highlighting the critical need to prevent HF in these patients. Treatment with a SGLT-2 inhibitor has been demonstrated to lower the risk of cardiovascular mortality and HF hospitalizations in patients with diabetes and may be an effective approach to mitigate many of the risks observed.31 Thiazolidinediones may specifically affect IS/TIA recurrence30 and coronary events32; GLP-1 agonists27 may affect specific cardiovascular outcomes are also considerations. An additional reason to envisage a more integrated management of IS and diabetes is a potential improvement of functional outcomes and reduction of health costs, as diabetes among patients with IS may be associated with higher expenditures. Our study points to the potential importance of adopting a more structured approach to the detection and long-term management of diabetes among those admitted with IS. While the management of hyperglycaemia in the acute phase of stroke is well integrated by neurologists, they are less prepared or armed to implement an active detection of undiagnosed cases of diabetes and long-term diabetes management. Current IS management guidelines do not mandate active detection of diabetes33,34; including with the measurement of HbA1C, which may help to detect pre-existing diabetes as post-stroke hyperglycaemia may be a transient phenomenon. This is particularly important as data indicate that up to 10% of patients admitted with IS and without previously known diabetes may in fact have the condition.35,36 A collaboration between neurologists and endocrinologists, especially for the post-discharge management of diabetes, may be necessary to achieve better outcomes.
The mechanistic pathways underlying the difference in outcomes of IS between patients with diabetes mellitus and those without diabetes mellitus remain to be explored. Our findings may reflect the possible diffuse atherosclerotic changes related to diabetes, including the cerebral vessels and other vascular beds, which may explain the higher rates of IS recurrence, composite of death and cardiovascular readmission. Indeed, diabetes is a well-known factor that contributes to the initiation and acceleration of the progression of atherosclerosis.5 Insulin resistance may have a central role in the process, as suggested by mechanistic studies37 and results from a recent clinical trial showing that improvement of insulin resistance led to better outcomes of IS.30 It is possible that the duration of diabetes matters in the pathogenic process, as suggested by findings of prior studies on the duration of diabetes and IS.38
There are limitations to our study. First, we primarily included IS patients aged ≥65 years, thus we were unable to assess the relationship between diabetes and IS outcomes in other age groups. Second, participation to the GWTG-Stroke registry is voluntary, thus not all USA hospitals are included. While the generalizability of our findings to non-GWTG-Stroke registry hospitals remains to be established, our findings are likely to represent routine clinical practice as the GWTG-Stroke registry is the largest stroke registry in the world. Third, we probably missed patients without a prior diagnosis of diabetes and who were not screened during their index hospitalization, especially as undiagnosed diabetes in frequent in the USA population.1 We also did not account for diabetes cases that arose during the follow-up period. An underestimation of diabetes frequency would bias the estimates of the diabetes and IS outcomes towards the null, as illustrated by our sensitivity analysis using an alternative and more inclusive definition of diabetes. Third, we did not have information on the duration of diabetes, which may influence outcomes of IS among patients with diabetes. We had limited information on HbA1C (only 48% of patients had data on HbA1C) and did not account for the various glucose lowering medications other than insulin therapy. The latter may influence IS outcomes, especially as some medications classes may be associated with better or worse IS outcomes.30,39 Fourth, we did not assess functional (e.g. long-term cognitive function) or quality of life outcomes. Finally, despite appropriate adjustment for confounders, residual confounding may have affected our estimates.
Conclusions
In a nationwide cohort of older patients with IS, among whom 30% had diabetes mellitus, a higher burden of mortality, recurrent events, and hospitalizations were observed in the diabetes group. This points to the potential importance of early detection and appropriate management of diabetes in IS patients, using specific therapies that have been demonstrated to improve outcomes. Further studies are warranted to clarify pathways through which diabetes affects outcomes of IS, as well as the best approaches (pharmacological and non-pharmacological) to mitigate more effectively diabetes in the setting of IS.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the Get with the Guidelines-Stroke (GWTG-Stroke) program, which is provided by the American Heart Association (AHA)/American Stroke Association. The GWTG-Stroke program is supported, in part, by a charitable contribution from Janssen Pharmaceutical Companies of Johnson & Johnson. GWTG-Stroke has been funded in the past through support from Boeringher-Ingelheim, Merck, Bristol-Myers Squib/Sanofi Pharmaceutical Partnership, and the AHA Pharmaceutical Roundtable (PRT). J.B.E. was awarded an American Heart Association/American Stroke Association (AHA/ASA) - Young Investigator Database Research Seed Grant in 2015 to conduct this study. J.B.E. is supported by the National Heart, Lung, and Blood Institute (NHLBI) grant T32 HL125232.
Conflict of interest: D.L.B. discloses the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. G.C. F. reports significant consulting for Novartis, and modest consulting for Amgen, Bayer, Gambro, Medtronic, and Janssen; G.C.F. is a member of the GWTG Steering Committee; G.C.F. holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and is also supported by the Ahmanson Foundation (Los Angeles, California). L.H.S. reports being the principal investigator of an investigator-initiated study of extended-window intravenous thrombolysis funded by the National Institutes of Neurological Disorders and Stroke (clinicaltrials.gov/show/NCT01282242) for which Genentech provides alteplase free of charge to Massachusetts General Hospital as well as supplemental per-patient payments to participating sites; serving as chair of the AHA/ASA GWTG stroke clinical work group and hospital accreditation Science Committee; serving as a stroke systems consultant to the Massachusetts Department of Public Health; serving as a scientific consultant to Medtronic (Victory AF and Stroke AF trials), and member of the data and safety monitoring board of the DeVOTE trial (novo Nordisk). All other authors (J.B.E.; H.X.; R.A.M.; Y.X.; E.E.S.; A.F.H.; and P.A.H) declared no conflict of interest. All authors had access to the data and a role in writing the manuscript.
Supplementary Material
Footnotes
See page 2387 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy033)
References
- 1. Menke A, Casagrande S, Geiss L, Cowie CC.. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 2. Koton S, Schneider ALC, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J.. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 3. Luitse MJA, Biessels GJ, Rutten GEHM, Kappelle LJ.. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 2012;11:261–271. [DOI] [PubMed] [Google Scholar]
- 4. Towfighi A, Markovic D, Ovbiagele B.. Current national patterns of comorbid diabetes among acute ischemic stroke patients. Cerebrovasc Dis 2012;33:411–418. [DOI] [PubMed] [Google Scholar]
- 5. Kanter JE, Johansson F, LeBoeuf RC, Bornfeldt KE.. Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques? Circ Res 2007;100:769–781. [DOI] [PubMed] [Google Scholar]
- 6. Mohan KM, Wolfe CDA, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP.. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489–1494. [DOI] [PubMed] [Google Scholar]
- 7. Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, Hernandez AF, Peterson ED, Schwamm LH.. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes 2010;3:291–302. [DOI] [PubMed] [Google Scholar]
- 8. Reeves MJ, Fonarow GC, Smith EE, Pan W, Olson D, Hernandez AF, Peterson ED, Schwamm LH.. Representativeness of the Get With The Guidelines-Stroke Registry: comparison of patient and hospital characteristics among medicare beneficiaries hospitalized with ischemic stroke. Stroke 2012;43:44–49. [DOI] [PubMed] [Google Scholar]
- 9. Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, Schwamm LH, Smith EE.. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J 2012;163:392–398.e1. [DOI] [PubMed] [Google Scholar]
- 10. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH.. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fonarow GC, Smith EE, Reeves MJ, Pan W, Olson D, Hernandez AF, Peterson ED, Schwamm LH.. Hospital-level variation in mortality and rehospitalization for medicare beneficiaries with acute ischemic stroke. Stroke 2011;42:159–166. [DOI] [PubMed] [Google Scholar]
- 12. Winell K, Pääkkönen R, Pietilä A, Reunanen A, Niemi M, Salomaa V.. Prognosis of ischaemic stroke is improving similarly in patients with type 2 diabetes as in nondiabetic patients in Finland. Int J Stroke 2011;6:295–301. [DOI] [PubMed] [Google Scholar]
- 13. Kammersgaard LP, Olsen TS.. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis 2006;21:187–193. [DOI] [PubMed] [Google Scholar]
- 14. Icks A, Claessen H, Morbach S, Glaeske G, Hoffmann F.. Time-dependent impact of diabetes on mortality in patients with stroke. Diabetes Care 2012;35:1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamalesh M, Shen J, Eckert GJ.. Long term postischemic stroke mortality in diabetes: a veteran cohort analysis. Stroke 2008;39:2727–2731. [DOI] [PubMed] [Google Scholar]
- 16. Liao C-C, Shih C-C, Yeh C-C, Chang Y-C, Hu C-J, Lin J-G, Chen T-L.. Impact of diabetes on stroke risk and outcomes. Medicine (Baltimore) 2015;94:e2282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sprafka JM, Virnig BA, Shahar E, McGovern PG.. Trends in diabetes prevalence among stroke patients and the effect of diabetes on stroke survival: the Minnesota Heart Survey. Diabet Med 1994;11:678–684. [DOI] [PubMed] [Google Scholar]
- 18. Rautio A, Eliasson M, Stegmayr B.. Favorable trends in the incidence and outcome in stroke in nondiabetic and diabetic subjects: findings from the Northern Sweden MONICA Stroke Registry in 1985 to 2003. Stroke 2008;39:3137–3144. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson M, Carlberg B, Eliasson M.. The disparity in long-term survival after a first stroke in patients with and without diabetes persists: the Northern Sweden MONICA study. Cerebrovasc Dis 2012;34:153–160. [DOI] [PubMed] [Google Scholar]
- 20. Gunarathne A, Patel JV, Potluri R, Gammon B, Jessani S, Hughes EA, Lip GYH.. Increased 5-year mortality in the migrant South Asian stroke patients with diabetes mellitus in the United Kingdom: the West Birmingham Stroke Project. Int J Clin Pract 2007;62:197–201. [DOI] [PubMed] [Google Scholar]
- 21. Putaala J, Liebkind R, Gordin D, Thorn LM, Haapaniemi E, Forsblom C, Groop PH, Kaste M, Tatlisumak T.. Diabetes mellitus and ischemic stroke in the young: clinical features and long-term prognosis. Neurology 2011;76:1831–1837. [DOI] [PubMed] [Google Scholar]
- 22. Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CDA.. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke 2003;34:1457–1463. [DOI] [PubMed] [Google Scholar]
- 23. Shou J, Zhou L, Zhu S, Zhang X.. Diabetes is an independent risk factor for stroke recurrence in stroke patients: a meta-analysis. J Stroke Cerebrovasc Dis 2015;24:1961–1968. [DOI] [PubMed] [Google Scholar]
- 24. Zhang C, Zhou Y-H, Xu C-L, Chi F-L, Ju H-N.. Efficacy of intensive control of glucose in stroke prevention: a meta-analysis of data from 59,197 participants in 9 randomized controlled trials. PLoS One 2013;8:e54465.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C.. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 2011;343:d4169.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zinman B, Inzucchi SE, Lachin JM, Wanner C, Fitchett D, Kohler S, Mattheus M, Woerle HJ, Broedl UC, Johansen OE, Albers GW, Diener HC.. Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke 2017;48:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T.. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 30. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu JHY, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B.. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2016;4:411–419. [DOI] [PubMed] [Google Scholar]
- 32. Young LH, Viscoli CM, Curtis JP, Inzucchi SE, Schwartz GG, Lovejoy AM, Furie KL, Gorman MJ, Conwit R, Abbott JD, Jacoby DL, Kolansky DM, Pfau SE, Ling FS, Kernan WN; IRIS Investigators. Cardiac outcomes after ischemic stroke or transient ischemic attackclinical perspective. Circulation 2017;135:1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 34. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 35. Fonville S, Zandbergen AAM, Vermeer SE, Dippel DWJ, Koudstaal PJ, Hertog HM. D.. Prevalence of prediabetes and newly diagnosed diabetes in patients with a transient ischemic attack or stroke. Cerebrovasc Dis 2013;36:283–289. [DOI] [PubMed] [Google Scholar]
- 36. Liu A, Carmichael KA, Schallom ME, Riley MJ, Klinkenberg WD.. Detecting and managing diabetes mellitus and prediabetes in patients with acute stroke. Diabetes Educ 2015;41:592–598. [DOI] [PubMed] [Google Scholar]
- 37. Mather KJ, Steinberg HO, Baron AD.. Insulin resistance in the vasculature. J Clin Invest 2013;123:1003–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banerjee C, Moon YP, Paik MC, Rundek T, Mora-Mclaughlin C, Vieira JR, Sacco RL, Elkind MSV.. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke 2012;43:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kunte H, Schmidt S, Eliasziw M, Zoppo GJ. D, Simard JM, Masuhr F, Weih M, Dirnagl U.. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke 2007;38:2526–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.