Abstract
Background
It is unknown whether disrupted tryptophan catabolism is associated with cardiovascular disease (CVD) in human immunodeficiency virus (HIV)–infected individuals.
Methods
Plasma tryptophan and kynurenic acid were measured in 737 women and men (520 HIV+, 217 HIV−) from the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. Repeated B-mode carotid artery ultrasound imaging was obtained from 2004 through 2013. We examined associations of baseline tryptophan, kynurenic acid, and kynurenic acid-to-tryptophan (KYNA/TRP) ratio, with risk of carotid plaque.
Results
After a 7-year follow-up, 112 participants developed carotid plaque. Compared to those without HIV infection, HIV-infected participants had lower tryptophan (P < .001), higher KYNA/TRP (P = .01), and similar kynurenic acid levels (P = .51). Tryptophan, kynurenic acid, and KYNA/TRP were correlated with T-cell activation (CD38+HLA-DR+) and immune activation markers (serum sCD14, galectin-3) but had few correlations with interleukin-6, C-reactive protein, or CVD risk factors (blood pressure, lipids). Adjusted for demographic and behavioral factors, each standard deviation (SD) increment in tryptophan was associated with a 29% (95% confidence interval [CI], 17%–38%) decreased risk of carotid plaque (P < .001), while each SD increment in kynurenic acid (P = .02) and KYNA/TRP (P < .001) was associated with a 34% (6%–69%) and a 47% (26%–73%) increased risk of carotid plaque, respectively. After further adjustment for CVD risk factors and immune activation markers, these associations were attenuated but remained significant.
Conclusions
Plasma tryptophan-kynurenine metabolites are altered in HIV infection and associated with progression of carotid artery atherosclerosis.
Keywords: association study, atherosclerosis, HIV infection, metabolite
In 2 human immunodeficiency virus (HIV) cohorts we report tryptophan catabolism and related metabolites in plasma were altered in HIV-infected individuals (decreased tryptophan and increased kynurenic acid-to-tryptophan ratio) and this disruption was associated with greater progression of carotid artery atherosclerosis.
Cardiovascular disease (CVD) has become a major concern for people who live with human immunodeficiency virus (HIV) infection [1]. Although prior studies have provided insights into the virologic, inflammatory, immunologic, treatment-related, and traditional risk factors that may affect HIV-related CVD risk, an understanding of its pathophysiology remains incomplete [2]. Recently, there has been an interest in examining the role of the tryptophan-kynurenine pathway, which is closely related to inflammation and immune activation, in the development of CVD [3]. Disrupted tryptophan catabolism has been noted in HIV infection and associated with HIV disease progression [4, 5]; however, its relationship with CVD risk in HIV infection has been explored only to a limited extent [6].
Tryptophan is an essential amino acid that is used for protein synthesis and the biosynthesis of serotonin and melatonin; it also serves as the sole source of kynurenine pathway metabolites [3]. In the kynurenine pathway, tryptophan is catabolized into kynurenine and several downstream metabolites (eg, kynurenic acid, anthranilic acid), mainly regulated by indoleamine 2,3-dioxygenase 1 (IDO-1), which is induced by Th1-type cytokines (eg, interferon-γ) during inflammation and immune activation [7]. In HIV-uninfected populations, several studies have linked plasma kynurenine-to-tryptophan (KYN/TRP) ratio, a measure that reflects IDO-1 activity, with carotid artery subclinical atherosclerosis [8–10] and CVD risk [11–14]. Little is known about this relationship in HIV-infected individuals. One small study of 105 participants showed an association between the declining plasma KYN/TRP ratio over 6 months of treatment and lower single time point measurement of carotid artery intima media thickness (cIMT) [6].
Our prior work in the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS) has demonstrated greater progression of carotid artery atherosclerosis in HIV-infected individuals, even in those with viral suppression during antiretroviral therapy (ART), compared to HIV-uninfected individuals [15]. In the present study, we profiled plasma levels of tryptophan and kynurenic acid in 737 WIHS women and MACS men, approximately 65% of whom were HIV infected, and examined their associations with risk of carotid artery plaque, assessed by repeated B-mode carotid artery ultrasound imaging over a 7-year period. Since generation of kynurenic acid rather than kynurenine has been suggested to reflect tryptophan consumption [16], we calculated the kynurenic acid-to-tryptophan (KYNA/TRP) ratio as an indicator of tryptophan catabolism [17] in the current study.
METHODS
Study Participants
Participants were from 2 prospective multicenter cohort studies of women and men with or at risk for HIV infection, the WIHS and MACS. Details on study design and methods have been described previously [18, 19]. Every 6 months, WIHS and MACS participants complete a core visit with a comprehensive physical examination; participants provide biological specimens and complete interviewer-administered questionnaires. Since 2004, the WIHS and MACS have collaborated on a uniform carotid artery imaging protocol [20], scanning the cohorts at 2- to 4-year intervals to ascertain progression of subclinical carotid artery atherosclerosis (the WIHS/MACS CVD study) [15]. In 2015, a plasma metabolomics study, which aimed to examine plasma metabolites in relation to cardiometabolic risk in the context of HIV infection, was initiated among participants aged ≥35 years who underwent carotid artery imaging for plaque assessment at a baseline visit (2004–2006) and at a follow-up visit (2011–2013) in the WIHS/MACS CVD study. As our primary outcomes included incident carotid artery plaque and incident diabetes, participants with prevalent carotid artery plaques and prevalent diabetes at baseline were excluded from plasma metabolomics profiling. In the current analysis, we included 737 women and men with data on plasma tryptophan and kynurenic acid and incident carotid artery plaque. Characteristics of included participants and those not included in the current study were generally similar and comparable (Supplementary Table 1), although participants who were included had lower prevalence of antihypertensive and lipid-lowering medication use compared to those not included. All individuals provided informed consent, and each site’s institutional review board approved the study.
Carotid Artery Plaque Ascertainment
Participants underwent high-resolution B-mode carotid artery ultrasound in order to image 6 locations in the right carotid artery: the near and far walls of the common carotid artery (CCA), carotid bifurcation, and internal carotid artery. A standardized protocol was used at all sites [15]. Focal plaque measures were obtained at a centralized reading center (University of Southern California). Coefficients of variation of repeated measurements of IMT at the CCA have been published [21], and replicate image acquisition and interpretation studies were repeated to ensure consistency over time. We defined a focal plaque as an area with localized IMT >1.5 mm in any of the 6 imaged carotid artery locations [22].
Plasma Tryptophan and Kynurenic Acid Measurement
Plasma tryptophan and kynurenic acid were profiled from stored frozen plasma specimens that had been collected at the core study visit closest to the baseline carotid artery imaging study visit using liquid chromatography-tandem mass spectrometry at the Broad Institute Metabolomics Platform (Cambridge, Massachusetts). Briefly, metabolites were profiled using a Nexera X2 Ultra-High Performance Liquid Chromatography (UHPLC)(Shimadzu Corp., Marlborough, Massachusetts) coupled to an Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, Massachusetts). Metabolite peaks were identified and confirmed using authentic reference standards. Metabolites were quantified using area-under-the-curve of the peaks. Raw data were processed using TraceFinder software (Thermo Fisher Scientific, Waltham, Massachusetts) and Progenesis QI (Nonlinear Dynamics, Newcastle upon Tyne, United Kingdom). Detailed methods of plasma metabolite measurement are described in the Supplementary Material.
Assessments of HIV Infection and Other Variables
Demographic, clinical, and laboratory variables were collected using standardized protocols at semiannual core study visits [15]. HIV infection was ascertained using enzyme-linked immunosorbent assay and confirmed using Western blot. HIV-specific parameters included CD4+ T-cell counts, HIV-1 viral load, and detailed information on ART. Hepatitis C virus (HCV) infection status was based on antibody and viral RNA testing. Inflammation and immune activation markers included serum-soluble [s]CD14, sCD163, galectin-3 [Gal-3], Gal-3 binding protein [Gal-3BP], C-reactive protein (CRP), and interleukin (IL)-6 [23]. CD4+ T-cell and CD8+ T-cell activation (CD38+HLA-DR+) and senescence (CD28-CD57+) markers were measured in a subsample of women using methods that have been previously described [24]. In the current analysis, these variables and biomarkers were assessed at baseline and were not updated at the time of the follow-up visit of carotid imaging.
Among HIV-infected participants, we defined persistent virologic suppression as consistent plasma HIV RNA levels <80 copies/mL simultaneous with continuous ART use over the study period (up to 16 measurements per participant). We allowed for 1 virologic “blip” during the period as long as it was <500 copies/mL, based on evidence that blips ≥500 copies/mL are associated with virologic rebound [25]. Participants were allowed to miss no more than 1 core visit to be eligible for being categorized as “persistently virologically suppressed.” In addition, HIV infection tests were also performed routinely for all HIV-uninfected participants during semiannual visits, and those included in the current analysis remained HIV uninfected over the study period.
Statistical Analyses
Characteristics of participants were compared by HIV serostatus in women and men separately. Raw values of tryptophan, kynurenic acid, and KYNA/TRP ratio were natural-log transformed to approximate a normal distribution before analysis. Linear regression was used to compare metabolite levels among HIV-uninfected, HIV-infected aviremic (undetectable viral load ≤80 copies/mL), and HIV-infected viremic (viral load >80 copies/mL) individuals, adjusting for age and sex. We calculated age- and sex-adjusted partial Spearman correlations of metabolite levels with CD4+ T-cell counts, viral load, inflammation and immune activation markers, markers of CD4+ T-cell and CD8+ T-cell activation and senescence, and conventional CVD risk factors. Poisson regression models with robust variance estimates were used to calculate risk ratios (RRs) and 95% confidence intervals (CIs) of carotid artery plaque per standard deviation (SD) increment in log-transformed metabolites. We tested interactions by sex and by HIV serostatus with generalized score statistics. We adjusted for age, sex, race/ethnicity, education, study site, current smoking, history of HIV infection, HIV serostatus, HIV treatment status and baseline viral load, body mass index (BMI), systolic blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, antihypertensive medication, lipid-lowering medication, sCD14, sCD163, Gal-3, Gal3-BP, and IL-6 in models. The associations between metabolites and incident carotid artery plaque were further examined in the following 3 strata: HIV-uninfected participants, HIV-infected participants with persistent virologic suppression, and HIV-infected participants without persistent virologic suppression, with generalized score statistics for the interaction tests. Analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, North Carolina), and a 2-sided P < .05 was considered statistically significant.
RESULTS
Participant Characteristics
At baseline, HIV-infected (291 women and 229 men) and HIV-uninfected (107 women and 110 men) groups were generally similar in terms of demographic and behavioral variables, although HIV-infected participants were more likely to have previously injected drugs and have a history of HCV infection (Table 1). HIV-infected participants were more likely to use antihypertensive medication (women only) and lipid-lowering medication and had lower BMI (women only) and lower total cholesterol and HDL cholesterol levels. The majority of HIV-infected individuals reported using highly active ART at baseline (74% in women and 83% in men), and 46% of women and 66% of men had undetectable HIV-1 viral load.
Table 1.
Baseline Characteristics of Participants by Human Immunodeficiency Virus Serostatus
| Characteristic | Women | Men | ||
|---|---|---|---|---|
| HIV+ (N = 291) | HIV− (N = 107) | HIV+ (N = 229) | HIV− (N = 110) | |
| Age, years | 42 (38–46) | 42 (38–47) | 46 (43–50) | 47 (45–54) |
| Race/ethnicity | ||||
| White/Other | 9 | 5 | 56 | 64 |
| Hispanic | 31 | 27 | 11 | 10 |
| African American | 60 | 68 | 33 | 26 |
| Education | ||||
| Less than high school | 40 | 33 | 9 | 5 |
| High school | 30 | 31 | 14 | 15 |
| College and above | 30 | 36 | 77 | 81 |
| Annual income <$30000 per year | 84 | 83 | 52 | 36 |
| Current smoking | 47 | 60 | 36 | 23 |
| Current alcohol use | ||||
| Abstainer | 57 | 42 | 22 | 10 |
| Light (<3 drinks/week, WIHS; 1–3, MACS) | 33 | 30 | 54 | 53 |
| Moderate (3–13, WIHS; 4–13, MACS) | 7 | 19 | 19 | 30 |
| Heavier (14+ drinks/week) | 3 | 9 | 5 | 7 |
| Current crack/cocaine use | 8 | 18 | 15 | 8 |
| History of injection drug use | 31 | 26 | 11 | 3 |
| History of hepatitis C infection | 34 | 22 | 15 | 6 |
| Body mass index, kg/m2 | 27.2 (24.1–31.5) | 29.1 (24.8–34.4) | 25.1 (22.6–28.1) | 25.8 (23.8–28.2) |
| Systolic blood pressure, mm Hg | 115 (106–124) | 116 (105–126) | 122 (115–129) | 126 (117–132) |
| Diastolic blood pressure, mm Hg | 74 (67–81) | 72 (66–80) | 73 (68–78) | 74 (69–80) |
| Total cholesterol, mg/dL | 175 (152–207) | 182 (160–203) | 187 (156–213) | 198 (172–222) |
| High-density lipoprotein cholesterol, mg/dL | 47 (38–57) | 56 (46–66) | 43 (36–52) | 49 (42–58) |
| Antihypertensive medication use | 17 | 10 | 16 | 17 |
| Lipid-lowering medication use | 4 | 0 | 23 | 14 |
| HIV-specific characteristics | ||||
| CD4+ T-cell count, cells/mm3 | 438 (289–619) | n/a | 519 (359–689) | n/a |
| HIV-1 viral load, copies/mL | 160 (80–4700) | n/a | 40 (40–1280) | n/a |
| Undetectable viral load (≤80 copies/mL) | 46 | n/a | 66 | n/a |
| Highly active ART use in past 6 months | 74 | n/a | 83 | n/a |
| Cumulative exposure of potent ART, years | 3.5 (2.0–6.5) | n/a | 4.9 (2.4–7.3) | n/a |
| of protease inhibitors, years | 2.5 (0–4.5) | n/a | 2.5 (0–6.1) | n/a |
| of nonnucleoside reverse transcriptase inhibitors, years | 1.0 (0–3) | n/a | 1.5 (0–4.3) | n/a |
| of nucleoside reverse transcriptase inhibitors, years | 5.5 (2.5–8.5) | n/a | 6.4 (3.1–8.8) | n/a |
Data are median (interquartile range) or %, assessed at baseline unless otherwise noted.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; n/a, not applicable; WIHS, Women’s Interagency HIV Study.
Tryptophan, Kynurenic Acid, and HIV Infection
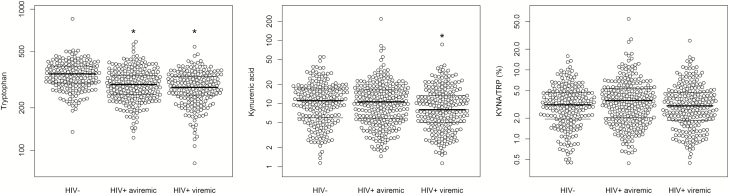
Plasma levels of tryptophan, kynurenic acid, and KYNA/TRP ratio were positively correlated with age (all P < .001), higher in men than in women (all P < .001), higher in HCV-infected participants than in those without HCV infection (all P < .001), and higher in HIV-infected participants than in those without HIV infection (all P < .001). After adjusting for age, sex, and HCV infection, HIV-infected participants had lower tryptophan (P < .001), higher KYNA/TRP ratio (P = .01), and similar kynurenic acid levels (P = .51) compared to those without HIV infection. We further examined these metabolites in HIV-infected aviremic (undetectable viral load ≤80 copies/mL) and HIV-infected viremic (viral load >80 copies/mL) individuals compared to those without HIV infection (Figure 1). There was a significantly decreased trend in levels of tryptophan and kynurenic acid across the 3 groups (both P < .001), with the lowest levels in HIV-infected viremic participants and the highest levels in HIV-uninfected participants.
Figure 1.
Plasma tryptophan, kynurenic acid and kynurenic acid-to-tryptophan (KYNA/TRP) ratio between human immunodeficiency virus (HIV)–infected and HIV-uninfected participants. Data are raw values (area under the curve of metabolite peaks) of plasma tryptophan, kynurenic acid, and KYNA/TRP ratio in a logarithmic scale among 217 HIV-uninfected, 290 HIV-infected aviremic (undetectable viral load ≤80 copies/mL), and 230 HIV-infected viremic (viral load >80 copies/mL) individuals. P for trend <0.001, <0.001, and = 0.86 for tryptophan, kynurenic acid, and KYNA/TRP ratio, respectively, across 3 groups. *P < .05 for pairwise comparison between HIV+ aviremic group or HIV+ viremic group vs HIV-uninfected group, controlling for multiple testing. Abbreviations: HIV, human immunodeficiency virus; KYNA/TRP, kynurenic acid-to-tryptophan.
Tryptophan, Kynurenic Acid, HIV Infection Parameters, Inflammation and Immune Activation Markers, and CVD Risk Factors
Among HIV-infected participants, CD4+ T-cell counts were positively correlated with tryptophan (r = 0.22; P < .001) and inversely correlated with KYNA/TRP ratio (r = −0.10; P = .006; Supplementary Figure 1). Tryptophan, kynurenic acid, and KYNA/TRP ratio were more closely correlated with specific monocyte activation and macrophage inflammation markers (serum sCD14, Gal-3), surface markers of CD4+ T-cell activation, and CD8+ T-cell activation and senescence, rather than global inflammation markers (IL-6, CRP) or conventional CVD risk factors (systolic blood pressure, total cholesterol, HDL cholesterol, BMI; Supplementary Figure 1).
Tryptophan, Kynurenic Acid, and Risk of Carotid Artery Plaque
After a median follow-up of 7 years (ranging from 6 to 9 years; 85% of participants had a follow-up of 6.5 to 7.5 years), 112 participants developed carotid artery plaques (IMT >1.5 mm) in any of the 6 imaged carotid artery locations. After adjustment for demographic and behavioral variables (model 1), higher plasma tryptophan was significantly associated with a lower risk of carotid artery plaque (RR, 0.71 [95% CI, 0.62, 0.84] per SD; P < .001), while higher plasma kynurenic acid (RR, 1.34 [95% CI, 1.06, 1.69] per SD; P = .02) and KYNA/TRP ratio (RR, 1.47 [95% CI, 1.26, 1.73] per SD; P < .001) were significantly associated with an increased risk of carotid artery plaque (Table 2). Results were consistent between women and men (all P for interaction > .05), and between HIV-infected and HIV-uninfected participants (all P for interaction > .05).
Table 2.
Associations of Plasma Tryptophan, Kynurenic Acid, and Kynurenic Acid-to-Tryptophan Ratio With Risk of Carotid Artery Plaque
| All | Women | Men | P for Interaction | HIV+ | HIV− | P for Interaction | ||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |||
| Number of participants | 112/737 | 45/398 | 67/339 | 90/520 | 22/217 | |||
| Tryptophan | ||||||||
| Model 1a | 0.71 (0.62, 0.84) | <.001 | 0.66 (0.54, 0.80) | 0.78 (0.63, 0.96) | .27 | 0.76 (0.63, 0.91) | 0.77 (0.51, 1.14) | .99 |
| Model 2b | 0.76 (0.65, 0.89) | <.001 | 0.69 (0.56, 0.84) | 0.84 (0.67, 1.04) | .20 | 0.76 (0.64, 0.90) | 0.77 (0.51, 1.14) | .96 |
| Model 3c | 0.82 (0.69, 0.97) | .02 | 0.77 (0.62, 0.96) | 0.87 (0.70, 1.08) | .45 | 0.84 (0.70, 1.01) | 0.67 (0.44, 1.02) | .38 |
| Model 4d | 0.81 (0.67, 0.99) | .04 | 0.78 (0.60, 1.00) | 0.85 (0.67, 1.09) | .56 | 0.82 (0.67, 1.02) | 0.75 (0.47, 1.19) | .72 |
| Kynurenic acid | ||||||||
| Model 1a | 1.34 (1.06, 1.69) | .02 | 1.49 (1.12, 1.98) | 1.13 (0.81, 1.59) | .25 | 1.32 (1.03, 1.68) | 1.42 (0.93, 2.17) | .76 |
| Model 2b | 1.33 (1.08, 1.64) | .007 | 1.40 (1.08, 1.81) | 1.23 (0.88, 1.70) | .54 | 1.31 (1.05, 1.65) | 1.42 (0.93, 2.17) | .70 |
| Model 3c | 1.27 (1.03, 1.55) | .02 | 1.32 (1.03, 1.68) | 1.18 (0.85, 1.64) | .60 | 1.27 (1.03, 1.58) | 1.23 (0.79, 1.90) | .86 |
| Model 4d | 1.29 (1.02, 1.63) | .03 | 1.37 (1.02, 1.83) | 1.16 (0.82, 1.66) | .48 | 1.32 (1.03, 1.70) | 1.13 (0.71, 1.79) | .54 |
| Kynurenic acid-to-tryptophan ratio | ||||||||
| Model 1a | 1.47 (1.26, 1.73) | <.001 | 1.54 (1.30, 1.83) | 1.33 (0.97, 1.82) | .42 | 1.40 (1.18, 1.67) | 1.57 (1.01, 2.44) | .64 |
| Model 2b | 1.41 (1.22, 1.63) | <.001 | 1.44 (1.23, 1.69) | 1.34 (0.98, 1.83) | .68 | 1.39 (1.19, 1.62) | 1.57 (1.01, 2.44) | .57 |
| Model 3c | 1.30 (1.11, 1.53) | .002 | 1.32 (1.10, 1.59) | 1.26 (0.93, 1.72) | .81 | 1.29 (1.09, 1.54) | 1.38 (0.88, 2.16) | .84 |
| Model 4d | 1.34 (1.10, 1.63) | .004 | 1.39 (1.10, 1.74) | 1.25 (0.90, 1.74) | .60 | 1.36 (1.11, 1.68) | 1.20 (0.74, 1.93) | .62 |
Data are adjusted RR and 95% CI on risk of carotid artery plaque per standard deviation increment in log-transformed metabolite variables.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; RR, risk ratio.
aAdjusted for age, sex, race/ethnicity, education, study site, current smoking, and history of hepatitis C virus.
bFurther adjusted for human immunodeficiency virus (HIV) serostatus, HIV treatment status, and baseline viral load level.
cFurther adjusted for systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medication use, lipid-lowering medication use, and body mass index (total sample size was reduced to 705 due to missing data on cardiovascular disease risk factors).
dFurther adjusted for serum levels of soluble(s) CD14, sCD163, galectin-3, Gal-3 binding protein, and interleukin-6 (total sample size was reduced to 673 due to missing data on inflammation and immune activation markers).
Further adjustment for HIV infection and related factors (model 2) did not change the significance of the results. After further adjustment for conventional CVD risk factors (model 3), the associations with risk of carotid artery plaque were slightly attenuated but remained significant (all P < .05). Even after additional adjustment for 5 inflammation and immune activation markers (model 4), the association results remained significant (all P < .05).
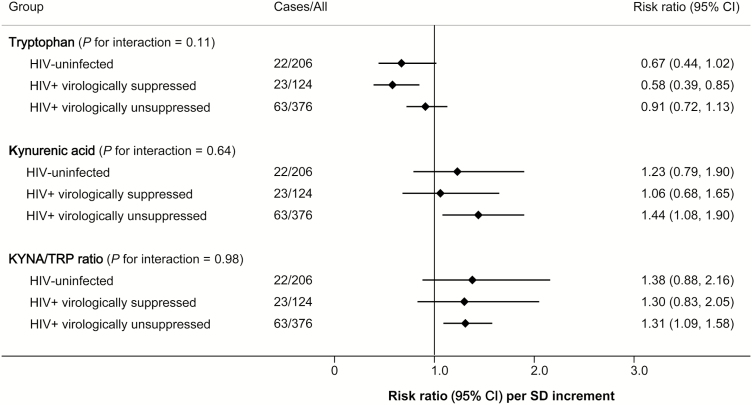
We further examined these associations in HIV-infected participants stratified by status of persistent virologic suppression over the study period, along with HIV-uninfected participants (Figure 2). Overall, the associations with risk of carotid artery plaque were generally consistent, in the same directions, across the 3 groups (all P for interaction > .05).
Figure 2.
Associations of plasma tryptophan, kynurenic acid, and kynurenic acid-to-tryptophan ratio with risk of carotid artery plaque in human immunodeficiency virus (HIV)–uninfected, HIV+ persistently virologically suppressed, and HIV+ virologically unsuppressed participants. Data are risk ratio (95% confidence interval) on risk of carotid artery plaque per standard deviation increment in log-transformed metabolite variables, adjusted for age, sex, race/ethnicity, education, study site, current smoking, history of hepatitis C virus, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medication use, lipid lowering medication use, and body mass index. HIV+ persistently virologically suppressed participants were defined as participants who had consistent plasma HIV RNA levels <80 copies/mL simultaneous with continuous antiretroviral therapy use over the study period. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; KYNA/TRP, kynurenic acid-to-tryptophan; SD, standard deviation.
DISCUSSION
While most previous studies have used KYN/TRP ratio [8–13], we evaluated KYNA/TRP ratio as an indicator of tryptophan catabolism [16, 17]. Despite this difference, our data led to the same conclusion of previous non-HIV population studies [8–13], namely, that the ratio of tryptophan and its metabolic products, which indicate tryptophan catabolism, is associated with CVD risk. Moreover, we found consistent association results for KYNA/TRP ratio and progression of carotid artery atherosclerosis between persistently virologically suppressed and unsuppressed individuals. This is in line with our prior work that suggested increased CVD risk in both virologically suppressed and unsuppressed HIV-infected individuals compared to those without HIV infection [15].
It is not surprising that the observed association between disrupted tryptophan catabolism and progression of carotid artery atherosclerosis only slightly changed after adjusting for traditional CVD risk factors, since plasma tryptophan, kynurenic acid, and KYNA/TRP ratio showed very weak correlations with these CVD risk factors. However, it should be noted that diabetes status, which is a risk factor for carotid artery atherosclerosis in this HIV-infected population [15], was not examined as a covariate in the current study, because participants with prevalent diabetes were excluded at baseline. Of note, diabetes status has been found to be associated with increased plasma levels of tryptophan and kynurenines in HIV-uninfected populations, and diabetes status may amplify the association between kynurenines and risk of CVD [26, 27].
We also found the independent association between tryptophan catabolism and carotid artery atherosclerosis after controlling for serum markers of monocyte activation and macrophage inflammation, which have themselves been associated with CVD risk in HIV infection [23, 28–30]. In this study, serum sCD14, a marker that potentially reflects monocyte and macrophage activation by host inflammatory cytokines and microbial components (eg, lipopolysaccharide) [31, 32], was positively correlated with KYNA/TRP ratio. This is in line with the fact that tryptophan catabolism can be regulated by both host inflammation and microbial exposure–induced IDO-1 activity [4]. Emerging evidence also suggests that gut microbiota altered during HIV infection might contribute to disrupted tryptophan catabolism through encoding tryptophan catabolism– related enzymes rather than through inducing host IDO-1 activity [33].
The role of tryptophan-kynurenine metabolites in the development of CVD remains unclear. As the precursor to serotonin and melatonin, tryptophan may contribute to CVD risk through depression and sleep disturbance [34, 35]. Kynurenic acid and other downstream kynurenines have been examined in few studies. In support of our results, Pedersen et al [26] found that higher plasma levels of kynurenic acid, along with the other 3 kynurenines, were associated with increased risk of acute myocardial infarction in patients with stable angina pectoris. However, in another study, it was reported that plasma kynurenine and 3-hydroxykynurenine, but not kynurenic acid or other kynurenines, were associated with risk of acute coronary events in community-dwelling elderly individuals [27]. Data from experimental studies are controversial [16, 36, 37]. A recent study demonstrated that kynurenic acid, but not kynurenine, may promote atherosclerotic plaque by inhibiting IL-10 production in mice, which may explain the relationship between high IDO-1 activity and atherosclerosis [16].
To the best of our knowledge, this is the first study in HIV-infected individuals to report significant associations between plasma levels of tryptophan catabolism–related metabolites and subclinical carotid atherosclerosis in a prospective analysis. Given the moderate magnitude of our effect estimates (eg, a 29% decreased risk of carotid plaque per SD increment in tryptophan; a 47% increased risk of carotid plaque per SD increment in KYNA/TRP ratio) and lack of data on incident CVD in this HIV-infected population, our findings should be interpreted with caution. A few previous studies in non-HIV populations have suggested that tryptophan catabolism–related metabolites serve as predictors for incident CVD [13, 14, 26, 27]. For example, among 2380 individuals with stable coronary artery disease, each SD increment of KYN/TRP ratio was associated with a 28% increased risk of major coronary events [14]. However, the stability of these metabolite levels in the prediction of CVD remains unclear as data on repeated measurements of these metabolites over time are limited. In a diet intervention trial, Yu et al [38] found that plasma levels of tryptophan and related metabolites changed after participants had been on a Mediterranean diet for 1 year. Furthermore, increases in tryptophan after 1-year diet intervention were associated with a lower risk of CVD. Thus, the possible utility of tryptophan and related metabolites as potential targets for interventions deserves further study. Indeed, in HIV-infected individuals, ART and niacin treatment have been found to reduce tryptophan catabolism and increase tryptophan levels [39], although it is unknown whether these changes are associated with CVD risk.
Our study has several limitations. Plasma kynurenine and several other downstream metabolites could not be measured using our liquid chromatography–mass spectrometry in the positive ion mode. However, it has been suggested that kynurenic acid, not kynurenine, may reflect tryptophan consumption and promote atherosclerosis [16]; kynurenic acid along with other downstream kynurenines showed consistent associations with CVD risk [26]. We did not have a case group with incident clinical CVD events, but our subclinical measure of carotid artery atherosclerosis has been validated as a surrogate outcome of clinical CVD events [20]. While our results were consistent in 2 cohorts of women and men with HIV infection, replication of our findings in other HIV cohorts would provide further validation. Although there was little evidence of metabolite–HIV interactions, our study did not have enough power to determine effect modification by HIV serostatus. Given the current sample size, we had 80% power to detect interaction RRs of 2.0, 2.3, and 2.2 for carotid artery plaque per SD increment in tryptophan, kynurenic acid, and KYNA/TRP ratio, respectively, with the statistical significance of 0.05. The observed differences in RRs of metabolites on carotid artery plaque between individuals with and without HIV infection were much smaller than those estimated in power calculation.
In summary, in this study from 2 HIV cohorts, we found that lower plasma tryptophan and higher levels of kynurenic acid and KYNA/TRP ratio were associated with progression of carotid artery atherosclerosis, suggesting a potential role of tryptophan catabolism and kynurenine pathway metabolites in atherogenesis and the development of CVD. Further studies are warranted to clarify underlying mechanisms and examine whether interventions that target these metabolites may help prevent CVD in HIV-infected individuals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Data for this article were collected as part of the WIHS.
Disclaimer. The contents of this article are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This study was supported by the National Heart, Lung, and Blood Institute (NHLBI; K01HL129892 to Q. Q.). Other funding sources for this study were provided to Q. Q. (R01HL140976), R. C. K. (R01 HL126543, R01 HL132794, R01HL083760, R01HL095140), W. S. P. (R01-HL-095129), D.B. H. (K01-HL-137557), and Q. Q. (Feldstein Medical Foundation Research Grant).
WIHS (principal investigators): University of Alabama at Birmingham and University of Mississippi (Mirjam-Colette Kempf and Deborah Konkle-Parker; U01-AI-103401); Atlanta (Ighovwerha Ofotokun and Gina Wingood; U01-AI-103408); Bronx (Kathryn Anastos; U01-AI-035004); Brooklyn (Howard Minkoff and Deborah Gustafson; U01-AI-031834); Chicago (Mardge Cohen and Audrey French; U01-AI-034993); Metropolitan Washington (Seble Kassaye; U01-AI-034994); Miami (Margaret Fischl and Lisa Metsch; U01-AI-103397); UNC (Adaora Adimora; U01-AI-103390); Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien; U01-AI-034989); WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub; U01-AI-042590); and Southern California (Joel Milam; U01-HD-032632) (WIHS I–WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute (NCI), the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects was also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. Support for WIHS data collection was also provided to University of California–San Francisco Clinical and Translational Science Awards (CTSA) (UL1-TR000004), Atlanta CTSA (UL1-TR000454), and University of North Carolina Center for AIDS Research (P30-AI-050410).
MACS (principal investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick; U01-AI35042); Northwestern University (Steven Wolinsky; U01-AI35039); University of California, Los Angeles (Roger Detels; U01-AI35040); University of Pittsburgh (Charles Rinaldo; U01-AI35041); and the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson; UM1-AI35043). The MACS is funded primarily by the NIAID at the NIH, with additional cofunding from the NCI. Targeted supplemental funding for specific projects was also provided by the NHLBI and the NIDCD. Support for MACS data collection was also provided to Johns Hopkins University CTSA (UL1-TR000424).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J 2013; 34:3538–46. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep 2016; 13:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu G, Chen S, Zhong J, Teng K, Yin Y. Crosstalk between tryptophan metabolism and cardiovascular disease, mechanisms, and therapeutic implications. Oxid Med Cell Longev 2017; 2017:1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Favre D, Mold J, Hunt PW, et al. . Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem 1998; 44:858–62. [PubMed] [Google Scholar]
- 6. Siedner MJ, Kim JH, Nakku RS, et al. . Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis 2016; 213:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991; 5:2516–22. [PubMed] [Google Scholar]
- 8. Pertovaara M, Raitala A, Juonala M, et al. . Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 2007; 148:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niinisalo P, Raitala A, Pertovaara M, et al. . Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 Study. Scand J Clin Lab Invest 2008; 68:767–70. [DOI] [PubMed] [Google Scholar]
- 10. Kato A, Suzuki Y, Suda T, et al. . Relationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patients. Hemodial Int 2010; 14:418–24. [DOI] [PubMed] [Google Scholar]
- 11. Wirleitner B, Rudzite V, Neurauter G, et al. . Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest 2003; 33:550–4. [DOI] [PubMed] [Google Scholar]
- 12. Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009; 204:309–14. [DOI] [PubMed] [Google Scholar]
- 13. Sulo G, Vollset SE, Nygård O, et al. . Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol 2013; 168:1435–40. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen ER, Midttun Ø, Ueland PM, et al. . Systemic markers of interferon-γ- mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2011; 31:698–704. [DOI] [PubMed] [Google Scholar]
- 15. Hanna DB, Post WS, Deal JA, et al. . HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Metghalchi S, Ponnuswamy P, Simon T, et al. . Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab 2015; 22:460–71. [DOI] [PubMed] [Google Scholar]
- 17. Zhao J. Plasma kynurenic acid/tryptophan ratio: a sensitive and reliable biomarker for the assessment of renal function. Ren Fail 2013; 35:648–53. [DOI] [PubMed] [Google Scholar]
- 18. Bacon MC, von Wyl V, Alden C, et al. . The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 20. Hodis HN, Mack WJ, LaBree L, et al. . The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128:262–9. [DOI] [PubMed] [Google Scholar]
- 21. Kaplan RC, Kingsley LA, Gange SJ, et al. . Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008; 22:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Touboul PJ, Hennerici MG, Meairs S, et al. . Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011 Basel, Switzerland: Cerebrovascular diseases, 2012; 34:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanna DB, Lin J, Post WS, et al. . Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis 2017; 215:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaplan RC, Sinclair E, Landay AL, et al. . T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011; 203:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grennan JT, Loutfy MR, Su D, et al. ; CANOC Collaboration Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012; 205:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedersen ER, Tuseth N, Eussen SJ, et al. . Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2015; 35:455–62. [DOI] [PubMed] [Google Scholar]
- 27. Eussen SJ, Ueland PM, Vollset SE, et al. . Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int J Cardiol 2015; 189:18–24. [DOI] [PubMed] [Google Scholar]
- 28. McKibben RA, Margolick JB, Grinspoon S, et al. . Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burdo TH, Lo J, Abbara S, et al. . Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shaked I, Hanna DB, Gleißner C, et al. . Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol 2014; 34:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crowe SM, Hoy JF. Are monocytes the canary in the coal mine for HIV-related atherosclerosis?J Infect Dis 2012; 206:1491–3. [DOI] [PubMed] [Google Scholar]
- 32. Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. . Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Badawy AA. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol 2013; 27:878–93. [DOI] [PubMed] [Google Scholar]
- 35. Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci 2017; 31:45–70. [DOI] [PubMed] [Google Scholar]
- 36. Zhang L, Ovchinnikova O, Jönsson A, et al. . The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J 2012; 33:2025–34. [DOI] [PubMed] [Google Scholar]
- 37. Cole JE, Astola N, Cribbs AP, et al. . Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A 2015; 112:13033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu E, Ruiz-Canela M, Guasch-Ferré M, et al. . Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevención con Dieta Mediterránea (PREDIMED) Study. J Nutr 2017; 147:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis 2003; 3:644–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.