AFF4, an essential core of SEC, was overexpressed in HNSCC tissue and cell lines. AFF4 promoted the proliferation, migration, invasion and tumor-initiation capacity by regulating SOX2 in HNSCC cells, indicating AFF4 may serve as a potential therapeutic target of HNSCC.
Abstract
Super elongation complex (SEC) controls gene transcription by releasing Pol II from pausing. Previous studies have shown that dysfunction of SEC was associated with multiple human cancers, such as leukemia and breast cancer. However, the role of SEC in head and neck squamous cell carcinoma (HNSCC) development remains largely unknown. In this study, we found expression of AF4/FMR2 family member 4 (AFF4), the core component of SEC, was upregulated dramatically in HNSCC cell lines and tumor tissues. By using siRNA-mediated depletion and overexpression of AFF4, we demonstrated AFF4 promoted proliferation, migration and invasion of HNSCC cells. Moreover, we found AFF4 enhanced the aldehyde dehydrogenase (ALDH) activity and sphere formatting activity and was required for the tumor-initiation capacity of stem-like cells in HNSCC cell lines. Mechanistically, we found the role of AFF4 in regulation of HNSCC cell behaviors was mainly mediated by sex-determining region Y box2 (SOX2), a critical regulator involved in development of several human cancers. SOX2 expression changed in parallel with AFF4 expression in response to depletion and overexpression of AFF4, respectively. More importantly, overexpression of SOX2 rescued the inhibited proliferation, migration, invasion and ALDH activity induced by knockdown of AFF4 in HNSCC cells, at least in part. Collectively, our findings indicate AFF4 may serve as a biomarker and a potential target of therapies for patients with HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) remains major health challenge as the seventh most common non-skin cancer worldwide (1,2). HNSCC accounts for more than 90% of head and neck cancers that arise from the mucosal surfaces of the oral cavity, oropharynx and larynx (3). More than new 550 000 cases are diagnosed annually that result in approximately 350 000 deaths every year (4). In addition to cigarette smoking and/or alcohol abuse, infection with high-risk human papillomaviruses (HPV) has been long considered as a key risk factor of HNSCC (3,5). In the USA, HPV-driven HNSCC is responsible for an approximately 25% increase in the incidence of HNSCC during the past decade, especially among middle-aged males (6). Current treatment paradigm of HNSCC includes surgery, radiation therapy, while chemotherapy may be used for palliative care (7). However, despite advances in therapeutic approaches, approximately half of all patients finally die of this disease. Recent studies on the molecular mechanisms that drive HNSCC development have provided a comprehensive landscape of genomic alterations in HNSCC (8–10). Several critical factors involved in homeostasis and differentiation of epithelial stem cells, such as sex-determining region Y box2 (SOX2), were found to be amplified and to promote HNSCC progression (8,11,12). However, the network controlling the expression of these genes is still not fully understood, which limits the development of targeted therapies for patients with HNSCC.
Super elongation complex (SEC) is essential for regulation of gene expression at transcriptional level, containing P-TEFb (positive transcription elongation factor), ELL (eleven-nineteen lysine-rich leukemia gene), AFF (AF4/FMR2 family member) and several other factors (13,14). In both mammalian and Drosophila cells, genome-wide mapping of (RNA polymerase II) Pol II has revealed that Pol II pauses at approximately +50 bp of the transcription start site of a majority of genes (15–17). SEC is capable of phosphorylating the C-terminal domain of Pol II and releasing it from the pausing for transcription. Recent studies have also shown that SEC is required for proper expression of HOX genes (a subset of homeotic genes) in early embryonic development but also contribute to misactivation of HOX genes in leukemia, highlighting a critical role of SEC in development and diseases (18,19).
AF4/FMR2 family member (AFF4) is a core component of SEC that functions as a scaffold to assemble the SEC by directly interacting with P-TEFb and AF9 (ALL1-fused gene from chromosome 9 protein) or ENL (eleven-nineteen-leukemia protein) (19,20). AFF4 is also required for SEC stability and activity (19). Like other three members in AFF family, AFF4 contains conserved N- and C-terminal domains, an ALF homology region and a serine-rich transactivation domain that was involved in transcriptional activation (21). Recent studies have found that translocation of AFF4 with MLL (mixed lineage leukemia) is implicated in acute lymphoblastic leukemia (19). And gain-of-function mutations in AFF4, which hindered the degradation of AFF4, led to CHOPS syndrome (C for cognitive impairment and coarse facies, H for heart defects, O for obesity, P for pulmonary involvement and S for short stature and skeletal dysplasia) (22). However, the role of AFF4 and SEC in HNSCC development remains largely unknown.
In this study, we examined the expression of SEC components in HNSCC cell lines and found AFF4 expression level was significantly upregulated, in comparison with human keratinocyte HaCaT cells. We then investigated the function of AFF4 in regulation of proliferation, migration and tumor-initiation capacity of HNSCC cells. Our findings indicate AFF4 may promote tumorigenesis and tumor-initiation capacity of HNSCC by regulating SOX2.
Materials and methods
Cell culture
Human head and neck SCC cell lines, SCC1 and SCC23 cells were a gift from Dr Thomas E. Carey, University of Michigan (Ann Arbor, MI). SCC1 cells are HPV negative, and SCC23 cells are HPV 31 positive (5). Both cell lines were authenticated by short tandem repeat DNA profiling analysis in 2017. SCC1 cells and SCC23 cells were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, plus 100 U/ml penicillin and 100 mg/ml streptomycin sulfate. Human-immortalized keratinocyte cell line, HaCaT, was kindly provided by Dr J. S. Gutkind (Moores Cancer Center, University of California, San Diego), which was recently verified by short tandem repeat test in 2017. HaCaT cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (all from Invitrogen, Grand Island, NY). All cells were maintained at a humidified condition at 37°C in 5 % CO2 and passed every 3–4 days.
Immunohistochemistry
A human HNSCC tissue array was purchased from Biomax (Rockville, MD). Immunohistochemistry was performed as described previously (23). Briefly, the tissues were dewaxed, rehydrated and incubated in 3% hydrogen peroxide for 10 min to block the endogenous peroxidase activity. After antigen retrieval, the tissues were incubated overnight at 4°C with the rabbit anti-AFF4 antibody (Abcam) at a dilution of 1:100. The expression of AFF4 was detected with an ABC kit (Vector Laboratories, Burlingame, CA). The stained tissues were scanned using Aperio Digital Pathology Systems (Leica Biosystems, Nussloch, Germany). Immunohistochemistry evaluation was then performed independently by two researchers as described previously (24,25). A semi-quantitative H-score for each specimen (ranged from 0 to 300) was calculated by multiplying the distribution areas (0–100%) by the intensities (0: negative; 1: weak staining; 2: moderate staining; 3: strong staining).
Transfection
siRNA-targeting AFF4 and a scrambled control siRNA (SCR) were purchased from Santa Cruz Biotechnology, CA. Transfection was performed using Lipofectamine RNAiMAX reagent (Invitrogen) according to manufacturer’s instructions. To implement a gene knockdown of AFF4 in a long term, SCC1 and SCC23 cells were transfected with lentiviruses carrying shRNAs targeting AFF4 (Santa Cruz Biotechnology) and were selected with 1 μg/ml puromycin dihydrochloride (Sigma–Aldrich, St. Louis, MO). For overexpression of SOX2, retroviruses expressing human SOX2 gene were purchased from Fulengen (Guangzhou, China). After selection, the transfection efficacy was confirmed by quantitative reverse transcription PCR (RT-qPCR) and western blot.
Western blot
Cells were harvested and lysed on ice for 30 min in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40), supplemented with protease inhibitor cocktail (Pierce Biotechnology). Aliquots of the lysates were subjected to dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were then transferred onto nitrocellulose membranes (Bio-Rad). The membranes were subsequently incubated with primary antibodies followed by a horseradish peroxidase-conjugated secondary antibody (Boster, Wuhan, China). Protein bands were detected using an enhanced chemiluminescence western blotting detection kit (Thermo). The following antibodies for western blot were used in this study: anti-AFF4 (Abcam, 1:1000), anti-SOX2 (Abcam, 1:2000), anti-HA-tag (Sigma–Aldrich, 1:2000), anti-α-tubulin (Sigma–Aldrich, 1:5000).
Quantitative reverse transcription PCR (RT-qPCR)
Quantitative RT-PCR experiments were carried out as described previously (26). The primer sequences used in this study were as following: 5′-TACAATGACGACAG AAACCTGC-3′ (forward) and 5′-GGCGATGAGTGTGAGACTTAGTA-3′ (reverse) for AFF1; 5′- CTGACAGCGAATCTAATGAGGC-3′ (forward) and 5′-CATTGGTTGGATGATTGGAGGA-3′ (reverse) for AFF2; 5′-GTCATCTCGTT GGAGTTCCCA-3′ (forward) and 5′-AGTGCCTCTCTTACTCTGCTG-3′ (reverse) for AFF3; 5′-AAAGGCCAGCATGGATCAGAA-3′ (forward) and 5′-GTGATTTGGAGCGTTGATGTTC-3′ (reverse) for AFF4; 5′-GTCGGAGAC GCCTGACTACT-3′ (forward) and 5′-TACTCGGCATTGAAGTCGTTC-3′ (reverse) for ELL. 5′-CATCACCGTACTGCATGTGAA-3′ (forward) and 5′-ACTGGATTGAAGGTCGAAAAGG-3′ (reverse) for ELL2; 5′-GCCCGGAC TAGCCTCTTACT-3′ (forward) and 5′-TGGGAGTCTCAGATACCCTCG-3′ (reverse) for ELL3; 5′-GAGGGGTTCACTCACGACTG-3′ (forward) and 5′-GGCATGATGAAGCCAGCGTA-3′ (reverse) for MLLT1; 5′-TTTGTGGAG AAAGTCGTCTTCC-3′ (forward) and 5′-GAGGTGATTCACTGGTGGATG-3′ (reverse) for MLLT3; 5′-ATGGCAAAGCAGTACGACTCG-3′ (forward) and 5′-GCAAGGCTGTAATGGGGAAC-3′ (reverse) for CDK9; 5′-GCTGCCAATGGC TCTAATGAA-3′ (forward) and 5′-TGCTGGGCATCGTAAGTATCTT-3′ (reverse) for BMI1; 5′-GCTTCAATGCTTCAGCTCCA-3′ (forward) and 5′-CCTG AAGTGCTGCTCCTTTC-3′ (reverse) for CD44; 5′-TTTGTGGGCCTGAA GAAAACT-3′ (forward) and 5′-AGGGCTGTCCTGAATAAGCAG-3′ (reverse) for NANOG; 5′-GCGAACCATCTCTGTGGTCT-3′ (forward) and 5′-GGAAAGTTGGG ATCGAACAA-3′ (reverse) for SOX2; 5′-ACAACTTTGGCATTGTGGAA-3′ (forward), and 5′-GATGCAGGGATGATGTTCTG-3′ (reverse) for GAPDH.
MTT assay
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay was performed to determine the proliferation rate of cells upon treatments. A total of 5 × 103 cells were seeded per well of a 96-well plate in triplicate and cultured for 1, 3, 5 and 7 days after transfection. Ten microliters of MTT (5 mg/ml) (Sigma) solution was added to each well and incubated for 4 h. The medium containing MTT was then removed and replaced with 100 μl DMSO to solubilize the formazan. Optical absorbance was measured at 570 nm with a microplate reader (Bio-Rad).
ALDH activity assay
Single cell suspension of SCC1 and SCC23 cells was generated, and the aldehyde dehydrogenase (ALDH) activity was examined using an Aldefluor assay kit (Stem Cell Technologies, Durham, NC), as described previously (27). For flow cytometry analysis, cells stained with a specific ALDH inhibitor diethylaminobenzaldehyde was used as negative control. The ALDH+ population was purified for further study.
Sphere formation assay and limiting dilution assay
To determine the self-renewal capacity in vitro, the ALDH+ cells were seeded in a six-well plate at a density of 1000 cells/well and cultured in serum-free media, supplemented with N-2 Supplement (Life Technologies, Carlsbad, CA), B27 Supplement (Life Technologies), 10 ng/ml EGF (Sigma-Aldrich), 10 ng/ml bFGF (Sigma-Aldrich), 5 μg/ml insulin (Life Technologies) (10). After 2 weeks of culture, the spheres with a diameter exceeding 10 µm were counted. Then, we performed limiting dilution assay, to test the self-renewal capacity of cells in vivo. The indicated number of cells was implanted subcutaneously at the dorsal sites of 6-week-old nude mice. Tumor incidence was observed for 2 months, and then the generated tumors were explanted and photographed. All the animal procedures were conducted according to the guidelines for the care and use of laboratory animals of State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, Sichuan University.
Wound-healing assay and transwell assay
Wound-healing assay was performed using culture inserts from Ibidi (Martinsried, Germany), according to the manufacturer’s protocol. Briefly, 1 × 104 cells were seeded into each of two cell culture reservoirs. After overnight culture, the wound was created by gently removing the silicon insert. Pictures were taken after 0, 8 and 12 h (as indicated), and the migrated distance was measured using ImageJ software. Transwell assay was performed using Matrigel invasion chambers (Corning, NY) as reported previously (28). Briefly, 1 × 105 siRNA-transfected cells were seeded into the invasion chambers. After 24 h, the invaded cells were stained with the HEMA-3 kit (Fisher) and counted under microscope (Olympus).
ChIP assay
Chromatin immunoprecipitation (ChIP) assay was performed using a Simple ChIP Assay Kit (Cell Signaling Technology, Danvers, MA) according to the manufacturer’s instruction. The precipitated DNA samples were purified and measured by Q-PCR. Results were shown as the percentage of input controls. The following antibodies were used: anti-AFF4 (Abcam, 1:500), anti-CDK9 (Abcam, 1:500) and anti-H3K4me3 (Millipore, 1:1000). The following primers were used: 5′- CGTCACATGGATGGTTGTCTAT-3′ (forward) and 5′- GGCTCAAACTTCTCTCCCTTTC-3′ (reverse) in the SOX2 promoter region; 5′- TGGTGCAAAAACATCTTGGA-3′ (forward) and 5′- TACCCAAGAACCAGGAGTGG-3′ at around 10 kb downstream of SOX2 transcription start sites.
Statistical analysis
All experiments were performed at least three times, unless otherwise noted. Student’s t-test or one-way ANOVA was used to determine the significance of different groups by SPSS software 19.0. For limiting dilution assay, statistical test was performed as described previously (29). Data were shown as mean ± standard error of the mean (SEM) of three independent experiments. Differences were considered significant at *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
AFF4 is upregulated in HNSCC
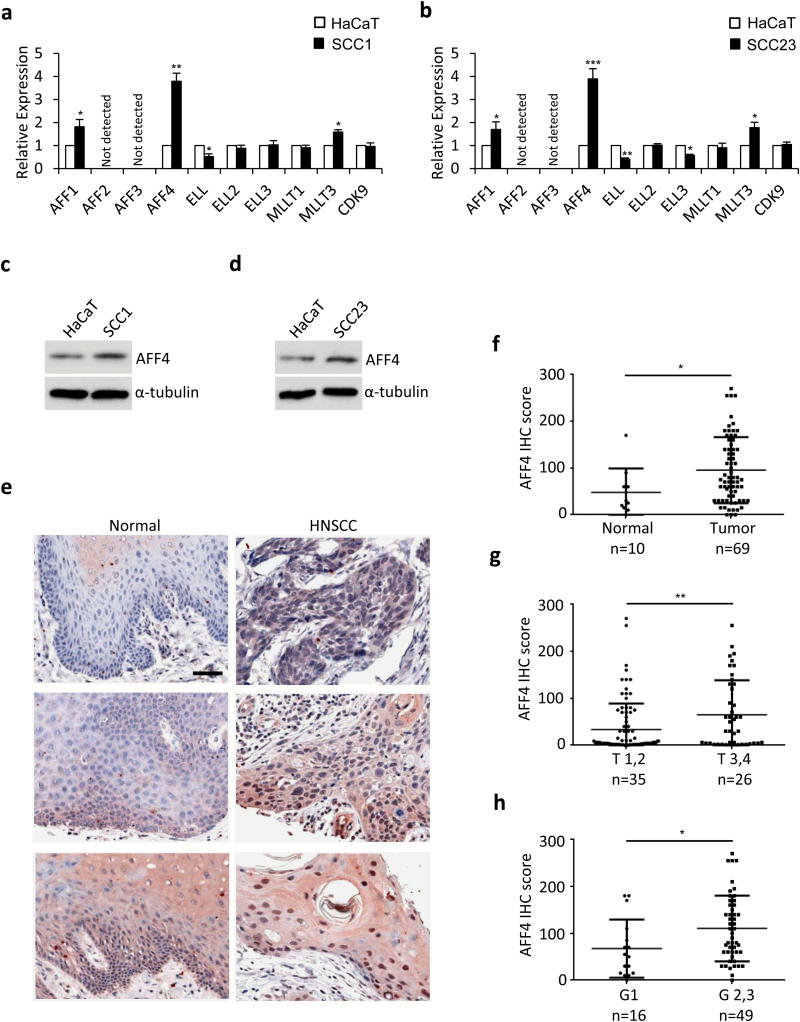
We first screened the expression of SEC components in human keratinocyte HaCaT cells and HNSCC cell lines, SCC1 and SCC23, by Q-PCR. As shown in Figure 1a and b, expression of AFF1, AFF4 and MLLT3 (encoding protein AF9) was significantly increased in SCC1 and SCC23 cells, compared with HaCaT cells. We also observed that ELL gene expression was decreased in both SCC1 and SCC23 cells, and ELL3 expression was downregulated in SCC23 cells but not in SCC1 cells. While AFF2 and AFF3 were not detected in all the three cell lines, the rest genes, such as ELL2, MLLT1 (encoding ENL) and CDK9, remained unchanged (Figure 1a and b). In addition, the upregulation of AFF4 was further confirmed by western blot (Figure 1c and d). As revealed by IHC, we further demonstrated the expression of AFF4 was elevated in HNSCC tissues, compared with normal tissues (Figure 1e and f). More importantly, AFF4 expression was correlated with tumor progression from tumor stage 1,2 (T1,2) to tumor stage 3,4 (T3,4) and from Grade 1 (G1) to Grade 2, 3 (G2,3) (Figure 1g and h). These results indicate SEC-containing AFF4 may play a role in promoting HNSCC progression.
Figure 1.
AFF4 is upregulated in HNSCC. (a, b) qPCR analysis of SEC component expression in SCC1 cells (a), SCC23 cells (b). The fold-change was normalized to the matched expression in HaCaT cells. n = 3. (c, d) Western blot analysis of AFF4 expression in HaCaT cells, SCC1 cells (c) and SCC23 cells (d). The a-tubulin was used as a loading control. n = 3. (e) Representative images of immunohistochemistry staining with AFF4 in normal human tongue mucosa and HNSCC tissues. Bar indicates 50 μm. (f) AFF4 expression was upregulated in HNSCC tissues (n = 69), compared with normal human tongue mucosa (n = 10). (g, h) AFF4 expression was correlated with tumor progression from TNM stage 1,2 (T1,2, n = 35) to TNM stage 3,4 (T3,4, n = 26) and from Grade 1 (G1, n = 16) to Grade 2, 3 (G2,3, n = 49). *P-value < 0.05; **P-value < 0.01.
AFF4 promotes proliferation of HNSCC cells
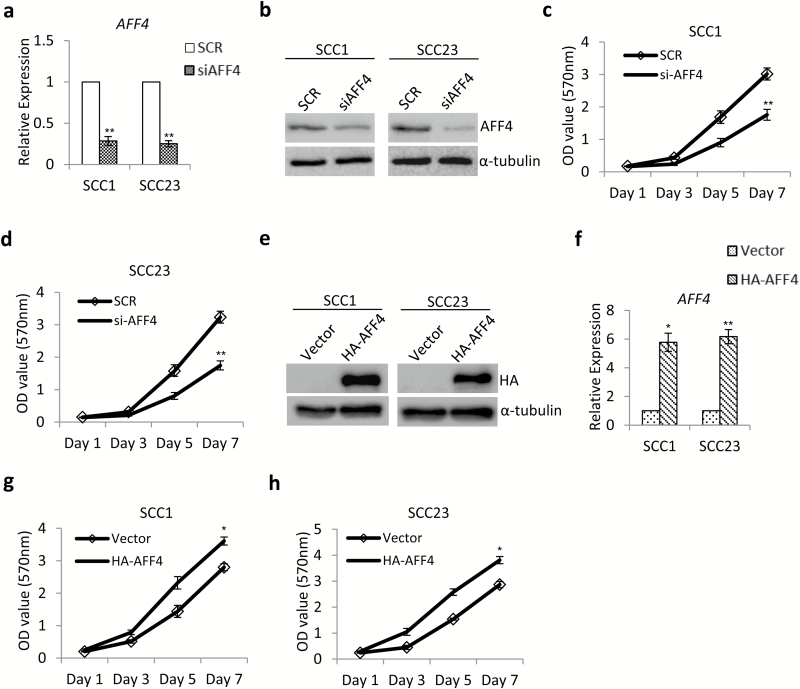
To explore the potential function of AFF4 in HNSCC cell lines, we examined whether AFF4 expression was associated with proliferation rate of HNSCC cells. siRNA-targeting AFF4 was used to knockdown AFF4 in SCC1 and SCC23 cells, and the efficiency was confirmed by Q-PCR and western blot (Figure 2a and b). We found that depletion of AFF4 inhibited proliferation of SCC1 and SCC23 cells at 3, 5 and 7 days, as determined by MTT assay (Figure 2c and d). We then generated SCC1 and SCC23 cell lines stably expressing HA-tagged AFF4 (Figure 2e and f). As expected, ectopic overexpression of AFF4 significantly enhanced proliferation of SCC1 and SCC23 cells (Figure 2g and h).
Figure 2.
AFF4 promotes proliferation of HNSCC cells. (a, b) SCC1 and SCC23 cells were transfected with scrambled control siRNA (SCR) and siAFF4, respectively. qPCR analysis (a) and western blot (b) was conducted to examine of AFF4 expression at 48 h post transfection. n = 3. (c, d) MTT assay results showed knockdown of AFF4 inhibited cell proliferation in SCC1 cells (c) and SCC23 cells (d). n = 3 at each time point. (e, f) SCC1 and SCC23 cells were transfected with retroviruses expressing HA-tagged AFF4. Cells transfected with empty vector were used as a control. n = 3. (e) Western blot and qPCR analysis (f) were conducted to examine expression of HA and AFF4 at 48 h post transfection respectively. n = 3. (g, h) MTT assay results showed overexpression of AFF4 enhanced cell proliferation in SCC1 cells (g) and SCC23 cells (h). n = 3 at each time point. *P-value < 0.05; **P-value < 0.01.
AFF4 enhances tumor-initiation capacity of HNSCC cells
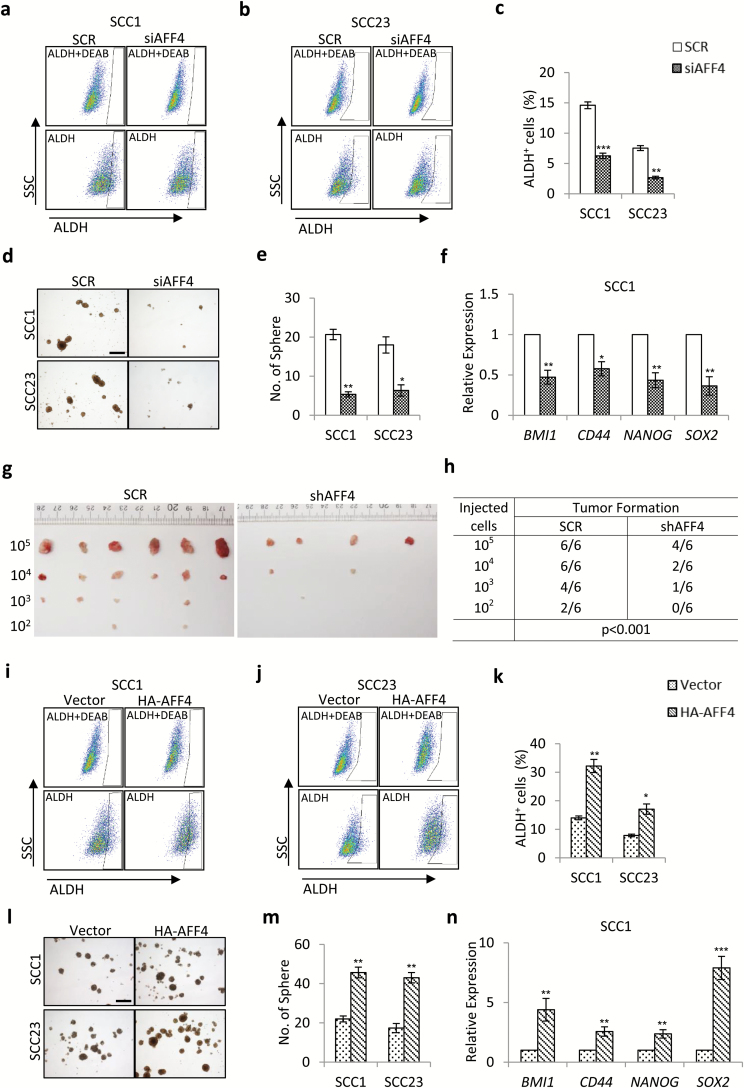
Since SEC was reported to promote cancer stem cell expansion, we next sought to investigate the role of AFF4 in regulating stemness of HNSCC cells (30). As determined by ALDH activity assay, depletion of AFF4 reduced the percentage of ALDH+ cells in SCC1 and SCC23 cells (Figure 3a–c) (31). We then isolated ALDH+ cells for sphere formation analysis. Consistently, sphere formation ability of SCC1 and SCC23 cells was significantly inhibited by depletion of AFF4 (Figure 3d and e). The expression levels of stemness-related genes, such BMI1, CD44, NANOG and SOX2, were also suppressed by depletion of AFF4 in SCC1 and SCC23 cells (Figure 3f; Supplementary Figure 1a, available at Carcinogenesis Online) (32,33). More importantly, AFF4 knockdown dramatically reduced the tumor-initiation capacity of SCC1 cells in vivo, as demonstrated by limiting dilution assay (Figure 3g and h). In contrast, overexpression of AFF4 resulted in a significant increase in ALDH and sphere-forming activity and promoted expression of stemness-related genes in SCC1 and SCC23 cells (Figure 3i–n; Supplementary Figure 1b, available at Carcinogenesis Online). Collectively, these findings suggest AFF4 may promote tumor progression by regulating stemness in HNSCC cells.
Figure 3.
AFF4 enhances tumor-initiation capacity of HNSCC cells. (a, b) Flow cytometry assay performed to examine the effects of AFF4 depletion on ALDH activity of SCC1 cells (a) and SCC23 cells (b). Cells stained with ALDH+DEAB, an ALDH inhibitor, served as the negative control. (c) Quantification of ALDH+ cells in a and b. n = 3. (d) Sphere formation assay conducted to examine the effects of AFF4 depletion on sphere formation capacity of SCC1 cells and SCC23 cells. Bar indicates 200 μm. (e) Quantification of formed spheres (diameter exceeding 10 µm) in d. n = 3. (f) qPCR analysis of the effects of AFF4 depletion on expression of BMI1, CD44, NANOG and SOX2 in SCC1 cells. n = 4. (g) In vivo limiting dilution assay conducted to examine the effects of AFF4 depletion on tumor-initiation capacity of SCC1 cells in vivo. n = 6 for each group. Total mice, 48. (h) Quantification of formed tumors in g. (i, j) Flow cytometry assay performed to examine the effects of AFF4 overexpression on ALDH activity of SCC1 cells (i) and SCC23 cells (j). (k) Quantification of ALDH+ cells in i and j. n = 3. (l) Sphere formation assay conducted to examine the effects of AFF4 overexpression on sphere formation capacity of SCC1 cells and SCC23 cells. Bar indicates 200 μm. (m) Quantification of formed spheres (diameter exceeding 10 µm) in k. n = 3. (n) qPCR analysis of the effects of AFF4 overexpression on expression of BMI1, CD44, NANOG and SOX2 in SCC1 cells. n = 4. *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
AFF4 promotes migration and invasion of HNSCC cells
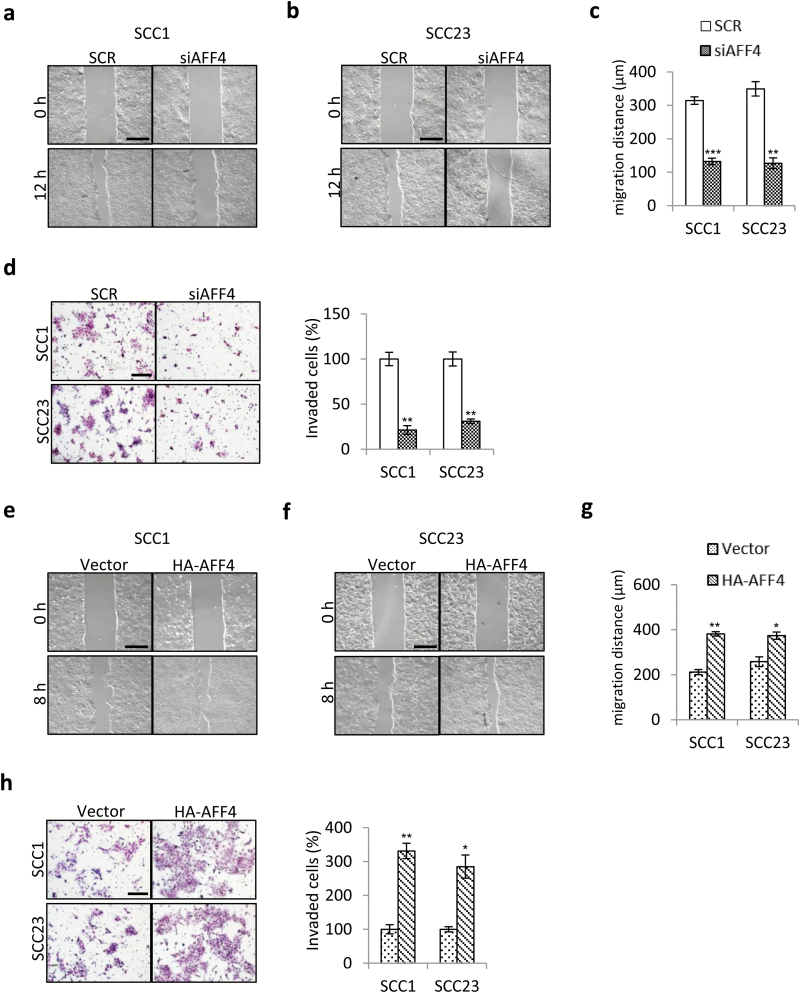
We then performed wound-healing assay and transwell assay to analyze whether AFF4 affects migration and invasion of HNSCC cells. As shown in Figure 4a–c, depletion of AFF4 caused a significant decrease in the migration distance 12 h post wound creation. The transwell assay also demonstrated knockdown of AFF4 inhibited the invasiveness of SCC1 and SCC23 cells (Figure 4d). In contrast, ectopic overexpression of AFF4 promoted migration and invasion in SCC1 and SCC23 cells in vitro (Figure 4e–h).
Figure 4.
AFF4 promotes migration and invasion of HNSCC cells. (a, b) Wound-healing assay performed to assess the effects of AFF4 depletion on migration activity of SCC1 cells (a) and SCC23 cells (b). Bar indicates 400 μm. (c) Quantification of migration distance in a and b. n = 3. (d) Transwell assay conducted to study the effect of AFF4 depletion on invasion activity of SCC1 cells and SCC23 cells. Bar indicates 200 μm. n = 3. (e, f) Wound-healing assay performed to assess the effects of AFF4 overexpression on migration activity of SCC1 cells (e) and SCC23 cells (f). Bar indicates 400 μm. (g) Quantification of migration distance in e and f. n = 3. (h) Transwell assay conducted to study the effect of AFF4 overexpression on invasion activity of SCC1 cells and SCC23 cells. Bar indicates 200 μm. n = 3. *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
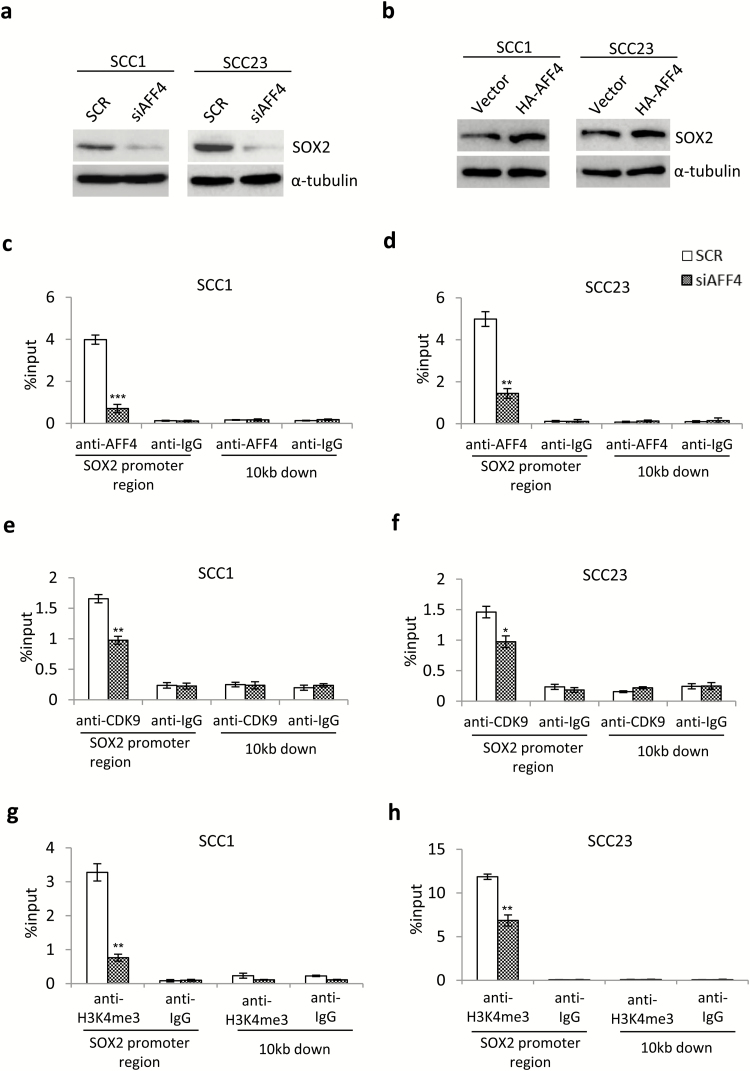
AFF4 promotes SOX2 expression and recruits CDK9 to SOX2 promoter
Recent studies have shown SOX2 plays a pivotal role in self-renewal and tumorigenicity of HNSCC cancer stem cells (11). To investigate whether SOX2 is regulated by AFF4, we further confirmed expression of SOX2 in response to AFF4 knockdown and overexpression by western blot, respectively. Interestingly, knockdown of AFF4 reduced SOX2 expression (Figure 5a), while overexpression of AFF4 elevated SOX2 expression in both SCC1 and SCC23 cells (Figure 5b). Furthermore, we found AFF4 directly bound to SOX2 promoter as determined by ChIP assay, which is almost abolished by knockdown of AFF4 (Figure 5c and d). In addition, CDK9 was also present at SOX2 promoter in SCC1 and SCC23 cells, while knockdown of AFF4 inhibited the recruitment of CDK9 to SOX2 promoter, indicating AFF4 is required for CDK9 binding to SOX2 promoter (Figure 5e and f). Finally, the level of histone H3 trimethylated at lysine 4 (H3K4me3), a transcription initiation marker, was inhibited by knockdown of AFF4 (Figure 5g and h), suggesting AFF4 is an essential factor for SOX2 transcription.
Figure 5.
AFF4 promotes SOX2 expression and recruits CDK9 to SOX2 promoter. (a) Western blot of SOX2 expression in response to depletion of AFF4 at 48 h post transfection. n = 3. (b) Western blot of SOX2 expression in response to overexpression of AFF4 at 48 h post transfection. n = 3. (c, d) ChIP assay was conducted to study the recruitment of AFF4 at SOX2 promoter in SCC1 cells (c) and SCC23 cells (d) transfected with SCR and siAFF4 at 48 h post transfection. n = 3. (e, f) ChIP assay was conducted to study the recruitment of CDK9 at SOX2 promoter in SCC1 cells (e) and SCC23 cells (f) transfected with SCR and siAFF4 at 48 h post transfection. n = 3. (g, h) ChIP assay was conducted to study the H3K4me3 levels at SOX2 promoter in SCC1 cells (g) and SCC23 cells (h) transfected with SCR and siAFF4 at 48 h post transfection, respectively. n = 3. *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001.
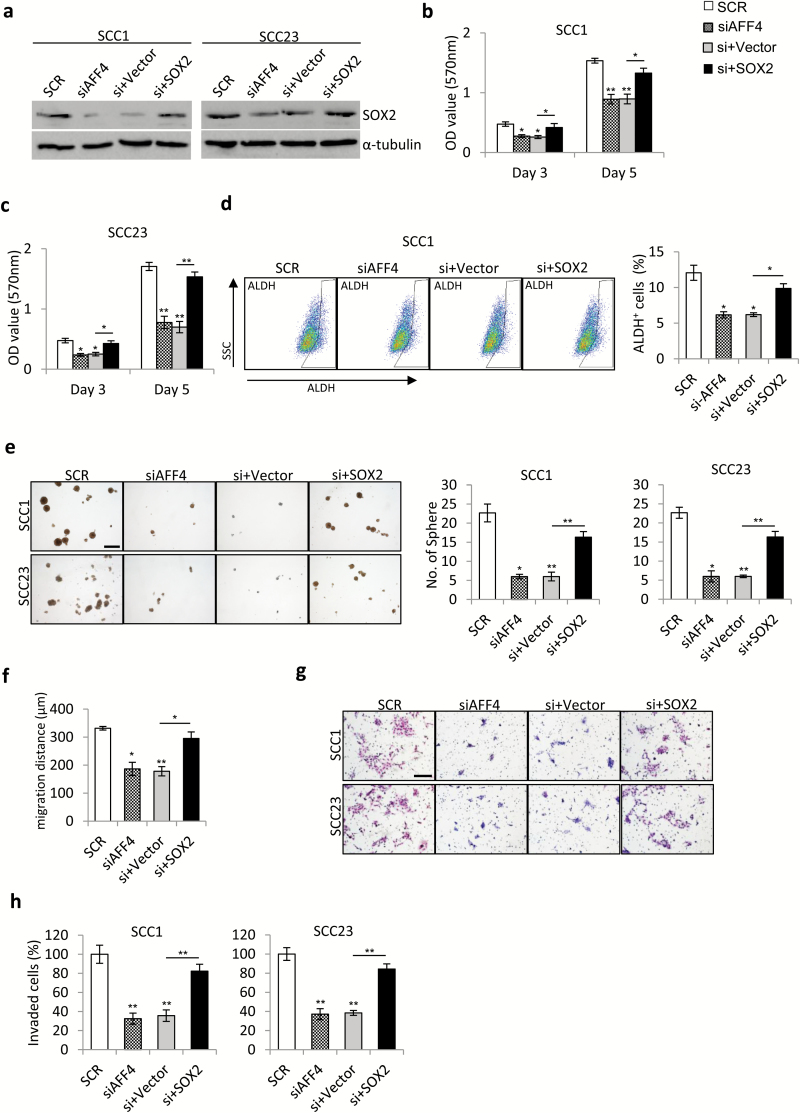
Overexpression of SOX2 rescued HNSCC cell behavior inhibited by AFF4 depletion
To investigate whether AFF4 regulates HNSCC cell behavior via SOX2, we stably overexpressed SOX2 in SCC1 and SCC23 cells. Introduction of exogenous SOX2 successfully restored the SOX2 expression suppressed by AFF4 depletion, compared with empty vector control (Figure 6a), and SOX2 overexpression rescued proliferation rate of SCC1 and SCC23 cells in response to AFF4 depletion (Figure 6b and c). Furthermore, the suppression of ALDH activity and sphere formation ability by AFF4 depletion were almost abrogated by SOX2 overexpression (Figure 6d and e; Supplementary Figure 2a, available at Carcinogenesis Online). In addition, overexpression of SOX2 also rescued the inhibited migration and invasion in AFF4 depleted SCC1 and SCC23 cells, at least in part, as revealed by wound-healing assay and transwell assay (Figure 6f–h; Supplementary Figure 2b, available at Carcinogenesis Online). Taken together, these results indicate the role of AFF4 in regulation of HNSCC cell behavior is mainly mediated by SOX2.
Figure 6.
Overexpression of SOX2 rescued the inhibitory effects on HNSCC cell behavior induced by AFF4 depletion. (a) Western blot analysis of SOX2 expression in SOX2-overexpressing SCC1 and SCC23 cells in response to AFF4 knockdown at 48 h post transfection. n = 3. (b, c) MTT assay for cell proliferation in SOX2-overexpressing SCC1 cells (b) and SCC23 cells (c) in response to AFF4 knockdown at 3, 5 days. n = 3 at each time point. (d) Flow cytometry assay for ALDH activity of SOX2-overexpressing SCC1 cells in response to AFF4 knockdown at 48 h post transfection. n = 3. (e) Sphere formation assay conducted to evaluate sphere formation capacity of SOX2-overexpressing SCC1 and SCC23 cells in response to AFF4 knockdown after 2 weeks of culture in suspension. Bar indicates 200 μm. n = 3. (f) Would healing assay for migration activity of SOX2-overexpressing SCC1 cells in response to AFF4 knockdown. n = 3. (g, h) Transwell assay for invasion activity SOX2-overexpressing SCC1 and SCC23 cells in response to AFF4 knockdown. n = 3. *P-value < 0.05; **P-value < 0.01.
Discussion
HNSCC remains a major health issue with a high morbidity and mortality profile. In this study, we found AFF4 expression was upregulated in HNSCC tissue and cell lines. We then demonstrated AFF4 promoted proliferation and migration and was required for the tumor-initiation capacity of HNSCC cells. Using ChIP assay, we found AFF4 was recruited to SOX2 promoter and promoted SOX2 expression. Furthermore, overexpression of SOX2 at least partially rescued the decrease in proliferation, migration and ALDH activity induced by knockdown of AFF4.
SEC is capable of promoting expression of target genes by overcoming Pol II pausing. Previous studies have shown that SEC complex could be a critical regulator in the pathogenesis of leukemia and breast cancer (19,30). However, to our knowledge, this is the first time that the function of SEC complex in HNSCC was identified. We screened the expression of SEC complex components in SCC1 and SCC23 cells, in comparison with HaCaT cells and found expression of AFF4, an essential component of SEC complex, was dramatically elevated. Since AFF4 function as the scaffold to assemble the SEC, we suspected SEC may play an important role in regulation of HNSCC progression. While knockdown of AFF4 inhibited proliferation of HNSCC cells, overexpression of AFF4 promoted cell growth, indicating AFF4 plays a role in regulation of proliferation of HNSCC cells. We also noticed that the cell numbers in the two groups were compatible at day 7 post transfection. It is possible that siAFF4 became inefficient after several days of cell culture or the inhibitory effect was overcome by alternative mechanisms.
Our results demonstrated AFF4 enhanced ALDH activity, sphere formation capacity and tumor-initiation capacity of HNSCC cells. HNSCC carries a poor prognosis, largely due to high rates of recurrence and metastasis (34,35). Recent studies have highlighted the role of stem-like cells in HNSCC progression and recurrence, which are capable of self-renewal and differentiating into non-tumorigenic and tumorigenic cancer cells (36). In this study, we found AFF4 expression levels are associated with ALDH activity and sphere formation capacity of HNSCC cells in vitro. Although the formed spheres were counted after 2 weeks of culture in suspension, we still suspect that the result of sphere formation assay might be slightly affected by the inhibition of proliferation induced by AFF4 knockdown in HNSCC cells. More importantly, by using limiting dilution assay, we demonstrated AFF4 was required for the tumor-initiation capacity of SCC1 cells in vivo. In addition, AFF4 enhanced migration and invasion capacity of HNSCC in vitro. Although stemness often associates with lower proliferation rates, like the quiescent hematopoietic stem cells in the niches (37,38), we found APP4 to enhance both proliferation and self-renewal of stem-like cells in HNSCC, like the role of SOX2 in HNSCC cells (11,39). In our opinion, the effect of stemness-related transcription factors on the proliferation and self-renewal of stem cells may largely depend on the cell type and microenvironment.
We also found the role of AFF4 in regulation of HNSCC cell behaviors was mainly mediated by SOX2. SOX2 is a key transcription factor for the self-renewal of both embryonic and adult stem cells and is necessary for reprograming of fibroblasts into an induced pluripotent stem cells (40). Recent studies have also found SOX2 was overexpressed in several human cancers, such as breast cancer, glioblastoma and skin SCC (41–44). In regard to HNSCC, the 3q26/28 region containing SOX2, TP63 and oncogene PIK3CA was often amplified in HNSCC tissues (8,45,46). In this study, we found AFF4 promoted SOX2 expression via binding to SOX2 promoter. Depletion of AFF4 significantly reduced H3K4me3 marks and recruitment of CDK9 at SOX2 promoters, indicating AFF4 is a key regulator of SOX2 expression in HNSCC cells. Furthermore, ectopic overexpression of SOX2, at least in part, rescued the inhibition in proliferation, migration and ALDH activity induced by AFF4 knockdown. However, SOX2 may not the only target gene of AFF4 in HNSCC. To fully understand the role of AFF4 in HNSCC development, more efforts are needed to investigate the binding profile of AFF4 at whole-genome level and the impact on genome-wide expression by targeting AFF4. It is also worthy to note that HNSCC is a heterogeneous disease. For example, HPV-positive HNSCC shows different epidemiology and survival rate with HPV-negative disease (9,47). Whether AFF4 and other SEC components play distinct roles in heterogeneous HNSCC still remains to be investigated in the future. In addition, due to the easy access to mucosa tissues, repeated sampling for longitudinal monitoring of HNSCC development can improve our understanding on the role of AFF4 in transformation of normal tissues. Initiation and progression of HNSCC are comprised of several stages. Understanding of the stages that AFF4 functions to promote HNSCC is essential for development of targeted therapies for AFF4-overexpressed HNSCC and possibly other cancers.
However, there are several limitations in this study. Firstly, the upstream signaling of AFF4 in HNSCC cells remains largely unknown. Since SCC1 cells are HPV negative and SCC23 cells are HPV 31 positive, HPV infection seems not be required for AFF4 upregulation. Secondly, whether AFF4 protein expression levels associated with prognosis of patients with HNSCC remains to be investigated. Finally, AFF4 is also expressed in normal tissues, although it is lower in comparison with HNSCC. It is possible that knockdown of AFF4 may have stronger effects on HNSCC tumor tissues than normal tissues. However, complete inhibition of AFF4 expression may also inhibit proliferation and self-renewal in normal tissues. How to reduce AFF4 expression in HNSCC cells back to an acceptable level without heavily disturbing normal homeostasis of normal tissue remains a major challenge.
In summary, we found AFF4 was upregulated in HNSCC cells, and AFF4 enhanced tumorigenesis and tumor-initiation capacity of HNSCC cells by regulating SOX2. Our findings indicate AFF4 may serve as a biomarker and a potential target of therapies for patients with HNSCC.
Supplementary material
Supplementary material can be found at Carcinogenesis online.
Funding
This project is supported by Foundation for the National Natural Science Foundation of China (No. 81600877, 81621062, 81520108009, 81472533) and 111 Project of MOE in China and China Postdoctoral Science Foundation (2016M600745).
Conflict of Interest Statement: None declared.
Abbreviations
- ALDH
aldehyde dehydrogenase
- ChIP
chromatin immunoprecipitation
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomaviruses
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide
- SEC
super elongation complex
References
- 1. Ferlay J., et al. (2010)Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer, 127, 2893–2917. [DOI] [PubMed] [Google Scholar]
- 2. Zhang C.Z., et al. (2016)Saliva in the diagnosis of diseases. Int. J. Oral Sci., 8, 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vigneswaran N., et al. (2014)Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. North Am., 26, 123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Burden of Disease Cancer Collaboration; Fitzmaurice C., et al. (2017)Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMAOncol., 3, 524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carol R.B., et al. (1991)Human papillomavirus DNA sequences in cell lines derived from head and neck squamous cell carcinomas. Otolaryngol. Head Neck Surg., 104, 303–310. [DOI] [PubMed] [Google Scholar]
- 6. Carvalho A.L., et al. (2005)Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int. J. Cancer, 114, 806–816. [DOI] [PubMed] [Google Scholar]
- 7. Cognetti D.M., et al. (2008)Head and neck cancer: an evolving treatment paradigm. Cancer, 113 (7 Suppl), 1911–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cancer Genome Atlas Network. (2015)Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, 517, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stransky N., et al. (2011)The mutational landscape of head and neck squamous cell carcinoma. Science, 333, 1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y.K., et al. (2014)Sphere-forming-like cells (squamospheres) with cancer stem-like cell traits from VX2 rabbit buccal squamous cell carcinoma. Int. J. Oral Sci., 6, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S.H., et al. (2014)SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br. J. Cancer, 111, 2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones K.B., et al. (2013)Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int. J. Oral Sci., 5, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo Z., et al. (2012)The super elongation complex family of RNA polymerase II elongation factors: gene target specificity and transcriptional output. Mol. Cell. Biol., 32, 2608–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo Z., et al. (2012)The super elongation complex (SEC) family in transcriptional control. Nat. Rev. Mol. Cell Biol., 13, 543–547. [DOI] [PubMed] [Google Scholar]
- 15. Dahlberg O., et al. (2015)P-TEFb, the super elongation complex and mediator regulate a subset of non-paused genes during early Drosophila embryo development. PLoS Genet., 11, e1004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeitlinger J., et al. (2007)RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat. Genet., 39, 1512–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marson A., et al. (2008)Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell, 134, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin C., et al. (2011)Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC). Genes Dev., 25, 1486–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin C., et al. (2010)AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol. Cell, 37, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mueller D., et al. (2009)Misguided transcriptional elongation causes mixed lineage leukemia. PLoS Biol., 7, e1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma C., et al. (1996)LAF-4 encodes a lymphoid nuclear protein with transactivation potential that is homologous to AF-4, the gene fused to MLL in t(4;11) leukemias. Blood, 87, 734–745. [PubMed] [Google Scholar]
- 22. Izumi K., et al. (2015)Germline gain-of-function mutations in AFF4 cause a developmental syndrome functionally linking the super elongation complex and cohesin. Nat. Genet., 47, 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ji N., et al. (2017)Synergistic effect of honokiol and 5-fluorouracil on apoptosis of oral squamous cell carcinoma cells. J. Oral Pathol. Med., 46, 201–207. [DOI] [PubMed] [Google Scholar]
- 24. Detre S., et al. (1995)A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol., 48, 876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H., et al. (2015)EZH2-mediated loss of miR-622 determines CXCR4 activation in hepatocellular carcinoma. Nat. Commun., 6, 8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W., et al. (2016)GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation. Nat. Commun., 7, 12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang P., et al. (2016)GINS2 regulates matrix metallopeptidase 9 expression and cancer stem cell property in human triple negative breast cancer. Biomed. Pharmacother., 84, 1568–1574. [DOI] [PubMed] [Google Scholar]
- 28. Peng L., et al. (2016)Ubiquitin specific peptidase 21 regulates interleukin-8 expression, stem-cell like property of human renal cell carcinoma. Oncotarget, 7, 42007–42016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu Y., et al. (2009)ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods, 347, 70–78. [DOI] [PubMed] [Google Scholar]
- 30. Ji X., et al. (2014)LARP7 suppresses P-TEFb activity to inhibit breast cancer progression and metastasis. Elife, 3, e02907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clay M.R., et al. (2010)Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck, 32, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen D., et al. (2017)Targeting BMI1+cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell, 20, 621–634.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan Z., et al. (2017)Prognostic value of cancer stem cell markers in head and neck squamous cell carcinoma: a meta-analysis. Sci. Rep., 7, 43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leemans C.R., et al. (2011)The molecular biology of head and neck cancer. Nat. Rev. Cancer, 11, 9–22. [DOI] [PubMed] [Google Scholar]
- 35. Feng X., et al. (2016)Overexpression of proteasomal activator PA28α serves as a prognostic factor in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res., 35, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frank N.Y., et al. (2010)The therapeutic promise of the cancer stem cell concept. J. Clin. Invest., 120, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boulais P.E., et al. (2015)Making sense of hematopoietic stem cell niches. Blood, 125, 2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crane G.M., et al. (2017)Adult haematopoietic stem cell niches. Nat. Rev. Immunol., 17, 573–590. [DOI] [PubMed] [Google Scholar]
- 39. Keysar S.B., et al. (2017)Regulation of head and neck squamous cancer stem cells by PI3K and SOX2. J. Natl. Cancer Inst., 109, djw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi K., et al. (2006)Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Pinilla S.M., et al. (2007)Sox2: a possible driver of the basal-like phenotype in sporadic breast cancer. Mod. Pathol., 20, 474–481. [DOI] [PubMed] [Google Scholar]
- 42. Fang X., et al. (2011)The SOX2 response program in glioblastoma multiforme: an integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genomics, 12, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boumahdi S., et al. (2014)SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature, 511, 246–250. [DOI] [PubMed] [Google Scholar]
- 44. Zhu F., et al. (2017)SOX2 is a marker for stem-like tumor cells in bladder cancer. Stem Cell Reports, 9, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schröck A., et al. (2014)Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis, 35, 1636–1642. [DOI] [PubMed] [Google Scholar]
- 46. Li Y., et al. (2015)Expression of p53, p21(CIP1/WAF1) and eIF4E in the adjacent tissues of oral squamous cell carcinoma: establishing the molecular boundary and a cancer progression model. Int. J. Oral Sci., 7, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Argiris A., et al. (2008)Head and neck cancer. Lancet, 371, 1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.