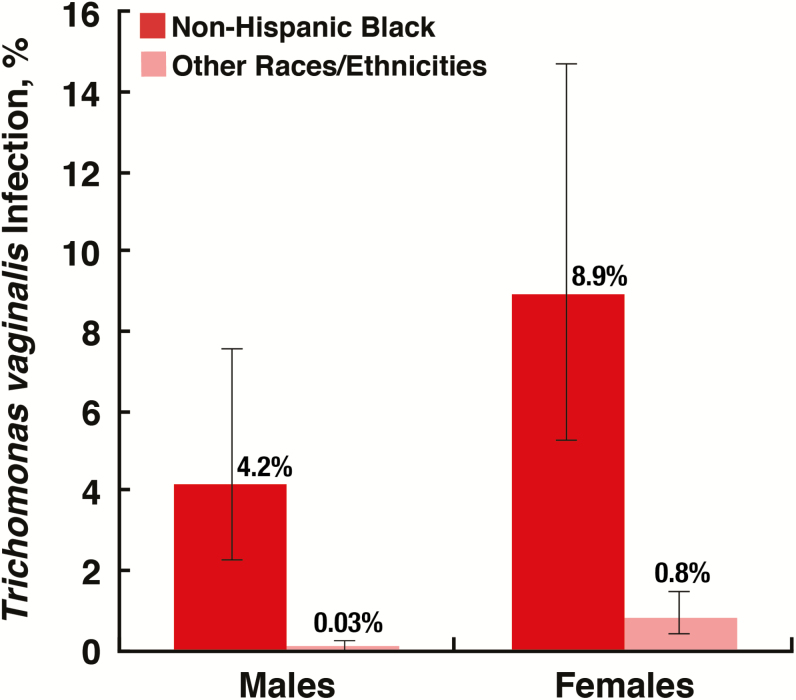
In a nationally representative sample of the civilian, noninstitutionalized US population aged 18–59 years, Trichomonas vaginalis infection was detected in 0.5% of males and 1.8% of females, and was highest among black males (4.2%) and black females (8.9%).
Keywords: NHANES, Trichomonas vaginalis, trichomoniasis, sexually transmitted infection, racial disparities
Abstract
Background
The epidemiology of Trichomonas vaginalis (TV) infection in the United States is poorly defined.
Methods
Males and females aged 18–59 years who participated in the 2013–2014 National Health and Nutrition Examination Survey and provided a urine specimen were tested for TV infection (n = 4057). Participants were also examined for Chlamydia trachomatis (CT) infection, genital human papillomavirus (HPV) infection, and herpes simplex virus type 2 serostatus. Weighted adjusted prevalence ratios (aPRs) were estimated by multivariable Poisson regression.
Results
TV infection prevalence was 0.5% and 1.8% among males and females, respectively. TV infection prevalence was 4.2% among black males, 8.9% among black females, and 0.03% and 0.8%, respectively, among males and females of other races/ethnicities. TV infection prevalence (aPR [95% confidence interval]) was positively associated with female sex (6.1 [3.3–11.3]), black race (vs other races/ethnicities; 7.9 [3.9–16.1]), older age (vs 18–24 years; 3.0 [1.2–7.1] for 25- to 39-year-olds and 3.5 [1.3–9.4] for 40- to 59-year-olds), having less than a high school education (vs completing high school or more; 2.0 [1.0–4.1]), being below the poverty level (vs at or above the poverty level; 4.0 [2.1–7.7]), and having ≥2 sexual partners in the past year (vs 0–1 sexual partners; 3.6 [2.0–6.6]). There were no TV and CT coinfections. Genital HPV detection was not independently associated with TV infection. Among persons aged 18-39 years, there was a significant racial disparity in all sexually transmitted infections examined, and this disparity was greatest for TV infection.
Conclusions
There is a high and disproportionate burden of urinary TV infection in the adult civilian, noninstitutionalized black population in the United States that warrants intervention.
(See the Editorial Commentary by Muzny on pages 218–20.)
Trichomonas vaginalis (TV) infection is the most common nonviral sexually transmitted infection (STI) worldwide [1]. Symptomatic TV infection is characterized by urethral discharge and dysuria in men, and vaginal discharge, dysuria, and abdominal pain in women [2, 3]. While most TV infections are asymptomatic in men and women [2, 4, 5], the pathologic sequelae from untreated and persistent TV infection is more severe than previously acknowledged. In men, TV infection is a cause of urethritis and is associated with prostatitis, balanoposthitis, and epididymitis [6, 7]. In women, TV infection can lead to urethritis and is associated with infertility, perinatal morbidity, cervical neoplasia, and pelvic inflammatory disease [7–11]. Finally, TV infection is strongly linked to an increased risk of human immunodeficiency virus (HIV) acquisition and transmission [12]. Despite the frequency and severity of TV infection, TV infection has received a limited public health response to date and has consequently been coined a “neglected” STI [13].
In the United States, TV infection is not notifiable and routine surveillance programs do not exist [7], thereby requiring sentinel surveillance to estimate the national burden of infection. Only 2 national population-based studies of TV infection have been conducted in the United States [4, 14]. In the 2001–2004 National Health and Nutrition Examination Survey (NHANES), it was first reported using swab-based polymerase chain reaction (PCR) testing that the prevalence of TV infection among females aged 14–49 years was 3.1% [4]. The 2001–2002 National Longitudinal Study of Adolescent Health utilized urine-based PCR testing and reported that TV infection prevalence was 1.7% and 2.8%, respectively, among males and females aged 18–26 years [14]. The national prevalence among older adult males has not been previously determined; in general, the epidemiology of TV infection is less characterized among males in comparison to females, partly due to historical challenges in diagnostic testing. It is difficult to make direct comparisons between studies due to differential sampling and testing methods, but studies have reported even higher estimates of TV infection prevalence in young urban populations [5], internet-recruited samples [15, 16], HIV/STI clinic populations [17, 18], incarcerated populations [19], people who use drugs [20, 21], and in other sentinel sites across the United States [22].
Although there are now US Food and Drug Administration (FDA)–cleared nucleic acid amplification tests (NAATs) and point-of-care diagnostic tests for males and females [3, 23], and TV infection is curable with effective short-course regimens (1 or 7 days) [2, 7], most asymptomatic TV infections remain unidentified and untreated. The US Centers for Disease Control and Prevention (CDC) supports TV testing in symptomatic women, but only provides a clear recommendation for TV screening (of symptomatic or asymptomatic individuals) in persons living with HIV [7]. The CDC does suggest, albeit with less assertion, that TV screening “might be considered” for individuals receiving care in settings with high prevalence (eg, prisons and sexually transmitted disease [STD] clinics) and for individuals engaged in high-risk behaviors (eg, multiple sex partners and illicit drug use) [7]. Additional population-based data are needed to help public health officials understand the burden of TV infection, identify at-risk populations, and appropriately direct disease control efforts.
In this study, we describe the prevalence and correlates of urinary TV infection in a nationally representative sample of the adult civilian, noninstitutionalized US population.
METHODS
Ethics Statement
Data collection for the study was approved by the CDC Research Ethics Review Board. All participants provided written informed consent. Data used in this analysis were de-identified and are publicly available. The present analysis was deemed exempt from review by the Johns Hopkins University School of Medicine Institutional Review Board.
Survey Design and Population
Administered by the National Center for Health Statistics (NCHS), the 2013–2014 NHANES is a complex survey of the US civilian, noninstitutionalized population. Respondents were interviewed in their homes and invited to a mobile examination center where a physical examination was performed. Respondents were eligible to undergo a blood draw, provide a urine sample and a self-administered penile/cervicovaginal swab, and complete a self-administered audio computer-assisted self-interview on sexual behavior. The examination response rate was 68.5% for all ages. This study was restricted to respondents aged 18–59 years who provided a urine sample and had valid TV results available for analysis.
Questionnaire Data
Sociodemographic and behavioral variables were collapsed into categories that maximized the stability of estimates while retaining epidemiological relevance. For instance, self-reported race/ethnicity was operationalized as “black” or “other races/ethnicities” in this analysis. The “black” group was solely composed of persons identifying as non-Hispanic black (single race). The “other races/ethnicities” group was composed of subgroups that were precategorized by NCHS: Hispanics, non-Hispanic whites, non-Hispanic Asians, and other non-Hispanic races (including non-Hispanic multiracial persons). The lifetime number of sex partners and number of sex partners in the past year included any sex partner regardless of type of sexual experience or their partner’s gender.
Laboratory Testing
The NHANES Laboratory Procedures Manual has detailed information on specimen collection, processing, storage and testing protocols [24]. Urinary TV infection status was determined using the Gen-Probe Aptima Combo Trichomonas vaginalis assay (Hologic, Gen-Probe, San Diego, California), which detects TV RNA by transcription-mediated amplification and has been FDA approved for use in urine from females. Age-eligible participants were also tested for Chlamydia trachomatis (CT) (age 18–39 years), herpes simplex virus type 2 (HSV-2) (age 18–49 years), and genital human papillomavirus (HPV) (age 18–59 years). Urinary CT infection status was determined using the Gen-Probe Aptima Combo 2 C. trachomatis assay (Hologic, Gen-Probe). HSV-2 serostatus was determined by the solid-phase enzymatic immunodot assay and the gG-2 monoclonal antibody inhibition assay [25]. Genital swabs were used to assess HPV infection status (≥1 of 37 genotypes) by the Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Indianapolis, Indiana).
Statistical Analysis
All reported prevalence estimates, measures of association, and 95% confidence intervals (CIs) were weighted to account for the unequal probabilities of selection, non-response to the medical examination, and noncoverage using weights provided by NCHS. Standard errors were computed by the Taylor series linearized (robust) variance estimator. Prevalence estimates with a relative standard error ≥30% are indicated and should be interpreted with caution.
In the primary analysis, TV infection prevalence was examined among all participants aged 18–59 years. Sociodemographic and behavioral correlates of TV infection were measured by prevalence ratios estimated by Poisson regression. All characteristics assessed in univariable models were considered for inclusion in the multivariable analysis, but only covariates that remained statistically significant in nested multivariable models were retained in the final multivariable model (P < .05). In separate models, associations between other STIs and TV infection were examined; two multivariable models were constructed for each STI, one including only sociodemographic characteristics and one including both sociodemographic characteristics and the number of sexual partners in the past year. A subgroup analysis was conducted among females.
In a supplemental analysis, prevalence of each STI was compared by race/ethnicity among participants aged 18–39 years, using methods previously described. To enhance direct comparability of effect sizes between infections, a sensitivity analysis was conducted limited to participants with complete data for all infections. It should be noted that binomial models yielded similar estimates (data not shown).
Two-sided P values were determined by design-adjusted Wald F tests. All analyses were conducted using Stata/SE software, version 14.2 (StataCorp, College Station, Texas).
RESULTS
Of the 4139 participants examined, 4057 participants had a urine specimen available for TV testing. Every urine specimen had valid TV results; 71 of 4057 participants in the analytic sample had detectable TV infection. The estimated prevalence of urinary TV infection in the civilian, noninstitutionalized US population aged 18–59 years was 1.2% (95% CI, .7%–2.0%).
TV Infection Prevalence by Sociodemographic and Behavioral Characteristics
In comparison to the age group 18–24 years (0.7%), TV infection prevalence was significantly higher among those aged 25–39 years (1.4%; P = .045) and nonsignificantly higher among those aged 40–59 years (1.2%; P = .254; Table 1). The prevalence of TV infection was significantly higher among females (1.8%) than males (0.5%; P < .001; Table 1). TV infection prevalence was also significantly different by race/ethnicity group with an estimate of 6.8% among the black population and 0.4% among the other races/ethnicities group (P < .001). This racial disparity in TV infection was observed among both males and females (P < .001 for both comparisons; Figure 1).
Table 1.
Prevalence of Urinary Trichomonas vaginalis Infection in the Civilian, Noninstitutionalized US Population Aged 18–59 Years, by Sociodemographic and Behavioral Characteristics
| Characteristic | No. | TV Prevalence, % (95% CI) |
Crude PR (95% CI) |
Adjusted PR (95% CI)a |
|---|---|---|---|---|
| Sex | ||||
| Male | 1942 | 0.5 (.2–1.0)b | Ref. | Ref. |
| Female | 2115 | 1.8 (1.1–3.1) | 3.8 (2.2–6.5) | 6.1 (3.3–11.3) |
| Age group, y | ||||
| 18–24 | 811 | 0.7 (.4–1.4)b | Ref. | Ref. |
| 25–39 | 1363 | 1.4 (.7–2.7)b | 2.0 (1.0–3.8) | 3.0 (1.2–7.1) |
| 40–59 | 1883 | 1.2 (.6–2.3)b | 1.7 (.7–4.3) | 3.5 (1.3–9.4) |
| Race/ethnicity | ||||
| Non-Hispanic black | 822 | 6.8 (4.0–11.2) | 16.8 (7.8–36.3) | 7.9 (3.9–16.1) |
| Other races/ethnicitiesc | 3235 | 0.4 (.2–.7) | Ref. | Ref. |
| Educational attainment | ||||
| High school or more | 3148 | 0.8 (.5–1.3) | Ref. | Ref. |
| Less than high school | 831 | 2.9 (1.5–5.6)b | 3.4 (2.0–5.9) | 2.0 (1.0–4.1) |
| Poverty status | ||||
| At or above poverty level (PID ≥1) | 2784 | 0.6 (.4–.9) | Ref. | Ref. |
| Below poverty level (PID <1) | 964 | 3.9 (2.4–6.4) | 6.8 (4.2–11.2) | 4.0 (2.1–7.7) |
| Marital status | ||||
| Married/living with partner | 2246 | 0.6 (.3–1.1)b | Ref. | … |
| Widowed/divorced/separated | 553 | 2.0 (1.1–3.9) | 3.5 (1.5–8.3) | … |
| Never married | 930 | 2.4 (1.3–4.3) | 4.1 (2.6–6.5) | … |
| Born in the United States | ||||
| No | 1157 | 0.4 (.2–1.1)d | 0.3 (.1–1.0) | … |
| Yes | 2898 | 1.3 (.8–2.3) | Ref. | … |
| Lifetime No. of sex partners | ||||
| 0–5 | 1916 | 0.8 (.4–1.8)d | Ref. | … |
| >5 | 1709 | 1.3 (.8–2.1) | 1.6 (.8–3.3) | … |
| No. of sex partners in the past 12 mo | ||||
| 0–1 | 2951 | 0.7 (.4–1.3) | Ref. | Ref. |
| ≥2 | 685 | 2.8 (1.7–4.7) | 4.1 (2.5–6.8) | 3.6 (2.0–6.6) |
Prevalence ratios of TV infection were estimated from Poisson regression models. P values were determined by the design-adjusted Wald F test. Estimates in bold had a P value <.05.
Abbreviations: CI, confidence interval; PID, poverty index ratio; PR, prevalence ratio; TV, Trichomonas vaginalis.
aMultivariable model included sex, age group, race/ethnicity, educational attainment, poverty status, and the number of sexual partners in the past 12 months.
bRelative standard error, ≥30 and <35%.
cIncludes Hispanics, non-Hispanic whites, non-Hispanic Asians, and persons of other non-Hispanic race (including multiracial persons).
dRelative standard error, ≥35 and <45%.
Figure 1.
Prevalence of urinary Trichomonas vaginalis infection in the civilian, noninstitutionalized US population aged 18–59 years, by sex and race/ethnicity. Error bars represent 95% confidence intervals; standard errors were estimated by Taylor series linearization. Prevalence was significantly different by race/ethnicity for each sex as estimated by design-adjusted Wald F tests (P < .01). The “Other races/ethnicities” group included persons identifying as multiracial. The estimate for males among the “Other races/ethnicities” group was based on one positive observation and should be interpreted with caution (relative standard error ≥30%).
TV infection prevalence was significantly higher among individuals below the poverty level (3.9%) than individuals at or above the poverty level (0.6%; P < .001; Table 1). Not obtaining a high school education was also significantly associated with a higher prevalence of TV infection (2.9%) in comparison to having at least completed high school or an equivalent education (0.8%; P < .001). Individuals born in the United States had a higher prevalence of TV infection (1.3%) than those born outside the United States (0.4%; P = .052). Prevalence of TV infection was similar between those who were never married (2.4%) and those who were widowed, divorced, or separated (2.0%; P = .692); however, both groups had a significantly higher prevalence of TV infection than individuals who were married or living with their partner (0.6%; P < .001 for both comparisons). Although a significant association was not detected between the number of lifetime sexual partners and TV infection prevalence (P = .176), having multiple sex partners in the past year was positively associated with TV infection (P < .001; Table 1).
In the multivariable model, female sex, black race, older age, being below the poverty level, not obtaining a high school education, and having multiple sex partners in the past year were independent factors associated with a higher TV infection prevalence (P < .05; Table 1). Correlates of TV infection prevalence observed in the overall sample were also observed among females (except educational attainment, P = .103; Supplementary Table 1).
STIs Associated With Urinary TV Infection Prevalence
Among participants aged 18–39 years, no one tested positive for infection with both CT and TV (Table 2). Among participants aged 18–49 years, there was a positive independent association between HSV-2 serostatus and TV infection (P < .01). Although HPV infection was associated with TV infection among participants aged 18–59 years in univariable analysis (P = .014), this association was completely attenuated in multivariable models (Table 2). These results were also seen among females when analyzed separately (Supplementary Table 2).
Table 2.
Association of Concurrent Sexually Transmitted Infections and Trichomonas vaginalis Infection in the Civilian, Noninstitutionalized US Population
| Infection | Age, y | No. | TV Prevalence, % (95% CI) | Crude PR (95% CI) |
Adjusted PR (95% CI)a |
Adjusted PR (95% CI)b |
|---|---|---|---|---|---|---|
| CT | ||||||
| Negative | 18–39 | 2115 | 1.2 (.6–2.1) | … | … | … |
| Positive | 59 | 0.0 | … | … | … | |
| HSV-2 | ||||||
| Seronegative | 18–49 | 2506 | 0.3 (.2–.5) | Ref. | Ref. | Ref. |
| Seropositive | 494 | 5.5 (3.3–9.1) | 18.6 (10.2–33.8) | 7.1 (3.8–13.3) | 6.0 (2.7–13.3) | |
| Genital HPV | ||||||
| Negative | 18–59 | 2079 | 0.7 (.3–1.5)c | Ref. | Ref. | Ref. |
| Positive | 1634 | 1.8 (1.1–2.9) | 2.6 (1.2–5.4) | 1.6 (.7–3.7) | 1.4 (.6–3.0) | |
This analysis reflects all available data for each individual infection. Prevalence ratios of TV infection were estimated from Poisson regression models. P values were determined by the design-adjusted Wald F test. Estimates in bold had a P value <.05.
Abbreviations: CI, confidence interval; CT, Chlamydia trachomatis; HPV, human papillomavirus; HSV-2, herpes simplex virus type 2; PR, prevalence ratio; TV, Trichomonas vaginalis.
aMultivariable model included sex, age, race/ethnicity, educational attainment, and poverty status.
bMultivariable model included sex, age, race/ethnicity, educational attainment, poverty status, and the number of sexual partners in the past 12 months.
cRelative standard error, 36.2%.
Comparative Prevalence of STIs
Prevalence of TV infection, CT infection, HSV-2 serostatus, and HPV infection among young adults aged 18–39 years is shown in Table 3 by sex and race/ethnicity group. Although the relative racial disparity (effect size) of each STI was partially attenuated after adjustment for sociodemographic factors and the number of recent sexual partners, the racial disparity appeared to be greatest for TV infection (Table 3). Similar trends were observed when limiting the analysis to participants with complete data for every infection (Supplementary Table 3).
Table 3.
Comparative Prevalence of Sexually Transmitted Infections in the Civilian, Noninstitutionalized US Population Aged 18–39 Years
| Infection | Prevalence, % (95% CI) | Crude PR (95% CI) |
Adjusted PR (95% CI)a |
Adjusted PR (95% CI)b |
||
|---|---|---|---|---|---|---|
| Overall | Non-Hispanic Black | Other Races/Ethnicities | ||||
| Males and females | ||||||
| TV infection | 1.2 (.6–2.1) | 7.0 (4.4–11.1) | 0.3 (.1–1.0)c | 23.7 (7.2–78.1) | 16.8 (5.4–52.7) | 10.6 (2.9–38.7) |
| CT infection | 2.0 (1.3–3.0) | 5.4 (3.7–7.9) | 1.5 (.8–2.8) | 3.6 (1.6–8.0) | 2.6 (1.1–6.3) | 2.1 (.8–5.4) |
| HSV-2 serostatus | 10.7 (8.8–13.1) | 31.3 (26.9–36.2) | 8.0 (6.2–10.2) | 3.9 (3.0–5.2) | 3.8 (2.8–5.1) | 3.8 (2.7–5.4) |
| Genital HPV infection | 43.6 (39.0–48.2) | 67.2 (60.3–73.5) | 40.1 (35.4–45.0) | 1.7 (1.4–2.0) | 1.7 (1.4–2.0) | 1.5 (1.3–1.7) |
| Males | ||||||
| TV infection | 0.3 (.1–.9)c | 2.7 (.9–7.9)c | 0.0d | … | … | … |
| CT infection | 1.8 (1.1–3.0) | 5.4 (3.2–8.9) | 1.3 (.6–2.9)e | 4.0 (1.5–10.6) | 3.8 (1.3–10.8) | 2.3 (.6–8.6) |
| HSV-2 serostatus | 7.2 (5.5–9.3) | 20.9 (15.4–27.8) | 5.5 (3.9–7.8) | 3.8 (2.4–5.8) | 3.9 (2.6–5.9) | 3.5 (2.0–6.0) |
| Penile HPV infection | 42.3 (37.5–47.2) | 69.5 (61.1–76.7) | 38.6 (33.4–44.0) | 1.8 (1.5–2.2) | 1.8 (1.6–2.1) | 1.6 (1.3–1.9) |
| Females | ||||||
| TV infection | 2.0 (1.1–3.5) | 10.5 (6.8–16.0) | 0.6 (.2–2.0)c | 17.6 (4.6–67.1) | 14.0 (3.9–50.2) | 8.3 (1.9–35.4) |
| CT infection | 2.2 (1.4–3.4) | 5.4 (3.2–9.1) | 1.7 (.9–3.1) | 3.2 (1.4–7.4) | 2.0 (.9–4.4) | 1.8 (.9–3.6) |
| HSV-2 serostatus | 14.3 (11.1–18.1) | 39.8 (34.9–45.0) | 10.5 (7.7–14.2) | 3.8 (2.7–5.4) | 3.7 (2.7–5.2) | 4.0 (2.8–5.7) |
| Vaginal HPV infection | 44.8 (39.6–50.1) | 65.3 (57.0–72.7) | 41.6 (36.4–47.0) | 1.6 (1.3–1.9) | 1.5 (1.2–1.8) | 1.4 (1.2–1.7) |
This analysis reflects all available data for each individual infection among persons aged 18–39 years since all participants in this age group were eligible to be tested for each infection shown. The overall unweighted sample size was 2174 for urinary TV and CT infections, 2077 for HSV-2 serostatus, and 2017 for genital HPV infection (see Supplementary Table 3 for results limited to persons with complete data for all infections). Prevalence ratios of each infection were estimated from Poisson regression models to compare prevalence estimates by race/ethnicity group. The “Other races/ethnicities” group composed of Hispanics, non-Hispanic whites, non-Hispanic Asians, and persons of other non-Hispanic race (including multiracial persons) was the reference group. Adjusted PRs were estimated from multivariable Poisson regression models. P values were determined by the design-adjusted Wald F test. Estimates in bold had a P value <.05.
Abbreviations: CI, confidence interval; CT, Chlamydia trachomatis; HPV, human papillomavirus; HSV-2, herpes simplex virus type 2; PR, prevalence ratio; TV, Trichomonas vaginalis.
aMultivariable model included sex, age group, race/ethnicity, educational attainment, and poverty status.
bMultivariable model included sex, age group, race/ethnicity, educational attainment, poverty status, and the number of sexual partners in the past 12 months.
cRelative standard error, ≥50 and <60%.
dThere were no males in the “Other races/ethnicities” group aged 18–39 years with detectable TV infection.
eRelative standard error, 36.5%.
DISCUSSION
In this nationally representative sample of the civilian, noninstitutionalized population aged 18–59 years, TV infection prevalence was estimated to be 0.5% among males and 1.8% among females. TV infection prevalence was highest among black males (4.2%) and black females (8.9%). The sex- and race-stratified estimates of TV infection prevalence in this study exceed estimates of TV burden in other high-income countries [26]. There is a clear need to eliminate the health disparities presented herein and improve the overall sexual health of the US population.
This is the first national study in the United States to examine TV infection in males and females up to 59 years of age. In multivariable analysis, TV infection was positively associated with age. This may be due to the detection of untreated, persistent TV infections in older age groups; however, due to the cross-sectional nature of this study, it is impossible to distinguish between incident and persistent TV infections. Long-term, prospective cohort studies are needed to better understand the natural history of TV infection across the life-span. The lower prevalence of TV infection in males compared to females in this study may in part be explained by sex differences in the susceptibility and host immune response to TV infection [6]. For instance, some studies have reported a relatively high rate of spontaneous resolution in males, which remains to be observed in females [6, 27]. The disparity in TV infection by sex in this study, however, may also be due to measurement error. Chernesky et al recently demonstrated that self-collected penile-meatal swabs are significantly more sensitive in detecting TV infection than first-catch urine [28], suggesting that TV infection prevalence was underestimated among males in this study.
The previous NHANES study noted a higher TV infection prevalence in black females compared to females of other races/ethnicities [4]. We highlight that this pronounced racial disparity in TV infection is present among males and females. A higher burden of TV infection in the black population may be due to a combination of factors: differences in sexual network characteristics (eg, assortative mixing—favoring sexual partners of the same race), differences in individual-level sexual risk behaviors (eg, higher numbers of sexual partners), biological differences in susceptibility to infection, and social and structural disparities (eg, inadequate healthcare) [29]. From a social justice lens, it cannot be overlooked that structural racism could be a contributing factor to the racial disparity in TV infection [30]. A previous study has shown that neighborhood social disorganization is significantly associated with TV infection [31]. In this study, the racial disparity in TV infection persisted despite adjustment for the number of sexual partners in the past year and appeared to exceed the relative racial disparity observed for other STIs, which supports the hypothesis that high individual-level sexual risk behavior likely does not fully explain the racial disparity in TV infection.
The unadjusted association between HPV infection and TV infection is consistent with a cohort study where HPV infections associated with concurrent TV infection exhibited slower rates of HPV clearance than among those without TV infection [32]. Interestingly, this association was completely attenuated in multivariable analysis. Disentangling the relationship between HPV infection and TV infection is important given their individual associations with the development of cervical neoplasia [8]. It is noteworthy that there were no CT/TV coinfections; while this could be due to limited power, this lack of association has also been observed elsewhere and may be due to differences in sexual exposure risk [17]. On the other hand, the strong association between HSV-2 serostatus and TV infection could be because HSV-2 serostatus is a biomarker of previous high-risk sexual behavior [25]. The epidemiology and consequences of TV coinfection require further study.
This study has limitations. NHANES is a cross-sectional design, so reported associations should not be interpreted in a causal manner. The relatively low prevalence of TV infection led to imprecise estimates and precluded further stratification, particularly among males. As demographic characteristics such as race/ethnicity were self-reported and operationalized using broad analytic categories, this may have resulted in misclassification of some individuals. In addition, several variables of interest with inadequate power (eg, HIV, pregnancy, sexual orientation), and those that were not available (eg, incarceration history, genital symptoms, and other coinfections), could not be included in this analysis. Continued surveillance of TV infection in additional cycles of NHANES will allow for a more representative sample and in-depth analysis. Even so, NHANES is prone to systematic errors, such as nonresponse and use of a sampling frame that does not capture high-risk populations (eg, homeless and incarcerated populations). Coupled with potential errors in biological sampling in males, as previously discussed, this study likely underestimated the true prevalence of TV infection. Finally, it is important to recognize that these data cannot speak to potential geographic heterogeneity in TV infection prevalence.
There has been much debate about whether TV infection should become a nationally notifiable condition. It is well established that TV infection meets 3 of the 7 CDC criteria needed to indicate an infection should be reportable: frequency, disparity, and communicability [33]. Although it was previously argued that TV infection does not meet the public interest criterion, a lack of public interest in TV infection cannot be assessed if there is inadequate public knowledge of TV [13]. For instance, a national survey of US women found that only 22% were very/somewhat familiar with TV infection [34]. Hoots et al suggested that it is unclear if TV infection meets the final 3 criteria: severity, costs associated with infection, and preventability [33]. Although severe outcomes directly attributable to TV infection are rare and/or difficult to determine, one model estimated that 746 new HIV cases occurring annually among US women are attributable to TV infection (lifetime medical costs: $167 million) [35]. It should be clarified that TV infection—like most STIs—is preventable. Correct and consistent condom use and voluntary medical male circumcision have been shown to prevent TV infection [36–38]. In addition, a community-randomized trial in Peru demonstrated that a combination prevention strategy can yield a significant reduction in TV infection prevalence [39]. It appears the question all the more so lies in the scalability of preventive interventions, which will require increased political will and financial support, and advocacy for it by the public health community.
Regardless of whether TV infection is eventually deemed to be notifiable, a stronger public health response to TV infection in the immediate future is warranted [13]. We are unaware of any national efforts to specifically control TV infection. To increase awareness and public knowledge of TV infection, public health campaigns already in place for HIV/STI prevention could easily begin to include educational information about TV infection. A tangible step clinicians could take to potentially reduce the racial disparity in TV infection is to routinely screen and subsequently treat men and women in high-prevalence communities irrespective of HIV status. If barriers to routine screening exist, clinicians should strongly consider screening high-risk persons in accordance with CDC guidelines [7]. NAAT-based diagnostic tests have already facilitated the implementation of routine screening programs for men and women in some STD and HIV clinics [17, 18, 40]. While such programs are commendable, the high burden of TV infection documented in this population-based study highlights the need to extend screening efforts beyond STD clinics. Acceptability of TV infection screening has already been demonstrated in nonclinic populations [15, 16]. Finally, per CDC guidelines, clinicians need to be made aware of the need for concurrent presumptive treatment of all sex partners to prevent reinfection and the need to retest the index patient for TV infection posttreatment [7]. While there may indeed be insufficient empirical evidence that these strategies will lead to epidemic control or a reduction in the racial disparity of TV infection in the United States, it would be an injustice to the black population to continue to ignore this STI until such evidence is available.
Despite accurate diagnostic tools and effective treatment options, TV infection remains a prevalent infection in the adult civilian, noninstitutionalized US population. Increased public health action is needed to eliminate the stark racial disparities in TV infection and achieve racial equity in sexual health. Continued surveillance of TV infection at the national and local level is warranted to guide the development and implementation of effective public health interventions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors sincerely thank the NHANES study team and participants, without whom this study would not have been possible.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by extramural funding from the National Institutes of Health (grant numbers 1R01AI120938 and 1R01AI128779 [to A. A. R. T] and U54 EB007958 and U01-068613 [to C. A. G.]) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Sexually transmitted infections Available at: http://www.who.int/mediacentre/factsheets/fs110/en/. Accessed 29 August 2017.
- 2. Meites E, Gaydos CA, Hobbs MM, et al. . A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis 2015; 61(Suppl 8):S837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Der Pol B. Clinical and laboratory testing for Trichomonas vaginalis infection. J Clin Microbiol 2016; 54:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45:1319–26. [DOI] [PubMed] [Google Scholar]
- 5. Rogers SM, Turner CF, Hobbs M, et al. . Epidemiology of undiagnosed trichomoniasis in a probability sample of urban young adults. PLoS One 2014; 9:e90548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995; 22:83–96. [PubMed] [Google Scholar]
- 7. Workowski KA, Bolan GA; Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol 1994; 23:682–90. [DOI] [PubMed] [Google Scholar]
- 9. Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis 2002; 34:519–22. [DOI] [PubMed] [Google Scholar]
- 10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. . The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis 2006; 33:747–52. [DOI] [PubMed] [Google Scholar]
- 11. Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014; 41:369–76. [DOI] [PubMed] [Google Scholar]
- 12. Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect 2013; 89:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor SN. Editorial commentary: Trichomonas vaginalis testing and screening in a high-risk population: is this a glimpse into the future?Clin Infect Dis 2014; 59:842–4. [DOI] [PubMed] [Google Scholar]
- 14. Miller WC, Swygard H, Hobbs MM, et al. . The prevalence of trichomoniasis in young adults in the United States. Sex Transm Dis 2005; 32:593–8. [DOI] [PubMed] [Google Scholar]
- 15. Gaydos CA, Hsieh YH, Barnes M, et al. . Trichomonas vaginalis infection in women who submit self-obtained vaginal samples after internet recruitment. Sex Transm Dis 2011; 38:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaydos CA, Barnes MR, Quinn N, Jett-Goheen M, Hsieh YH. Trichomonas vaginalis infection in men who submit self-collected penile swabs after internet recruitment. Sex Transm Infect 2013; 89:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muzny CA, Blackburn RJ, Sinsky RJ, Austin EL, Schwebke JR. Added benefit of nucleic acid amplification testing for the diagnosis of Trichomonas vaginalis among men and women attending a sexually transmitted diseases clinic. Clin Infect Dis 2014; 59:834–41. [DOI] [PubMed] [Google Scholar]
- 18. Muzny CA, Burkholder GA, Fry KR, Austin EL, Schwebke JR. Trichomonas vaginalis nucleic acid amplification testing at an urban HIV clinic. Sex Transm Dis 2016; 43:483–8. [DOI] [PubMed] [Google Scholar]
- 19. Sutcliffe S, Newman SB, Hardick A, Gaydos CA. Prevalence and correlates of Trichomonas vaginalis infection among female US federal prison inmates. Sex Transm Dis 2010; 37:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller M, Liao Y, Gomez AM, Gaydos CA, D’Mellow D. Factors associated with the prevalence and incidence of Trichomonas vaginalis infection among African American women in New York City who use drugs. J Infect Dis 2008; 197:503–9. [DOI] [PubMed] [Google Scholar]
- 21. Hearn LE, Whitehead NE, Dunne EM, Latimer WW. Correlates of Trichomonas vaginalis among middle age and older adults who use drugs. Subst Use Misuse 2015; 50:1501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ginocchio CC, Chapin K, Smith JS, et al. . Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50:2601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaydos CA, Klausner JD, Pai NP, Kelly H, Coltart C, Peeling RW. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect 2017; 93:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) MEC laboratory procedures manual Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2013–2014/manuals/2013_mec_laboratory_procedures_manual.pdf. Accessed 29 August 2017.
- 25. Xu F, Sternberg MR, Kottiri BJ, et al. . Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 296:964–73. [DOI] [PubMed] [Google Scholar]
- 26. Field N, Clifton S, Alexander S, et al. . Trichomonas vaginalis infection is uncommon in the British general population: implications for clinical testing and public health screening. Sex Transm Infect 2016. doi:10.1136/sextrans-2016-052660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Price MA, Zimba D, Hoffman IF, et al. . Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex Transm Dis 2003; 30:516–22. [DOI] [PubMed] [Google Scholar]
- 28. Chernesky M, Jang D, Smieja M, et al. . Urinary meatal swabbing detects more men infected with Mycoplasma genitalium and four other sexually transmitted infections than first catch urine. Sex Transm Dis 2017; 44:489–91. [DOI] [PubMed] [Google Scholar]
- 29. Kraut-Becher J, Eisenberg M, Voytek C, Brown T, Metzger DS, Aral S. Examining racial disparities in HIV: lessons from sexually transmitted infections research. J Acquir Immune Defic Syndr 2008; 47(Suppl 1):S20–7. [DOI] [PubMed] [Google Scholar]
- 30. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017; 389:1453–63. [DOI] [PubMed] [Google Scholar]
- 31. Ford JL, Browning CR. Neighborhood social disorganization and the acquisition of trichomoniasis among young adults in the United States. Am J Public Health 2011; 101:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shew ML, Fortenberry JD, Tu W, et al. . Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med 2006; 160:151–6. [DOI] [PubMed] [Google Scholar]
- 33. Hoots BE, Peterman TA, Torrone EA, Weinstock H, Meites E, Bolan GA. A Trich-y question: should Trichomonas vaginalis infection be reportable?Sex Transm Dis 2013; 40:113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Sexual Health Association. Trichomoniasis Available at: http://www.ashasexualhealth.org/stdsstis/trichomoniasis. Accessed 13 September 2017.
- 35. Chesson HW, Blandford JM, Pinkerton SD. Estimates of the annual number and cost of new HIV infections among women attributable to trichomoniasis in the United States. Sex Transm Dis 2004; 31:547–51. [DOI] [PubMed] [Google Scholar]
- 36. Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, Lissouba P, Puren A, Auvert B. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sex Transm Infect 2009; 85:116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gray RH, Kigozi G, Serwadda D, et al. . The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2009; 200:42 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crosby RA, Charnigo RA, Weathers C, Caliendo AM, Shrier LA. Condom effectiveness against non-viral sexually transmitted infections: a prospective study using electronic daily diaries. Sex Transm Infect 2012; 88:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. García PJ, Holmes KK, Cárcamo CP, et al. ; Peru PREVEN Study Team Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community-randomised controlled trial. Lancet 2012; 379:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis A, Dasgupta A, Goddard-Eckrich D, El-Bassel N. Trichomonas vaginalis and human immunodeficiency virus coinfection among women under community supervision: a call for expanded T. vaginalis screening. Sex Transm Dis 2016; 43:617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.