Summary
Context
Unlike pituitary macroadenomas, microadenomas (micros) are not commonly associated with hypopituitarism. In clinical practice, we have observed that patients with ACTH-secreting micros have a higher than expected prevalence of central hypothyroidism (HT), and we speculated that this effect might be because of glucocorticoid-induced suppression of the hypothalamic–pituitary–thyroid axis.
Objective
To determine whether there is a difference in the prevalence of central HT among ACTH micros compared to other types of microadenoma, and if so, to investigate whether this is directly related to the degree of hypercortisolism.
Design, setting and patients
Retrospective study of 149 newly diagnosed patients with pituitary micros: 34 ACTH-secreting, 72 prolactin-secreting (PRLomas) and 43 clinically nonfunctioning adenomas (NFAs).
Main outcomes measures
Prevalence of central HT, correlation between normalized free T4 or TSH vs normalized urinary free cortisol (UFC) or salivary cortisol.
Results
The prevalence of central HT was significantly higher in the ACTH compared to the non-ACTH adenomas: 18% (ACTH), 1% (PRL) and 0% (NFAs). The mean normalized free T4 was lower in the ACTH micros compared to the non-ACTH micros (1·29 ± 0·06 vs 1·50 ± 0·23, P = 0·0001). There was no correlation between the degree of hypercortisolism, as reflected by 24-h urine free cortisol and salivary cortisol, and free T4 or TSH levels among the ACTH adenomas. Similarly, there were no differences in mean UFC or salivary cortisol between ACTH adenomas with and without central HT. Following transsphenoidal adenomectomy, central HT recovered in three of six patients with ACTH micros.
Conclusions
These findings suggest that patients with ACTH-secreting microadenomas should always, at a minimum, undergo testing for central HT. However, given the potential for recovery of thyroid function following cure of Cushing disease, we recommend withholding thyroid hormone replacement until after pituitary surgery.
Introduction
Pituitary macroadenomas (macros) may result in varying degrees of hypopituitarism, while limited observational studies and clinical experience suggest that microadenomas (micros) are unlikely to compromise pituitary function.1 Raised intrasellar pressure causes hypopituitarism likely by compressing the portal blood supply and interrupting delivery of hypothalamic hormones to the anterior pituitary.2 With respect to pituitary micros, there are no specific data regarding the prevalence of hypopituitarism as it could occur along a continuum of tumour size (i.e. smaller vs larger micros). As larger micros may resemble macros in their ability to compromise anterior pituitary function, recent Endocrine Society guidelines recommend testing for hypopituitarism in all patients with an incidentally discovered pituitary adenoma, regardless of size.1 Based on combined data of micros and macros (weighted heavily towards macros), when hypopituitarism does occur, GH and gonadotrophin deficiency are the most likely to be present, followed by ACTH and TSH deficiency.3–6
We have noted that among our patients with micros, those with ACTH-secreting adenomas seem to have a higher prevalence of central hypothyroidism (HT) compared to other micro types (both hormone-secreting and clinically nonfunctioning) of comparable size. We speculated that this might be related to the effects of hypercortisolism on the hypothalamic–pituitary–thyroid axis. The goal of our study was to determine whether there was a difference in the prevalence of central HT among ACTH micros compared to other types of microadenoma, and if so, to investigate whether this was directly related to the degree of hypercortisolism. As there are limited data regarding the prevalence of hypopituitarism specifically among micros, a secondary goal of our study was to report our experience on anterior pituitary function (albeit limited to central HT) in this patient population.
Patients and methods
With approval from our Institution’s Review Board, we performed a retrospective electronic chart review of 149 newly diagnosed patients with pituitary micros. There were 34 ACTH-secreting (ACTHomas), 72 prolactin-secreting (PRLomas) and 43 clinically nonfunctioning adenomas (NFAs). The patients with ACTHomas were seen by two of the authors (R.S. and G.S. W.) in their endocrinology clinical practices over a 12-year period (2000–2012). Because of the higher prevalence of PRLomas and NFAs, these patients were selected sequentially from the practice of one author (R.S.) from 2008 to 2012. With the exception of the patients’ self-reported heights, all of the data were collected from the electronic medical records and patient charts. For calculations involving tumour size, only those tumours that had specific maximal dimensions on the radiology report were included.
Given that surgery itself poses a risk of hypopituitarism, we reviewed only the pretreatment biochemical evaluation. The majority of PRLomas were treated with dopamine agonists, most NFAs were followed over time and required no intervention and all ACTHomas were treated surgically. As most GH-secreting tumours are macros at presentation, we did not have a sufficient number of acromegalic patients with micros to include in our study. Given the aim of our study, we did not evaluate TSH-secreting adenomas.
For all patients with micros, we identified those who fulfilled the criteria for a diagnosis of central HT prior to treatment: a low free T4 in conjunction with a low or inappropriately normal TSH level. We excluded those patients with an antecedent diagnosis of primary HT or those NFAs and PRLomas with a history of glucocorticoid use within 3 months prior to initial endocrine evaluation. While most patients had concurrent free T4 and TSH values, we included subjects who had only a free T4 value as long as it was normal, as this was considered sufficient to exclude central HT. The proportion of patients who had free T4 or simultaneous free T4 and TSH that were drawn at our institution was: 51% and 44% for NFAs, 56% and 50% for PRLomas and 42% and 39% for ACTHomas, respectively. Between 2000 and 2004 in our hospital, free T4 was measured using the AIA-PACK FT4 assay, and since 2005, the ST AIA-PACK FT4 assay has been used (both assays from Tosoh Bioscience, San Francisco, CA, USA). From 2000 to 2004, the Tosoh Bioscience AIA-PACK TSH third-generation assay was used in our institution, and since 2005, the ST AIA-PACK TSH assay was used. The only difference between the AIA and ST AIA assays is a shorter assay time for the latter, with nearly identical assay results (r2 = 0·9954 for ST AIA-PACK vs AIA-PACK FT4 and r2 = 0·9983 ST AIA-PACK TSH vs AIA-PACK TSH third generation). The assay sensitivities for ST AIA-PACK FT4 and ST AIA-PACK TSH are 1·3 pM and 0·01 μIU/l, respectively. For ST AIA-PACK FT4, the coefficient of variation for inter- and intra-assay runs at concentration of 7·2 pM is 4·9% and 4·0%, respectively. For ST AIA-PACK TSH, the coefficient of variation for inter- and intra-assay runs at a concentration of 2·19 μIU/l are 5·0% and 2·9%, respectively. Among our patients, 29% of the ACTHoma patients had free T4 and/or TSH obtained before 2005 (using AIA-PACK assays) and 71% after 2004 (using ST AIA-PACK assays). Although various commercial assays were used for free T4, the lower limit of normal was defined to be 9·0 pM for 92% of cases.
To test the hypothesis that hypercortisolism rather than tumoural mass effect was contributing to central HT, we divided the patients into two groups: ACTH-secreting and non-ACTH-secreting, with the latter group consisting of the PRLomas and NFAs. We then sought to test whether the degree of hypercortisolaemia in ACTH adenomas (as reflected by urinary free cortisol (UFC) and salivary cortisol measurements) was different between those patients with and without central HT. Various commercial assays were used in this study for UFC and salivary cortisol measurements. The most commonly used commercial UFC assay utilized high-performance liquid chromatography from 2000 to 2005, and after 2005, has utilized liquid chromatography/mass spectrometry. To overcome the issue of interlaboratory variability, we normalized the salivary cortisol and UFC values by dividing them by the upper limit of the reference range provided by the performing laboratory. Similarly, we normalized the free T4 by dividing the values by the lower limit of reference range, and the TSH by dividing the values by the upper limit of reference range. Using this approach, none of the normalized free T4 values <1 (indicative of HT) corresponded to normalized TSH values >1 (indicative of adequate TSH response), and therefore, all were consistent with central HT.
As cortisol excess can also lead to gonadotrophin deficiency, we determined the prevalence of amenorrhoea/oligomenorrhoea in premenopausal women, excluding those with a history of oophorectomy or hysterectomy. We did not have a sufficient number of pretreatment IGF-1 levels in our Cushing disease cohort to study the relationship between hypercortisolism and GH deficiency.
Statistical analysis
GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA, USA) was employed to perform statistical analysis. T-test was used to compare means between the groups, χ2-test (Fisher’s exact test, two-sided) was used for comparison of proportions and linear regression was used to correlate continuous variables. Data are expressed as means ± SEM.
Results
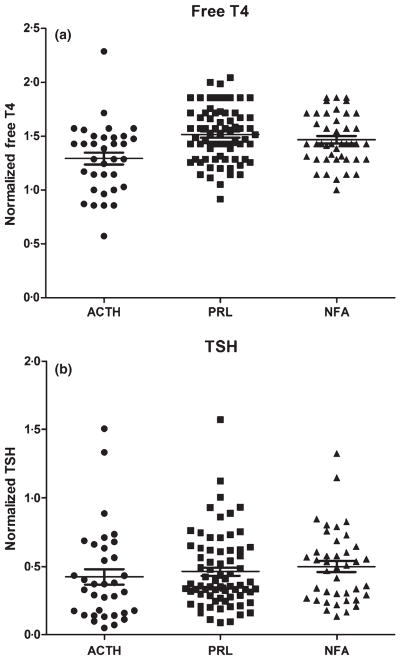
Consistent with the female predominance in Cushing disease, we observed a significantly higher proportion of women in the ACTH group compared to the non-ACTH group, with a similar age distribution between groups (Table 1). There was no difference in mean tumour diameter in the ACTH vs non-ACTH groups. The mean normalized free T4 was significantly lower in the ACTH vs non-ACTH group. However, there was no difference in mean normalized TSH between the groups (Fig. 1). Central HT was present in six of 34 ACTH adenomas (18%), one of 72 PRLomas (1%) and 0 of 43 NFAs (0%). This corresponded to an odds ratio of 11·30 (2·169–58·87, 95% confidence interval) of central HT in ACTH adenomas compared to non-ACTH adenomas. The PRLomas and NFAs did not differ with respect to patient demographics, tumour size and results of biochemical testing for HT (Table 1).
Table 1.
Comparison of patient demographics, tumour size and baseline biochemical evaluation of thyroid function in adenomas according to type
|
P-value
|
|||||||
|---|---|---|---|---|---|---|---|
| All adenomas (n = 149) | ACTH adenomas (n = 34) | Non-ACTH adenomas (n = 115) | PRLomas (n = 72) | NFAs (n = 43) | ACTH vs Non-ACTH | PRL vs NFA | |
| No. female/male (%) | 120/26 (81/19) | 33/1 (97/3) | 87/25 (76/24) | 59/13 (82/18) | 28/12 (65/35) | 0·009 | 0·16 |
| Mean age (years) | 35·6 ± 1·2 | 38·4 ± 2·2 | 34·8 ± 1·3 | 33·7 ± 1·4 | 36·5 ± 2·6 | 0·18 | 0·30 |
| Maximal tumour diameter (mm) | 5·26 ± 0·18 (n = 123) | 5·82 ± 0·39 (n = 28) | 5·11 ± 0·20 (n = 95) | 5·18 ± 0·24 (n = 62) | 4·98 ± 0·33 (n = 33) | 0·09 | 0·63 |
| Normalized free T4 | 1·45 ± 0·02 (n = 149) | 1·29 ± 0·06 (n = 34) | 1·50 ± 0·23 (n = 115) | 1·52 ± 0·03 (n = 72) | 1·47 ± 0·04 (n = 43) | 0·0001 | 0·30 |
| Normalized TSH | 0·46 ± 0·02 (n = 145) | 0·42 ± 0·06 (n = 34) | 0·47 ± 0·03 (n = 111) | 0·46 ± 0·03 (n = 71) | 0·50 ± 0·04 (n = 40) | 0·36 | 0·47 |
PRLomas, prolactinomas; NFA, nonfunctioning adenomas. Bold font indicates statistically significant P-values.
Fig. 1.
(a) Normalized free T4 values (Free T4/lower limit of reference range for assay) according to adenoma type. (b) Normalized TSH values (TSH/upper limit of reference range for assay).
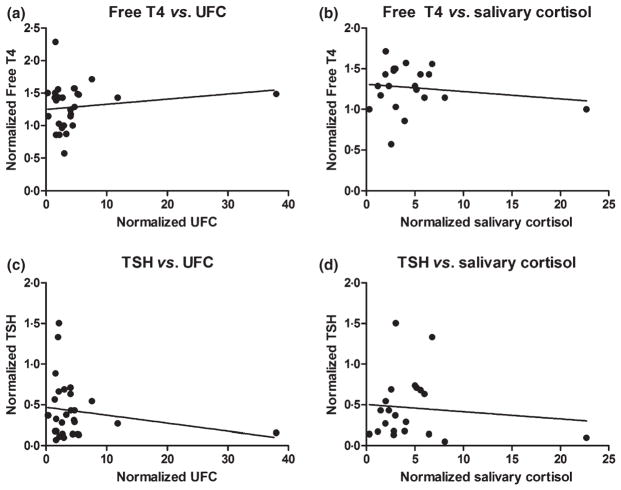
Among the ACTH adenoma patients, those with central HT were younger in age, but otherwise had similar BMI and tumour size (Table 2). The mean normalized free T4 was significantly lower in the group with central HT compared to those without. We did not find a direct correlation between measures of hyper-cortisolism (normalized UFC and salivary cortisol) and either normalized free T4 or TSH (Fig. 2).
Table 2.
Patient demographics, tumour characteristics and biochemical profile of ACTHomas
| ACTH adenomas (n = 34)
|
|||
|---|---|---|---|
| With central HT (n = 6) | Without central HT (n = 28) | P-value (with vs without central HT) | |
| No. female/male (%) | 6/0 (100/0) | 27/1 (96/4) | 1·0 |
| Mean age (years) | 28·8 ± 4·5 | 40·4 ± 2·2 | 0·04 |
| BMI (kg/m2) | 42·7 ± 6·1 (n = 5) | 37·3 ± 2·6 (n = 19) | 0·38 |
| Prevalence of amenorrhoea (n = premenopausal women) | 100% (n = 5) | 78% (n = 18) | 0·54 |
| Maximal tumour diameter (mm) | 5·83 ± 0·95 (n = 6) | 5·81 ± 0·44 (n = 22) | 0·98 |
| Normalized free T4 | 0·83 ± 0·05 (n = 6) | 1·39 ± 0·04 (n = 28) | <0·0001 |
| Normalized TSH | 0·28 ± 0·09 (n = 6) | 0·45 ± 0·07 (n = 28) | 0·28 |
| Normalized UFC | 2·42 ± 0·28 (n = 6) | 5·10 ± 1·59 (n = 23) | 0·40 |
| Normalized salivary cortisol | 3·45 ± 0·67 (n = 5) | 5·01 ± 1·30 (n = 16) | 0·52 |
HT, hypothyroidism; UFC, urinary free cortisol. Bold font indicates statistically significant P values.
Fig. 2.
Correlation between (a) normalized free T4 vs normalized urinary free cortisol (UFC), n = 29, r2 = 0·02, P = 0·42. (b) Normalized free T4 vs salivary cortisol, n = 21, r2 = 0·02, P = 0·51. (c) Normalized TSH vs normalized UFC, n = 29, r2 = 0·04, P = 0·32. (d) Normalized TSH vs normalized salivary cortisol, n = 21, r2 = 0·01, P = 0·65.
Recovery of thyroid function following transsphenoidal surgery was seen in three of the six Cushing disease patients with baseline central HT. The time to recovery was documented to be 2 weeks for two of the patients. One individual was lost to follow-up and returned to clinic over a year later. At that time, she had been off levothyroxine for several months and had documented normal free T4 and TSH levels. The one PRLoma patient with central HT was unfortunately lost to follow-up after starting levothyroxine and cabergoline, so his long-term thyroid status could not be determined. The three patients who had recovery of thyroid function were also deemed to be in remission from Cushing disease at the time of their evaluation. For the three patients who had persistent central HT following surgery, one was deemed to be in remission of Cushing disease, while the other two had persistent hypercortisolism requiring additional treatment (pituitary irradiation for both and bilateral adrenalectomy for one).
Discussion
We found that ACTH micros are associated with significantly lower mean free T4 levels and a higher prevalence of central HT (18%) compared to other types of microadenoma (<1%). This effect does not appear to be mediated by tumour compression on the normal pituitary, as there was no difference in tumour size between the ACTH and non-ACTH groups, nor was there any difference in tumour size in the ACTH group with central HT compared to the group with normal thyroid function. However, our data do not support our hypothesis that hypercortisolism is the mechanism behind central HT in these patients, as we found no correlation between free T4 or TSH levels and the degree of hypercortisolism as measured by UFC and salivary cortisol.
Glucocorticoids have long been known to alter thyroid function at the level of the hypothalamus and pituitary.7–9 In healthy subjects, administration of high doses of glucocorticoids leads to a 50% decrease in serum TSH levels, 19% decrease in serum total T3 levels, but no significant changes in serum free T4 levels.10 The disparate effect on serum T3 and T4 levels is believed to be because of glucocorticoid-mediated changes in peripheral thyroid hormone metabolism, specifically increased reverse T3 production because of type 1 deiodinase inhibition in the liver.10,11 It has also been shown that exogenous glucocorticoids alter hypothalamic control by reducing mRNA expression of thyrotropin releasing hormone (TRH).12 In vitro studies have recently identified a role for annexin 1, a protein produced by the folliculostellate cell of the pituitary, which is believed to play a paracrine role in the modulation of TSH and other anterior pituitary hormones.13
In individuals with Cushing syndrome, cortisol excess results in decreased 24-h secretion of TSH because of diminished pulsatility of the hormone, an effect which can be reversed after surgical correction of the source of hypercortisolism (pituitary or adrenal adenomectomy).13 In agreement with our study, Roelfsema et al.13 showed that free T4 levels were reduced specifically in patients with pituitary-dependent Cushing syndrome compared to controls, but not in adrenal Cushing. Transsphenoidal adenomectomy has been shown to restore the dynamic secretory patterns of ACTH, cortisol, TSH, GH and PRL.14,15 Indeed, in our study, half of the Cushing disease patients with central HT had recovery of thyroid function following curative transsphenoidal surgery. One of the three patients with persistent postoperative central HT was actually deemed to be in remission from Cushing disease, raising the possibility of surgically induced HT in her case.
In light of the above-described relationship between hypercortisolism and thyroid function, we were surprised by the lack of correlation between the extent of hypercortisolism and baseline free T4 levels, or the diagnosis of central HT. As ‘cortisol burden’ is a combination of degree and duration of hypercortisolism, it is possible that we failed to identify a correlation between central HT and hypercortisolism because the impact of disease duration on cumulative cortisol exposure cannot be readily determined. It is also possible that the strict definition of central HT (low free T4 and inappropriately normal or low TSH) may underestimate the prevalence of mild central HT in all the adenomas, as we do not know whether a TSH level is appropriate for patients whose free T4 levels are at the low-normal end (at or around the cut-off of normal). Given the logarithmic relationship between TSH and free T4, a normal TSH in the face of low-normal free T4 levels may actually represent a deficient pituitary response for some individuals. Such cases would only be recognizable with a TRH stimulation test. It is also conceivable that the local effect of high ACTH may influence the synthesis or secretion of TSH, independent of the serum cortisol level.
An alternative explanation is that the impaired thyroid function in Cushing disease may simply reflect a sick euthyroid syndrome status. It has been shown that patients with Cushing disease have higher levels of intrapituitary cytokines, which are known to modulate both the adrenal and thyroid axes.16,17 Despite the immunosuppressive effects of cortisol, Cushing syndrome is considered a proinflammatory state, and it is plausible that the pattern of thyroid function tests seen in Cushing disease individuals may indicate an adaptive response to chronic illness rather than true TSH deficiency.17,18
In addition to the suppressive effects on TSH secretion, gluco-corticoids negatively affect gonadotrophins, GH, PRL and vasopressin.19 The high prevalence of amenorrhoea/oligomenorrhoea in our ACTHoma patients suggests the presence of gonadotrophin deficiency in these individuals.19 Although an inverse relationship between degree of hypercortisolism and GH secretion has been observed in patients with Cushing disease, GH secretion is usually preserved except in cases of severe hypercortisolism.20 Differences between GH and PRL secretory dynamics were not observed when comparing patients with ACTH micros vs macros, and it has been speculated that the negative suppressive effects of cortisol or an intrapituitary paracrine mechanism may be the cause of impaired GH/PRL secretion.20 Given the retrospective nature of our study, we were unable to study the impact of hypercortisolism on GH and PRL.
Interestingly, the only tumour associated with central HT in the non-ACTH group was a very small (2 mm) PRLoma. None of the NFAs, including those >6 mm, were associated with central HT. This observation is in contrast to a study that showed a prevalence of hypopituitarism in 50% of 38 nonfunctioning micros, of which 39% had central HT.4 In this study, the mean tumour size of the NFAs was similar to ours (4·2 vs 5·0 mm), excluding size as the reason for the discrepancy.4 Our findings are in agreement with the data of Orija et al.,21 who implied that the prevalence of partial hypopituitarism in over 100 nonfunctioning micros was low. Clearly, further prospective studies are needed to establish the prevalence of hypopituitarism in micros.16
There are several limitations to our study. First, given the rarity of Cushing disease, we had a relatively small sample size of ACTH micros, which may, just by chance, overestimate the prevalence of central HT in this population. As a retrospective study, there is an obvious selection bias in that only patients for whom biochemical testing was available were included. As Cushing’s syndrome can be diagnosed on the basis of two of three diagnostic tests, we did not have UFC and salivary cortisol values for all patients. Also, our study was limited to PRLomas, ACTHomas and NFAs, so the results may not be applicable to GH-secreting micros.
In conclusion, while currently there is a lack of clear evidence to support testing for hypopituitarism in microadenomas, our study suggests that patients with ACTH-secreting micros may be at higher risk for central hypothyroidism than other micros. Until larger, more rigorous prospective studies determine whether a size threshold should guide testing for hypopituitarism, we suggest that screening for central hypothyroidism be considered in all patients with ACTH micros at the time of diagnosis. However, as the pattern of thyroid dysfunction seen in Cushing disease may actually reflect a sick euthyroid state that may normalize after hypercortisolism is resolved, we recommend withholding thyroid hormone replacement until after pituitary surgery.
Footnotes
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism. 1996;96:894–904. doi: 10.1210/jc.2010-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arafah BM, Prunty D, Ybarra J, et al. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. Journal of Clinical Endocrinology & Metabolism. 2000;85:1789–1793. doi: 10.1210/jcem.85.5.6611. [DOI] [PubMed] [Google Scholar]
- 3.Cury ML, Fernandes JC, Machado HR, et al. Non-functioning pituitary adenomas: clinical feature, laboratorial and imaging assessment, therapeutic management and outcome. Arquivos Brasileiros de Endocrinologia e Metabologia. 2009;53:31–39. doi: 10.1590/s0004-27302009000100006. [DOI] [PubMed] [Google Scholar]
- 4.Yuen KC, Cook DM, Sahasranam P, et al. Prevalence of GH and other anterior pituitary hormone deficiencies in adults with nonsecreting pituitary microadenomas and normal serum IGF-1 levels. Clinical Endocrinology. 2008;69:292–298. doi: 10.1111/j.1365-2265.2008.03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante E, Ferraroni M, Castrignano T, et al. Nonfunctioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. European Journal of Endocrinology. 2006;155:823–829. doi: 10.1530/eje.1.02298. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostis P, Adamidou F, Polyzos SA, et al. Pituitary incidentalomas: a single-centre experience. International Journal of Clinical Practice. 2011;65:172–177. doi: 10.1111/j.1742-1241.2010.02537.x. [DOI] [PubMed] [Google Scholar]
- 7.Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Practice & Research: Clinical Endocrinology & Metabolism. 2009;23:793–800. doi: 10.1016/j.beem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoloff JT, Fisher DA, Appleman MD., Jr The role of glucocorticoids in the regulation of thyroid function in man. The Journal of Clinical Investigation. 1970;49:1922–1929. doi: 10.1172/JCI106411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. The Journal of Clinical Investigation. 1969;48:2096–2103. doi: 10.1172/JCI106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuels MH, McDaniel PA. Thyrotropin levels during hydrocortisone infusions that mimic fasting-induced cortisol elevations: a clinical research center study. The Journal of Clinical Endocrinology and Metabolism. 1997;82:3700–3704. doi: 10.1210/jcem.82.11.4376. [DOI] [PubMed] [Google Scholar]
- 11.Chopra IJ, Williams DE, Orgiazzi J, et al. Opposite effects of dexamethasone on serum concentrations of 3,3′,5′-tri-iodothyronine (reverse T3) and 3,3′,5-triiodothyronine (T3) Journal of Clinical Endocrinology & Metabolism. 1975;41:911–920. doi: 10.1210/jcem-41-5-911. [DOI] [PubMed] [Google Scholar]
- 12.Alkemade A, Unmehopa UA, Wiersinga WM, et al. Glucocorticoids decrease thyrotropin-releasing hormone messenger ribonucleic acid expression in the paraventricular nucleus of the human hypothalamus. Journal of Clinical Endocrinology & Metabolism. 2005;90:323–327. doi: 10.1210/jc.2004-1430. [DOI] [PubMed] [Google Scholar]
- 13.Roelfsema F, Pereira AM, Biermasz NR, et al. Diminished and irregular TSH secretion with delayed acrophase in patients with Cushing’s syndrome. European Journal of Endocrinology. 2009;161:695–703. doi: 10.1530/EJE-09-0580. [DOI] [PubMed] [Google Scholar]
- 14.Veldman RG, Frolich M, Pincus SM, et al. Apparently complete restoration of normal daily adrenocorticotropin, cortisol, growth hormone, and prolactin secretory dynamics in adults with Cushing disease after clinically successful transsphenoidal adenomectomy. Journal of Clinical Endocrinology & Metabolism. 2000a;85:4039–4046. doi: 10.1210/jcem.85.11.6967. [DOI] [PubMed] [Google Scholar]
- 15.Kuwayama A, Kageyama N, Nakane T, et al. Anterior pituitary function after transsphenoidal selective adenomectomy in patients with Cushing disease. Journal of Clinical Endocrinology & Metabolism. 1981;53:165–173. doi: 10.1210/jcem-53-1-165. [DOI] [PubMed] [Google Scholar]
- 16.Paoletta A, Arnaldi G, Papa R, et al. Intrapituitary cytokines in Cushing disease: do they play a role? Pituitary. 2011;14:236–241. doi: 10.1007/s11102-010-0285-9. [DOI] [PubMed] [Google Scholar]
- 17.Pappa TA, Vagenakis AG, Alevizaki M. The non-thyroidal illness syndrome in the non-critically ill patient. Euro-pean Journal of Clinical Investigation. 2011;41:212–220. doi: 10.1111/j.1365-2362.2010.02395.x. [DOI] [PubMed] [Google Scholar]
- 18.Kristo C, Godang K, Ueland T, et al. Raised serum levels of interleukin-8 and interleukin-18 in relation to bone metabolism in endogenous Cushing’s syndrome. European Journal of Endocrinology. 2002;146:389–395. doi: 10.1530/eje.0.1460389. [DOI] [PubMed] [Google Scholar]
- 19.Carlson HE. Drugs and pituitary function. In: Melmed S, editor. Pituitary. Elsevier Inc; Oxford: 2011. pp. 413–430.pp. 421 [Google Scholar]
- 20.Veldman RG, Frolich M, Pincus SM, et al. Growth hormone and prolactin are secreted more irregularly in patients with Cushing disease. Clinical Endocrinology. 2000b;52:625–632. doi: 10.1046/j.1365-2265.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 21.Orija IB, Weil RJ, Hamrahian AH. Pituitary incidentaloma. Best Practice & Research: Clinical Endocrinology & Metabolism. 2012;26:47–68. doi: 10.1016/j.beem.2011.07.003. [DOI] [PubMed] [Google Scholar]