Abstract
Sugar-sweetened beverage (SSB) consumption is a risk factor for childhood obesity. Including this measure in electronic health records (EHR) could enhance clinical care and facilitate research on this topic. We implemented a single-item, EHR screening question for SSB and 100% fruit juice at 8 pediatric practices affiliated with a North Carolina academic medical center. From March–December 2017, we evaluated SSB screening of children 6 months–17 years of age. In a sub-sample of screened patients, we also conducted a telephone-based validation survey, comparing EHR-based responses to a lengthier beverage questionnaire, using Spearman rank coefficients and Kappa statistic. 22,626 children (91% of all seen) were screened for SSB intake. The screened population was diverse – 35% non-Hispanic White, 26% African-American, and 30% Hispanic. Consistent with national estimates, reported intake was typically higher than recommended: 41% (n = 9220) reported consuming SSB or fruit juice >1×/day in the past month, and consumption was higher among race/ethnic minorities. Of 201 validation survey respondents, direct correlation between their beverage survey and EHR screener responses was moderate, with a Spearman's rank correlation coefficient of 0.41 (p < 0.001) and Kappa statistic of 0.42 (95% CI 0.24–0.60). EHR-based screening for SSBs and fruit juice was successfully implemented, generating a large volume of SSB consumption data in a diverse patient population. Inclusion of patient-reported dietary measures in the EHR is feasible and could be useful for clinical care and research. Planned modifications may improve the correlation of such a screener with lengthier dietary instruments.
Keywords: Electronic health record, Childhood obesity, Sugar-sweetened beverages
1. Background/introduction
Sugar-sweetened beverage (SSB) consumption is a modifiable risk factor for childhood obesity, (de Ruyter et al., 2012; Ebbeling et al., 2012; Malik et al., 2013; Dong et al., 2015; Hu, 2013; Trumbo and Rivers, 2014; Millar et al., 2014; Pan et al., 2014) and has been linked to adverse health outcomes such as cardiometabolic disease, in both children and adults.(Ambrosini et al., 2013; Loh et al., 2017; Van Rompay et al., 2015; Kosova et al., 2013; Malik et al., 2014; Stelmach-Mardas and Walkowiak, 2016; Campos et al., 2015; Berentzen et al., 2015) Even overconsumption of 100% fruit juice in children is not benign – it is associated with subsequent greater SSB intake and adiposity.(Shefferly et al., 2015; Sonneville et al., 2015) Consumption of SSBs in the United States has declined in recent years; however, intake still exceeds recommended levels.(Kit et al., 2013; Ford et al., 2016; Briefel et al., 2015; Barlow and Expert, 2007) Furthermore, over-consumption and early initiation of SSBs are especially common among lower-income and racial/ethnic minority children.(Richmond et al., 2013; Dodd et al., 2013; Han and Powell, 2013; Taveras et al., 2013)
Clinical guidelines set strict limits on children's daily intake of sweet drinks: only small amounts of 100% fruit juice, with general avoidance of all SSBs.(Heyman and Abrams, 2017; Vos et al., 2017) Guidelines also universally recommend that medical providers ask about and counsel on SSB intake as part of a comprehensive approach to pediatric obesity prevention and treatment.(Davis et al., 2007; Krebs et al., 2007; Spear et al., 2007) Unfortunately, this information is not easily gathered in systematic ways that can be tracked over time. Providers may not consistently adhere to SSB screening guidelines due to lack of prompts to screen. Even practices that do routinely screen for SSB consumption often rely on paper questionnaires or free text in progress notes to document patient responses, rendering SSB data unusable for population management, easy historical reference on an individual patient, or research purposes. There exists an increasing emphasis on electronic health record (EHR) capture of social and behavioral determinants of health.(Adler and Stead, 2015) Yet, the handling of dietary behavior information differs from other elements of the patient chart, such as weight, allergies, or medications, all of which can be easily searched or filtered in the EHR.
To increase the routine and standardized screening of children for SSB intake, and enable health systems to track such intake in their patient populations, we created and implemented a single-item, point-of-care SSB and fruit juice screening question to be administered using the EHR. The aims of this study were two-fold: (1) To understand the feasibility of implementing an EHR-based SSB screener in busy clinical practices, using measures of screening rates over time, and examining encounter and patient-level predictors of whether or not screening took place, and (2) To test the validity of self-reported SSB consumption data recorded in the EHR using our screener. To accomplish the second aim, we surveyed a subsample of the EHR-screened population, using a lengthier beverage questionnaire that included items from the 2013–2014 National Health and Nutrition Examination Survey (NHANES) and compared these responses to their EHR-documented SSB consumption.
2. Methods
2.1. Setting
We worked with the informatics team at Wake Forest Baptist Medical Center in North Carolina, to implement an EHR-based SSB screening question in 8 affiliated primary care pediatric (n = 7) or family medicine (n = 1) practices. Practices ranged in size from 2 to 5 physicians, and served a diverse group of patients in both rural and urban locations. All practices used the same, enterprise-wide EHR platform (Epic®), for documentation and clinical care. The institutional IRB approved the project.
2.2. Patient population
Patients 6 months through 17 years of age were eligible to be screened for SSB intake at any in-person clinic visit beginning March 20, 2017. Data for this analysis spans the 56,211 clinical encounters that occurred through December 20, 2017.
2.3. Intervention
We created a screening question intended to estimate a child's recent frequency of consumption, similar to beverage items in the 2013–14 NHANES dietary screener. However, to enhance screening rates in a busy clinical setting, we combined all SSB types and fruit juice (FJ) into a single item. We also chose to include flavored/sweetened milks as an SSB example.(Afeiche et al., 2018) A time frame of “the past month” was specified because clinical staff administered the screening question during all encounter types. In the case of urgent/sick visits, the days immediately prior would not reflect typical dietary behavior.
The final screener read, “In the past month, how often did (child's name) drink a sugar-sweetened beverage or 100% fruit juice? Sugar-sweetened beverages include things like fruit-flavored drinks, juice from concentrate, punch, Kool Aid, soda, sports drinks, sweet tea or flavored milks.” Respondents selected one of the following categorical response options: “Never”, “Once per week or less”, “Several per week but not every day”, “1 per day”, “2 per day”, “3 per day”, “4 or more per day” or “Refused”.
Medical assistants and nursing staff were prompted to conduct SSB screening while “rooming” patients. Upon electronically entering vital signs for an eligible patient, the SSB question automatically appeared on their computer monitor, formatted as a yellow “Best Practice Alert” (BPA) box. Response choices appeared as click boxes within the BPA. Staff were instructed read the question aloud to parents or caregivers for children ≤12 years of age, and to the patient directly for 13–17 year-olds. For Spanish-speaking patients/families, a Spanish-language paper version of the question was provided (Supplement).
If a screening response indicated more SSB/FJ consumption than recommended for a patient's age, an educational paragraph automatically inserted in the after-visit-summary (AVS) document provided following the clinic encounter (Supplement). For infants under 12 months of age, the AVS paragraph on sugary drinks appeared if any SSB/FJ intake was reported. For children 1 year and older, the paragraph was included only if frequency exceeded 1 per day. The paragraph differed based on the age group of the child and appeared in English or Spanish depending on the language preference recorded in the EHR.
Because SSB/FJ intake may change over time, the screening BPA repeated at 90-day intervals for each child to capture longitudinal information. Capturing change over time is important because SSB consumption becomes more frequent as children age, (Han and Powell, 2013) and because individual-level trends over time may help to identify patients in need of a more dedicated intervention to reduce consumption. The 90-day minimum for repeat screening was chosen to prevent excessive screening burden among children who visit the pediatrician frequently for acute or chronic illness.
2.4. Staff training, monitoring and incentives
Prior to activating the SSB screener in the EHR, our research team conducted on-site trainings for clinical office staff. The trainings included education about the health importance of SSBs and details regarding screening implementation. We reviewed a suggested workflow for the screener, instructing staff to read the exact wording of the question as provided in the BPA, and to read answer choices aloud to respondents. Physicians were welcomed, but not required, to attend these trainings.
Research staff followed up with brief weekly visits at each practice for the first month of SSB screening, and monthly thereafter. At monthly visits, we provided practice managers with a personalized report detailing their site's screening performance. To avoid penalizing lower volume clinics, we measured site performance using the percent of eligible encounters in compliance with screening. On a quarterly basis, all staff members at locations where screening compliance averaged ≥70% (project benchmark for implementation) received $10 gift cards.
2.5. EHR data extraction
We extracted EHR data weekly on all pediatric encounters at participating practices. Data elements included patient demographics (age, sex, race/ethnicity, language), encounter information (type, location, date), and responses to SSB screening.
2.6. Validation survey
We conducted a telephone validation survey on a stratified random sample of 201 patients (n = 50 infants, n = 101 1–12 year olds, n = 50 13–17 year olds) who underwent EHR-based screening. The survey served 2 main purposes: (de Ruyter et al., 2012) to collect a timely, validated measure of child SSB consumption against which our EHR-based measure could be compared, and (Ebbeling et al., 2012) to assess patient and parental impressions of the EHR-based SSB screening at the recent pediatric visit.
We conducted telephone surveys in English or Spanish within one week of the clinical encounter. For children ≤12 years, only the caregiver present at the recent visit responded to the survey; teenagers responded to questions along with their parent/caregiver. We obtained verbal consent from parents/caregivers, with assent from teens. The survey lasted approximately 10 min and included questions about recall of and comfort with SSB screening at the pediatrician's office, recall of receiving information about SSBs in the AVS document, assessment of whether SSB intake changed as a result of screening/AVS, and detailed questions about the patient's beverage consumption, from the 2013–14 NHANES questionnaire. We separately assessed unflavored and flavored milk, soda, juice, coffee/tea, sweetened fruit drinks, and sport/energy drinks. Demographic questions included child race/ethnicity, parental educational level, and household structure.
2.7. Analysis
2.7.1. SSB screening & implementation data
We created patient and encounter-level summary statistics to describe encounters compliant with and not compliant with screening. Since one child could have multiple encounters, we used marginal models incorporating generalized estimating equations to examine for differences between the two groups of encounters (compliant vs. not). Among patients screened at least once, we described SSB/FJ intake and examined characteristics of higher versus lower consumers, using Chi Square testing.
2.7.2. Validation study
Response types to the EHR screener (categorical) and NHANES questions (continuous) differed. To allow for comparison between measures, we transformed the response data. For NHANES items, we created a summary variable for total SSB/FJ intake by converting all measures to servings/day and summing responses to the 5 component sugary drink/juice questions. This resulted in a continuous variable ranging from 0 to a maximum of 7.2 per day. For the EHR-based SSB/FJ screener, we converted responses to numeric values to represent daily intake as follows: “Never” or “less than 1/week” = 0, “Several/Week” = 0.5, “1 per day” = 1, “2 per day” = 2, “3 per day” = 3 and “4 or more per day” = 4.
We then compared transformed survey respondent values from NHANES questions and the EHR screener using a paired t-test. We also calculated a Spearman's rank correlation coefficient for the measures overall and by parental educational status. We calculated a Kappa statistic for agreement by dichotomizing source data for both the EHR SSB screener and the NHANES questions around “Never” (or zero) versus any intake. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Encounter-level findings & screening compliance
Of 56,211 patient encounters in 9 months, 49,104 (87%) included up-to-date SSB screening responses. Encounters where screening was missed (non-compliant) had a higher proportion of Spanish speakers (28.0% vs. 19.5%) (Table 1). Non-compliant encounters also had a much higher proportion of nurse/injection visits (22.2% vs. 5.0%).
Table 1.
Encounter-level characteristics and SSB screening compliance for 56,211 patient encountersa.
| Variable | Compliant encounters (n = 49,104/87%) | Non-compliant encounters (n = 7107/13%) |
p-Valueb |
|---|---|---|---|
| Age group | |||
| 6 m – <1 yr | 3943 (8.0%) | 632 (8.9%) |
0.01 |
| 1–4 yr | 15,160 (30.9%) | 2050 (28.8%) | |
| 5–9 yr | 12,912 (26.3%) | 1961 (27.6%) | |
| 10–12 yr | 7344 (15.0%) | 1073 (15.1%) | |
| 13–17 yr | 9745 (19.9%) | 1391 (19.6%) | |
| Sex | |||
| Male | 25,309 (51.5%) | 3540 (49.8%) |
0.04 |
| Female | 23,654 (48.2%) | 3552 (50.0%) | |
| Sex not documented | 141 (0.3%) | 15 (0.2%) | |
| Race/ethnicity | |||
| Non-Hispanic White | 19,730 (40.2%) | 2452 (34.5%) |
<0.0001 |
| African American | 11,288 (23.0%) | 1416 (19.9%) | |
| Hispanic | 13,561 (27.6%) | 2521 (35.5%) | |
| Other | 4525 (9.2%) | 718 (10.1%) | |
| Language | |||
| English | 39,173 (79.8%) | 5032 (70.8%) |
<0.0001 |
| Spanish | 9561 (19.5%) | 1993 (28.0%) | |
| Other | 370 (0.8%) | 82 (1.1%) | |
| Visit type | |||
| Well child | 16,417 (33.4%) | 2167 (30.5%) |
<0.0001 |
| Urgent | 4641 (9.5%) | 439 (6.2%) | |
| Return issue | 21,659 (44.1%) | 2367 (33.3%) | |
| Nurse/injection | 2439 (5.0%) | 1576 (22.2%) | |
| Other | 3948 (8.0%) | 558 (7.9%) |
p-Value was calculated using a marginal model incorporating generalized estimating equations to account for potential multiple visits from the same subject across encounters.
Encounters took place between March and December 2017, at 8 affiliated primary care pediatric and family medicine practices within a large academic medical center in North Carolina.
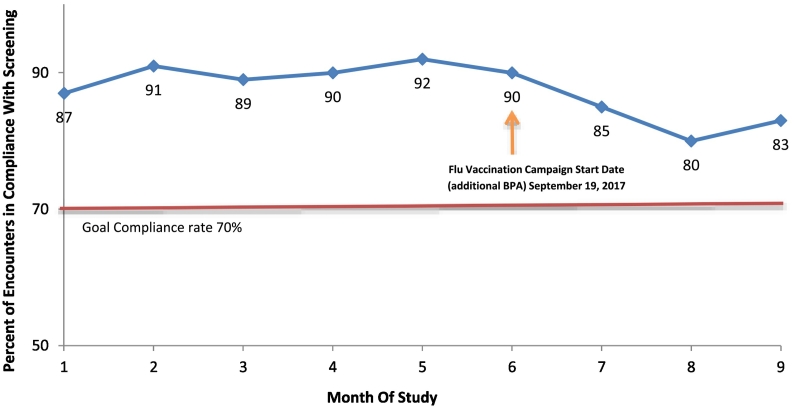
Throughout the study, average screening compliance across practices exceeded our 70% benchmark (Fig. 1), ranging from a low of 80% in month 8 to a high of 92% in month 5. We observed a decline in screening rates beginning in month 7 of the study period, coincident with the onset of our health system's annual “Flu Vaccination Campaign”.
Fig. 1.
Average screening compliance over time across 8 practices affiliated with Wake Forest Baptist Health.
Encounter was considered “compliant” if either: (a) screening took place, or (b) screening was not done, but was not indicated due to patient age (<6 months or >17 years), or due to SSB screening already having been completed in prior 90 days. Monthly compliance presented rates here are averaged across all 8 participating practices. Month 1 is calendar month spanning March 20 through April 19, month 9 is November 20–December 20. A decline in compliance was observed coincident with the start of our annual Flu Vaccine campaign.
3.2. Patient-level findings: screened population
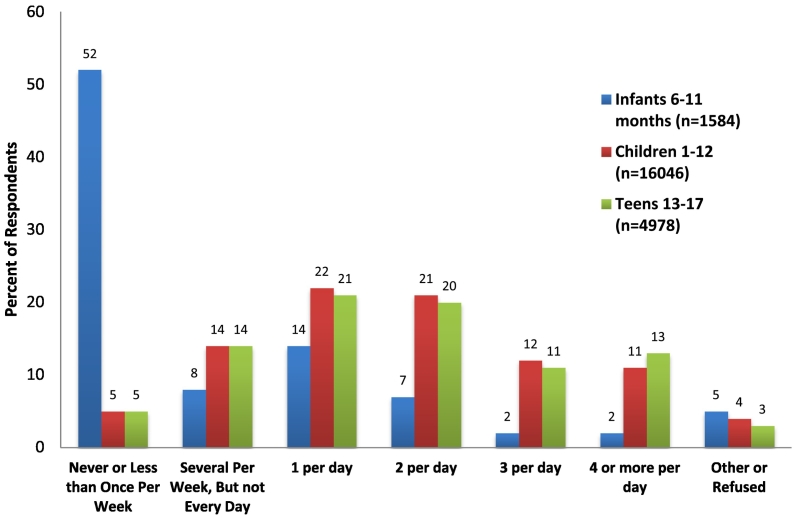
A total of 22,626 patients (91% of 24,873 patients seen) were screened at least once for SSB intake, and 5250 children (21%) had at least 2 separate measures captured. The screened population was balanced between male and female, and was race/ethnically diverse, with 35% identified as non-Hispanic White, 26% African-American, 30% Hispanic, and 9% other race or ethnicity. Most patients reported a valid frequency response for SSB/FJ consumption – only 76 (0.3%) refused, and 765 (3%) had a free text response entered (e.g. “did not screen for clinical reasons”). Forty-one percent of patients (9220) reported consuming SSB or FJ more than once per day in the month prior to screening (Fig. 2) and consumption patterns varied by age group.
Fig. 2.
Frequency of SSB/100% fruit juice consumption in last month as reported in EHR for 22,626 pediatric patients*.
** Encounters took place between March and December 2017, at 8 affiliated primary care pediatric and family medicine practices within a large academic medical center in North Carolina; data represent screening response at first SSB screening for each child (i.e. multiple measures per child not included in this figure).
Because recommended consumption varies according to child age, we separately examined infants and children according to screening response category. Among 1445 infants with a recorded SSB/FJ intake frequency, 882 (61%) of their parents reported that the infant “never” consumed SSBs. Race/ethnicity of infant SSB/FJ consumers was different than non-consumers (e.g. 28% of SSB/FJ consuming infants were African-American, vs. 15% of non-consuming infants); there were no differences by infant sex or parental language preference (Table 2). Similarly, of 20,152 children aged ≥1 year, the group reporting higher consumption (>1 SSB or FJ per day; n = 9032) had a larger proportion of African-American children (34% vs 20%) (Table 3).
Table 2.
Comparison of infants (6–11 months) whose parents report they consumed any SSB or fruit juice in prior month, versus those reporting no consumptiona.
| Variable | N/% reporting any SSB/FJ intake (n = 563, 39%) |
N/% reporting no SSB/FJ intake (n = 882, 61%) |
p-Valueb |
|---|---|---|---|
| Sex | |||
| Female | 248 (44%) | 439 (50%) | 0.07 |
| Male | 315 (56%) | 442 (50%) | |
| Sex not documented | 0 (0%) | 1 (0.1%) | |
| Race/ethnicity | |||
| Non-Hispanic White | 164 (29%) | 395 (45%) | <0.0001 |
| African American | 155 (28%) | 131 (15%) | |
| Hispanic | 147 (26%) | 190 (21%) | |
| Other | 97 (17%) | 166 (19%) | |
| Language | |||
| English | 473 (84%) | 767 (87%) | 0.12 |
| Spanish | 88 (16%) | 108 (12%) | |
| Other | 2 (0.4%) | 7 (1%) |
Encounters took place between March and December 2017, at 8 affiliated primary care pediatric and family medicine practices within a large academic medical center in North Carolina.
Chi-Square testing.
Table 3.
Comparison of children ≥1 year with more (≥2 per day) versus less (≤1) SSB and fruit juice consumption reported in prior montha.
| Variable | N/% reporting 2 or more per day (n = 9032, 45%) |
N/% reporting ≤1 per day (n = 11,120, 55%) |
p-Valueb |
|---|---|---|---|
| Age group | |||
| 1–4 yr | 2223 (25%) | 3087 (28%) | <0.0001 |
| 5–9 yr | 2942 (33%) | 3351 (30%) | |
| 10–12 yr | 1644 (18%) | 2052 (18%) | |
| 13-17 yr | 2223 (25%) | 2630 (24%) | |
| Sex | |||
| Male | 4731 (52%) | 5475 (49%) | <0.0001 |
| Female | 4252 (47%) | 5566 (50%) | |
| Sex not documented | 49 (0.5%) | 79 (0.7%) | |
| Race/ethnicity | |||
| Non-Hispanic White | 2739 (30%) | 4527 (41%) | <0.0001 |
| African American | 3100 (34%) | 2218 (20%) | |
| Hispanic | 2634 (29%) | 3387 (30%) | |
| Other | 559 (6%) | 988 (9%) | |
| Language | |||
| English | 6985 (77%) | 8572 (77%) | 0.03 |
| Spanish | 1954 (22%) | 2469 (22%) | |
| Other | 93 (1%) | 79 (0.7%) |
Encounters took place between March and December 2017, at 8 affiliated primary care pediatric and family medicine practices within a large academic medical center in North Carolina.
Chi-Square testing.
3.3. Validation survey results
Two-hundred and one respondents completed our telephone survey (76% response rate). Relative to our overall patient population, a higher proportion of phone survey respondents self-identified as Hispanic (41% vs. 28%) (Table 4). The majority of surveyed caregivers were mothers (88%), and 69% were in two-parent households.
Table 4.
Characteristics of 201 participants in telephone-based validation surveys.
| N/% | |
|---|---|
| Language used for interview | |
| English | 141 (70%) |
| Spanish | 60 (30%) |
| Race/ethnicity of child | |
| Non-Hispanic White | 61 (31%) |
| African-American | 40 (20%) |
| Hispanic | 82 (41%) |
| Other/refused | 18 (9%) |
| Identity of caregiver completing survey | |
| Mother | 176 (88%) |
| Father | 17 (9%) |
| Grandparent or other caregiver | 8 (3%) |
| Caregiver household status | |
| In co-parenting household | 138 (69%) |
| Single with full custody | 42 (21%) |
| Single with Shared custody | 10 (5%) |
| Refused | 11 (5%) |
| Number of other children ≤ 17 years in home | |
| 0 | 63 (31%) |
| 1–2 | 103 (50%) |
| 3 or more | 32(18%) |
| Refused | 3 (1%) |
| Caregiver self-reported educational attainment | |
| Less than HS | 36 (18%) |
| HS diploma or GED | 79 (40%) |
| Some college/2 y degree | 43 (22%) |
| 4-y college degree or higher | 39 (20%) |
| Refused | 3 (2%) |
Just over half (53%, n = 105) of respondents indicated that they recalled SSB screening at their recent pediatric visit. Among those, 92% (n = 97) were either somewhat or extremely comfortable with it, and 40% (n = 42) stated that they had tried to alter their child's SSB intake (or, for teens, their own intake) as a result.
Among respondents who should have received an after-visit summary document on SSBs (n = 88), 56% (n = 49) did not recall receiving it. However, among those who reported that they did receive and read this information, 64% (n = 25) stated that doing so resulted in trying to change SSB or FJ intake behavior.
Survey responses to NHANES beverage questions were summarized separately for three age groups (e-Tables 1–3). Mean(SD) SSB/FJ frequency for EHR screening responses among survey respondents was 1.2(1.2)/day versus 1.3(1.4)/day for NHANES-based responses. The distribution of responses to the two measures were similar (e-Fig. 1), although the NHANES-based measure had a higher maximum because the categorical EHR measure was capped at “≥4/day”. A paired t-test on the distribution of differences between EHR and NHANES responses was not statistically different from zero (p = 0.53). The Spearman's rank correlation coefficient comparing the two measures was 0.41 (p < 0.0001). When this measure was calculated separately by parental education level, it was 0.53 (moderate correlation, p < 0.001) for those with some college or greater, and 0.29 (weak correlation, p < 0.001) for those with high school/GED or less. A Kappa statistic was 0.42 (moderate agreement, 95%CI 0.24–0.60, e-Fig. 2) for agreement between the 2 methods for discriminating between “any” or “no” SSB/Fruit Juice intake.
4. Discussion
In this study of a novel EHR-based SSB screening technique, we observed high uptake of the screener across participating practices, resulting in the rapid accumulation of large-scale data on a key dietary risk factor for childhood obesity. Using the EHR to screen for SSB intake allowed for uniform screening and data aggregation and population monitoring, as well as individual tracking of children. Buy-in from clinical staff, the brevity of the screening process, and automated prompts to screen children at 90-day intervals all likely contributed to successful implementation in eight busy clinical practices.
There is a recognized need to enhance the utility of EHRs for obesity-related patient care,(Bronder et al., 2015) and we are not the first to implement routine screening for relevant patient behaviors. In Kaiser Permanente's “Exercise as a Vital Sign” program, patients are asked about physical activity at each primary care visit, with responses stored in the EHR;(Sallis, 2011; Coleman et al., 2012) a practice that has increased health behavior counseling and improved health outcomes.(Grant et al., 2014) Similarly, Inter-Mountain Healthcare implemented an EHR-based physical activity assessment that identifies adults not meeting recommended levels of physical activity.(Ball et al., 2016) Our study expands the collection of patient-reported measures in the EHR to include an important modifiable dietary factor.
Using the Re-AIM (Reach, Effectiveness, Adoption, Implementation and Maintenance) framework, (Glasgow et al., 1999) we have identified successes, challenges, and opportunities for future improvement of our SSB screener. The “Reach” of our intervention refers to the proportion of the target population who participated in screening. Ninety-one percent of eligible patients were screened, resulting in the collection of dietary behavior data on a large and diverse group of children. To enhance the reach of SSB screening, we elected to sacrifice detail in the dietary information collected in favor of brevity. We felt it was more important to have a short screener conducted on more people than a lengthy dietary screener that could disrupt clinical workflows and perhaps lead to a biased sample of respondents.
Our telephone-based SSB intake estimates displayed only moderate correlation with the single-item EHR screener. Possible reasons for the observed discrepancy between NHANES and EHR estimates include: the time elapsed between the measures, impact of repeated screening on behavior and response, and the different settings/methods in which the measures were administered. Using a larger number of questions to assess beverage consumption tends to result in higher reported intake, because respondents may underestimate intake when asked to consider all beverage types in a single question.(Lundeen et al., 2017) We are in the process of transitioning to a 2-item screener that separately asks about SSB and 100% fruit juice in the hopes that it will further improve the validity of our estimates.
The “Efficacy” of our SSB screening question and any accompanying intervention will ultimately be measured with longitudinal studies examining whether reported levels of intake and obesity prevalence decline over time. We had hoped that the inclusion of educational information in the after-visit summary (AVS) would influence beverage consumption. Unfortunately, our telephone survey respondents indicated that the AVS was not typically read. Among those parents who did read and recall the information, many verbalized at least an intent to decrease their child's sugary drink intake. Moving forward, it will be critical to develop and evaluate more effective educational interventions that reduce child SSB intake while not disrupting clinical workflows.
“Adoption” of the SSB screener was successful for several reasons. First, we elected to have the screening performed by clinical support staff, who are accustomed to asking about similar aspects of a child's medical and social history (e.g. tobacco exposure) while rooming patients. These staff members may be better suited to ask such questions than medical providers who have competing demands during a clinical encounter. Second, we engaged health system clinical staff and leadership in a sustained fashion throughout the implementation period. This was accomplished using regular face-to-face contacts with staff and consistent communication with practice managers at each site, who served as local champions for SSB screening. We also employed some friendly between-site competition and small rewards for staff that had sustained high levels of screening. To our surprise, this translated to giving rewards for staff at all sites, in all quarters, as they consistently exceeded our target screening rate of 70%. The potential for this type of screening to be replicated in other health systems is high, with broader adoption an important measure for these later phases of work. Related to adoption, we have not yet undertaken work to study how/whether physicians are using the SSB data in the EHR. We intend for it to be helpful in both population management and for flagging of at-risk children, and suspect this is a fruitful area for future study.
In the Re-AIM framework, “Implementation” is akin to the fidelity of an intervention – was it rolled out as intended when faced with the messy real world of clinical practice? We designed with ease-of-implementation in mind. Our “intervention” was a single-item screener and accompanying educational information in the AVS, both automated by the EHR. We are not certain whether the screening question was consistently read word-for-word by staff vs. paraphrased, which could have implications for data quality. We are planning further work, including focus groups with staff, to address this topic.
“Maintenance” of SSB screening has continued after the study period at all 8 practices with completion rates still well above the 70% threshold. We observed a distinct drop-off in screening compliance in early fall (Fig. 1) coincident with the start of the health system's annual flu vaccine campaign. This raised concerns that competing demands of clinical staff could inhibit SSB screening at times. However, the visit type used for flu shots (RN vists/injections) also had the lowest overall compliance. Upon reviewing with practice managers, we learned that workflow for nurse visits differs from physician visits: vitals are often not taken, or visits are not conducted in front of an open EHR window, potentially causing the SSB BPA to be missed during many of these encounters. Notably, we are observing a rebound in SSB screening compliance rates as flu vaccine season winds down.
5. Conclusion
A recent National Academy of Medicine report called for more routine capture of patient-reported measures by health systems, in part to facilitate behavior change counseling and tracking.(Adler and Stead, 2015) Our experience with implementing an EHR-based measure of SSB intake suggests that such capture is feasible for patient dietary behaviors. Future work should explore the use of expanded screening questions to more closely mirror results from validated instruments like NHANES, and explore ways to maximize the data's clinical utility. If a standardized SSB screener can be implemented in other settings, we envision enhanced population management for childhood obesity prevention, as well as the creation of large comparative datasets for population surveillance and observational research on a key risk factor for childhood obesity.
Conflict of interest statement
The authors have no financial relationships or conflict of interests relevant to this article to disclose.
Funding information
This research was supported through a grant from Healthy Eating Research, a national program of the Robert Wood Johnson Foundation (Grant Number 74370).
Acknowledgments
This research was supported through a grant from Healthy Eating Research, a national program of the Robert Wood Johnson Foundation (Grant Number 74370). We would like to acknowledge the enormous effort of clinical staff at our 8 participating practices, as well as that of our Project Manager, Beatriz Ospino-Sanchez, and IT “gurus” Adam Moses and Wendell Futrell. This project also would not have been possible without the support and vision of clinical leadership at Wake Forest Baptist Medical Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2018.06.007.
Appendix A. Supplementary data
Supplementary Tables and Figures: Validation Survey
References
- Adler N.E., Stead W.W. Patients in context—EHR capture of social and behavioral determinants of health. N. Engl. J. Med. 2015;372(8):698–701. doi: 10.1056/NEJMp1413945. [DOI] [PubMed] [Google Scholar]
- Afeiche M.C., Koyratty B.N.S., Wang D., Jacquier E.F., Le K.A. Intakes and sources of total and added sugars among 4 to 13-year-old children in China, Mexico and the United States. Pediatr. Obes. 2018;13(4):204–212. doi: 10.1111/ijpo.12234. [Epub 2017 Sep 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G.L., Oddy W.H., Huang R.C., Mori T.A., Beilin L.J., Jebb S.A. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am. J. Clin. Nutr. 2013;98(2):327–334. doi: 10.3945/ajcn.112.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T.J., Joy E.A., Gren L.H., Shaw J.M. Concurrent validity of a self-reported physical activity "vital sign" questionnaire with adult primary care patients. Prev. Chronic Dis. 2016;13 doi: 10.5888/pcd13.150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow S.E., Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Berentzen N.E., van Stokkom V.L., Gehring U. Associations of sugar-containing beverages with asthma prevalence in 11-year-old children: the PIAMA birth cohort. Eur. J. Clin. Nutr. 2015;69(3):303–308. doi: 10.1038/ejcn.2014.153. [DOI] [PubMed] [Google Scholar]
- Briefel R.R., Deming D.M., Reidy K.C. Parents' perceptions and adherence to children's diet and activity recommendations: the 2008 feeding infants and toddlers study. Prev. Chronic Dis. 2015;12 doi: 10.5888/pcd12.150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronder K.L., Dooyema C.A., Onufrak S.J., Foltz J.L. Electronic health records to support obesity-related patient care: results from a survey of United States physicians. Prev. Med. 2015;77:41–47. doi: 10.1016/j.ypmed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Campos V., Despland C., Brandejsky V. Sugar- and artificially sweetened beverages and intrahepatic fat: a randomized controlled trial. Obesity (Silver Spring) 2015;23(12):2335–2339. doi: 10.1002/oby.21310. [DOI] [PubMed] [Google Scholar]
- Coleman K.J., Ngor E., Reynolds K. Initial validation of an exercise "vital sign" in electronic medical records. Med. Sci. Sports Exerc. 2012;44(11):2071–2076. doi: 10.1249/MSS.0b013e3182630ec1. [DOI] [PubMed] [Google Scholar]
- Davis M.M., Gance-Cleveland B., Hassink S., Johnson R., Paradis G., Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics. 2007;120(Suppl. 4):S229–S253. doi: 10.1542/peds.2007-2329E. [DOI] [PubMed] [Google Scholar]
- de Ruyter J.C., Olthof M.R., Seidell J.C., Katan M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012;367(15):1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- Dodd A.H., Briefel R., Cabili C., Wilson A., Crepinsek M.K. Disparities in consumption of sugar-sweetened and other beverages by race/ethnicity and obesity status among United States schoolchildren. J. Nutr. Educ. Behav. 2013;45(3):240–249. doi: 10.1016/j.jneb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Dong D., Bilger M., van Dam R.M., Finkelstein E.A. Consumption of specific foods and beverages and excess weight gain among children and adolescents. Health Aff. (Millwood) 2015;34(11):1940–1948. doi: 10.1377/hlthaff.2015.0434. [DOI] [PubMed] [Google Scholar]
- Ebbeling C.B., Feldman H.A., Chomitz V.R. A randomized trial of sugar-sweetened beverages and adolescent body weight. N. Engl. J. Med. 2012;367(15):1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford C.N., Ng S.W., Popkin B.M. Ten-year beverage intake trends among US preschool children: rapid declines between 2003 and 2010 but stagnancy in recent years. Pediatr. Obes. 2016;11(1):47–53. doi: 10.1111/ijpo.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R.W., Schmittdiel J.A., Neugebauer R.S., Uratsu C.S., Sternfeld B. Exercise as a vital sign: a quasi-experimental analysis of a health system intervention to collect patient-reported exercise levels. J. Gen. Intern. Med. 2014;29(2):341–348. doi: 10.1007/s11606-013-2693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E., Powell L.M. Consumption patterns of sugar-sweetened beverages in the United States. J. Acad. Nutr. Diet. 2013;113(1):43–53. doi: 10.1016/j.jand.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman M.B., Abrams S.A. Section on gastroenterology H, nutrition, committee on N. Fruit juice in infants, children, and adolescents: current recommendations. Pediatrics. 2017;139(6) doi: 10.1542/peds.2017-0967. [DOI] [PubMed] [Google Scholar]
- Hu F.B. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013;14(8):606–619. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit B.K., Fakhouri T.H., Park S., Nielsen S.J., Ogden C.L. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999-2010. Am. J. Clin. Nutr. 2013;98(1):180–188. doi: 10.3945/ajcn.112.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosova E.C., Auinger P., Bremer A.A. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J. Acad. Nutr. Diet. 2013;113(2):219–227. doi: 10.1016/j.jand.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs N.F., Himes J.H., Jacobson D., Nicklas T.A., Guilday P., Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- Loh D.A., Moy F.M., Zaharan N.L., Jalaludin M.Y., Mohamed Z. Sugar-sweetened beverage intake and its associations with cardiometabolic risks among adolescents. Pediatr. Obes. 2017;12(1):e1–e5. doi: 10.1111/ijpo.12108. [Epub 2016 Feb 4] [DOI] [PubMed] [Google Scholar]
- Lundeen E.A., Park S., Dooyema C., Blanck H.M. Total sugar-sweetened beverage intake among US adults was lower when measured using a 1-question versus 4-question screener. Am. J. Health Promot. 2017 doi: 10.1177/0890117117736957. (890117117736957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik V.S., Pan A., Willett W.C., Hu F.B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A.H., Akram Y., Shetty S., Malik S.S., Yanchou Njike V. Impact of sugar-sweetened beverages on blood pressure. Am. J. Cardiol. 2014;113(9):1574–1580. doi: 10.1016/j.amjcard.2014.01.437. [DOI] [PubMed] [Google Scholar]
- Millar L., Rowland B., Nichols M. Relationship between raised BMI and sugar sweetened beverage and high fat food consumption among children. Obesity (Silver Spring) 2014;22(5):E96–103. doi: 10.1002/oby.20665. [DOI] [PubMed] [Google Scholar]
- Pan L., Li R., Park S., Galuska D.A., Sherry B., Freedman D.S. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics. 2014;134(Suppl. 1):S29–S35. doi: 10.1542/peds.2014-0646F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T.K., Spadano-Gasbarro J.L., Walls C.E. Middle school food environments and racial/ethnic differences in sugar-sweetened beverage consumption: findings from the healthy choices study. Prev. Med. 2013;57(5):735–738. doi: 10.1016/j.ypmed.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br. J. Sports Med. 2011;45(6):473–474. doi: 10.1136/bjsm.2010.083469. [DOI] [PubMed] [Google Scholar]
- Shefferly A., Scharf R.J., DeBoer M.D. Longitudinal evaluation of 100% fruit juice consumption on BMI status in 2-5-year-old children. Pediatr. Obes. 2015 doi: 10.1111/ijpo.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville K.R., Long M.W., Rifas-Shiman S.L., Kleinman K., Gillman M.W., Taveras E.M. Juice and water intake in infancy and later beverage intake and adiposity: could juice be a gateway drink? Obesity (Silver Spring) 2015;23(1):170–176. doi: 10.1002/oby.20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B.A., Barlow S.E., Ervin C. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- Stelmach-Mardas M., Walkowiak J. Dietary interventions and changes in cardio-metabolic parameters in metabolically healthy obese subjects: a systematic review with meta-analysis. Nutrients. 2016;8(8) doi: 10.3390/nu8080455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras E.M., Gillman M.W., Kleinman K.P., Rich-Edwards J.W., Rifas-Shiman S.L. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167(8):731–738. doi: 10.1001/jamapediatrics.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo P.R., Rivers C.R. Systematic review of the evidence for an association between sugar-sweetened beverage consumption and risk of obesity. Nutr. Rev. 2014;72(9):566–574. doi: 10.1111/nure.12128. [DOI] [PubMed] [Google Scholar]
- Van Rompay M.I., McKeown N.M., Goodman E. Sugar-sweetened beverage intake is positively associated with baseline triglyceride concentrations, and changes in intake are inversely associated with changes in HDL cholesterol over 12 months in a multi-ethnic sample of children. J. Nutr. 2015;145(10):2389–2395. doi: 10.3945/jn.115.212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M.B., Kaar J.L., Welsh J.A. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation. 2017;135(19):e1017–e1034. doi: 10.1161/CIR.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables and Figures: Validation Survey