Abstract
Introduction
Current models posit a sequence of amyloid β (Aβ), tau, atrophy, and cognitive change leading to Alzheimer's disease, but ambiguities remain. We examined these sequences via serial mediations.
Methods
We studied normal controls, early mild cognitive impairment, and late mild cognitive impairment individuals from the Alzheimer's Disease Neuroimaging Initiative 2 database for the mediation of baseline cerebrospinal fluid Aβ effects on 2-year cognitive change via regional longitudinal atrophy rate (AR) alone or AR and tau.
Results
In normal controls, Aβ correlated directly with regional ARs and memory loss, with no mediations. In early mild cognitive impairment, tau and lateral temporal ARs serially mediated the influence of Aβ on memory while Aβ affected memory via hippocampal AR. Late mild cognitive impairment consistently showed serial mediations of tau followed by atrophy. However, Aβ effects on memory also continued to be specifically mediated by medial temporal ARs without intermediate tau.
Discussion
Biomarker sequences vary by region and disease state, suggesting the need to refine current cascade models.
Keywords: Alzheimer's disease, MCI, Amyloid β, Tau, Cognitive aging, Longitudinal atrophy
1. Background
Recent biomarker cascade models [1], [2], [3] depict biomarker evolution as a sequence of sigmoid abnormality curves, in which amyloid β (Aβ) abnormality precedes abnormality in tau, which in turn leads to elevated brain degeneration and accelerated cognitive decline (changes in cognition [ΔCog]). The earliest model [1] featured a strict succession of abnormality curves, with Aβ always in the lead, but a later refinement acknowledged that preexisting tau abnormality might occur before Aβ while still remaining below threshold levels of detection [2]. Alternatively, a combined neurodegeneration category of tau with other markers—magnetic resonance imaging (MRI) atrophy and [18]fluorodeoxyglucose (FDG) measures of hypometabolism—might exist in levels that are barely detectable before Aβ abnormality [3]. Although these models acknowledge that tau may be independently deposited in brainstem, locus coeruleus, and medial temporal lobe regions (MTL), all models nonetheless make explicit predictions about biomarker sequences. The first is that Aβ is the necessary inducer of increasingly elevated tau/neurodegeneration [3]. Second, to the extent that Alzheimer's disease (AD)–related brain atrophy and FDG decline can be distinguished from effects of aging [3], these will not occur without abnormal tau. Finally, cognitive decline will not be present without abnormal neurodegeneration (see Fig. 5 in [2] and Fig. 2A–C in [3]). These predictions therefore posit a very clear ordering, in which the only possible deviation may be the presence of age-related medial temporal tau or neurodegeneration before Aβ abnormality.
There have been several studies investigating the sequential predictions of these models [4], [5], [6], [7], [8], [9]. The main difficulty for definitive verification is that there does not exist a dataset with sufficiently long follow-up to monitor longitudinal changes, given that the buildup of brain Aβ is postulated to take decades [10]. In response, studies to date have relied implicitly or explicitly on the concept of mediation—the direct effects of baseline Aβ on cognition could be largely explained or attenuated by one or more intervening variables such as tau, MRI atrophy, or FDG—using cross-sectional or longitudinal study designs.
Mediation effects have been inferred using hierarchical models [4], [5], in which variables are successively introduced one at a time to see if they diminish the effects of variables which were significant in a preceding model. Alternatively, an explicit mediation model incorporating a pathway of the form A → B → C (see Fig. 1C in [11]) estimates whether the product of the effects A → B times B → C significantly reduces the direct unmediated effect A → C. Studies using explicit modeling have investigated the roles of cortical atrophy [8], regional atrophy and FDG [7] or regional baseline and change in FDG [6] as mediators of effects of Aβ or tau on cognitive change.
The full sequential hypothesis of Aβ → tau → atrophy → ΔCog has been previously investigated [5]. These authors found partial support for the full sequence but also some unexpected deviations. For example, cerebrospinal fluid (CSF) Aβ and tau had independent effects on hippocampal baseline volume and longitudinal atrophy as well as on ventricle baseline volume and longitudinal enlargement. Meanwhile, CSF tau had an independent effect on baseline cognition. The study of the partial sequence Aβ → tau → atrophy for hippocampus, precuneus, and (as a control) the precentral gyrus [4] also found some deviations; for example, in normals, Aβ directly predicted hippocampal atrophy without the mediation of tau. Meanwhile, all the explicit mediation studies [6], [7], [8] found regionally significant mediation effects of regional cortical atrophy [7], [8] or FDG decline [6], [7] for the effects of baseline Aβ or tau on cognition.
This brief survey of current literature suggests that a systematic study of the full biomarker sequence, including regional variation of atrophy rates in different diagnostic categories, may be useful to clarify the extent of applicability for the posited succession of events [1], [2], [3]. Serial mediation models—incorporating pathways of the format A → B1 → B2 → C and all possible subpathways (see [11], Fig. 1D)—offer the means to simultaneously test alternative mediations of the effects of Aβ on ΔCog via selected regional atrophy, with and without the influence of tau, and of tau, independent of regional atrophy. This allows evaluation of competing hypotheses. By comparison, previous mediation studies [6], [7], [8] did not incorporate all these factors and thus provided only partial tests of the full biomarker cascade, whereas the hierarchical model analysis [5] examined only a few regions of interest (ROIs). Our models included 2-year atrophy rates of 10 selected brain regions known to be involved in early tau deposition independent of Aβ [12], [13] as well as of others known to be associated with the trajectory of cognitive decline in AD [7], [14], [15], [16], [17].
2. Methods
2.1. Study design
Data were obtained from the database of the Alzheimer's Disease Neuroimaging Initiative (ADNI) (adni.loni.usc.edu). The National Institute of Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations launched ADNI in 2003 as a public-private partnership. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD.
The principal investigator of ADNI is Michael Weiner, MD, VA Medical Center and University of California, San Francisco. For current information on ADNI, see www.adni-info.org.
2.2. Study participants
The study population was drawn from nondemented participants in the ADNI-2 database (Table 1). Inclusion/exclusion criteria are described at www.adni-info.org. Briefly, subjects in ADNI-2 are between the ages of 55 and 90 years at enrollment, have completed at least six years of education, and are free of any significant neurological disease other than AD. Normal controls (CNs) are distinguished from MCI categories by the Clinical Dementia Rating [18] score of 0 versus 0.5, respectively. The early mild cognitive impairment (EMCI) group differed from late mild cognitive impairment (LMCI) group only based on education-adjusted scores for the delayed paragraph recall subscore on the Wechsler Memory Scale–Revised Logical Memory II [19]; EMCI subjects were intermediate between normal subjects and LMCI.
Table 1.
Participant characteristics
| Category | CN | EMCI | LMCI | P value |
|---|---|---|---|---|
| N | 80 | 85 | 64 | |
| Age (yrs) | 73.9 (6.3)B | 71.6 (7.0) | 70.6 (7.5) | .013 |
| Gender (% male) | 50 | 52 | 48 | .921 |
| Education (yrs) | 17.2 (2.4)A | 15.4 (2.6)B | 16.7 (2.7) | <.001 |
| APOE ε4+ (%) | 25A,B | 55.2 | 53.1 | <.001 |
| CSF Aβ (pcg/mL) | 207.4 (54.6)A,B | 171.6 (54.0) | 166.7 (48.2) | <.001 |
| CSF T-tau (pcg/mL) | 75.6 (41.1)B | 85.2 (51.0) | 99.2 (58.2) | .021 |
| MEM baseline | 1.14 (0.54)A,B | 0.42 (0.51)B | 0.06 (0.63) | <.001 |
| ΔMEM (2 yrs) | 0.13 (0.32)B | −0.02 (0.44) | −0.17 (0.48) | <.001 |
| EF baseline | 0.97 (0.74)A,B | 0.43 (0.70) | 0.21 (0.81) | <.001 |
| ΔEF (2 yrs) | 0.06 (0.54) | −0.04 (0.62) | −0.11 (0.67) | .253 |
| ΔMTL (2 yrs, %) | −0.01 (0.02)A,B | −0.02 (0.02)B | −0.03 (0.03) | <.001 |
| ΔLTR (2 yrs, %) | −0.01 (0.01)B | −0.01 (0.02)B | −0.02 (0.03) | <.001 |
Abbreviations: Aβ, amyloid β; CN, normal controls; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; LTR, lateral temporal lobe; MTL, medial temporal lobe; t-tau, total tau.
NOTE. Format: mean (standard deviation). Units are specified for variables where appropriate. Cognitive measures (MEM and EF) are dimensionless. ΔMTL means medial temporal lobe atrophy rate, and ΔLTR means lateral temporal lobe atrophy rate. Column five gives P values for group comparisons from an analysis of variance (continuous variable) or chi-square test (categorical variable). For significant overall group results, pairwise tests were performed at α = 0.05, using Tukey-Kramer post hoc tests. A (in CN column): significantly different from EMCI; B (in CN or EMCI columns): significantly different from LCMI.
Owing to the longitudinal aims of our analysis, we selected subjects from the ADNI-2 database having baseline CSF Aβ and total tau (t-tau) measurements together with baseline and 2-year cognitive measurements and structural MRI scans. Selection was made a priori from ADNI-2 subjects based on the availability of complete data including longitudinal imaging and measures of cognition.
2.3. Cognitive measures
For measures of memory and executive function, we used composite scores available in the ADNI database: ADNI-MEM [20] and ADNI-EF [21], hereafter referred to as MEM and EF. Composite scores are advantageous for summarizing results of multiple tests in related domains. They afford greater precision than the individual component tests, are robust to floor and ceiling effects, and have a normal distribution with the same unit size [21] which enables direct comparisons. The MEM score accounts for version differences of its component tests [20].
2.4. CSF Aβ and T-tau
We used continuous baseline measurements of CSF Aβ and CSF t-tau. Baseline CSF t-tau and phosphorylated-tau concentrations were highly correlated (r = 0.70), and therefore, CSF t-tau concentrations were used for analysis. Moreover, a recent study has shown that both tau measures are significantly and linearly associated with entorhinal cortex (ERC) T807 uptake within a cognitively normal group, whereas Aβ is not [22], raising the question of the prevalence of non–amyloid-associated pathology (primary age-related tauopathy [PART] [23]) within our subjects. To further investigate this issue, we therefore examined the relationship between CSF t-tau and CSF Aβ, plotting distributions and using cluster analyses for putative clusters of low brain (high CSF) Aβ–low CSF t-tau (i.e., normal in both ranges), low brain Aβ–high t-tau (PART), and high brain Aβ–high t-tau (AD pathology).
2.5. Structural MRI acquisition and image processing
Baseline and 2-year structural MRIs were acquired on 3-Tesla scanners using the standardized ADNI protocol [24]. Voxel-wise longitudinal atrophy rates for the serial MRI scans from each participant were computed using a tensor-based morphometry (TBM) method designed to enhance sensitivity and specificity for biological change by incorporating knowledge of likely tissue boundary locations [25], [26] using an in-house processing pipeline described previously [27]. Briefly, we linearly align images at time 1 and time 2 to a “halfway space” to avoid interpolation biases when only one image is transformed [28]. Each brain scan is then corrected for field inhomogeneities using an atlas-based technique [29] and finally tissue-segmented using an algorithm sensitive to edge presence [30]. The ensuing TBM registration combines the segmented images with intensity gradients to enhance the likelihood of real edge detection and suppress noise [26]. TBM is performed in both directions, and the results are constrained to be inverses of each other [31]. The log-transformed determinant of the 3 × 3 Jacobian matrix of the TBM deformation at each voxel quantifies local brain change [32]. These will be referred to as log-Jacobians.
2.6. Brain atrophy rates in specified ROIs
We aimed to test mediation effects on Aβ predictions of cognitive change (either MEM or EF) by baseline t-tau and regional longitudinal atrophy. This approach was based, in part, on testing alternative mediation models of biomarker cascade hypotheses [1], [3] while accounting for regional atrophy rates that may be differentially affected by CSF Aβ and t-tau concentrations because of the topographical nature of Alzheimer's pathology [15], [16].
To examine regional differences in brain atrophy and their associations with CSF Aβ, CSF t-tau, and ΔCog, we selected a set of relevant ROIs a priori for specific analysis, which included the amygdala; entorhinal cortex; parahippocampal gyrus (PHG); inferior, middle, and superior lateral temporal ROIs (combined and designated as lateral temporal lobe [LTR] ROI); hippocampus; thalamus; splenium; and posterior cingulum bundle. In addition, because of recent findings of association with cognitive decline, we added the insula [7] and superior longitudinal fasciculus, parieto-temporal branch [17].
The posterior cingulum bundle, splenium, thalamus, and insula were drawn in-house by an experienced anatomist and have been used in previous publications from our laboratory [27], [33]. The amygdala, ERC, PHG, and LTR ROIs were derived from the Desikan-Killiany Atlas of gray matter parcellations [34]. The superior longitudinal fasciculus, parieto-temporal white matter ROI was derived from the Johns Hopkins Atlas [35]. The hippocampi were segmented in each participant's native brain by an automatic atlas-based technique [36].
We computed subject regional atrophy rates as the mean log-Jacobian values over specific ROI masks.
2.7. Statistical analysis
2.7.1. Approach
We first characterized the participants in each diagnostic group by demographics, presence of the APOE ε4 allele, baseline Aβ, t-tau, and cognitive function as well as simple estimates of 2-year change in cognition, defined as the difference between the 2-year score and the baseline score. We also summarized group 2-year atrophy rates in MTL and LTR. The diagnostic groups were compared using analysis of variance, followed by Tukey-Kramer post-hoc tests for continuous variables (all of which met the assumption of normality) and chi-square test for categorical variables.
Subsequent analyses explored the univariate interrelationships between the CSF markers, regional atrophy rates, and cognitive change measures. Mixed effects regression models using all cognitive assessments taken over a period of 2 years for each subject were used for analyses with cognitive function as the outcome. Individual slopes and intercepts were accounted for using random effects. Linear regression was used for all other outcomes. Relevant covariates of age, gender, and educational achievement were included as covariates of the univariate relationships to assess the need for inclusion in subsequent analyses. All analyses were performed separately for each diagnostic group. The final analysis phase evaluated mediations of Aβ → ΔCog.
2.7.2. Mediation analysis
2.7.2.1. Serial mediation models of biomarker cascade hypotheses
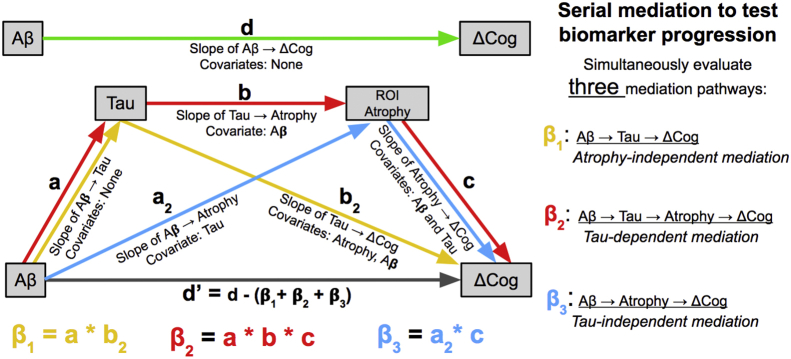
We used a four-factor, serial mediation model [11] illustrated in Fig. 1. Three mediation tests were performed simultaneously. They were the triangle pathways Aβ → t-tau → ΔCog (β1), Aβ → ROI atrophy → ΔCog (β3), and the 4-pathway Aβ → t-tau → ROI atrophy → ΔCog (β2). Each edge weight in Fig. 1 was derived by linear or mixed effects of regression (depending on outcome) of an outcome box against one or more covariates from input boxes, in models controlling for age, gender, and education. The weights of edges in a mediation pathway were multiplied to give the effect of the mediation [11]. Thus, in Fig. 1, β1 = a*b2, β2 = a*b*c, and β3 = a2*c.
Fig. 1.
Serial mediation model. Diagram of mediation model pathways showing unmediated effect of Aβ on cognitive change (upper diagram, green arrow) and serial mediation model including three mediation pathways (lower diagram, paths color coded). The strength of a mediation pathway (i.e., β1, β2, β3) is the multiplicative product of the component edge weights in the pathway, as indicated at the bottom of the figure. Abbreviations: Aβ, amyloid β; ΔCog, changes in cognition.
Effect sizes for mediation pathways express the amount of change in cognition (in units of standard deviation [20], [21]) per unit difference in Aβ (pcg/mL). For atrophy regression, the effects express log-Jacobian difference (in approximate volume change percentage) per change of CSF Aβ.
The β1 pathway Aβ → t-tau → ΔCog indicates that t-tau mediates the effects of Aβ on cognition via some factor other than the ROI atrophy included in that model. Conversely, its nonsignificance would suggest that much of the effect of Aβ is mediated by pathways (with or without tau) that include the ROI in question. The β2 pathway Aβ → t-tau → Atrophy → ΔCog models the serial biomarker cascade proposed by Jack et al. [3] for specific ROI atrophy rates. Thus, β2 refines the biomarker cascade hypothesis by examining effects in local brain regions. Alternatively, the β3 pathway Aβ → Atrophy → ΔCog tests the mediation effect of ROI atrophy while controlling for t-tau. The effects β2 and β3 together provide complementary evaluations of the biomarker cascade hypothesis—comparing the full biomarker cascade with one involving atrophy but independent of t-tau—for a single selected ROI.
2.7.2.2. Computing the significance of mediation effects
Mediation pathway significance was rigorously tested using a bootstrap [37] resampling scheme of the original data. All pathways were tested simultaneously. To estimate 95% and 99% confidence intervals for mediation effects [11], we resampled the dataset with replacement 10,000 times, using the same resampling to compute all regression and mediation effects at each iteration. Analyses and computations were performed using R, version 3.2.4 [38]. If both the upper and lower bounds of a confidence interval had the same sign, then the mediation or regression effect was considered significant (i.e., not 0) at the corresponding level of confidence.
3. Results
3.1. Participant characteristics
Participant characteristics appear in Table 1 for CN (N = 80), EMCI (N = 85), and LMCI (N = 64). Subtle but significant group differences were present for age (P = .01) and education (P < .0001), with CNs being the oldest and most educated. There were striking between-group differences for prevalence of at least one APOE ε4 allele between the CN and MCI groups (25% in CN vs. 55% for EMCI and 53% for LMCI).
A similar group difference was found for CSF Aβ, with mean values above the 192 pcg/mL cutoff [39] in CN group (i.e., CN mean was Aβ−) but below in both the MCI groups (P < .001). There was no significant difference in CSF Aβ between EMCI and LMCI groups. For CSF t-tau, CN and EMCI groups both had mean values below the cutoff of 92 pcg/mL [39] (i.e., they were t-tau−), whereas the LMCI t-tau mean was above the cutoff. CN and LMCI t-tau means differed significantly (P = .015 for all-pairs comparison using Tukey-Kramer), but EMCI did not significantly differ from either.
A final observation is relevant for the robustness of our results. As seen in Table 1, variability of key components in the mediation pathway models—Aβ, t-tau, and regional atrophy—is present in all groups. A large amount of t-tau variability exists in the CN and MCI groups. Meanwhile, for representative regional atrophy rates for MTL and LTR, the standard deviations in all diagnostic groups equal or exceed the mean values.
3.2. Cognitive performance
As expected, baseline and 2-year change in MEM and EF scores all become more negative with progression from CN to EMCI and LMCI groups (Table 1).
3.3. Associations between Aβ, t-tau, regional atrophy, and cognitive decline
Preliminary regression analyses by cognitive group showed increasing strength of associations between baseline CSF Aβ and t-tau proceeding from CN (β = −0.13, P = .22) to EMCI (β = −0.41, P < .001) and LMCI (β = −0.64, P < .001) in models controlling for age, gender, and education.
Then, we tested for mediations. We recall that our serial model simultaneously tested three mediation pathways: β1, with tau mediating the effects of Aβ on cognition, independent of regional atrophy; β2, showing the full serial mediation of Aβ, tau, regional atrophy, and cognition; and β3, with regional atrophy mediating the effects of Aβ without tau.
Among CNs, we found no significant mediations of Aβ → ΔCog. We then tested simple regressions using the bootstrap sampling with 10,000 iterations. CSF Aβ correlated significantly with atrophy in several regions such as the amygdala, ERC, hippocampus, LTR, PHG, superior longitudinal fasciculus, parieto-temporal, and thalamus. The amygdala, hippocampus, and PHG (all regions within the MTL) remained significant at the 99% confidence level. Aβ also significantly predicted ΔMEM (which remained significant at the 99% confidence level) but not ΔEF. These relations are displayed in Tables 2 and 3.
Table 2.
Associations of Aβ with regional atrophy rates in CN
| ROI atrophy | β (95% CI) |
|---|---|
| Amygdala | 0.00011* [0.00005–0.000165] |
| ERC | 0.000118 [0.000002–0.00022] |
| Hippocampus | 0.000091* [0.000028–0.000152] |
| Insula | 0.000048 [0.0–0.000094] |
| LTR | 0.000066 [0.000015–0.000121] |
| PHG | 0.000081* [0.000029–0.000133] |
| Post Cing | 0.000039 [−0.000001 to 0.000085] |
| SLF PT | 0.000053 [0.000007–0.0001] |
| Splenium | 0.000041 [−0.000001 to 0.00192] |
| Thalamus | 0.000057 [0.000003–0.000104] |
Abbreviations: Aβ, amyloid β; CI, confidence interval; CN, normal controls; ERC, entorhinal cortex; LTR, lateral temporal lobe; PHG, parahippocampal gyrus; Post Cing, posterior cingulum bundle; ROIs, regions of interest; SLF PT, superior longitudinal fasciculus, parieto-temporal.
NOTE. Regression coefficients are computed by bootstrap sampling with 10,000 iterations, controlling for age, gender, and education in CN group (N = 80). β coefficients and 95% confidence intervals are displayed. Coefficients significant at 95% confidence level are in bold. Significance at 99% confidence level is further indicated with an asterisk.
Table 3.
Associations of Aβ with cognitive change and t-tau in CN
| Outcome | β [95% CI] |
|---|---|
| ΔEF | 0.000768 [−0.000888 to 0.002353] |
| ΔMEM | 0.000993* [0.000295–0.001707] |
| CSF t-tau | −0.134486 [−0.314519 to 0.033084] |
Abbreviations: CI, confidence interval; t-tau, total tau.
NOTE. Regression coefficients are computed by bootstrap sampling with 10,000 iterations, controlling for age, gender, and education in CN group (N = 80). β coefficients and 95% confidence intervals are displayed. Coefficients significant at 95% confidence level are in bold. Significance at 99% confidence level is further indicated with an asterisk.
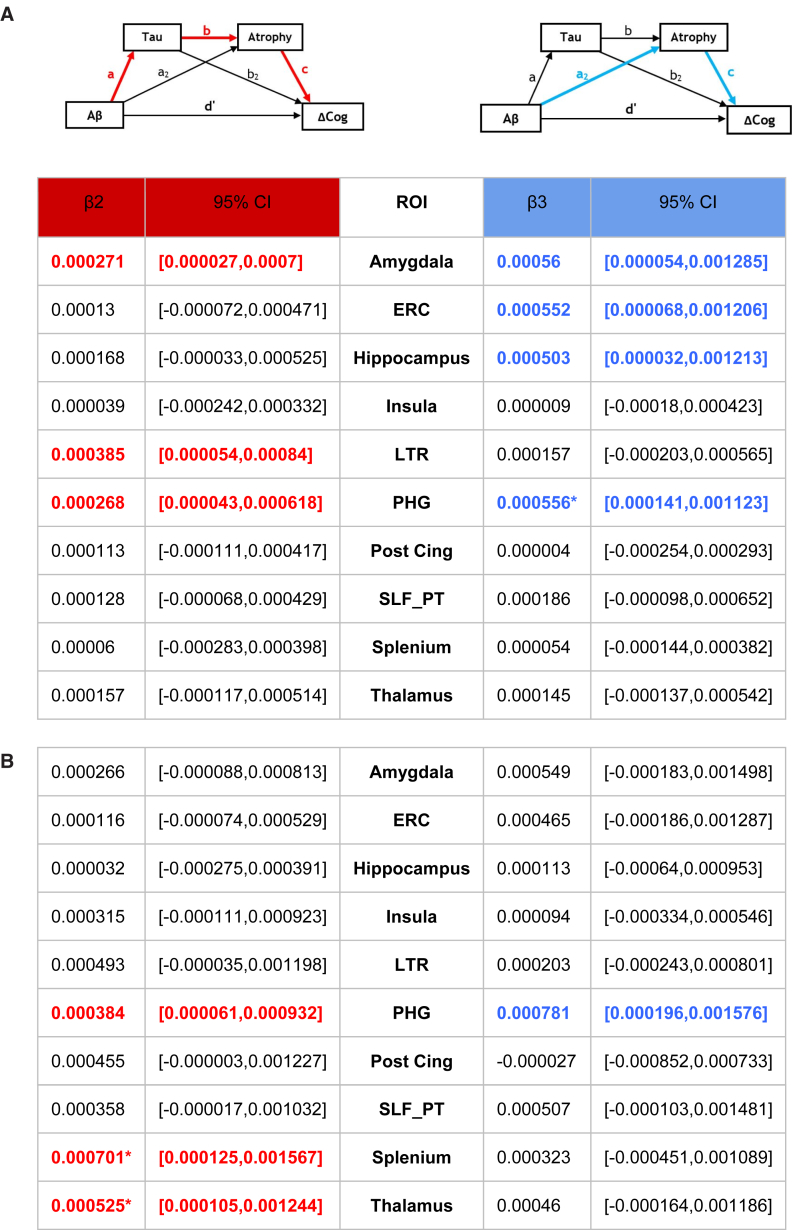
In EMCI, we found a single significant β2 mediation (by the sequence of t-tau and LTR regional atrophy) of Aβ → ΔMEM and a single β3 mediation by hippocampal atrophy without t-tau. There were no significant mediations of Aβ → ΔEF. Mediation effects for EMCI appear in Fig. 2.
Fig. 2.
Mediation effects of Aβ → ΔMEM in EMCI. Small diagrams above the table indicate β2 pathways (red color code) and β3 (blue). β2 and β3 effects express change in cognitive outcomes (units of standard deviation) per unit differences in CSF Aβ (pcg/mL). A decrease of Aβ correlates with exacerbated cognitive loss, so β2 and β3 are both positive effects. All mediation effects and significance are computed by bootstrap sampling with 10,000 iterations. Effect sizes and 95% confidence intervals are displayed for each ROI. Mediations significant at 95% confidence level are in bold and color coded by pathway. Significance at 99% confidence level is further indicated by an asterisk. Abbreviations: Aβ, amyloid β; CI, confidence interval; EMCI, early mild cognitive impairment; ERC, entorhinal cortex; LTR, lateral temporal lobe; PHG, parahippocampal gyrus; ROIs, regions of interest; SLF PT, superior longitudinal fasciculus, parieto-temporal.
We also found four significant β1 mediations for the models involving atrophy in the insula, posterior cingulate, splenium, and thalamus, relative to the effect of Aβ on MEM in EMCI (data not shown). These pathways suggest that for atrophy in those ROIs, tau mediated the effects of Aβ on MEM via some other means, either through atrophy in other ROIs or directly rather than through the mechanism of regional atrophy. This was the only diagnostic group that had any significant β1 pathways.
Among LMCI, we found significant β2 mediations of Aβ → ΔMEM by LTR, amygdala, and PHG atrophy. The amygdala and PHG also had additional significant β3 mediations of ΔMEM. For each of these two regions, the β3 effect was roughly twice the magnitude of the β2 effect. In addition, the PHG β3 mediation remained significant at the 99% confidence level. For ΔMEM, there were also significant β3 mediations by atrophy in ERC and hippocampus.
In LMCI, there were also mediations of Aβ → ΔEF. There were significant β2 effects for splenium and thalamus atrophy, and these remained significant at the 99% confidence level. Meanwhile, PHG was involved in both β2 and β3 mediations, with β3 magnitude roughly twice as large.
Mediation effects for LMCI appear in Fig. 3.
Fig. 3.
LMCI patterns of mediation. Small diagrams above the table indicate β2 pathways (red color code) and β3 (blue) (see Fig. 1). β2 and β3 effects express change in cognitive outcomes (units of standard deviation) per unit differences in CSF Aβ (pcg/ml). A decrease of Aβ correlates with exacerbated cognitive loss, so β2 and β3 are both positive effects. All mediation effects and significance are computed by bootstrap sampling with 10,000 iterations. Effect sizes and 95% confidence intervals are displayed for each ROI. Mediations significant at 95% confidence level are in bold and color coded by pathway. Significance at 99% confidence level is further indicated by an asterisk. (A) The mediation of Aβ → ΔMEM. (B) The mediation of Aβ → ΔEF. Abbreviations: Aβ, amyloid β; CI, confidence interval; ERC, entorhinal cortex; LMCI, late mild cognitive impairment; LTR, lateral temporal lobe; PHG, parahippocampal gyrus; ROIs, regions of interest; SLF PT, superior longitudinal fasciculus, parieto-temporal.
Finally, Supplementary Fig. 1 shows the results of scatterplots for each diagnostic group, examining the prevalence of CSF Aβ and t-tau levels in CN, EMCI, and LMCI. We performed 3-means clustering, and in each group, we found clusters roughly representing the ranges of normal Aβ with normal t-tau as well as abnormal Aβ coincident with both normal and abnormal t-tau. However, the incidence of normal Aβ with high t-tau or PART was low to absent. We found 11 such participants in CN group (14%), but only three of these had t-tau levels greater than 20% above the cutoff. We found no PART participants in EMCI and only two (3%) in LMCI.
4. Discussion
To our knowledge, this is the first study using serial mediation to test predictions of biomarker sequence hypotheses. Novel findings include evidence that the succession of biomarker abnormality varies both by anatomical region—distinguishing between medial temporal and neocortical locations—and by disease state (i.e., CN vs. EMCI vs. LMCI). Aβ has a direct effect on regional atrophy in normals, prominently and most significantly in MTL regions, and also in some neocortical and white matter ROIs and independently on ΔMEM (Tables 2 and 3). Furthermore, Aβ has continued direct effects via atrophy without tau in EMCI and LMCI, as represented by the significant β3 mediations of ΔMEM by atrophy of MTL ROIs (Figs. 2 and 3). However, in EMCI, the sequence Aβ → t-tau → LTR atrophy → ΔMEM also occurs, and in LMCI, this expands to other regions for both ΔMEM and ΔEF. These β2 mediations are consistent with the predictions [1], [2], [3].
These results are consistent with, and extend, an earlier study that examined temporal relations of CSF Aβ and tau, longitudinal MRI change in hippocampal and ventricle volumes, and cognition, using hierarchical modeling [5]. The findings of early influence of Aβ on MTL atrophy in normals also corroborate the previous study examining CSF biomarker associations with volume change in hippocampus and precuneus [4]. The independence of tau and amyloid processes in normal individuals is supported by a recent study of the relations of CSF Aβ and tau with T807 brain tau uptake among a group of cognitively normal individuals [22]. The authors found significant associations between CSF tau and local ERC tau uptake as well as between CSF Aβ and PiB cortical SUVR, but the association of CSF Aβ and T807 in MTL regions was not significant [22].
Among the previous explicit mediation studies, our study is closest to the approach [6] which modeled baseline and longitudinal FDG change as mediators for effects of baseline CSF Aβ and tau on change in cognition. The other two studies [7], [8] used cross-sectional brain measures to estimate mediation of regional cortical thickness [8] for effects of Aβ on memory or mediation of regional gray matter volumes and FDG [7] of Aβ effects on cognitive baseline and change. As in our study, they found regional variation of the strengths of mediation effects. However, none of the previous studies used serial mediation and hence did not evaluate the full sequence of biomarker predictions or the competing subsequences simultaneously.
According to recent imaging results [12], [22], [40] and established literature [13], [23], tau deposition in MTL regions may initially be independent of Aβ but may propagate to neocortical regions under the influence of Aβ and is thus more strongly associated with it in these locations. Consistent with this picture, our findings suggest two scenarios for mediation of the effects of Aβ on ΔCog. First, by the effect of Aβ directly on regional atrophy without t-tau (β3 pathway; Fig. 1) in MTL regions until late MCI and later, by the effect of Aβ on t-tau leading to atrophy (β2 pathway) in neocortex ROIs, starting among EMCI in LTR and expanding to posterior limbic (thalamus and splenium) among LMCI. Regions of the β3 scenario correspond to the locations of the early Braak stages (I and II) of neurofibrillary tangle deposition, whereas those of the β2 scenario correspond to neurofibrillary tangle propagation in Braak stages III and above [16].
Our findings therefore suggest the following hypothesis. In MTL regions, Aβ is the determining factor for atrophy because it is interacting with t-tau that is already present. This favors β3 mediation, whereas β2 mediation is weaker or nonsignificant because tau deposition in MTL is independent of Aβ [12], [22], [40]. In terms of our model in Fig. 1, β2 is small because the regression coefficient “a” of Aβ → t-tau is weak, whereas β3 is large because the coefficient “a2” is strong. Conversely, in neocortical regions, tau may be propagated there by Aβ. There, the presence of tau depends on Aβ and effectively absorbs the effect of Aβ on local atrophy, leading to β2 significance. In terms of our Fig. 1, “a” is strong, and β2 is large. Our findings of increasing association between Aβ and t-tau in EMCI and LMCI also support this hypothesis.
The low incidence of PART in CN and its virtual absence in EMCI and LMCI favor the view that this is occurring in the AD trajectory rather than from nonamyloid PART. In particular, Supplementary Fig. 1 suggests that the weak association of CSF Aβ and t-tau in CN is not due to PART (i.e., subjects with normal Aβ and high t-tau) but rather is due to high variability of t-tau among subjects with abnormal Aβ. That being said, there are nonetheless some individuals in the PART category among the normals who could be weakening our results, and so caution is appropriate.
Because MTL tau deposition has been shown to be independent of CSF Aβ in cognitively normal individuals [22] and because abnormal CSF t-tau (and hence MTL tau deposition [22]) occurs mainly in our subjects with abnormal Aβ, our data likely reflect the early effects of abnormal Aβ in the AD spectrum with and without early MTL tau. We conclude therefore that our hypothesis about Aβ interacting with preexistent tau early in the AD spectrum is plausible.
The atrophy rates significant for the β2 and β3 mediations may therefore reflect the dual nature of Aβ′s influence to synergize with already-present t-tau in MTL areas while catalyzing the propagation of t-tau to neocortical areas. This is consistent with the hypothesized role of Aβ in late-onset AD (see [3], Fig. 2B,C) and also with recent findings based on cross-sectional analysis [40] suggesting that Aβ interacts with hippocampal and cortical tau deposition to produce neurodegeneration and also that in the absence of Aβ, hippocampal tauopathy alone may not lead to neurodegeneration.
4.1. Limitations
Besides atrophy, at least two recent studies have also looked at mediation effects of FDG [6], [7]. This is indeed an important topic for investigation, but in the interest of simplicity, we omitted any focus of FDG in this article. This also has some justification in that the more recent biomarker model refinements combine structural MRI atrophy and FDG under the umbrella category of neurodegeneration [2], [3].
Lack of positron emission tomography imaging of local t-tau and Aβ deposition is another limitation to our study. Our findings and their interpretation, therefore, require further confirmation. That noted, cross-sectional t-tau imaging data are consistent with our findings [12], [22], [40]. Furthermore, recent publications lend support to the hypothesis that global indices of Aβ and t-tau may be used to make regional inferences without imaging of local deposition. First, CSF t-tau is significantly correlated with MTL tau deposition in normals [22]. Second, cortical uptake of Aβ in widely separated brain regions has been shown to be nonlocally associated with increased tau deposition, especially in temporal and frontal-parietal regions [41]. Third, image-based tau staging [42] suggests that in vivo spreading of tau deposition follows Braak neurofibrillary tangle stages and that these stages depend on global measures of tau. Consequently, CSF measures of Aβ and t-tau may be representative of predictable brain states, in which (1) the level of CSF Aβ is more crucial than regional deposition and (2) the level of CSF t-tau is correlated with predictable locations of tau deposition in the Braak staging order. However, a caveat is warranted. Although CSF Aβ levels may develop over decades, changes in CSF t-tau may be faster, which calls for caution in using it as a measure of a static brain state over the period of observation.
A third limitation relates to the unusually low incidence of PART in our cohort. Although our results are consistent with our hypothesis that in CN, high brain amyloid is enhancing toxicity of tau which is already present for subjects in the early AD spectrum, confirmation is needed. Larger studies of cognitively normal individuals, using combined tau and amyloid positron emission tomography imaging and including populations where PART is more common, will likely clarify the impact of PART on longitudinal atrophy and cognitive measures. We must also caution that PART was not entirely absent in our CN group, and so it still could have reduced the association between Aβ, tau, atrophy, and cognition in this relatively small group of subjects.
A fourth concern may be that our models combined baseline measures for Aβ and t-tau with longitudinal values for regional atrophy and cognitive change. Because Aβ deposition takes place on a time scale of decades [10], while early MTL deposition of tau may be age dependent [12], mediation studies of change in these variables must await the availability of decades-long datasets. In the meantime, for reasons cited in a previous paragraph, we propose that baseline measurements of CSF Aβ variables are not likely to evolve much over a 2-year period and so can validly test predicted sequences of biomarker abnormality within each group studied (i.e., CN, EMCI, and LMCI). However, changes in CSF t-tau over the lag interval between CSF measurements and our structural scans may not be negligible, and this suggests caution in the interpretation of our results.
Longitudinal atrophy and cognitive decline, in contrast to change in CSF measurements of Aβ and tau, are robustly measurable over a 2-year interscan interval, and changes in brain structure may be more strongly correlated with future cognitive changes than baseline measurements [43]. Thus, a considerable strength of our study is the incorporation of longitudinal regional atrophy and cognitive change in serial mediations to simultaneously test alternative pathways between Aβ and cognition. Furthermore, longitudinal analyses within diagnostic categories emphasize the dynamic nature of progression along the hypothesized biomarker pathways and are, by definition, less susceptible to limitations introduced by group differences into cross-sectional analyses commonly used as the basis for various sequential biomarker models.
Our study may also reflect a limitation from reduced statistical power because of smaller samples sizes necessitated by analyzing each diagnostic group separately. We acknowledge this issue but note the importance of testing for patterns in each group to capture the time (i.e., sequential degree of cognitive impairment) dimension of biomarker progression. We also underline that the cognitive groups were defined by very precise changes in diagnostic scores (Clinical Dementia Rating = 0.5 for both MCI, and in addition, reduced scores for LMCI on the Wechsler Memory Scale–Revised Logical Memory II). Our results therefore imply that the specific changes in these cognitive measures correlate with a progressive unfolding of biomarker sequences. A limitation may also result from the fact that because of ADNI exclusion criteria, this cohort may not accurately reflect a broader population with respect to white matter lesions and vascular disease. For these reasons, future studies are necessary to test our results on larger and differing samples.
Finally, because we performed separate mediation analyses for atrophy in each ROI and each cognitive domain for each cognitive group, resulting in a large number of fitted models, it would be natural to incorporate a penalty for multiple comparisons to correct for potential false indications of significance (type I errors). We did not do so, however, for two reasons. First, bootstrapping [11] does not provide actual P values for the effect sizes but rather the distribution of their estimated values, enabling confidence intervals that estimate the significance for each mediation. As a result, we do not have the ability to rank the significances by P value as is typically done in corrections based on false discovery or family-wise error rates. Second, multiple comparisons corrections may be undesirable because the regions and cognitive domains were strategically chosen to be those primarily affected by AD. Although corrections may reduce type I errors, they can also increase type II errors in situations where patterns of associations exist [44]. Nonetheless, our estimation of two confidence intervals at 95% and 99% was designed to give a sense of the hierarchy of effect strengths for our associations and mediations.
4.2. Conclusion
We have found that serial mediation analyses show regional differences in the interaction of Aβ, t-tau, brain atrophy, and cognitive decline. These findings support the prominent biomarker cascade model in neocortical areas while suggesting that Aβ remains an important or even determining factor in MTL atrophy leading to loss of cognition. Future biomarker models, therefore, should take into account both temporal (disease state) and spatial (anatomical regions) dimensions.
Research in Context.
-
1.
Systematic review: The literature for studies involving mediation of the effects of amyloid β (Aβ) on cognition was reviewed. Two earlier results demonstrated that brain structure and function mediates the effects of Aβ. However, no study has used serial mediation to evaluate current biomarker sequences.
-
2.
Interpretation: Serial mediation supports biomarker models of Aβ → tau → regional atrophy → cognitive decline in early and late mild cognitive impairment for lateral temporal regions and also in late mild cognitive impairment for other neocortical and white matter regional atrophy. However, medial temporal atrophy alone continues to mediate the effects of Aβ. Furthermore, Aβ directly correlates with regional atrophy without tau in normal participants. This pattern suggests an early and continued prominent role of Aβ independent of tau. It also suggests regional specificity for current biomarker models.
-
3.
Future directions: The present study's results need to be confirmed by data with local tau imaging.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The study was funded by NIH (P30 AG010129). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering as well as through generous contributions from AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; Euroimmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research and Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
This study was funded by the NIH (P30 AG010129), which had no role in any aspect of the study, including study design, data collection, analysis, or writing.
Statistical analysis conducted by Teresa Jenica Filshtein, PhD, Danielle Harvey, PhD, and Dan Mungas, PhD, all at University of California, Davis.
Search Terms: Alzheimer's disease; MCI; Amyloid β; T-tau; Cognitive aging; longitudinal atrophy.
Authors' contributions: E.F. contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting/revising manuscript for content. T.J.F. and D.H. contributed to analysis and interpretation of data, statistical analysis, and drafting/revising of the manuscript for content. A.R. contributed to study concept and design and analysis and interpretation of data. D.M. also worked for analysis and interpretation of data, drafting/revising manuscript for content, and statistical analysis. C.D.C. contributed to study concept and design, drafting/revising manuscript for content, and study supervision.
Disclosure: All the authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.04.001.
Supplementary data
References
- 1.Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack Clifford R., Holtzman David M. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stricker N.H., Dodge H.H., Dowling N.M., Han S.D., Erosheva E.A., Jagust W.J. CSF biomarker associations with change in hippocampal volume and precuneus thickness: implications for the Alzheimer's pathological cascade. Brain Imaging Behav. 2012;6:599–609. doi: 10.1007/s11682-012-9171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han S.D., Gruhl J., Beckett L., Dodge H.H., Stricker N.H., Farias S. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer's disease. Brain Imaging Behav. 2012;6:610–620. doi: 10.1007/s11682-012-9177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowling N.M., Johnson S.C., Gleason C.E., Jagust W.J. The mediational effects of FDG hypometabolism on the association between cerebrospinal fluid biomarkers and neurocognitive function. NeuroImage. 2015;105:357–368. doi: 10.1016/j.neuroimage.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson N., Aisen P.S., Jagust W., Mackin S., Weiner M. Brain structure and function as mediators of the effects of amyloid on memory. Neurology. 2015;84:1136–1144. doi: 10.1212/WNL.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villeneuve S., Reed B.R., Wirth M., Haase C.M., Madison C.M. Cortical thickness mediates the effect of b -amyloid on episodic memory. Neurology. 2014;82:761–767. doi: 10.1212/WNL.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormino E.C., Kluth J.T., Madison C.M., Rabinovici G.D., Baker S.L., Miller B.L. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 11.Hayes A.F. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- 12.Schöll M., Lockhart S.N., Schonhaut D.R., Schwimmer H.D., Rabinovici G.D., Correspondence W.J.J. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price J.L., Morris J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Nestor P.J., Fryer T.D., Smielewski P., Hodges J.R. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann Neurol. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- 15.Thal D.R., Rüb U., Orantes M., Braak H. Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 16.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 17.Douaud G., Menke R.L., Gass A., Monsch A.U., Rao A., Whitcher B. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. J Neurosci. 2013;33:2147–2155. doi: 10.1523/JNEUROSCI.4437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris J.C. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9:173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Psychological Corporation; San Antonio, Texas: 1987. WMS-R: Wechsler memory Scale-Revised. [Google Scholar]
- 20.Crane P.K., Carle A., Gibbons L.E., Insel P., Mackin R.S., Gross A. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons L.E., Carle A.C., Mackin R.S., Harvey D., Mukherjee S., Insel P. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chhatwal J.P., Schultz A.P., Marshall G.A., Boot B., Gomez-Isla T., Dumurgier J. Temporal T807 binding correlates with CSF tau and phospho-tau in normal elderly. Neurology. 2016;87:920–926. doi: 10.1212/WNL.0000000000003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crary J.F., Trojanowski J.Q., Schneider J.A., Abisambra J.F., Abner E.L., Alafuzoff I. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack C.R., Jr., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher E., Knaack A., Singh B., Lloyd E., Wu E., Carmichael O. Combining boundary-based methods with tensor-based morphometry in the measurement of longitudinal brain change. IEEE Trans Med Imaging. 2013;32:223–236. doi: 10.1109/TMI.2012.2220153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher E. Using prior information to enhance sensitivity of longitudinal brain change computation. In: Chen C.H., editor. Frontiers of Medical Imaging. World Scientific Publishing; Singapore: 2014. pp. 63–81. [Google Scholar]
- 27.Fletcher E., Villeneuve S., Maillard P., Harvey D., Reed B., Jagust W. Beta-amyloid, hippocampal atrophy and their relation to longitudinal brain change in cognitively normal individuals. Neurobiol Aging. 2016;40:173–180. doi: 10.1016/j.neurobiolaging.2016.01.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S.M., De Stefano N.D., Jenkinson M., Matthews P. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr. 2001;25:466–475. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher E., Carmichael O., DeCarli C. 2012. MRI non-uniformity correction through interleaved bias estimation and B-spline deformation with a template; pp. 106–109. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher E., Singh B., Harvey D., Carmichael O., DeCarli C. 2012. Adaptive image segmentation for robust measurement of longitudinal brain tissue change; pp. 5319–5322. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen G.E., Johnson H.J. Consistent image registration. IEEE Trans Med Imaging. 2001;20:568–582. doi: 10.1109/42.932742. [DOI] [PubMed] [Google Scholar]
- 32.Hua X., Leow A.D., Parikshak N., Lee S., Chiang M.-C., Toga A.W. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer's disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage. 2008;43:458–469. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D.Y., Fletcher E., Carmichael O.T., Singh B., Mungas D., Reed B. Sub-regional hippocampal injury is associated with fornix degeneration in Alzheimer's disease. Front Aging Neurosci. 2012;4:1. doi: 10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Zhang J., Oishi K., Faria A.V., Jiang H., Li X. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aljabar P., Heckemann R.A., Hammers A., Hajnal J.V., Rueckert D. Multi-atlas based segmentation of brain images: atlas selection and its effect on accuracy. NeuroImage. 2009;46:726–738. doi: 10.1016/j.neuroimage.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 38.R-Core-Team . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ Available at: [Google Scholar]
- 39.Jack C.R., Vemuri P., Wiste H.J., Weigand S.D., Aisen P.S., Trojanowski J.Q. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68:1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Benzinger T.L., Su Y., Christensen J., Friedrichsen K., Aldea P. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. JAMA Neurol. 2016;63110:1–8. doi: 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockhart S.N., Schöll M., Baker S.L., Ayakta N., Swinnerton K.N., Bell R.K. Amyloid and tau PET demonstrate region-specific associations in normal older people. NeuroImage. 2017;150:191–199. doi: 10.1016/j.neuroimage.2017.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho H., Choi J.Y., Hwang M.S., Kim Y.J., Lee H.M., Lee H.S. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer's disease spectrum. Ann Neurol. 2016;80:1–12. doi: 10.1002/ana.24711. [DOI] [PubMed] [Google Scholar]
- 43.Raz N., Rodrigue K.M. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.