Abstract
We evaluated the antioxidant and porcine pancreatic lipase inhibition (PPLI) activities of 90 plants extracts. The antioxidant activity was measured using the free-radical scavenging capacity (DPPH) and reducing power (RP) assays. The pancreatic lipase inhibition assay was used to determine the PPLI activity of plant extracts. Among the 90 plant extracts examined, 41.0 % crude extracts showed antilipase activity of more than 50%. The most active plants by means of IC50 value were Camellia sinensis (0.5 mg/ml), Ceratonia siliqua (leaves) (0.8 mg/mL), Curcuma longa (0.8 mg/mL), Sarcopoterium spinosum (1.2 mg/mL), and Mentha spicata (1.2 mg/mL). The antioxidant activity of plant extracts using the DPPH and RP assays reveals comparable results. The most active antioxidant extracts using both assays were the leaves and fruit epicarp of Rhus coriaria, areal parts of Sarcopoterium spinosum, and leaves of Ceratonia siliqua. Our results suggest natural resources that possess strong antioxidant and pancreatic lipase inhibitory activities with potential applications in the treatment and prevention of obesity and overweight. The extracts of Camellia sinensis, Ceratonia siliqua, Curcuma longa, Sarcopoterium spinosum, and Mentha spicata were proved to have a great potential as antioxidants and antiobesity agents.
1. Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. Body mass index (BMI) is a crude population measure of obesity that is commonly used to classify overweight and obesity in adults; it is defined as a person's weight in kilograms divided by the square of their height in meters (kg/m2). A person with a BMI of 30 or more is generally considered obese, while a person with a BMI equal to or more than 25 is considered overweight [1].
The prevalence of obesity differs from one country to another and depends on several factors including gender, age, educational accomplishment, annual household income, employment status, and social class [2, 3]. Obesity is considered an extremely costly health problem, the direct medical cost of overweight and obesity combined is approximately 5.0% to 10% of US health care spending [4].
According to the recent WHO report, more than 1.9 billion adults, 18 years and older, were overweight in 2016, of these over 650 million were obese. Over 340 million children and adolescents aged 5-19 were overweight or obese, while 41 million children under the age of 5 were overweight or obese in 2016. Most of the world's population lives in countries where overweight and obesity kill more people than underweight; obesity has reached epidemic proportions globally, with about 2.8 million people dying each year as a result of being overweight or obese [1].
Overweight and obesity were once considered a high-income countries problem; however, it is now also prevalent in low- and middle-income countries [1]. The factors leading to this widespread increase in obesity have been suggested to include economic growth, modernization, westernization of lifestyles (high calorie diet including processed foods higher in fats and refined sugars and decrease in exercise levels), and the globalization of food markets [5, 6]. Other factors may also contribute to this problem including familial susceptibility, endocrine disorders, and environmental factors [7], with women being suggested to be especially at risk [8, 9].
In Palestine, the prevalence of overweight and obesity was estimated as 58.7% and 71.3% among men and women, respectively [10]. This can be attributed to several factors including decreased physical activity, increased consumption of high caloric foods, particularly with an increase in energy coming from fat, smoking, and urbanization [11]. A cross-sectional study was carried out in Palestine to investigate the prevalence of overweight and obesity among school children aged from 6-12 years. The study revealed that the prevalence of overweight and obesity among male students was 13.3% and 7.9%, respectively, while it was 13.6% and 4.9% among female students, respectively [12].
Different powerful synthetic chemical drugs used against obesity are available in the pharmaceutical market; the FDA has approved five prescription medications for long-term use for the treatment of overweight and obesity, including orlistat, lorcaserin, phentermine-topiramate, naltrexone-bupropion, and liraglutide [13]. However, the cost of these products generally keeps them out of reach of most people; more importantly, many of these products have adverse side effects including cardiovascular events and strokes, coughing, dizziness, mouth dryness, anxiousness, fatigue, flatulence, headache, insomnia, leakage of oily stools, nausea, and hepatic adverse effects [14–17]. That is the reason for the withdrawal from the market of some older drugs such as Sibutral, rimonabant, isomeride, ponderal, and Xenical [16, 18]. Hence, the use of naturally occurring inhibitors is considered to be safer [19].
Pancreatic lipase plays a key role in the efficient digestion of triglycerides [20]. Lipases are involved in the hydrolysis of glycerides to glycerol and free fatty acids. The enzyme inhibition is one of the approaches used to treat obesity due to the fact that 50-70 % of total dietary fat hydrolysis was performed by pancreatic lipase [21].The mechanism involves inhibition of dietary triglyceride absorption, as this is the main source of excess calories [22]. Besides, pancreatic lipase inhibition does not alter any central mechanism which makes it an ideal approach for obesity treatment [23]. This enzyme has been widely used for the determination of the potential efficacy of natural products as antiobesity agents [15]. Orlistat is the synthetic clinically approved drug used for obesity management. This molecule acts by the inhibition of PL activity and reduction of triglyceride absorption, and its long-term administration accompanying an energy restricted diet results in weight loss [24].
The impact of overweight and obesity from a public health perspective is enormous and continue to increase. Numerous studies have verified the association of overweight and obesity in the development of different metabolic disorders including diabetes mellitus, atherosclerosis, cardiovascular diseases, hypertension, and cancer [25–27]. It is therefore essential to develop ways of preventing more people from becoming obese by finding natural inhibitors of digestion and absorption of dietary lipids which reduce energy intake through gastrointestinal mechanisms without altering any main mechanisms [22].
Plant derived products (fruits and vegetables) constitute an important part of the human diet all over the world and provide nutrients that are essential for life. Plants contain nonnutrient biologically active phytochemicals including polyphenols and anthocyanins, which are involved in a wide range of health benefits such as decreasing the rates of cardiovascular diseases, lowering the risk of cancer, stroke, and type 2 diabetes, and preventing obesity [28, 29]. Antioxidant-rich fruits help in the prevention of free-radical induced oxidative stress [30–32].
Plant derived products may also contain phytochemicals that act as enzyme inhibitors [33]. These compounds have the ability to bind to enzymes and inhibit their activity. The presence of inhibitors of carbohydrate and glycerides hydrolyzing enzymes such as α-glucosidase, α-amylase, and lipase in plant derived products helps in the control of blood glucose level in patients with type 2 diabetes and to prevent obesity [20, 34].
Knowledge of herbs has been inherited and transferred from generation to generation for thousands of years. In Palestine as in many other countries herbal medicine is still the primary form of therapy in the practiced traditional medical systems [35–39]. Three hundred ninety-six plant species have been reported to be used in Traditional Arabic Palestinian Herbal Medicine (TAPHM) for the treatment of various diseases [40]; of these 39 plants were reported to be used for the treatment of obesity [41] (Table 1). Accordingly, the aim of this study was to evaluate in vitro the antioxidant and PPL inhibitory activities of some Palestinian medicinal plants that have been reported in TAPHM for the treatment of obesity and other chronic diseases and to explore newly potent and safe natural therapeutic agents for the treatment of obesity.
Table 1.
Plants used in Traditional Arabic Palestinian Herbal Medicine for the treatment of obesity.
| Plant Derived Product (Family) | English Common Name | Plant Part | Mode of Preparation |
|---|---|---|---|
| Alchemilla xanthochlora Rothm. (Alchemilla vulgaris auct.) (Rosaceae) | Lion's Foot, Lady's – Mantle | AP | (i) A decoction is prepared from the plant; take 1 cup twice daily. (ii) A decoction is prepared from plant mixed with rosemary, and wild thyme. 1-2 cups are taken daily |
|
| |||
| Allium cepa L. (Liliaceae) | Onion | FLE | (i) Add 1 teaspoon of the plant juice to a cup of fruit juice to sweeten the taste, and drink 1 cup daily |
|
| |||
| Allium sativum L. (Liliaceae) | Garlic | GLV | (i) Eaten raw before breakfast |
|
| |||
| Brassica oleracea L. (Brassicaceae) | Wild Cabbage | AP | (i) Eaten raw as salad, or cooked as vegetables. (ii) A decoction is prepared from the plant leaves. 1 cup is taken daily for 4-5 months |
|
| |||
| Brassica oleracea var. botrytis L. (Brassicaceae) | Cauliflower | FR | (i) Eaten raw as salad |
|
| |||
| Camellia sinensis (L.) Kuntze (Theaceae) | Tea | LE | (i) A standard decoction is prepared from the plant. Take 2 cups daily |
|
| |||
| Cassia senna L. (Fabaceae) | Senna | LE, SD | (i) Mix equal amounts of the leaves of senna, cumin, fennel, and anise leaves; eat one teaspoon daily after lunch. (ii) A decoction is prepared from the leaves or the seeds; 1 cup is taken daily. |
|
| |||
| Cichorium pumilum Jacq. (Asteraceae) | Dwarf Chicory | AP | (i) A decoction is prepared from the leaves. Take 3 cups daily. (ii) Smash 5 teaspoons of the roots, mix with 1 L of water, and boil for 15 minutes. 1 cup is taken before meals |
|
| |||
| Cinnamomum verum J. Presl (Lauraceae) | Cinnamon Tree | BRK | (i) A standard decoction is prepared from the plant. Take 2 cups daily |
|
| |||
| Citrullus lanatus (Thunb.) Matsum & Nakai (Cucurbitaceae) | Watermelon | FR | (i) Eaten raw between meals |
|
| |||
| Citrus limon (L.) Burm. Fil (Rutaceae) | Lime, Limon Tree | FR | (i) A juice is prepared from the fruit; one teaspoon is taken daily before breakfast (ii) A standard decoction is prepared from the leaves. 1 cup is taken daily before breakfast. |
|
| |||
| Citrus paradisi Macfad. (Rutaceae) | Grapefruit | FR | (i) Fruits are eaten raw or drunk as a juice 1- 2 cups daily before breakfast |
|
| |||
| Crataegus aronia (L.) Bosc. ex DC. (Rosaceae) | Hawthorn | LE | (i) A decoction is prepared from the leaves. 1-3 cups are taken daily |
|
| |||
| Cucumis sativus L. (Cucurbitaceae) | Cucumber | FR | (i) Eaten raw between meal |
|
| |||
| Cuminum cyminum L. (Apiaceae) | Cumin | SD | (i) Mix equal amounts of the seeds of cumin, fennel, anise, and senna leaves; eat one teaspoon daily after lunch. (ii) Soak the ground seeds overnight in boiled water and lemon. Drink the filtrate in the morning before breakfast for a month |
|
| |||
| Cynara scolymus L. (Asteraceae) | Artichoke | FR | (i) Fruits are eaten raw as salad |
|
| |||
| Eriobotrya japonica L. (Rosaceae) | Medlar Tree | LE | (i) A decoction is prepared from the plant leaves and olive leaves. Drink 1 cup twice a day. |
|
| |||
| Eruca sativa Miller (Brassicaceae) | Garden Rocket | AP | (i) Eaten raw with salad |
|
| |||
| Foeniculum vulgare Miller (Apiaceae) | Fennel | LE | (i) Leaves are eaten with salad. (ii) Seeds are boiled in water for 20 minutes; drink 2 cups daily for 3-4 weeks |
|
| |||
| Lactuca sativa L. (Asteraceae ) | Lettuce | AP | (i) Eaten fresh with salad. |
|
| |||
| Lupinus albus L. (Fabaceae) | Lupine | SD | (i) Seeds are soaked in water for few days and then eaten raw. (ii) 1 teaspoon of the grinded seeds is taken daily. (iii) The ground seeds are mixed with fenugreek seeds and honey; 1 teaspoon is taken daily. |
|
| |||
| Malva sylvestris L. (Malvaceae) | Common Mallow | LE | (i) Cooked as vegetables |
|
| |||
| Matricaria aurea (L.) Sch. Bip. (Asteraceae ) | Golden Cotula | AP | (i) Leave are soaked in hot water for 2 hours. Drink 1 cup twice daily. (ii) Add 3 teaspoons of the flowers to boiling water, filtrate, and have 1 cup twice a day for 1 month |
|
| |||
| Mentha spicata L. (Lamiaceae) | Peppermint | AP | (i) A decoction is prepared from the plant. 1 cup is taken when needed |
|
| |||
| Olea europaea L. (Oleaceae) | Olive | LE | (i) A decoction is prepared from the plant leaves and meddler tree leaves. Drink 1 cup twice a day. |
|
| |||
| Origanum syriacum L. (Lamiaceae) | Wild Thyme, Mother of Thyme | AP | (i) A decoction is prepared from the plant; take 1 cup twice daily. (ii) A decoction is prepared from plant mixed with Lion's Foot, rosemary, and wild thyme. 1-2 cups are taken daily |
|
| |||
| Paronychia argentea Lam. (Caryophyllaceae) | Silvery Whitlow- Wart | AP | (i) A decoction is prepared from plant mixed with lion's foot, rosemary, and wild thyme. 1-2 cups are taken daily |
|
| |||
| Petroselinum sativum Hoffm. (Apiaceae) | Parsley | AP | (i) A standard decoction is prepared from the plant; take 1-3 cups/day. (ii) Eaten raw with salad |
|
| |||
| Pimpinella anisum L. (Apiaceae) | Anise | SD | (i) Mix equal amounts of the seeds of anise, cumin, fennel, and senna; eat one teaspoon daily after lunch. |
|
| |||
| Pistacia palaestina Boiss. (Anacardiaceae) | Palestinian pistachio | LE | (i) A standard decoction is prepared from the plant; take 2 cups three time a day |
|
| |||
| Portulaca oleracea L. (Portulacaceae) | Purslane | SD | (i) A standard decoction is prepared from the seeds; take 1-2 cups daily |
|
| |||
| Punica granatum L. (Punicaceae) | Pomegranate | HS | (i) The husk is dried, ground, and then soaked in water. 1 cup is taken daily. |
|
| |||
| Pyrus malus L. (Rosaceae) | Apple | FR | (i) Add one teaspoon of apple vinegar to a glass of water; drink 3 times daily after meals. (ii) Drink 1 teaspoon of apple vinegar before breakfast. |
|
| |||
| Rosmarinus officinalis L. (Lamiaceae) | Rosemary | AP | (i) A decoction is prepared from the plant; take 1 cup twice daily. (ii) A decoction is prepared from plant mixed with Lion's Foot, Silvery Whitlow-Wart, and wild thyme. 1-2 cups are taken daily |
|
| |||
| Salvia fruticosa Mill. (Lamiaceae) | Common Sage | LE | (i) A standard decoction is prepared from the plant. Take 2-3 cups daily for a month. |
|
| |||
| Teucrium capitatum L. (T. polium L.) (Lamiaceae) | Cat Thyme | LE | (i) A standard decoction is prepared from the plant; take 1 cup/day when needed. |
|
| |||
| Trigonella berythea Boiss. & Blanche(T. foenum- graecum L.) (Fabaceae) | Fenugreek | SD | (i) A decoction is prepared from 1 teaspoon of the seeds in 500 ml of water; take 1-2 cups daily. (ii) The ground seeds are mixed with fenugreek seeds and honey; 1 teaspoon is taken daily. |
|
| |||
| Varthemia iphionoides Boiss & Blanche (Chiliadenus iphionoides)(Asteraceae) | Common Varthemia | AP | (i) A standard decoction is prepared from the plant. 1 cup is taken twice a day |
|
| |||
| Zingiber officinale Rose. (Zingiberaceae) | Ginger | Rz | (i) A decoction is prepared from the rhizomes. 1 cup is taken daily |
∗RZ: rhizome; HS: husk; AP: aerial parts; LE: leave; FR: fruits; SD: seeds; BRK: bark; FLE: fleshy leaves; GLV: gloves.
2. Materials and Methods
2.1. Plant Materials
Plants material were either collected from Nablus area in Northern Palestine or purchased from traditional drug stores (Attarin Shops) in Nablus city. Taxonomic identification of the plant materials was kindly confirmed by Professor M. S. Ali-Shtayeh, plant biologist, BERC. The voucher specimens have been deposited at the Herbarium of the Biodiversity & Biotechnology Research Institute, BERC, Til, Nablus, Palestine.
Seventy-eight plant species used in TAPHM, of which 39 species were reported to be used for the treatment of obesity (Table 1) [41], have been analyzed in this study for their antioxidant and pancreatic lipase inhibition potentials. Plant parts were dried in the shade, ten grams from each dried material was ground and incubated separately with 100 ml of 70% ethanol at 35°C for 3 hours. The extracts were then filtered and evaporated under vacuum at 50°C to dryness. The powdered extracts were stored at −20°C for further analyses.
2.2. Pancreatic Lipase Inhibition Assay
2.2.1. Lipase Preparation
The enzyme solution was prepared immediately before use following the method described by Bustanji et al. [42] with some modifications. Crude porcine pancreatic lipase type II (Sigma, USA, EC 3.1.1.3) was suspended in 20 mM tris-HCl buffer pH 8 to give a concentration of 10 mg/ml. The suspension was mixed using a stirrer for 15 min and centrifuged at 1500 g for 10 min and the clear supernatant was recovered.
2.2.2. Lipase Inhibition Reaction
The ability of the plant extracts to inhibit PPL was measured using the modified method previously reported by Bustanji et al. [42]. The lipase activity was determined by measuring the hydrolysis of p-nitrophenol butyrate (pNPB) to p-nitrophenol at 410 nm using UV-VIS spectrophotometer. Lipase assays were performed by incubating 200 μl of plant extract (5mg/ml ethanol) with 100 μl of PPL solution for 5 min at 37°C; then 10μL of the pNPB substrate (100 mM in acetonitrile) was added. The volume was completed to 1 mL using the buffer. The release of pNPB is estimated as the increment increase in absorbance against blank. The percentage of residual activity of PL was determined by comparing the lipase activity in the presence and the absence of the tested inhibitors. Orlistat (200 μg/ml) was used as a positive standard inhibitor control, whereas plant extract was replaced by ethanol to be used as negative control. All experiments were repeated twice.
Inhibition of the lipase activity was expressed as the percentage decrease in the activity when PPL was incubated with the test compounds. Lipase inhibition (%) was calculated according to the following formula:
| (1) |
2.2.3. Minimum Inhibitory Concentration IC50
The concentration of most active plant extracts giving 50% lipase inhibition (IC50) was performed using several concentrations of the extracts, ranging from 0.156 to 5.0 mg/mL and the percentages of residual activity of PL data were used to evaluate the IC50 values. The IC50 value of the extract was calculated from the least squares regression line of the semilogarithmic plot against percentage inhibition curve using Microsoft Excel version 10 software. All assays were repeated twice on different occasions, and the calculated inhibition percentages were the mean of 2 observations.
2.3. Antioxidant Capacity Assays
2.3.1. DPPH (α, α-Diphenyl-β-picrylhydrazyl) Assay for Scavenging Activity
DPPH radical scavenging activity was determined according to the method described by Choi et al. [43]. Briefly, 1 ml of the ethanolic plants extract (1mg/ml) or standards (BHT and ascorbic acid) were mixed with 1.5 ml (0.02 %) of DPPH solution in methanol. The mixture was incubated in the dark for 30 min, and the absorbance was measured at 517 nm in the spectrophotometer (6800 UV-VIS spectrophotometer) using methanol as blank. The percentage of scavenging of DPPH radicals was calculated by using the following formula [27]:
| (2) |
where AS is the absorbance of the sample; AC is the absorbance of the negative control (methanol without the sample).
The IC50 value, which is defined as the concentration of extract (mg mL-1) required to scavenge 50 % of DPPH, was calculated using the graph by plotting inhibition percentage against serial extract concentration (1.0-0.016 mg/ml) [44]. The antioxidant activity index (AAI) was calculated by dividing the DPPH final concentration (0.1 mg/ml) by the IC50 of the plant extract [45].
2.3.2. Reducing Power Capacity Assessment
The reducing power (RP) was evaluated following the method of Oyaizu [46]. In this technique, 1.0 ml of various concentrations of plants extracts (1.0-0.016 mg/ml) were mixed with 2.5 ml of potassium buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide [K3Fe (CN) 6] (1 %) solution. The mixture was incubated for 30 min at 50°C, and then 2.5 ml of trichloro acetic acid (10 %) solution was added to the mix. The total mixture was centrifuged at 3000 g for 10 min. Then 2.5 ml supernatant solution was withdrawn from the mixture and mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (0.1 %) solution. Blank sample was similarly prepared by replacing extract with 60% ethanol, to calibrate the instrument (6800 UV-VIS spectrophotometer). The absorbance of the solution was measured at 700 nm; higher absorbance indicates higher RP. BHT and ascorbic acid were used as standards.
The effective concentration at which the absorbance was 0.5 for reducing power (EC50) was obtained by interpolation from linear regression analysis [47]. Increased absorbance of the mixture reaction indicates increasing RP [48].
2.4. Statistical Analysis
All results were expressed as mean ± standard deviation (n=3). Significance of differences from the control was determined by Duncan's test and a p value < 0.05 was considered significant.
3. Results
Table 2 summarizes the results of antioxidant and antilipase inhibitory assays using ethanolic extracts from 78 medicinal plants (90 plants extracts) commonly administrated orally or topically to treat diverse diseases.
Table 2.
Antioxidant and pancreatic lipase inhibition activity of Palestinian plants used in Traditional Arabic Palestinian Herbal Medicine (TAPHM).
| Scientific name (Family) | Common name | Plant part | Arabic name | Antioxidant capacity | Lipase inhibition | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | Reducing Power | |||||||||
| % Inhibition (1mg/ml) | IC50 (mg/ml) | AAI | ABS-700 nm | EC50 (mg/ml) | % Inhibition (5mg/ml) | IC50 (mg/ml) | ||||
| Camellia sinensis (L) Kuntze (Theaceae) | Tea | LE | Shai | 86.2±4.1 | 0.10±0.00 | 1±0.02 | 2.26±0.1 | 0.11±0.0 | 99.0±1.5 | 0.45±0.2 |
| Ceratonia siliqua L. (Caesalpiniaceae) | Carob | LE | Kharrob | 91.2±3.1 | 0.04±0.00 | 2.63±0.1 | 2.6±0.1 | 0.05±0.1 | 95.4±8.0 | 0.76±0.4 |
| Curcuma longa L. (Zingiberaceae) | Turmeric | RZ | Kurkum | 79.5±0.9 | 0.39±0.10 | 0.26±0.05 | 0.87±0.03 | 0.59±0.1 | 97.4±0.8 | 0.82±0.3 |
| Sarcopoterium spinosum (L.) Spach. (Rosaceae) | Shrubby Barnet | AP | Natesh | 68.2±7.7 | 0.02±0.01 | 4.08±0.9 | 2.56±0.03 | 0.04±0.0 | 95.3±15.0 | 1.17±0.2 |
| Mentha spicata L. (Lamiaceae) | Peppermint | AP | Na'na' | 74.0±0.2 | 0.21±0.03 | 0.47±0.05 | 2.04±0.03 | 0.15±0.02 | 92.5±2.0 | 1.19±1.0 |
| Rhus coriaria L. (Anacardiaceae) | Sicilian Sumac | LE | Summaq | 95.1±3. | 0.02±0.01 | 4.67±0.1 | 2.61±0.03 | 0.005±0.0 | 95.1±7.6 | 1.24±0.2 |
| Dittrichia viscosa (L.) Greuter (Asteraceae) | Inula | AP | Irq tayyon | 86.5±0. | 0.11±0.01 | 0.94±0.1 | 2.33±0.1 | 0.13±0.01 | 78.5±14.8 | 1.28±0.3 |
| Rosmarinus officinalis L. (Lamiaceae) | Rosemary | AP | Hasalban | 89.9±0.5 | 0.09±0.02 | 1.11±0.3 | 2.28±0.08 | 0.16±0.01 | 94.9±4.1 | 1.31±0.6 |
| Eruca sativa Miller (Brassicaceae) | Garden Rocket | AP | Jarjeer | 21.7±0.5 | NA∗∗ | NA | 0.8±0.03 | NA | 74.9±2.6 | 1.45±0.3 |
| Elaeagnus angustifolia L. (Elaeagnaceae) | Narrow-Leaved Oleaster | LE | Zaizafon | 51.9±1.8 | NA | NA | 0.46±0.1 | NA | 96.8±2.2 | 1.51±0.2 |
| Teucrium capitatum L. (T. polium L.) (Lamiaceae) | Cat Thyme | AP | Je'det Alsobian | 85.3±6.1 | 0.12±0.01 | 0.82±0.1 | 1.66±0.02 | 0.41±0.01 | 79.2±14.5 | 1.65±0.5 |
| Coridothymus capitatus (L.) Reichb. (Lamiaceae) | Capitate Thyme | LE | Za'tar Farsi | 88.0±0.2 | 0.07±0.01 | 1.39±0.2 | 2.43±0.02 | 0.14±0.01 | 78.1±22.2 | 1.92±0.1 |
| Malva sylvestris L. (Malvaceae) | Common Mallow | AP | Khubeizeh | 21.3±1.2 | NA | NA | 0.37±0.01 | NA | 72.7±5.6 | 1.97±0.9 |
| Ziziphus sativa Gaertn. (Rhamnaceae) | Jujube | LE | Inab | 84.9±0.2 | 0.10±0.01 | 0.96±0.1 | 1.87±0.4 | 0.18±0.1 | 84.0±8.1 | 1.99±0.8 |
| Punica granatum L. (Punicaceae) | Pomegranate | HS | Rumman | 78.7±3.30 | 0.05±0.01 | 2.04±0.4 | 2.62±0.01 | 0.03±0.0 | 92.5±5.8 | 2±0.2 |
| Cassia senna L. (Fabaceae) | Senna | LE | Sanamaki | 31.2±0.12 | NA | NA | 0.47±0.02 | NA | 81.8±12.8 | 2.32±0.5 |
| Citrus limon (L.) Burm. Fil (Rutaceae) | Lime, Limon Tree | LE | Laimoun | 84.7±2.10 | 0.43±0.10 | 0.23±0.03 | 0.52±0.06 | 1.19±0.02 | 82.2±10.8 | 2.46±0.2 |
| Cynara scolymus L. (Asteraceae) | Artichoke | FR | Kharshouf | 3.8±0.51 | NA | NA | 0.13±0.01 | NA | 73.5±1.7 | 2.68±0.2 |
| Crataegus aronia (L.) Bosc. ex DC. (Rosaceae) | Hawthorn | LE | Za'rour Sha'ek | 85.0±0.31 | 0.51±0.06 | 0.2±0.1 | 0.73±0.03 | 0.71±0.02 | 72.7±8.9 | 2.73±0.04 |
| Portulaca oleracea L. (Portulacaceae) | Purslane | SD | Baqleh | 88.9±0.71 | 0.41±0.08 | 0.25±0.03 | 1.28±0.01 | 0.33±0.01 | 72.2±4.8 | 2.77±0.6 |
| Juglans regia L. (Juglandaceae) | Walnut | LE | Jouz | 82.2±3.22 | 0.14±0.04 | 0.71±0.2 | 1.68±0.03 | 0.39±0.03 | 69.1±8.3 | 2.81±0.2 |
| Arum palaestinum Boiss. (Araceae) | Palestinian Arum | LE | Lufe | 83.8±0.58 | 0.49±0.03 | 0.2±0.0 | 0.67±0.01 | 0.77±0.00 | 76.5±9.2 | 2.88±0.8 |
| Quercus calliprinos Webb (Fagaceae) | Kermes Oak | LE | Sendian | 76.1±3.20 | 0.08±0.06 | 1.23±1.5 | 2.66±0.01 | 0.05±0 | 71.2±9.7 | 2.88±0.1 |
| Cichorium pumilum Jacq. (Asteraceae) | Dwarf Chicory | LE | Hindba' | 82.7±1.60 | 0.6±0.07 | 0.17±0.03 | 0.79±0.01 | 0.7±0.02 | 71.3±10.2 | 2.94±0.8 |
| Foeniculum vulgare Miller (Apiaceae ) | Fennel | LE | Shoumer | 74.9±0.7 | 0.37±0.03 | 0.27±0.1 | 0.74±0.02 | 0.69±0.01 | 83.2±8.5 | 3.01±0.2 |
| Cuminum cyminum L. (Apiaceae ) | Cumin | SD | Kamoon | 45.0±0.5 | NA | NA | 0.46±0.01 | NA | 72.9±5.2 | 3.01±0.0 |
| Ziziphus spina-christi (L.) Desf. (Rhamnaceae) | Christ's Thorn Jujube | LE | Seder | 86.2±2.6 | 0.12±0.02 | 0.82±0.2 | 1.51±0.13 | 0.4±0.02 | 70.1±6.9 | 3.11±0.1 |
| Cynara scolymus L. (Asteraceae) | Artichoke | FR | Kharshouf | 8.1±0.2 | NA | NA | 0.17±0.03 | NA | 75.7±2.4 | 3.16±0.5 |
| Portulaca oleracea L. (Portulacaceae) | Purslane | AP | Baqleh | 82.9±0.9 | 0.53±0.07 | 0.19±0.01 | 1.21±0.02 | 0.46±0.00 | 72.9±11.8 | 3.21±0.3 |
| Satureja thymbra L. (Lamiaceae) | Summer Savory | AP | Za'tar | 82.9±2.8 | 0.34±0.29 | 0.29±0.4 | 1.93±0.09 | 0.18±0.01 | 60.9±9.5 | 3.31±0.1 |
| Ceratonia siliqua L. (Caesalpiniaceae) | Carob | SD | Kharrob |
89.0±1.4 | 0.60±0.02 | 0.17±0.01 | 1.42±0.11 | 0.35±0.03 | 68.7±6.2 | 3.3±0.4 |
| Alchemilla xanthochlora Rothm. (Alchemilla vulgaris auct.) (Rosaceae) | Lion's Foot, Lady's – Mantle | LE | Rijl Alasad, Kaf Alasad | 19.0±0.23 | NA | NA | 0.43±0.01 | Na | 77.4±19.1 | 3.36±0.1 |
| Salvia fruticosa Mill. (Lamiaceae) | white sage, Common Sage, Garden Sage | AP | Maryemeyeh | 91.0±0.6 | 0.13±0.00 | 0.76±0.02 | 1.51±0 | 0.32±0.1 | 65.8±2.1 | 3.45±0.4 |
| Psidium guajava L. (Myrtaceae) | Guava | LE | Guava | 69.6±0.2 | 0.09±0.01 | 1.11±0.1 | 2.29±0.02 | 0.12±0.02 | 55.4±2.8 | 3.47±1.6 |
| Trigonella berythea Boiss. & Blanche(T. foenum- graecum L.) (Fabaceae) | Fenugreek Seed | SD | Hilbeh | 60.2±7.6 | 1.02±0.04 | 0.1±0.0 | 0.22±0.01 | 63.8±5.3 | 3.67±0.1 | |
| Paronychia argentea Lam. (Caryophyllaceae) | Silvery Whitlow- Wart | AP | Rijl Alhamameh | 78.7±0.7 | 0.38±0.02 | 0.26±0.1 | 1.05±0.01 | 0.43±0.01 | 68.4±17.1 | 4.23±1.1 |
| Ficus carica L. (Moraceae) | Fig Tree | LE | Teen | 82.2±0.3 | 0.29±0.00 | 0.34±0.0 | 0.71±0.02 | 0.88±0.09 | 49.5±7.9 | NA |
| Capparis spinosa L. (Capparaceae) | Caper Bush, Egyptian Caper | LE | Kappar | 90.47±4.0 | 0.24±0.15 | 0.42±0.3 | 1.09±0.02 | 0.52±0.07 | 49.0±7.7 | NA |
| Solanum nigrum L. (Solanaceae) | Nightshade | AP | Samweh | 83.0±4.0 | 0.44±0.02 | 0.23±0.01 | 0.78±0.13 | 0.8±0 | 46.3±4.5 | NA |
| Origanum majorana L. (Lamiaceae) | Sweet-Marjoram | AP | Mardaqoush | 82.1±0.1 | 0.23±0.12 | 0.44±0.3 | 2.06±0.02 | 0.26±0.02 | 46.1±5.6 | NA |
| Euphorbia hierosolymitana Boiss. (Euphorbiaceae) | Spurge | AP | Halabloub | 90.5±4.7 | 0.33±0.08 | 0.3±0.1 | 0.85±0.02 | 0.59±0.02 | 45.8±2.8 | NA |
| Eucalyptus camaldulensis Dehn. (Myrtaceae) | Red River Gum | LE | Kina | 87.9±4 | 0.09±0.03 | 1.11±0.4 | 2.37±0.02 | 0.15±0.01 | 44.3±5.4 | NA |
| Origanum syriacum L. (Lamiaceae) | Wild Thyme, Mother of Thyme | AP | Za'tar bari | 81.4±1.8 | 0.3±0.08 | 0.33±0.1 | 1.02±0.03 | 0.57±0.02 | 41.5±1.6 | NA |
| Cinnamomum zeylanicum Blume. (Lauraceae) | Cinnamon Tree | BRK | Qerfeh | 86.4±1.9 | 0.06±0.02 | 1.54±0.4 | 2±0.18 | 0.24±0.02 | 40.5±8.6 | NA |
| Matricaria aurea (L.) Sch. Bip. (Asteraceae) | Golden Cotula | AP | Babounej | 75.1±14.6 | 0.39±0.06 | 0.26±0.04 | 0.82±0.02 | 0.69±0.09 | 38.5±2.7 | NA |
| Nigella ciliaris DC. (Ranunculaceae) | Nigella, Black Cumin | SD | Qezha | 34.4±9.0 | NA | NA | 0.19±0.03 | NA | 38.4±3.2 | NA |
| Syzygium aromaticum (L.) Merr. and Perry. (Myrtaceae) | Clove | FLW | Kabsh Koronful | 80.2±2.3 | 0.15±0.01 | 0.65±0.04 | 2.46±0.06 | 0.09±0.01 | 37.1±0.9 | NA |
| Olea europaea L. (Oleaceae) | Olive | LE | Zaitoun | 91.1±2.5 | 0.29±0.02 | 0.35±0.02 | 1.02±0.05 | 0.54±0.04 | 36.8±1.3 | NA |
| Pimpinella anisum L. (Apiaceae ) | Anise | SD | Yansoon | 83.3±4.0 | 0.47±0.01 | 0.21±0.0 | 0.63±0.04 | 1.06±0.12 | 35.4±2.0 | NA |
| Pinus halepensis Mill. (Pinaceae) | Aleppo Pine | AP | Sonawabar halabi | 46.5±7.7 | NA | NA | 0.48±0.06 | NA | 32.5±6.1 | NA |
| Passiflora incarnata L. (Passifloraceae) | Passion flower | LE | Pasiflora | 79.2±6.0 | 0.74±0.04 | 0.13±0.01 | 0.53±0.24 | 1.6±0.61 | 32.3±2.8 | NA |
| Myrtus communis L. (Myrtaceae) | Common Myrtle | LE | Habaq | 81.1±0.5 | 0.17±0.01 | 0.59±0.02 | 1.92±0.24 | 0.25±0.02 | 30.2±0.1 | NA |
| Pistacia palaestina Boiss. (Anacardiaceae) | Palestinian pistachio, Terebinth | LE | Botom Falastini | 89.98±1.1 | 0.17±0.02 | 0.57±0.1 | 1.37±0.12 | 0.32±0 | 29.0±0.2 | NA |
| Retama raetam (Forssk.) Webb (Fabaceae) | Retama, White Broom | AP | Retem | 70.4±4.9 | 0.4±0.06 | 0.23±0.03 | 0.48±0.16 | NA | 28.6±3.9 | NA |
| Rhus coriaria L. (Anacardiaceae) | Sicilian Sumac | EPC | Summaq | 92.8±1.3 | 0.02±0.01 | 4.37±1.32 | 2.66±0.06 | 0.06±0 | 25.5±3.2 | NA |
| Rhus coriaria L. (Anacardiaceae) | Sicilian Sumac | SD | Summaq | 30.2±2.2 | NA | NA | 0.46±0.18 | NA | 23.4±2.3 | NA |
| Lepidium sativum L. | Cress | SD | Rashad | 92.2±0.7 | 0.60±0.08 | 0.18±0.03 | 0.65±0.21 | 0.94±0.06 | 22.9±2.6 | NA |
| Laurus nobilis L. (Lauraceae) | Laurel, Sweet Bay | LE | Ghar | 85.9±0.5 | 0.50±0.01 | 0.2±0.0 | 0.73±0.05 | 0.82±0.07 | 22.5±2.6 | NA |
| Varthemia iphionoides Boiss & Blanche (Chiliadenus iphionoides) (Asteraceae) | Common Varthemia | AP | Kteileh | 84.6±0.9 | 0.40±0.01 | 0.2±0.0 | 1.12±0.01 | 0.4±0.03 | 20.6±5.4 | NA |
| Capparis spinosa L. (Capparaceae) | Caper Bush, Egyptian Caper | FR | Kappar | 20.1±0.2 | NA | NA | 0.43±0.13 | NA | 19.9±19.6 | NA |
| Lupinus albus L.(Fabaceae) | Lupine | SD | Tormous Mor | 16.2±4.1 | NA | NA | 0.06±0.01 | NA | 19.9±6.8 | NA |
| Ceratonia siliqua L. (Caesalpiniaceae) | Carob | FR | Kharrob | 27.0±0.6 | NA | NA | 0.28±0.17 | NA | 17.5±2.5 | NA |
| Ruta chalepensis L. (Rutaceae) | Rue | AP | Figan | 73.8±0.4 | 0.34±0.24 | 0.29±0.28 | 0.35±0.1 | NA | 17.1±2.5 | NA |
| Zingiber officinale Rose. (Zingiberaceae) | Ginger | RZ | Zanjabeel | 79.0±7.8 | 0.77±0.00 | 0.13±0.0 | 0.45±0.01 | NA | 16.7±7.6 | NA |
| Foeniculum vulgare Miller (Apiaceae ) | Fennel | SD | Shoumer | 87.0±0.6 | 0.45±0.00 | 0.22±0.0 | 0.75±0.22 | 0.87±0.08 | 16.5±1.2 | NA |
| Morus nigra L. (Moraceae) | Mulberry | FR | Toot | 69.4±14.3 | 1.47±0.35 | 0.1±0.02 | 0.18±0.01 | NA | 16.1±2.3 | NA |
| Elaeagnus angustifolia L. (Elaeagnaceae) | Narrow-Leaved Oleaster | FR | Zaizafon | 8.6±0.8 | NA | NA | 0.17±0.01 | NA | 15.7±2.4 | NA |
| Cupressus sempervirens L. (Cupressaceae) | Cypress | LE | Saro | 91.48±1.2 | 0.26±0.02 | 0.4±0.03 | 1.5±0.02 | 0.33±0.01 | 15.2±1.0 | NA |
| Thuja occidentalis L. (Cupressaceae) | Tree Of Life | LE | Afs | 82.0±7.1 | 0.33±0.11 | 0.3±0.1 | 0.75±0.23 | 0.58±0.02 | 14.3±11.5 | NA |
| Phaseolus vulgaris L. (Fabaceae) | Common Bean | SD | Fasolia' | 25.0±2.7 | NA | NA | 0.32±0.04 | NA | 13.7±1.1 | NA |
| Cucumis sativus L. (Cucurbitaceae) | Cucumber | FR | Khiar | 1.0±0.0 | NA | NA | 0.07±0.00 | NA | 13.5±2.0 | NA |
| Punica granatum L. (Punicaceae) | Pomegranate | SD | Rumman | 42.9±0.2 | NA | NA | 0.19±0.01 | NA | 12.4±2.7 | NA |
| Petroselinum sativum Hoffm. (Apiaceae ) | Parsley | AP | Baqdounes | 27.7±0.3 | NA | NA | 0.12±0.01 | NA | 12.4±0.7 | NA |
| Citrullus lanatus (Thunb.) Matsum & Nakai (Cucurbitaceae) | Watermelon | FR | Bateekh | 2.8±0.32 | NA | NA | 0.04±0.00 | NA | 11.6±0.5 | NA |
| Brassica oleracea var. botrytis L. (Brassicaceae) | Cauliflower | FR | Qernabeet | 2.0±0.8 | NA | NA | 0.22±0.05 | NA | 11.2±1.1 | NA |
| Brassica oleracea var. botrytis L. (Brassicaceae) | Cauliflower | FR | Qernabeet | 2.0±0.8 | NA | NA | 0.22±0.05 | NA | 11.2±1.1 | NA |
| Aloe vera (L.) Burm. f. (Xanthorrhoeaceae) | Aloe | LE | Sabrah murrah | 31.9±12.0 | NA | NA | 0.18±0.03 | NA | 10.1±0.9 | NA |
| Rubus sanctus Schreb. (Rosaceae) | Blackberry | LE | Oliq | 88.1±4.6 | 0.4±0.16 | 0.24±0.1 | 0.49±0.01 | NA | 10.1±2.8 | NA |
| Cichorium pumilum Jacq. (Asteraceae) | Dwarf Chicory | RT | Hindba' | 4.9±0.2 | NA | NA | 0.13±0.01 | NA | 10.1±0..0 | NA |
| Coriandrum sativum L. (Apiaceae ) | Coriander | SD | Kozbareh | 85.9±5.6 | 0.6±0.02 | 0.16±0.01 | 0.42±0.16 | NA | 9.7±2.2 | NA |
| Sesamum indicum L. (Pedaliaceae) | Sesame | SD | Semsem | 11.0±3.2 | NA | NA | 0.11±0 | NA | 9.5±7.4 | NA |
| Lactuca sativa L. (Asteraceae) | Lettuce | AP | Khass | 28.6±6 | NA | NA | 0.18±0.04 | NA | 7.4±0.4 | NA |
| Brassica oleracea L. (Brassicaceae) | Wild Cabbage | AP | Malfouf | 40.0±10.9 | NA | NA | 0.27±0.06 | NA | 6.3±5.4 | NA |
| Hibiscus sabdariffa L. (Malvaceae) | Roselle | FLW | Karkadaih | 89.1±5.1 | 0.5±0.05 | 0.19±0.02 | 0.62±0.07 | 0.98±0.1 | 6.3±0.4 | NA |
| Citrus paradisi Macfad. (Rutaceae) | Grapefruit | FR | Laimoon Aljeneh | 2.5±0.3 | NA | NA | 0.1±0.01 | NA | 4.4±0.2 | NA |
| Allium cepa L. (Amaryllidaceae) | Onions | FLE | Basal | 18.0±8.1 | NA | NA | 0.1±0.02 | NA | 2.5±1.6 | NA |
| Citrus limon (L.) Burm. Fil (Rutaceae) | Limon Tree | FR | Laimoun | 52.5±0.9 | 3.6±1.9 | 0.03±0.02 | 0.4±0.06 | NA | 2.3±1.2 | NA |
|
Vitis vinifera L. (Vitaceae) |
Grape | FR | Inab | 87.5±0.1 | 0.5±0.0 | 0.2±0.00 | 1.1±0.08 | 0.34±0.1 | 2.0±1.6 | NA |
| Allium sativum L. (Amaryllidaceae) | Garlic | GLV | Thoum | 3.1±5.2 | NA | NA | 0.1±0.04 | NA | 2.0±0.2 | NA |
|
Pyrus malus L. (Rosaceae) |
Apple | FR | Tuffah | 26.1±7.6 | NA | NA | 0.1±0.07 | NA | 1.7±2.4 | NA |
| Orlistat (200 ug/ml) | 92±0.2 | 0.1±0.0 | ||||||||
| Ascorbic acid (100ug/ml) | 89.2±0 | 0.03±0.0 | 3.9±0.01 | 1.5±0.08 | 0.06±0.02 | |||||
| BHT (100ug/ml) | 93.4±0.57 | 0.03±0.0 | 3.2±0.51 | 0.8±0.05 | 0.06±0.00 | |||||
∗RZ: rhizome; HS: husk; AP: aerial parts; LE: leave; FR: fruits; SD: seeds; BRK: bark; FLW: flowers; EPC: epicarp; RT: roots; FLE: fleshy leaves; GLV: gloves. ∗∗NA: not applicable.
Using the pancreatic lipase inhibition assay, the pancreatic lipase inhibition activity was determined by measuring the hydrolysis of pNPB to p-nitrophenol at 410 nm [42]. The antioxidant activity was measured using the free-radical scavenging capacity (DPPH) and RP assays. The RP was evaluated by measuring absorbance at 700 nm after mixing the samples with ferric compounds; higher absorbance indicates higher RP. The scavenging effects on DPPH radicals were determined by measuring the decay in absorbance at 517 nm due to the DPPH radical reduction, indicating the antioxidant activity of the compounds in a short time [49, 50].
Pancreatic lipase inhibition was expressed in percentage (%) and IC50, whereas antioxidant activity was expressed as AAI and EC50 using DPPH and RP capacity assessment techniques, respectively.
Of the 90 ethanolic crude extracts, 31 were prepared from leaves, 23 from aerial parts, 14 from seeds, 12 from fruits, two from each rhizomes, fruit husk, and flowers, and one from the epicarp, root, gloves, and bark (Figure 1). The selection of plants part was primarily dependent on the significant role in traditional medication or cooking purposes [41, 51].
Figure 1.
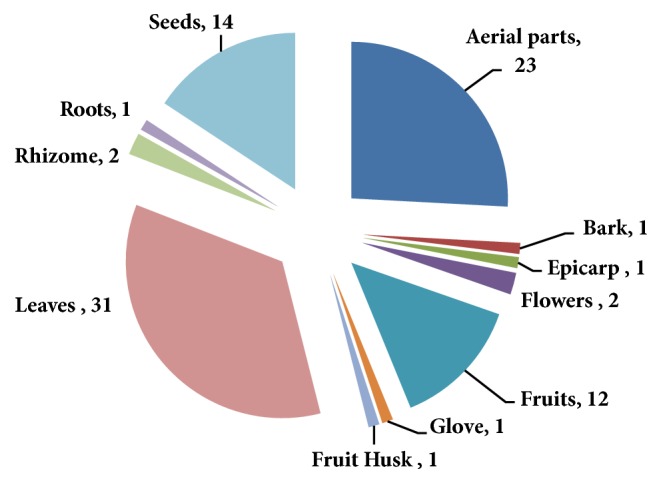
Plant parts used in the study.
3.1. Antioxidant Activity
Antioxidant activity of plant extracts using the DPPH assay ranged between 1.0 and 95.1% for plant extracts at a concentration of 1 mg/ml. Sixty-one extracts exhibited antioxidant activity ≥ 50 %. The IC50 values for the leaves and epicarp of Rhus coriaria, aerial parts of Sarcopoterium spinosum, leaves of Ceratonia siliqua, and the fruit husk of Punica granatum were 0.02, 0.02, 0.02, 0.04, and 0.05 mg/ml (Figure 2), respectively. Twenty-one plant extracts exhibited AAI > 0.5; the antioxidant activity indices (AAIs) for Rhus coriaria leaves and epicarp, Sarcopoterium spinosum, and Ceratonia siliqua were higher or comparable to the AAI of ascorbic acid (3.85) (Table 2). This indicates the high potential for free-radical scavenging of these extracts.
Figure 2.
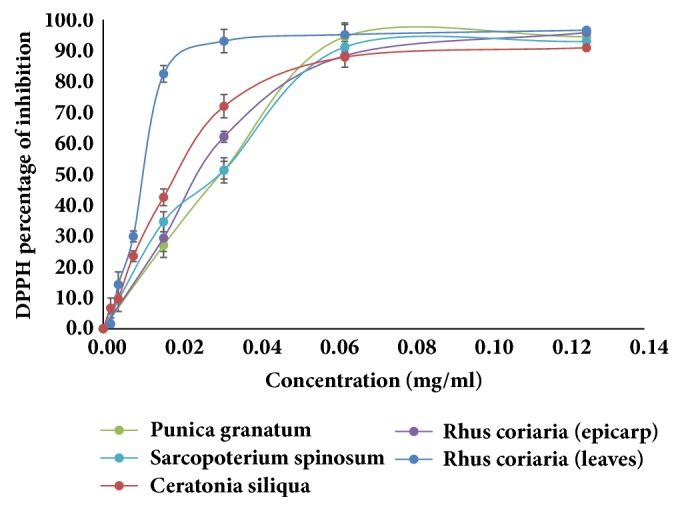
Antioxidant activity of the five most active plants' ethanolic extract using DPPH. Experiments have been performed in triplicate.
The RP was measured by direct electron donation in the reduction of Fe3+(CN−)6–Fe2+(CN−)6. The product was visualized by forming the intense Prussian blue color complex and then the absorbance was measured at the 700 nm; a higher absorbance value indicates a stronger RP of the samples.
Using ferric reducing power assay, the EC50 value for the ethanolic extracts ranged between 1.6 and 0.005 mg/ml. The EC50 of the leaves of Rhus coriaria, fruit husk of Punica granatum, areal parts of Sarcopoterium spinosum, leaves of Quercus calliprinos, leaves of Ceratonia siliqua, and epicarp of Rhus coriaria were 0.005, 0.03, 0.04, 0.05, and 0.06 mg/ml, respectively, whereas the EC50 value for the positive controls ascorbic acid and BHT was 0.057 mg/ml for both controls (Figure 3, Table 2). These results indicate the relatively high ferric reducing activity of these medicinal plant extracts compared to the pure standards ascorbic acid and BHT.
Figure 3.
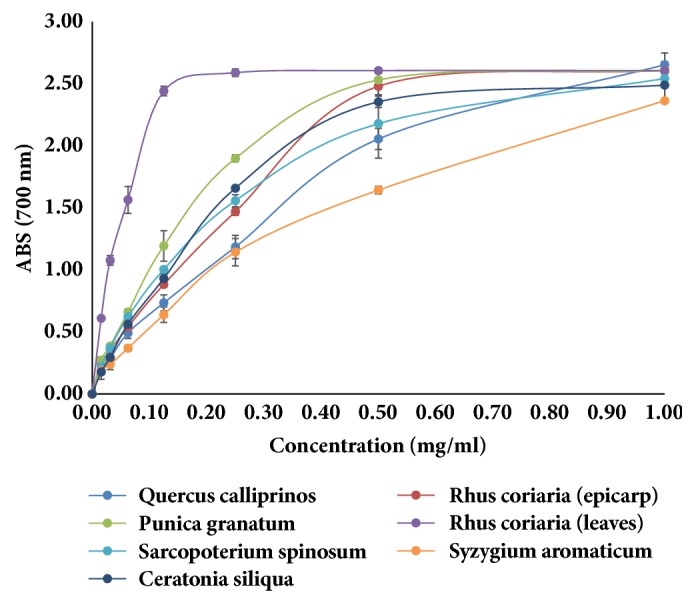
Antioxidant activity of the seven most active plants extracts using reductive potential. Experiments have been performed in triplicate.
3.2. Pancreatic Lipase Enzyme Inhibition Activity
Ninety crude extracts were prepared from natural plant species, either collected from the Nablus area or purchased from traditional drug shops, and their antilipase activity was investigated at a concentration of 5 mg/mL for PPL inhibition. A variety of the tested plant extracts shows a strong inhibitory potential to the pancreatic lipase digestive enzyme (Table 2); the PPL inhibitory activity of the tested plant extracts ranged between 1.7 and 98.97 %. The plant extracts were divided into three categories, which were low (< 30%) inhibition, medium (30-70%) inhibition, and high (> 70%) inhibition when incubated with PPL for 10 min at 37°C. The PPL inhibition assay screening detected 29 extracts exhibiting high (> 70%) inhibition while 23 extracts with medium (30-70%) inhibition and the remaining 38 plant extracts showed low inhibition (< 30%). All plants extracts were set at 5 mg/ml as this concentration could give a consistent result with low standard deviation in the assay.
The significant inhibition of PPL was observed up to 99.0 % with Camellia sinensis leaves, 97.4% with Curcuma longa rhizomes, and 96.8 of Elaeagnus angustifolia leaves. Treatment with orlistat (at final concentration 200 μg/mL) as a positive control, a well-known antilipase agent, significantly inhibited the PPL activity up to 92.0 %. Orlistat, a hydrogenated derivative of lipstatin, is the only pancreatic lipase inhibitor currently approved for a long-term treatment of obesity.
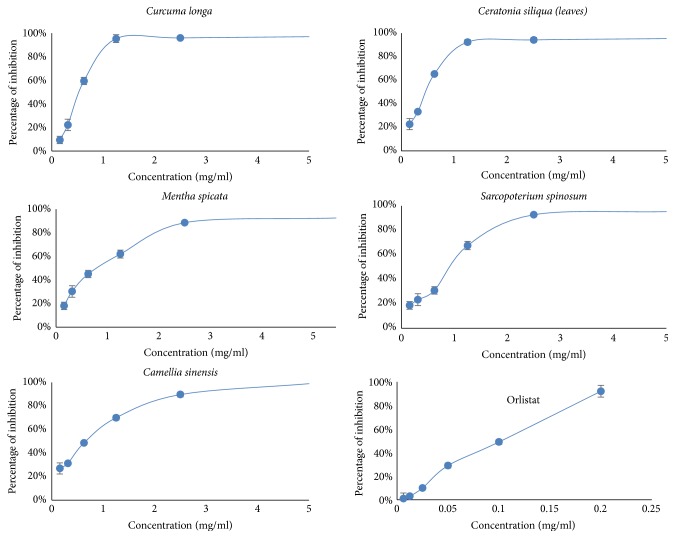
Among the 90 plant extracts examined, 37 (41 %) crude extracts from natural plant species showed antilipase activity of more than 50%. These were further investigated for their PPL inhibitory effects at different concentrations, and a dose-response curve was obtained. Figure 4 presents the top 5 plant extracts in comparison with orlistat as a control. The most active plants by means of IC50 value were Camellia sinensis (0.45 mg/ml), Ceratonia siliqua (leaves) (0.76 mg/mL), Curcuma longa (0.82 mg/mL), Sarcopoterium spinosum (1.17 mg/mL), and Mentha spicata (1.19 mg/mL).
Figure 4.
Porcine pancreatic lipase inhibitory (PPLI) activity of Camellia sinensis, Ceratonia siliqua, Curcuma longa, Sarcopoterium spinosum, and Mentha spicata. Orlistat was used as a positive control. Experiments have been performed in triplicate.
Of the 41 plant extracts used by Palestinian in TAPHM for the treatment of obesity 16 plant extracts (39%) have shown possessing a strong PPL inhibitory activity > 70%, 7 with medium PPL inhibitory activity, while the rest (18) exhibited weak activity < 30 % (Table 2).
4. Discussion
With more people avoiding chemical drugs for the management of overweight and obesity, due to the fear of health adverse side effects, tendency is now towards natural-based products; thus the development of new antiobesity molecules from natural products has become a necessity. This seems doable because, in traditional herbal medicine, several plants are used for their weight- reducing effects. The plant bioactive constituents are expected to act as natural inhibitors of digestive lipases [22, 52]. Antioxidant and in vitro porcine pancreatic lipase, PPL, inhibitory tests were conducted on seventy-six plants, of which thirty-nine species with weight-reducing or related potential were used in Palestinian traditional medicine, to find new crude antiobesity products from natural sources.
In this study 59.3 % and 68.6 % of the plants extracts have shown possessing antioxidant activity using the RP and DPPH assays, respectively. The percentages of DPPH free-radical scavenging activity and ferric reducing power were found to be maximum at 1 mg/mL of the plant extract. The antioxidant activity of the plant extracts has shown being comparable using the DPPH and the RP assays (Table 2); both assays revealed similar antioxidant activity of the tested extracts except for a very small number of extracts (5 extracts, 5.5 %). The leaves and epicarp of Rhus coriaria exhibited the highest antioxidant activity in both assays. However, only 5 plant extracts, Rubus sanctus, Coriandrum sativum, Zingiber officinale, Ruta chalepensis, and Retama raetam, have shown possessing strong scavenging capacity by DPPH, while they exhibited low RP activity. Both DPPH and RP are known to be related to the nature of polyphenolics contributing to their electron transfer/hydrogen donating ability [53]. Thus, it is preferred to assess the antioxidant capacity of a plant extract by more than one method, as different methods can assess scavenging capacity of different free radicals and this can explain the difference in the antioxidant activity of the tested plant extracts using DPPH and RP assays. Free radicals are involved in many disorders like cancer, diabetes mellitus, and neurodegenerative and inflammatory diseases. Antioxidants due to their scavenging activity are useful for the management of those diseases [54, 55].
All plants analyzed in this study have been reported in TAPHM to be used for the treatment of one or more ailments including chronic diseases [40]. However, 39 of these plants which possess high antioxidant activity (> 50%) by either method, DPPH or RP, have been reported by the Palestinian people to be used as food, either tea (53.8 %), raw (43.6%), or seasoning (41.0 %). This would indicate the safety of using these plants as natural antioxidant agents for therapeutical uses.
Five basic mechanisms have been suggested for the weight-reducing effects of antiobesity natural products including inhibiting pancreatic lipase activity [56], promoting lipolysis [57], preventing adipogenesis [58], promoting thermogenesis and lipid metabolism [58], and controlling appetite [59]. However, pancreatic lipase inhibitory effect is one of the most widely studied mechanisms to determine the potential activity of natural products as obesity modulating agents [60].
Several studies have been conducted to evaluate the pancreatic lipase inhibitory activity of plant extracts; these studies have led to the identification of several plants and the isolation of natural compounds with PPL inhibitory activity [15, 27]. In our study, the most active PPLI plant extracts by means of minimum inhibitory concentration (IC50) were Camellia sinensis, Ceratonia siliqua, Curcuma longa, Sarcopoterium spinosum, Mentha spicata, Rhus coriaria L. (Anacardiaceae), Dittrichia viscosa (L.) Greuter (Asteraceae), Rosmarinus officinalis L. (Lamiaceae), Eruca sativa Miller (Brassicaceae), and Elaeagnus angustifolia L. (Elaeagnaceae) (Table 2).
In the following, we discuss some of these plants and their possible action mechanisms.
Camellia sinensis (tea) was among the most active plant extracts as PPLI in this study by means of IC50. Our results are in accordance with those of other researches which demonstrated that a tea-based drug (Exolise) standardized with the active component catechin at 25% was proposed as a natural antiobesity agent, showing strong PPLI activity and increase in thermogenesis [61].
A randomized, double-blind, placebo-controlled clinical trial was conducted to explore the effect of the consumption of high-dose of green tea extract on weight reduction [62]. The study revealed a significant weight loss, reduced waist circumference, and a consistent decrease in total cholesterol and LDL plasma levels without any side or adverse effects in women with central obesity [62]. Different types of tea are among the most widely investigated natural materials for pancreatic lipase inhibition. Various polyphenols (e.g., L-epicatechin, epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate, EGCG) isolated from tea leaves showed strong inhibitory activity against pancreatic lipase [63]. The efficacy of tea polyphenols (e.g., catechins: epigallocatechin gallate, EGCG) in regulating multiple targets concludes in inhibiting pancreatic lipase activity, reducing nutrient absorption, impairing adipogenesis, suppressing appetite, enhancing cellular oxidation defenses, activating AMP-activated protein kinase in the skeletal muscle, adipose tissues, and liver, and improving systemic inflammation, hyperglycemia, and insulin resistance [56–59]. Through these diverse mechanisms tea catechins reduce obesity and decrease the adverse health effects of obesity and related complications [59, 61].
Carob (Ceratonia siliqua) is a large leguminous tree of the Mediterranean area, belonging to the Caesalpiniaceae family. The plant is used in TAPHM for the treatment of abdominal pain, diarrhea, stomach ulcer, diabetes, chest pain, cough, and urinary tract infections [40, 41]. Carob is utilized mainly for the industrial conversion of the seeds, to obtain locust bean gum or carob gum and germ flour as a by-product, which are used in food preparations as a thickening agent and a source of protein, respectively [64]. The locust bean gum has been shown to possess lipid-lowering effects and to inhibit acylated ghrelin, the hunger hormone. This hormone has effect on making subjects more satiated at meals and making satiation last a long time, decreasing postprandial responses of triglycerides and nonesterified fatty acids, and altering respiratory amount, suggesting a change towards enhanced fatty acid oxidation [65, 66].
In this study the leaves, pods, and seeds of carob were analyzed for their PPLI activity; the leaves and seeds of the plant have shown possessing strong PPLI activity with IC50 of 0.8 and 3.3 mg/mL, respectively. This can be attributed to the fact that carob products have been found to contain an array of bioactive compounds including polyphenols and flavonoids that possess antiobesity properties [67]. On the other hand, the seeds of the plant have been shown to contain various bioactive flavonoid compounds including epicatechin, epicatechin gallate, and myricetin with higher concentrations than in pods [67, 68].
A study performed by Vaya & Mahmood [69] has shown that carob leaves contain nine different flavonoids, with myricetin as the major constituent. Interestingly, Su et al. [70] showed that consumption of myricetin may help to prevent obesity and obesity-related metabolic complications. They found myricetin treatment to effectively decrease the size and number of epididymal adipocytes and hepatocytes and reduce fat mass compared with the high-fat diet- (HFD-) induced obesity in mice without myricetin. It was suggested that that myricetin may suppress the expression of adipogenic and lipogenic genes, thereby regulating the onset of obesity [71, 72].
In vivo studies on the effects of polyphenol-rich infusion from carob leaves on inflammation associated with obesity and dextran sulfate sodium- (DSS-) induced ulcerative colitis in Swiss mice showed that carob leaf infusion decreased inflammation severity linked with HFD-induced obesity and dextran sulfate sodium- (DSS-) induced acute colitis designated by reduction in proinflammatory cytokines expression in adipose tissue, colon, and spleen [73]. The anti-inflammatory effect of carob leaves was attributed to its polyphenols which might relieve inflammation severity linked with obesity and colitis.
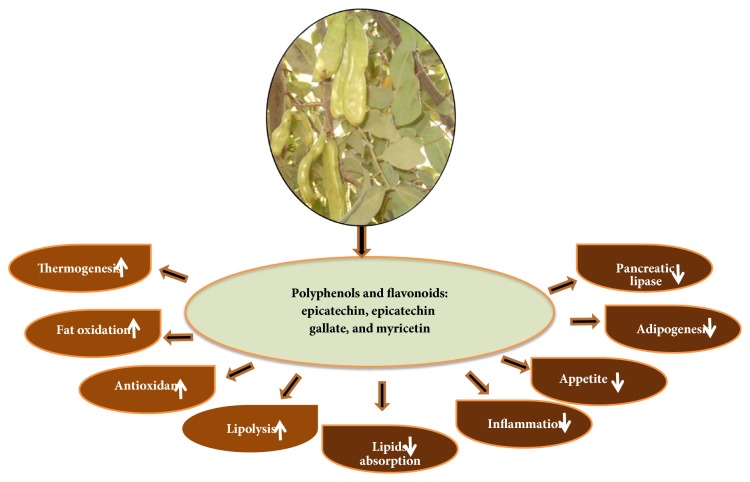
The present results as well as a review of the available literature have led us to believe that carob products can exert their antiobesity effect through the following main basic mechanisms (Figure 5): inhibiting pancreatic lipase activity, impairing adipogenesis, promoting lipolysis, increasing thermogenesis and fat oxidation, suppressing chronic low-grade inflammation, enhancing cellular antioxidant defenses, reducing absorption of lipids, and controlling appetite. However, the relative role of these mechanisms would depend on the type of carob product and diet consumed by individuals as well as the dietary conditions. Our results indicate that carob products might be a powerful lipase inhibitor and could be used as a weight control in obese patients.
Figure 5.
Antiobesity mechanisms and related beneficial effects of Ceratonia siliqua extracts and their bioactive constituents.
Curcuma longa (turmeric) is a rhizomatous herbaceous perennial plant of the ginger family, Zingiberaceae. Turmeric is a commonly used spice and is well documented for its medicinal effects in Indian and Chinese medical systems; the plant is recognized for its anti-inflammatory, antiangiogenic, antioxidant, wound healing, and anticancer properties and health promoting properties [74, 75]. In TAPHM the rhizomes of the plant have been reported to be used for the treatment of several ailments including flatulence, intestinal worms, hepatitis, atherosclerosis, and skin wounds and burns [41].
In the present study, the plant rhizomes exhibited strong PPLI activity (97.4 %, IC50 0.8 mg/mL). This is in agreement with those of Yadav and Chaudhurym [76] and Witkin and Li [77] who also reported a significant antiobesity activity of the plant. Curcumin is the main constituent of the plant; it promotes weight loss and reduces the incidence of obesity-related diseases by inhibiting preadipocyte differentiation, inhibiting macrophage expansion and infiltration in white adipose tissue, suppressing inflammatory adipokine secretion from white adipose tissue, and promoting cytoprotective antioxidant expression [78].
Mentha spicata (spearmint) is belonging to the Lamiaceae family. Its extracts (juice and leaf oil) are known to possess several pharmacologic activities, including antioxidant [79], antimicrobial [80], antitumor [81], anti-inflammatory [82], and diuretic [83] properties. It is commonly used in TAPHM in the form of tea as a home remedy to help alleviate stomach pain, to stimulate digestion, notably of fats, and to treat obesity, muscle spasm, headache, menstrual pain, and other digestive system ailments [41]. Mint was among the 39 plants reported in TAPHM to be utilized for weight loss. The PPLI results of the mint extract in this study are in accordance with its traditional use in Palestine and other countries for the treatment of obesity [41].
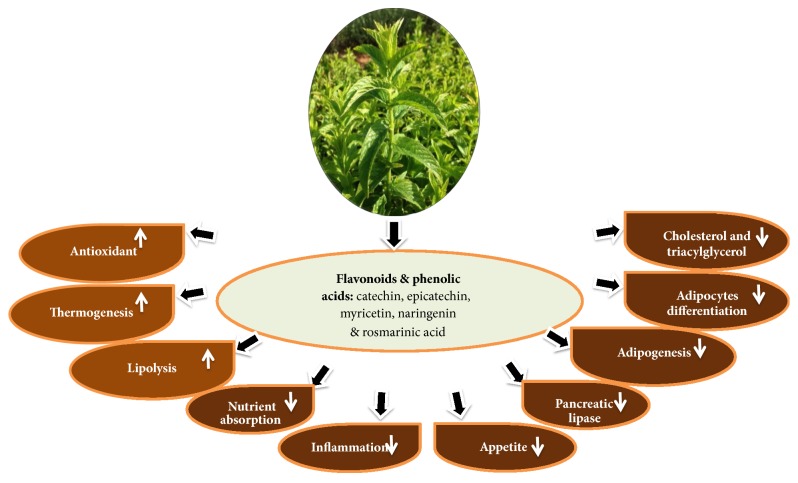
The M. spicata extract contains several bioactive ingredients with known antiobesity properties and is potentially useful in treatment of obesity and overweight related complications (e.g., diabetes and cardiovascular diseases). These compounds include flavonoids such as catechin, epicatechin, rutin, myricetin, luteolin, apigenin, naringenin, kaempferol, and quercetin and phenolic acids such as rosmarinic, gallic, chlorogenic, and caffeic acids [84–88]. Different mechanisms can therefore be proposed to explain the weight-reducing effects of M. spicata including inhibiting pancreatic lipase activity, promoting lipolysis, decreasing nutrient absorption, reducing adipocytes differentiation, stimulating thermogenesis and lipid metabolism, inhibiting oxidative stress and inflammation, inhibiting synthesis of plasma, hepatic cholesterol, and triacylglycerol, and controlling appetite (Figure 6).
Figure 6.
Antiobesity mechanisms and related beneficial effects of Mentha spicata extracts and their bioactive constituents.
Sarcopoterium spinosum is a widely distributed chamaephyte of the Rosaceae family growing in the eastern Mediterranean region. The shrub is mentioned as a medicinal plant in several ethnobotanical surveys, documenting the use of S. spinosum aqueous root extract by traditional medicinal practitioners for the treatment of diabetes and pain relief (e.g., toothache), as well as cancer therapy [41, 89–92]. The glucose lowering properties of the plant's roots, leaves, and fruits have been demonstrated by Elyasiyan et al. [93]. The plant's glucose lowering agents (bioactive polyphenols, e.g., catechin) target insulin affected tissues (e.g., liver, muscle, and adipose tissue) leading to inhibiting carbohydrate digestion, enhancing insulin secretion, and improving the transmission of insulin signaling in adipocyte and myotypes, leading to enhancing glucose uptake, metabolism, and regulation of whole body energy balance [93, 94].
In our study the plant aerial parts extracts were among the top 5 most active PPLI plant extracts by means of IC50 (1.2 mg/mL). This can be attributed to the fact that the Sarcopoterium extracts contain an array of bioactive ingredients including polyphenols and flavonoids known as catechins, which include (−)-epigallocatechin gallate, (−)-epicatechin gallate, (−)-epicatechin, (−)-epigallocatechin [92, 95, 96], and triterpenoids such as tormentic acid [97, 98] and α-tocopherol [99] that possess antiobesity properties [93]. Our results are in concordance with those of Tovit et al. [100] and Rozenberg et al. [94] which also suggest that the bioactive plant extracts have various beneficial antiobesity effects such as inhibiting lipolysis in an adipocyte, inducing glucose uptake in a cell, reducing plasma glucose levels, preventing atherosclerosis, decreasing weight gain, decreasing food consumption, and/or reducing or preventing obesity.
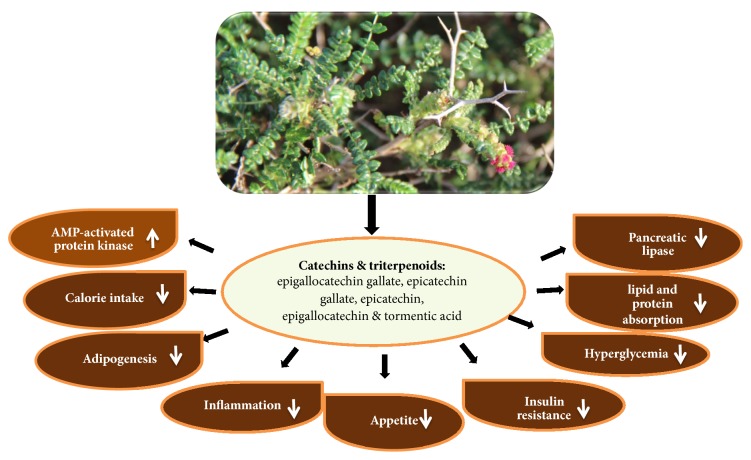
Sarcopoterium-derived polyphenols are expected to reduce obesity and decrease the adverse health effects of obesity and related complications by producing different physiological effects, including weight-reducing effects through the following basic mechanisms (Figure 7): inhibiting pancreatic lipase activity and decreasing absorption of lipids and proteins in the intestine, thus reducing calorie intake, impairing adipogenesis, suppressing appetite, enhancing the activation of AMP-activated protein kinase in the skeletal muscle, adipose tissues, and liver, and improving systemic inflammation, hyperglycemia, and insulin resistance [56, 58, 59, 93].
Figure 7.
Antiobesity mechanisms and related beneficial effects of Sarcopoterium spinosum extracts and their bioactive constituents.
Table 2 shows that antilipase activity is not necessarily associated with antioxidant activity; however, there were interesting coincidences in the case of Camellia sinensis, Ceratonia siliqua, and Curcuma longa. Eight plant extracts which show strong PPL inhibitory activity have shown possessing weak antioxidant activity by both methods. These include Elaeagnus angustifolia, Alchemilla xanthochlora, Cassia senna, Malva sylvestris, Cuminum cyminum, Cynara scolymus (fruit bulb and husk), and Eruca sativa; these plants can be considered as potential PPL inhibitors but not as antioxidants. On the other hand, the epicarp of Rhus coriaria, the seeds of Lepidium sativum, and the leaves of Cupressus sempervirens and Olea europaea could be considered as alternatives to antioxidants, but not as antilipase sources.
It is worth noting that 76.9 % of the plants analyzed in this study were reported to be used in Palestine as food either in the form of tea, salad, and condiment or in cooked form [51]; of these plants 41.7 % ( 25 plant extracts) possess PPLI activity of more than 50 %. The use of these plants as food suggests that they are safe and can be used in the management of obesity and as natural antioxidants. All plant species analyzed in this study for their antioxidant and PPLI potentials were mentioned to be used in TAPHM for the treatment of several human ailments [40]. On the other hand, of the 41 plant extracts reported by the Palestinians to be used for the treatment of obesity (Table 1), 18 plant extracts have shown to possess PPLI activity of > 50 %. Our results support these ethnobotanical studies and suggest that these plants may serve as the basis for the development of novel antioxidants and antiobesity pharmaceuticals and nutraceuticals/functional foods.
5. Conclusions
Our results suggest natural resources that possess strong antioxidant and pancreatic lipase inhibitory activities with potential applications in the treatment and prevention of obesity and overweight problem. The extracts of Camellia sinensis, Ceratonia siliqua, Curcuma longa, Sarcopoterium spinosum, and Mentha spicata have shown possessing strong antioxidants and PPLI potentials. However, future studies are needed for screening in-depth phytochemical, clinical, and possible studies on molecular mechanism of action and identification of the constituents responsible for the antioxidant and PPLI activities. At the same time, efforts should be made to normalize the plant extracts with potent antioxidants and PPLI activities and formulate best alternative herbal products in order to substitute manmade drugs which are presently in use.
Acknowledgments
This research was funded by the Biodiversity & Environmental Research Center (BERC).
Abbreviations
- AAI:
Antioxidant activity index
- AC:
Absorbance of the negative control
- AS:
Absorbance of the sample
- BMI:
Body mass index
- DPPH:
α, α-Diphenyl-β-picrylhydrazyl
- DSS:
Dextran sulfate sodium
- EC50:
The effective concentration at which the absorbance was 0.5
- ECG:
L-Epicatechin, epicatechin gallate
- EGC:
Epigallocatechin
- EGCG:
Epigallocatechin gallate
- HFD:
High-fat diet
- pNPB:
p-Nitrophenol butyrate
- PPL:
Porcine pancreatic lipase
- RP:
Reducing power
- TAPHM:
Traditional Arabic Palestinian Herbal Medicine.
Data Availability
The data sets supporting the results of this article will be freely available upon request to the corresponding author (msshtayeh@yahoo.com) for noncommercial use only.
Disclosure
An earlier version of this work was presented at the “International Conference on Science and Society: Biopiracy and Phytomedicine (ICSS 2017),” 2017.
Conflicts of Interest
All authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.World Health Organization (WHO) World Health Organization Factsheets, Obesity and overweight. 2017. http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 2.Cheung C. K., Wyman J. F., Halcon L. L. Use of complementary and alternative therapies in community-dwelling older adults. The Journal of Alternative and Complementary Medicine. 2007;13(9):997–1006. doi: 10.1089/acm.2007.0527. [DOI] [PubMed] [Google Scholar]
- 3.Ndao-Brumblay S. K., Green C. R. Predictors of complementary and alternative medicine use in chronic pain patients. Pain Medicine. 2010;11(1):16–24. doi: 10.1111/j.1526-4637.2009.00767.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsai A. G., Williamson D. F., Glick H. A. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obesity Reviews. 2011;12(1):50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerkadi A. Evaluation of nutritional status of united arab emirates university female students. Emirates Journal of Food and Agriculture. 2003;15(2):42–50. doi: 10.9755/ejfa.v15i2.5005. [DOI] [Google Scholar]
- 6.Hawkes C. Uneven dietary development: linking the policies and processes of globalization with the nutrition transition, obesity and diet-related chronic diseases. Globalization and Health. 2006;2(4) doi: 10.1186/1744-8603-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennert A. L. Hoodia gordonii (Masson) Sweet ex Decne: Une plante d’Afrique du Sud, de son utilisation traditionnelle vers un éventuel avenir thérapeutique. [These de doctorat] France: Université de Lorraine; 2012. (Fre). [Google Scholar]
- 8.Monteiro C. A., Moura E. C., Conde W. L., Popkin B., Barry M. Socioeconomic status and obesity in adult populations of developing countries: a review. Bulletin of the World Health Organization. 2004;82(12):940–946. [PMC free article] [PubMed] [Google Scholar]
- 9.Kalter-Leibovici O., Atamna A., Lubin F., et al. Obesity among Arabs and Jews in Israel: A population-based study. Israel Medical Association Journal. 2007;9(7):525–530. [PubMed] [Google Scholar]
- 10.Husseini A., Abu-Rmeileh N. M., Mikki N., et al. Cardiovascular diseases, diabetes mellitus, and cancer in the occupied Palestinian territory. The Lancet. 2009;373(9668):1041–1049. doi: 10.1016/s0140-6736(09)60109-4. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Rahim H. F., Holmboe-Ottesen G., Stene L. C. M., et al. Obesity in a rural and an urban Palestinian West Bank population. International Journal of Obesity. 2003;27(1):140–146. doi: 10.1038/sj.ijo.0802160. [DOI] [PubMed] [Google Scholar]
- 12.Isbaih M. Prevalence of Overweight and Obesity among School-Age Children in Nablus City [MSc thesis] An-Najah National University; 2009. [Google Scholar]
- 13.Yanovski S. Z., Yanovski J. A. Long-term drug treatment for obesity: a systematic and clinical review. Journal of the American Medical Association. 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viner R. M., Hsia Y., Tomsic T., Wong I. C. K. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obesity Reviews. 2010;11(8):593–602. doi: 10.1111/j.1467-789x.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 15.Karthiga T., Venkatalakshmi P., Vadivel V., Brindha P. In vitro anti-obesity, antioxidant and anti-inflammatory studies on the selected medicinal plants. International Journal of Toxicological and Pharmacological Research. 2016;8(5):332–340. [Google Scholar]
- 16.Claire C., Ludivine M., Alina R., et al. Traitement pharmacologique de l’obésité, Médecine. The Journal of Clinical Endocrinology & Metabolism. 2012;61:1–12. (Fre). [Google Scholar]
- 17.Kang J. G., Park C. Y. Anto-obesity Drugs: A review about their effects and safety. Diabetes & Metabolism. 2012;36:13–25. doi: 10.4093/dmj.2012.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y., Ma X., Wu C., et al. Effect of Anti-Obesity Drug on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2012;7(6):p. e39062. doi: 10.1371/journal.pone.0039062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J., Song J., Lee J. Optimal Extraction Conditions of Anti-obesity Lipase Inhibitor from. Mycobiology. 2010;38(1):p. 58. doi: 10.4489/MYCO.2010.38.1.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe M. E. The triglyceride lipases of the pancreas. Journal of Lipid Research. 2002;43(12):2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 21.Birari R. B., Bhutani K. K. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discovery Therapy. 2007;12(19-20):879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Thomson A. B. R., De Pover A., Keelan M., Jarocka-Cyrta E., Clandinin M. T. Inhibition of lipid absorption as an approach to the treatment of obesity. Methods in Enzymology. 1997;286:3–44. doi: 10.1016/S0076-6879(97)86003-X. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y., Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nature Reviews Drug Discovery. 2004;3(8):695–710. doi: 10.1038/nrd1469. [DOI] [PubMed] [Google Scholar]
- 24.Neovius M., Johansson K., Rössner S. Head-to-head studies evaluating efficacy of pharmaco-therapy for obesity: A systematic review and meta-analysis. Obesity Reviews. 2008;9(5):420–427. doi: 10.1111/j.1467-789X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 25.Lien D. N., Quynh N. T., Quang N. H., Phuc D. V., Thi N., Ngan T. Anti-obesity and body weight reducing effect of Fortunellajaponica peel extract fractions in experimentally obese mice. KKU Science. 2009;37:96–104. [Google Scholar]
- 26.Khader Y., Batieha A., Ajlouni H., El-Khateeb M., Ajlouni K. Obesity in Jordan: Prevalence, associated factors, comorbidities, and change in prevalence over ten years. Metabolic Syndrome and Related Disorders. 2008;6(2):113–120. doi: 10.1089/met.2007.0030. [DOI] [PubMed] [Google Scholar]
- 27.Alias N., Leow T. C., Ali M. S. M., et al. Anti-obesity potential of selected tropical plants via pancreatic lipase inhibition. Advances in Obesity, Weight Management & Control. 2017;6(4) doi: 10.15406/aowmc2017.06.00163. [DOI] [Google Scholar]
- 28.Alakolanga A. G. A. W., Kumar N. S., Jayasinghe L., Fujimoto Y. Antioxidant property and α-glucosidase, α-amylase and lipase inhibiting activities of Flacourtia inermis fruits: characterization of malic acid as an inhibitor of the enzymes. Journal of Food Science and Technology. 2015;52(12):8383–8388. doi: 10.1007/s13197-015-1937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T., Tang Q., Gao Z., et al. Blueberry and Mulberry Juice Prevent Obesity Development in C57BL/6 Mice. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0077585.e77585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W., Su H., Xu Y., Bao T., Zheng X. Protective effect of wild raspberry (Rubus hirsutus Thunb.) extract against acrylamide-induced oxidative damage is potentiated after simulated gastrointestinal digestion. Food Chemistry. 2016;196:943–952. doi: 10.1016/j.foodchem.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Shen Y., Su H., Zheng X. Hispidin derived from Phellinus linteus affords protection against acrylamide-induced oxidative stress in Caco-2 cells. Chemico-Biological Interactions. 2014;219:83–89. doi: 10.1016/j.cbi.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Bao T., Chen W. Comparison of the protective effect of black and white mulberry against ethyl carbamate-induced cytotoxicity and oxidative damage. Food Chemistry. 2018;243:65–73. doi: 10.1016/j.foodchem.2017.09.106. [DOI] [PubMed] [Google Scholar]
- 33.Shivaraj B., Pattabiraman T. N. Natural plant enzyme inhibitors. Characterization of an unusual alpha-amylase/trypsin inhibitor from ragi (Eleusine coracana Geartn.). Biochemical Journal. 1981;193(1):29–36. doi: 10.1042/bj1930029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp H., Hollinshead J., Bartholomew B. B., Oben J., Watson A., Nash R. J. Inhibitory effects of Cissus quadrangularis L. Derived components on lipase, amylase and α-glucosidase activity in vitro. Natural Product Communications (NPC) 2007;2(8):817–822. [Google Scholar]
- 35.Ali-Shtayeh M. S., Jamous R. M., Jamous R. M. Herbal preparation use by patients suffering from cancer in Palestine. Complementary Therapies in Clinical Practice. 2011;17(4):235–240. doi: 10.1016/j.ctcp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Ali-Shtayeh M. S., Jamous R. M., Jamous R. M. Complementary and alternative medicine use amongst Palestinian diabetic patients. Complementary Therapies in Clinical Practice. 2012;18(1):16–21. doi: 10.1016/j.ctcp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Ali-Shtayeh M. S., Jamous R. M., Jamous R. M., Salameh N. M. Y. Complementary and alternative medicine (CAM) use among hypertensive patients in Palestine. Complementary Therapies in Clinical Practice. 2013;19(4):256–263. doi: 10.1016/j.ctcp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Ali-Shtayeh M. S., Jamous R. M., Jamous R. M. Plants used during pregnancy, childbirth, postpartum and infant healthcare in Palestine. Complementary Therapies in Clinical Practice. 2015;21(2):84–93. doi: 10.1016/j.ctcp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Ali-Shtayeh M. S., Jamous R. M., Salameh N. M. Y., Jamous R. M., Hamadeh A. M. A. Complementary and alternative medicine use among cancer patients in Palestine with special reference to safety-related concerns. Journal of Ethnopharmacology. 2016;187:104–122. doi: 10.1016/j.jep.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 40.Ali-Shtayeh M. S., Jamous R. M., Abu-Zeitoun S. Y. National list of medicinal plants in Palestine - west bank and Gaza strip. Til, Nablus, Palestine, Biodiversity and Environmental Research Center (BERC) 2014 [Google Scholar]
- 41.Ali-Shtayeh M. S., Jamous R. M. Traditional Arabic Palestinian Herbal Medicine, TAPHM. Til, Nablus, Palestine, Biodiversity and Environmental Research Center, BERC; 2008. [Google Scholar]
- 42.Bustanji Y., Al-Masri I. M., Mohammad M., et al. Pancreatic lipase inhibition activity of trilactone terpenes of Ginkgo biloba. Journal of Enzyme Inhibition and Medicinal Chemistry. 2011;26(4):453–459. doi: 10.3109/14756366.2010.525509. [DOI] [PubMed] [Google Scholar]
- 43.Choi H. Y., Jhun E. J., Lim B. O. Application of flow injection-chemilumineacence to the study of radical scavenging activity in plants. Phytother Res. 2000;14:250–253. doi: 10.1002/1099-1573(200006)14:4<250::AID-PTR587>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 44.Elzaawely A. A., Xuan T. D., Tawata S. Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. and their antioxidant activity. Food Chemistry. 2007;103(2):486–494. doi: 10.1016/j.foodchem.2006.08.025. [DOI] [Google Scholar]
- 45.Scherer R., Godoy H. T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chemistry. 2009;112(3):654–658. doi: 10.1016/j.foodchem.2008.06.026. [DOI] [Google Scholar]
- 46.Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 47.Piaru S. P., Mahmud R., Abdul Majid A. M. S., Mahmoud Nassar Z. D. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pacific Journal of Tropical Medicine. 2012;5(4):294–298. doi: 10.1016/S1995-7645(12)60042-X. [DOI] [PubMed] [Google Scholar]
- 48.Singh R., Shushni M. A. M., Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arabian Journal of Chemistry. 2015;8(13):322–328. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 49.Cheung L. M., Cheung P. C. K., Ooi V. E. C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chemistry. 2003;81(2):249–255. doi: 10.1016/S0308-8146(02)00419-3. [DOI] [Google Scholar]
- 50.Mau J. L., Lin H. C., Song S. F. Antioxidant properties of several speciality mushrooms. Food Research International. 2002;35(6):519–526. doi: 10.1016/S0963-9969(01)00150-8. [DOI] [Google Scholar]
- 51.Ali-Shtayeh M. S., Jamous R. M., Al-Shafie' J. H., et al. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. Journal of Ethnobiology and Ethnomedicine. 2008;4, article no. 13 doi: 10.1186/1746-4269-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kazemipoor M., Radzi C. J., Cordell G. A., Yaze I. Potential of traditional medicinal plants for treating obesity: A review. Proceedings of the International Conference on Food Science and Nutrition; 2012; pp. 1–6. [Google Scholar]
- 53.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 54.Valko M., Rhodes C. J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Willcox J. K., Ash S. L., Catignani G. L. Antioxidants and prevention of chronic disease. Critical Reviews in Food Science and Nutrition. 2004;44(4):275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 56.Seyedan A., Alshawsh M. A., Alshagga M. A., Koosha S., Mohamed Z. Medicinal plants and their inhibitory activities against pancreatic lipase: a review. Evidence-Based Complementary and Alternative Medicine. 2015;2015:13. doi: 10.1155/2015/973143.973143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y. S., Lee Y., Kim J., et al. Inhibitory activities of Cudrania tricuspidata leaves on pancreatic lipase in vitro and lipolysis in vivo. ECAM. 2012 doi: 10.1155/2012/878365.878365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen Y., Song S. J., Keum N., Park T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evidence-Based Complementary and Alternative Medicine. 2014;2014:12. doi: 10.1155/2014/971890.971890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang C. S., Zhang J., Zhang L., Huang J., Wang Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Molecular Nutrition & Food Research. 2016;60(1):160–174. doi: 10.1002/mnfr.201500428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abumrad N. A., Nassi F., Marcus A. Fordtran’s Gastrointestinal and Liver Disease. 10th. Philadelphia, PA, USA: Elsevier Saunders; 2016. Digestion and absorption of dietary fat, carbohydrate, and protein. [Google Scholar]
- 61.Chantre P., Lairon D. Recent findings of green tea extract AR25 (exolise) and its activity for the treatment of obesity. Phytomedicine. 2002;9(1):3–8. doi: 10.1078/0944-7113-00078. [DOI] [PubMed] [Google Scholar]
- 62.Chen I., Liu C., Chiu J., Hsu C. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clinical Nutrition. 2016;35(3):592–599. doi: 10.1016/j.clnu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Thielecke F., Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome—a review. Phytochemistry. 2009;70(1):11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Santos M., Rodrigues A., Teixeira J. A. Production of dextran and fructose from carob pod extract and cheese whey by Leuconostoc mesenteroides NRRL B512(f) Biochemical Engineering Journal. 2005;25(1):1–6. doi: 10.1016/j.bej.2005.01.022. [DOI] [Google Scholar]
- 65.Birketvedt G. S., Berwin P. A. Northern white kidney bean extract and Ceratonia siliquaextract in combination with green tea extract in the treatment of excess weight and obesity. US Patent; 2016. (2016/0310552Al). [Google Scholar]
- 66.Gruendel S., Garcia A. L., Otto B., et al. Carob pulp preparation rich in insoluble dietary fiber and polyphenols enhances lipid oxidation and lowers postprandial acylated ghrelin in humans. Journal of Nutrition. 2006;136(6):1533–1538. doi: 10.1093/jn/136.6.1533. [DOI] [PubMed] [Google Scholar]
- 67.Mahtout R., Zaidi F., Saadi L. O., Boudjou S., Oomah B. D., Hosseinian F. Carob (Ceratonia siliqua L.) supplementation affects kefir quality and antioxidant capacity during storage. International Journal of Engineering and Techniques. 2016;2(4):p. 168. [Google Scholar]
- 68.Rtibi K., Selmia S., Gramia Dh., et al. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomedicine Pharmacotherapy. 2017;93:522–528. doi: 10.1016/j.biopha.2017.06.088. [DOI] [PubMed] [Google Scholar]
- 69.Vaya J., Mahmood S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.) BioFactors. 2006;28(3-4):169–175. doi: 10.1002/biof.5520280303. [DOI] [PubMed] [Google Scholar]
- 70.Su H.-M., Feng L.-N., Zheng X.-D., Chen W. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. Journal of Zhejiang University SCIENCE B. 2016;17(6):437–446. doi: 10.1631/jzus.B1600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou Y., Xue P., Bai Y., et al. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein β during adipogenesis. Free Radical Biology & Medicine. 2012;52(2):462–472. doi: 10.1016/j.freeradbiomed.2011.10.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joseph S. B., Laffitte B. A., Patel P. H., et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. The Journal of Biological Chemistry. 2002;277(13):11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 73.Aboura I., Nani A., Belarbi M., et al. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomedicine & Pharmacotherapy. 2017;96:1022–1035. doi: 10.1016/j.biopha.2017.11.125. [DOI] [PubMed] [Google Scholar]
- 74.Ramadan G., Al-Kahtani M. A., El-Sayed W. M. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation. 2011;34(4):291–301. doi: 10.1007/s10753-010-9278-0. [DOI] [PubMed] [Google Scholar]
- 75.Araújo C. A. C., Leon L. L. Biological activities of Curcuma longa L. Memórias do Instituto Oswaldo Cruz. 2001;96(5):723–728. doi: 10.1590/S0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 76.Yadav K. D., Chaudhury A. K. Anti-obesity mechanism of Curcuma longa L. - An over view. Indian Journal of Natural Products and Resources (IJNPR) 2016;7(2):99–106. [Google Scholar]
- 77.Witkin J. M., Li X. Curcumin, an active constiuent of the ancient medicinal herb Curcuma longa L.: some uses and the establishment and biological basis of medical efficacy. CNS & Neurol Disord—Drug Targets. 2013;12(4):487–497. doi: 10.2174/1871527311312040007. [DOI] [PubMed] [Google Scholar]
- 78.Saad B., Zaid H., Shanak S., Kadan S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals: Safety, Efficacy, and Action Mechanisms. Springer International Publishing AG; 2017. Chapter 2. [DOI] [Google Scholar]
- 79.Brahmi F., Khodir M., Mohamed C., Pierre D. Chemical Compostion and Biological Activities of Mentha Species. In: El-Shemy H. A., editor. Aromatic and Medicinal Plants – Back to Nature. Rijeka, Croatia: InTech; 2017. [Google Scholar]
- 80.Kizil S., Hasimi N., Tolan V., Kilinç E., Yuksel U., Kilinç E. Mineral content, essential oil compo- nents and biological activity of two Mentha species (M. piperita L., M. spicata L.) Turkish Journal of Field Crops. 2010;15(6):148–153. [Google Scholar]
- 81.Hajighasemi F., Hashemi V., Khoshzaban F. Cytotoxic effect of mentha spicata aqueous extract on cancerous cell lines in vitro. Journal of Medicinal Plant Research. 2011;5(20):5142–5147. [Google Scholar]
- 82.Arumugam P., Priya N. G., Subathra M., Ramesh A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environmental Toxicology and Pharmacology. 2008;26(1):92–95. doi: 10.1016/j.etap.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Abbaszadeh B., Valadabadi S. A., Farahani H. A., Darvishi H. H. Studying of essential oil variations in leaves of Mentha species. African Journal of Plant Science. 2009;3(10):217–221. [Google Scholar]
- 84.Brahmi F., Hauchard D., Guendouze N., et al. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.) M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae) Industrial Crops and Products. 2015;74:722–730. [Google Scholar]
- 85.Yamamura S., Ozawa K., Ohtani K., Kasai R., Yamasaki K. Antihistaminic flavones and aliphatic glycosides from Mentha spicata. Phytochemistry. 1998;48(1):131–136. doi: 10.1016/S0031-9422(97)01112-6. [DOI] [Google Scholar]
- 86.Proestos C., Chorianopoulos N., Nychas G.-J. E., Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. Journal of Agricultural and Food Chemistry. 2005;53(4):1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- 87.Papageorgiou V., Mallouchos A., Komaitis M. Investigation of the antioxidant behavior of air- and freeze-dried aromatic plant materials in relation to their phenolic content and vegetative cycle. Journal of Agricultural and Food Chemistry. 2008;56(14):5743–5752. doi: 10.1021/jf8009393. [DOI] [PubMed] [Google Scholar]
- 88.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. Regulation of lipolysis in adipocytes. Annual Review of Nutrition. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yaniv Z., Dafni A., Friedman J., Palevitch D. Plants used for the treatment of diabetes in Israel. Journal of Ethnopharmacology. 1987;19(2):145–151. doi: 10.1016/0378-8741(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 90.Dafni A., Yaniv Z., Palevitch D. Ethnobotanical survey of medicinal plants in northern Israel. Journal of Ethnopharmacology. 1984;10(3):295–310. doi: 10.1016/0378-8741(84)90017-5. [DOI] [PubMed] [Google Scholar]
- 91.Ali-Shtayeh M. S., Yaniv Z., Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. Journal of Ethnopharmacology. 2000;73(1-2):221–232. doi: 10.1016/s0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 92.Bachrach Z. Y. Ethnobotanical studies of Sarcopoterium spinosum in Israel. Israel Journal of Plant Sciences. 2007;55(1):111–114. doi: 10.1560/IJPS.55.1.111. [DOI] [Google Scholar]
- 93.Elyasiyan U., Nudel A., Skalka N., et al. Anti-diabetic activity of aerial parts of Sarcopoterium spinosum. BMC Complementary and Alternative Medicine. 2017;17(1) doi: 10.1186/s12906-017-1860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rozenberg K., Smirin P., Sampson S. R., Rosenzweig T. Insulin-sensitizing and insulin-mimetic activities of Sarcopoterium spinosum extract. Journal of Ethnopharmacology. 2014;155(1):362–372. doi: 10.1016/j.jep.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 95.Sipahi H., Korkmaz B., Avsar C., Fandakli S., Elmas E. Volatile Profiles, Antioxidant, and Antimicrobial Activities of Essential Oils and Extracts of Different Parts from Sarcopoterium spinosum (L.) Spach Growing Wild in Sinop (Turkey) Journal of Essential Oil Bearing Plants. 2017;20(3):688–700. doi: 10.1080/0972060X.2017.1342567. [DOI] [Google Scholar]
- 96.Smirin P., Taler D., Abitbol G., et al. Sarcopoterium spinosum extract as an antidiabetic agent: In vitro and in vivo study. Journal of Ethnopharmacology. 2010;129(1):10–17. doi: 10.1016/j.jep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 97.Reher G., Reznicek G., Baumann A. Triterpenoids from Sarcopoterium spinosum and Sanguisorba minor. Planta Medica. 1991;57(5):p. 506. doi: 10.1055/s-2006-960189. [DOI] [PubMed] [Google Scholar]
- 98.Reher G., Buděšínský M. Triterpenoids from plants of the sanguisorbeae. Phytochemistry. 1992;31(11):3909–3914. doi: 10.1016/S0031-9422(00)97552-6. [DOI] [Google Scholar]
- 99.Hudifa L. K., Alnouri A. S., Aburjai T. A., Hudaib M. M. Chemical Composition of the Essential Oil from Roots of Sarcopoterium spinosum (L.) (Rosaceae) Grown in Syria. Journal of Essential Oil Bearing Plants. 2013;16(3):412–416. doi: 10.1080/0972060X.2013.813267. [DOI] [Google Scholar]
- 100.Tovit R., Zohar K., Dvir D. Pharmaceutical compositions comprising extracts of Sarcopoteriumspinosum, components thereof, and uses thereof. US Patent; 2012. (PCT/IB2010/052551). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets supporting the results of this article will be freely available upon request to the corresponding author (msshtayeh@yahoo.com) for noncommercial use only.