Abstract
Mumps, a common childhood disease in the pre-vaccine era that causes swelling of the parotid salivary glands, can lead to orchitis, viral meningitis, and sensorineural deafness. While the incidence of disease decreased dramatically after the vaccine was added to standard vaccination schedules, the disease has made a substantial resurgence in recent years. As a result, it becomes critical to examine the factors involved in recurring outbreaks. Although low and incomplete vaccination coverage may be a key reason, it does not fully explain the issue due to the high rate of occurrence in populations with high vaccination coverage rates. Multiple studies suggest that waning immunity and secondary vaccine failure play a large role, the effects of which were previously masked by subclinical boosting. Significant knowledge gaps persist around the exact role and mechanism of waning immunity and demonstrate the need for more research in this area, as well as a reevaluation of mumps vaccine policy.
Keywords: Mumps, Mumps Vaccine, Mumps virus, Measles-Mumps-Rubella Vaccine, Polymorphism, Genetic, Cytokines, Receptors, Cytokine, Antibodies, Immunity, Humoral
Introduction
Mumps causes fever, muscle pain, and swelling of the parotid salivary glands. Severe complications can arise from mumps infection, leading to sensorineural deafness, viral meningitis in children, and orchitis and oophoritis, with orichitis occuring in up to 30% of cases [1]. Though a closely related virus has been isolated in bats, [2] humans are the only reservoir of mumps. Mumps spreads through respiratory droplets and contact. Initial vaccines contained inactivated virus and while effective, did not induce long-lasting protection [3]. Cases of mumps in the United States dropped sharply after the introduction of the attenuated mumps vaccine in 1967. [4] The monovalent vaccine was combined with the measles and rubella vaccines, creating the MMR-I vaccine. In 1971, a new version of the vaccine, MMR-II, was approved by the FDA for use [5]. Although only one dose was initially required, recurrent outbreaks of measles led to the establishment of a second dose of MMR-II vaccine for all children in 1989, resulting in a drop of mumps cases [6]. Mumps (R0=4–7) is not as highly contagious as measles (R0=12–18) and most of the current outbreaks occur in settings such as schools and military dormitories, indicating that close contact is required for outbreaks to occur in highly vaccinated populations. Epidemiological calculations suggest immunization coverages of 79–100% may be necessary to achieve herd immunity [5, 7]; however, outbreaks continue to occur in countries like the US, where vaccine coverage has remained >90%, suggesting that our understanding of the factors influencing mumps transmission is incomplete.
Despite the availability of a vaccine and recommendations for its use, outbreaks of mumps continue to occur in industrialized countries with high vaccine coverage rates and excellent healthcare systems [1–4]. Since the early 2000s, a surge of mumps cases in vaccinated populations has occurred worldwide; outbreaks have been documented across the United States, the Netherlands, the United Kingdom, Sweden, Belgium, and elsewhere [8–11]. In the United States, a large outbreak occurred in 2006 and then subsided. In 2012 and 2014, further mumps outbreaks occurred, with the Centers for Disease Prevention and Control (CDC) reporting 229 and 1,151 cases, respectively. The number of cases has subsequently ballooned over the last two years: 5,833 and 5,629 mumps cases were reported in 2016 and 2017, respectively, in the United States(as of December 31, 2017). These are reported cases, and the actual number of cases is unknown due to under-reporting and asymptomatic infections. These outbreaks largely affected professional sports teams, students on college campuses, religious communities, military populations, and adolescent/young adult populations with typically high vaccine coverage [12]. Given the trend depicted in Figure 1, it is likely the United States will continue to experience large-scale outbreaks.
Figure 1. Reported Cases of Mumps in the United States by Year.
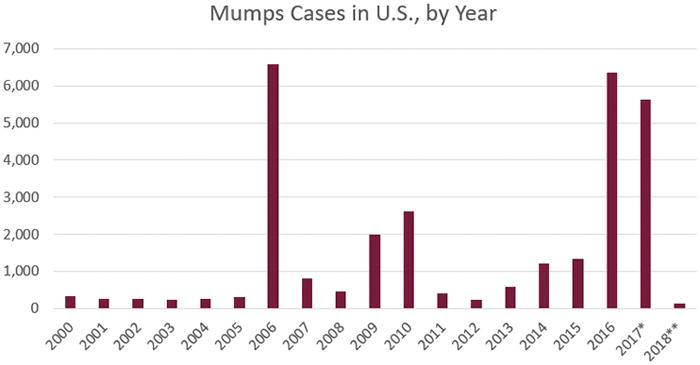
This figure, from the Centers for Disease Control and Prevention, outlines the number of mumps cases by year. *Case count is preliminary. **Cases as of January 27, 2018.
While measles and rubella have been the subject of considerable research efforts, mumps has not been studied as thoroughly—likely because of historically small numbers of cases in the vaccination era and the perceived lack of significant morbidity and mortality compared to that of measles and rubella. However, given the dramatic increases in mumps cases worldwide with associated morbidity even in highly vaccinated populations, this perspective is now changing. The scientific community increasingly recognizes the existence of substantial knowledge gaps in the generation and long-term maintenance of immune responses to mumps vaccine. These knowledge gaps hinder the ability of public health systems to protect populations against mumps outbreaks. The increasing appearance of mumps outbreaks necessitates a reprioritization of mumps vaccine research to help explain why mumps outbreaks continue to occur in healthy, highly vaccinated populations. Research to date suggests that this trend is likely due to a combination of low/incomplete vaccine coverage, primary vaccine failure, and secondary vaccine failure; the effects of which were likely suppressed due to subclinical boosting when mumps virus widely circulated. Below we explore the effect of each of these factors.
Potential Factors Contributing to Mumps Outbreaks
Low/incomplete vaccine coverage
Many factors have been suggested as possible causes of mumps outbreaks, with low vaccine coverage emerging as an obvious concern. Many mumps outbreaks have occurred in populations with either low or incomplete vaccine coverage. During a 2004 outbreak in the United Kingdom, almost 70% of the mumps cases occurred in those who had not received the vaccination at all [13]. This trend was also observed in outbreaks in Sweden and Canada [10, 14].
Vaccine hesitancy and resistance has grown in recent decades, in part due to misinformation spread by the anti-vaccine movement [15], contributing to a decrease in vaccine confidence and coverage in many communities. The impact of vaccine hesitancy can be seen most clearly in Japan, where the vaccine was removed in 1993 from standard vaccination schedules due to concerns about adverse events after administration of the MMR vaccine. It is worth noting that Japan used the Urabe strain, which has been associated with aseptic meningitis. Since then, mumps cases in Japan have skyrocketed [16], and significantly higher numbers of mumps cases have continued compared to countries that have retained the MMR vaccine as a part of the immunization schedule. Japan serves as an important case study for the rest of the world, demonstrating the significant impact of low vaccine coverage on a population.
Low vaccine coverage is unlikely to be a major contributing factor to recent outbreaks of mumps disease in the United States. Vaccination rates for the MMR vaccine in the United States are generally above 90%, and yet outbreaks have occurred in highly vaccinated communities. In recent mumps outbreaks (Table 1), 10 to 99% of the mumps cases had previously received two doses of MMR vaccine [17–27]. For example, a 2005 outbreak in the Czech Republic, which caused more than 5,000 cases of mumps, occurred in a population where vaccination coverage was 99.6%(at least one dose), and 70.6% (two doses)[27]. A 2004 outbreak in the Netherlands further demonstrates this problem—93% of the affected population had received at least one dose of the MMR vaccine [8]. Even more striking are the cases from a 2006 outbreak at a Kansas university and an outbreak at a camp in New York in 2005, in which more than 95% of subjects in these two outbreaks had received two doses of the MMR vaccine [28, 29]. Thus, low vaccine coverage does not fully explain mumps resurgence. These outbreaks in highly vaccinated populations indicate other contributing effects exist and should be explored to fully understand public health prevention needs.
Table 1.
Selection of Recent Mumps Outbreaks (MMR-II-Jeryl Lynn Strain Vaccine)
| Year | Location | # Cases | % vaccinated | Reference |
|---|---|---|---|---|
| 2017 | United States – 47 states and District of Columbia | 5,629 (as of Dec. 31, 2017) | Data not yet available | [12] |
| 2016-Feb 1, 2017 | United States – Arkansas | 2,706 | 66% 2 dose, 7% 1 dose | [17] |
| 2015–2016 | United States – Illinois | 317 | 16% 3 dose, 73% 2 dose, 4% 1 dose | [18] |
| 2014 | United States | 1,151 | Varies across outbreak locations | [12, 52] |
| 2012–2013 | Belgium | 4,061 | 69% 2 dose, 30% 1 dose | [19] |
| 2009–2011 | Jerusalem | 3,130 | 46.9% 2 doses, 28.3% 1 dose | [20] |
| 2013 | United Kingdom | 28 | 84% 2 doses, 8% 1 dose | [21] |
| 2013 | Poland | 2,436 | 10.3% 2 doses, 43.2% 1 dose | [22] |
| 2009 | United States – New York | 1,521 | 75% 2 doses, 13% 1 dose | [23, 24] |
| 2006 | Austria | 214 | 10.7% 2 doses, 30.2% 1 dose | [25] |
| 2006 | United States | 6,584 | 62.5% 2 doses, 24.8% 1 dose | [26] |
| 2005–2006 | Czech Republic | 5,998 | 70.6% 2 doses, 1.1% 1 dose | [27] |
Primary Vaccine Failure
Primary vaccine failure is another contributor to the resurgence of mumps. Multiple studies have implicated primary vaccine failure in epidemic settings.[30, 31] The Jeryl Lynn vaccine strain of mumps virus—used in the United States, Western Europe, and many other developed countries—induced seroconversion in 95% of recipients in a 20-month follow-up of seronegative children after a randomized clinical trial [32]. However, clinical effectiveness for the Jeryl Lynn strain (as determined by secondary attack rates between immunized and non-immunized children) ranges from 62–91% [32]. Vaccine failure was also apparent with the Rubini and Urabe mumps virus vaccine strains widely used in other areas of the world [32]. This presents a significant issue for public health officials dealing with outbreaks, where high vaccine effectiveness is crucial to preventing the spread of the disease and recurrent outbreaks.
Recurrent outbreaks of measles in the 1980s led to the addition of a second dose of the MMR vaccine to standard vaccination schedules in 1989. Multiple studies compared the effectiveness of one-dose versus two-dose vaccination regimens of the MMR vaccine, and found that while differences were not statistically significant, raw percentages suggested that two doses were more effective than a single dose to combat mumps [9, 10, 29]. Assessments of outbreaks in the United Kingdom, Canada, and Sweden determined that the affected populations tended to have only one dose of the vaccine, further suggesting that a prime-boost regimen may be useful to maintain long-term protection [10, 12, 14].
Other mumps vaccines strains, such as the Urabe and Leningrad-Zagreb strains, are also highly immunogenic;[32] however, these strains are associated with higher levels of adverse events(e.g., aseptic meningitis) than vaccines containing the Jeryl Lynn strain [26, 32, 33]. Because of this, it is unlikely these strains would be used to replace the Jeryl Lynn strain in the United States and Europe.
Antigenic Variation
Antigenic variation has been suggested as another possible factor in mumps vaccine failure. Wild-type mumps virus strains(genotype G) identified in outbreaks (in North America and Europe)have tended to differ phylogenetically [34, 35] from the Jeryl Lynn strain (genotype A) used in the MMR-II vaccine. While antibody responses elicited by the vaccine do cross-neutralize strains from other genotypes, antigenic difference do exist and the titer required for similar levels of viral neutralization is not the same [36]. It has therefore been suggested that a new vaccine that targets multiple genotypes of the virus may be necessary to improve efficacy and fully control these outbreaks [37]. In the influenza field, researchers have identified a phenomenon called ‘antigenic sin’, where the immune response to an initial exposure restricts the host’s ability to generate new responses to a closely related pathogen. This effect is believed to be due to the preferential expansion of the memory response that formed against the initial pathogen which interferes with the ability of naïve responses to develop against the new pathogen. Mumps has typically been considered a pathogen with low antigenic variability and therefore antigenic sin as a result of MMR-elicited response to mumps (genotype A) has not been studied. In fact, studies testing the ability of Jeryl-Lynn vaccine-induced antibodies to neutralize mumps virus of different genotypes demonstrated that neutralization of m[38]umps viruses still occurred even with a genotype mismatch [38, 39]. Vaccine-induced neutralizing antibody titers were indeed lower for some strains; however, these differences were judged too small to significantly impact immunity. Thus, it is unlikely that genotype mismatch explains either the reduced effectiveness of the vaccine in recent years or the recent outbreaks. Furthermore, a vaccine targeting currently circulating genotypes may not provide effective immunity for genotypes circulating in the future.
Secondary Vaccine Failure / Waning Immunity
A number of studies conducted in the last two decades have demonstrated an increased risk of mumps disease as time after vaccination increases, implying a waning of immunity in vaccinated individuals to sub-protective levels. Indeed, high school and college age populations have generally demonstrated a greater likelihood of contracting the disease [11]. Aretrospective evaluation of the large 2006 outbreak in the Midwestern United States found that mumps cases were more likely to occur in individuals who had received their second MMR dose 10 or more years prior to exposure [26]. Studies of outbreaks in France and Belgium demonstrated that the chance of developing mumps increases by 10–27% with every year after vaccination, which is highly suggestive of waning immunity [11, 40]. An increase in mumps susceptibility over time has been observed in numerous other population-based studies [11, 41, 42]. Furthermore, a 2017 study of over 20,000 college students in Iowa after an outbreak determined that the attack rate jumps from 1.6 cases per 1,000 for those who received the second dose of the vaccine within two years of the outbreak, to 11.3 cases per 1,000 for those who received it over 13 years before [43]. Taken together, these data consistently point to waning immunity as the major contributor to the increasing incidence of mumps in highly vaccinated communities.
These recent findings are in contrast to early studies of the mumps vaccine that found no link between immunity and time after administration. These early studies found a primary failure rate of about 5.5% for the Jeryl Lynn strain of mumps vaccine, and antibody persistence for at least 7–9 years[44, 45]. However, these early studies were conducted 5–10 years after the first release of the vaccine, often during outbreak conditions, failing to consider that vaccine efficacy may have been confounded by subclinical boosting of immunity in vaccinated individuals through exposure to circulating wild-type mumps virus strains—an effect that would disappear over time as vaccination reduced the incidence of circulating virus.
It is likely that this subclinical boosting is a major reason behind potential overestimation of vaccine-induced protection from mumps disease in the early years of mumps vaccination -a factor no longer operative at a widespread level over the recent decades. Only a few studies have directly or even indirectly investigated the possibility or impact of subclinical boosting of mumps vaccine responses. A few studies demonstrated that in vaccinated, wild-type-exposed populations, measuring mumps-specific IgG levels alone as indicators of vaccine-induced mumps immunity artificially inflates measures of vaccine efficacy [46, 47]. Additionally, a study of vaccine failure in Belarus found that in a cohort of people who contracted wild-type mumps despite previous mumps vaccination, only a small minority of their overall high mumps IgG antibody levels were specific to the vaccine-strain virus [48] indicating that mumps vaccine alone does not account for high levels of mumps antibodies relative to the boosting provided by natural infection. This illustrates the potential impact of subclinical boosting within a vaccinated population. Since circulation of wild-type virus has decreased significantly since the decade immediately following release of the vaccine, it becomes increasingly more important to examine how impactful subclinical boosting was on early measures of vaccine efficacy, and to reevaluate efficacy in current populations.
With less subclinical boosting, the effect of waning immunity plays an increasingly critical role in the reemergence of the disease. As little research has been dedicated to determining when vaccine-induced immunity to mumps wanes over time, and why individuals and populations eventually become susceptible to disease, more information must be gathered regarding the mechanism of waning, and determining potential methods of overcoming such obstacles.
Discussion
As discussed above, multiple factors have contributed to the reemergence of mumps. While the impacts of low vaccine coverage, primary vaccine failure, and antigenic variation have been studied, these factors do not explain the dramatically increasing number of recent mumps cases in highly vaccinated populations. Secondary vaccine failure appears to be playing the dominant role in the re-emergence of mumps outbreaks in highly vaccinated populations. This failure is a result of long-term waning immunity, a phenomenon which may have been previously masked by subclinical boosting through exposure to wild-type mumps virus.
Although secondary vaccine failure is likely to be the reason behind continuing mumps outbreaks in vaccinated populations, research regarding mechanisms of waning immunity to mumps after vaccination has been minimal. The knowledge gaps surrounding secondary vaccine failure significantly hinder prospects for eradication of mumps. Understanding the role of waning immunity is critical to developing a better vaccine; however, only a handful of studies have collected data that examines mumps immunity. This creates knowledge gaps about correlates of protection, when vaccine-induced immunity peaks and subsequently decreases, and the role of humoral vs. cellular immunity in long-term protection. The uncertainty over the mechanisms of waning immunity in mumps susceptibility, and how to correct or prevent secondary vaccine failure, demonstrates the need for considerable further research in this area.
Administration of a third dose of the MMR vaccine has also been proposed as a possible solution to combat outbreaks and a handful of studies support this idea. The 2015 mumps outbreak at the University of Iowa provided an ideal cohort to examine the impact of administering a third dose. Just prior to the peak of the outbreak, the university implemented a massive campaign to vaccinate students, with 94% of students receiving a third MMR-II dose. Individuals who received a third dose of MMR had a 78% lower risk of contracting mumps. Administration of a third dose during an outbreak appears to reduce disease risk and will likely help contain the spread of the disease. The study found also boost in mumps-reactive antibody levels for up to three months after administration, and this boost in Ab titer is likely the reason behind the reduced disease risk. However, one year after vaccination antibody titers ad mostly returned to pre-vaccination levels, indicating that this boost to immunity is temporary [43]. Another study also demonstrated that a third dose of MMR-II only temporarily increases humoral immunity, followed by a rapid return to pre-third dose antibody levels [49]. Given the transient nature of the boosted immune response, a third MMR dose fails to solve the problem of apparent secondary vaccine failure over the long-term, and may best serve as a stopgap measure in outbreak situations. Notably, the Advisory Committee on Immunization Practices (ACIP) recently voted in favor of using a third dose of mumps vaccine in the context of a mumps outbreak.[18]
Most of this review has focused on humoral immunity as neutralizing antibody responses are considered protective. Mumps vaccines doe elicit T cell responses that eliminate virus-infected cells. Mumps-specific cellular immunity has been measured by lymphoproliferation, cytokine secretion (typically IFNg and IL-10), and by IFNg ELISPOT assay after in vitro stimulation with mumps virus. These responses are detectable for years and even decades after vaccination and in some cases are detected in the absence of humoral immunity to mumps [5, 50]. There is some correlation between humoral and cellular immune responses to mumps;[51] however, the factors controlling each response are not known. While cellular immune responses are thought to be important for resolution of disease they are not believed to materially contribute to protection against infection. A caveat to this is that robust humoral immunity may rely on T cell help. Further studies investigating cellular immunity to mumps using modern tools to assess Both CD4 and CD8 T cell numbers, phenotype, and function may provide additional insights into the contribution of these cells to mumps immunity.
Conclusion
Since the development of the mumps-containing vaccine nearly 50 years ago, the incidence of mumps has been drastically reduced; however, recurring outbreaks of mumps despite vaccination mandate improvements to the mumps-component of the MMR vaccine. Encouraging vaccination in populations only prevents outbreaks caused by low immunization rates and does not address primary and secondary vaccine failure. Few studies have focused on understanding how waning immunity occurs, in whom, and at what point persons become susceptible to disease. Filling these critical knowledge gaps will require comprehensive, long-term studies of the mumps component of the MMR vaccine. The results of these studies could elucidate the immunologic and genetic mechanisms behind waning immunity and inform the development of new vaccine candidates that are more effective at protecting populations from the mumps virus. In geographic areas where subclinical circulation and subclinical wild virus boosting is increasingly unlikely, a full understanding of all the factors impacting mumps vaccine-induced immunity is necessary for the protection of populations and progress toward worldwide eradication of mumps disease. In this regard, funding of research studies to understand, at a systems-level, the generation and maintenance of long-term immunity is critical—as are studies aiming to develop improved mumps vaccines.
Acknowledgments
Funding:
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award number R01AI127365. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors wish to thank Caroline L. Vitse for her editorial assistance during the writing of this manuscript.
Footnotes
Disclosures:
Dr. Poland is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc., Avianax, Adjuvance, Alopexx, Sanofi Pasteur, GlaxoSmithKline, and Emergent Biosolutions. Dr. Poland holds four patents related to vaccinia and measles peptide research. Dr. Kennedy has received funding from Merck Research Laboratories to study waning immunity to mumps vaccine. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.Masarani M, Wazait H, Dinneen M. Mumps orchitis. Journal of the Royal Society of Medicine. 2006 Nov;99(11):573–5. doi: 10.1258/jrsm.99.11.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drexler JF, Corman VM, Muller MA, Maganga GD, Vallo P, Binger T, et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012 Apr 24;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilleman MR. Past, present, and future of measles, mumps, and rubella virus vaccines. Pediatrics. 1992;90(1 Pt 2):149–53. [PubMed] [Google Scholar]
- 4.Mumps--United States, 1985–1986. MMWR Morbidity and mortality weekly report. 1987 Mar 20;36(10):151–5. [PubMed] [Google Scholar]
- 5.Rubin S. Mumps Vaccines. In: Plotkin S, Orenstein W, Offit P, Edwards KM, editors. Plotkin’s VACCINES. 7. Philadelphia, PA: Elsevier; 2017. pp. 663–87. [Google Scholar]
- 6.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports. 2013 Jun 14;62(RR-04):1–34. [PubMed] [Google Scholar]
- 7.Deeks SL, Lim GH, Simpson MA, Gagne L, Gubbay J, Kristjanson E, et al. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2011 Jun 14;183(9):1014–20. doi: 10.1503/cmaj.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockhoff HJ, Mollema L, Sonder GJ, Postema CA, van Binnendijk RS, Kohl RH, et al. Mumps outbreak in a highly vaccinated student population, The Netherlands, 2004. Vaccine. 2010;28(17):2932–6. doi: 10.1016/j.vaccine.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerging Infectious Diseases. 2007 Jan;13(1):12–7. doi: 10.3201/eid1301.060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartorius B, Penttinen P, Nilsson J, Johansen K, Jonsson K, Arneborn M, et al. An outbreak of mumps in Sweden, February–April 2004. Euro Surveill. 2005 Sep;10(9):191–3. [PubMed] [Google Scholar]
- 11.Vandermeulen C, Roelants M, Vermoere M, Roseeuw K, Goubau P, Hoppenbrouwers K. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine. 2004;22(21–22):2713–6. doi: 10.1016/j.vaccine.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. [Date accessed: May 7 2018];Mumps Cases and Outbreaks. 2017 http://www.cdc.gov/mumps/outbreaks.html.
- 13.Mumps epidemic--United kingdom, 2004–2005. MMWR MorbMortalWklyRep. 2006;55(7):173–5. [PubMed] [Google Scholar]
- 14.Public Health Agency of Canada. Supplement: Guidelines for the Prevention and Control of Mumps Outbreaks in Canada. Canada Communicable Disease Report. 2010 doi: 10.14745/ccdr.v36i00as1. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10pdf/36s1-eng.pdf. [DOI] [PMC free article] [PubMed]
- 15.Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. NEnglJ Med. 2011;364(2):97–9. doi: 10.1056/NEJMp1010594. [DOI] [PubMed] [Google Scholar]
- 16.Honda H, Shimizu Y, Rutter M. No effect of MMR withdrawal on the incidence of autism: a total population study. J Child Psychol Psychiatry. 2005 Jun;46(6):572–9. doi: 10.1111/j.1469-7610.2005.01425.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith N. [Date accessed: May 7, 2018];Update on Mumps Outbreak: Arkansas. 2017 https://www.hhs.gov/sites/default/files/Smith_16x9_Update%20on%20Mumps%20Outbreak%2C%20Arkansas-remediated.pdf.
- 18.Albertson JP, Clegg WJ, Reid HD, Arbise BS, Pryde J, Vaid A, et al. Mumps Outbreak at a University and Recommendation for a Third Dose of Measles-Mumps-Rubella Vaccine - Illinois, 2015–2016. MMWR Morbidity and mortality weekly report. 2016 Jul 29;65(29):731–4. doi: 10.15585/mmwr.mm6529a2. [DOI] [PubMed] [Google Scholar]
- 19.Braeye T, Linina I, De Roy R, Hutse V, Wauters M, Cox P, et al. Mumps increase in Flanders, Belgium, 2012–2013: results from temporary mandatory notification and a cohort study among university students. Vaccine. 2014 Jul 31;32(35):4393–8. doi: 10.1016/j.vaccine.2014.06.069. [DOI] [PubMed] [Google Scholar]
- 20.Zamir CS, Schroeder H, Shoob H, Abramson N, Zentner G. Characteristics of a large mumps outbreak: Clinical severity, complications and association with vaccination status of mumps outbreak cases. Hum Vaccin Immunother. 2015;11(6):1413–7. doi: 10.1080/21645515.2015.1021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aasheim ET, Inns T, Trindall A, Emmett L, Brown KE, Williams CJ, et al. Outbreak of mumps in a school setting, United Kingdom, 2013. Hum Vaccin Immunother. 2014;10(8):2446–9. doi: 10.4161/hv.29484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korczynska MR, Rogalska J. Mumps in Poland in 2013. Przegl Epidemiol. 2015;69(2):209–12. [PubMed] [Google Scholar]
- 23.Update: mumps outbreak-New York and New Jersey, June 2009–January 2010. MMWR MorbMortalWkly Rep. 2010;59(5):125–9. [PubMed] [Google Scholar]
- 24.Barskey AE, Schulte C, Rosen JB, Handschur EF, Rausch-Phung E, Doll MK, et al. Mumps outbreak in Orthodox Jewish communities in the United States. The New England Journal of Medicine. 2012 Nov;367(18):1704–13. doi: 10.1056/NEJMoa1202865. [DOI] [PubMed] [Google Scholar]
- 25.Schmid D, Pichler AM, Wallenko H, Holzmann H, Allerberger F. Mumps outbreak affecting adolescents and young adults in Austria, 2006. Euro Surveill. 2006;11(6):E060615 1. [PubMed] [Google Scholar]
- 26.Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. NEnglJ Med. 2008;358(15):1580–9. doi: 10.1056/NEJMoa0706589. [DOI] [PubMed] [Google Scholar]
- 27.Boxall N, Kubinyiova M, Prikazsky V, Benes C, Castkova J. An increase in the number of mumps cases in the Czech Republic, 2005–2006. Euro Surveill. 2008 Apr 17;13(16) [PubMed] [Google Scholar]
- 28.Cortese MM, Jordan HT, Curns AT, Quinlan PA, Ens KA, Denning PM, et al. Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis. 2008;46(8):1172–80. doi: 10.1086/529141. [DOI] [PubMed] [Google Scholar]
- 29.Schaffzin JK, Pollock L, Schulte C, Henry K, Dayan G, Blog D, et al. Effectiveness of previous mumps vaccination during a summer camp outbreak. Pediatrics. 2007;120(4):e862–e8. doi: 10.1542/peds.2006-3451. [DOI] [PubMed] [Google Scholar]
- 30.Weibel RE, Stokes J, Jr, Buynak EB, Whitman JE, Jr, Hilleman MR. Live, attenuated mumps-virus vaccine. 3. Clinical and serologic aspects in a field evaluation. New England Journal of Medicine. 1967;276:245–51. doi: 10.1056/NEJM196702022760501. [DOI] [PubMed] [Google Scholar]
- 31.Sugg WC, Finger JA, Levine RH, Pagano JS. Field evaluation of live virus mumps vaccine. The Journal of pediatrics. 1968 Apr;72(4):461–6. doi: 10.1016/s0022-3476(68)80334-8. [DOI] [PubMed] [Google Scholar]
- 32.Peltola H, Kulkarni PS, Kapre SV, Paunio M, Jadhav SS, Dhere RM. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clinical Infectious Diseases. 2007;45(4):459–66. doi: 10.1086/520028. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet MC, Dutta A, Weinberger C, Plotkin SA. Mumps vaccine virus strains and aseptic meningitis. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 34.Utz S, Richard JL, Capaul S, Matter HC, Hrisoho MG, Muhlemann K. Phylogenetic analysis of clinical mumps virus isolates from vaccinated and non-vaccinated patients with mumps during an outbreak, Switzerland 1998–2000. Journal of Medical Virology. 2004 May;73(1):91–6. doi: 10.1002/jmv.20064. [DOI] [PubMed] [Google Scholar]
- 35.Afzal MA, Buchanan J, Dias JA, Cordeiro M, Bentley ML, Shorrock CA, et al. RT-PCR based diagnosis and molecular characterisation of mumps viruses derived from clinical specimens collected during the 1996 mumps outbreak in Portugal. Journal of Medical Virology. 1997 Aug;52(4):349–53. doi: 10.1002/(sici)1096-9071(199708)52:4<349::aid-jmv1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Rubin SA, Qi L, Audet SA, Sullivan B, Carbone KM, Bellini WJ, et al. Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect Dis. 2008;198(4):508–15. doi: 10.1086/590115. [DOI] [PubMed] [Google Scholar]
- 37.May M, Rieder CA, Rowe RJ. Emergent lineages of mumps virus suggest the need for a polyvalent vaccine. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2018 Jan;66:1–4. doi: 10.1016/j.ijid.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Santak M, Lang-Balija M, Ivancic-Jelecki J, Kosutic-Gulija T, Ljubin-Sternak S, Forcic D. Antigenic differences between vaccine and circulating wild-type mumps viruses decreases neutralization capacity of vaccine-induced antibodies. Epidemiology and Infection. 2013 Jun;141(6):1298–309. doi: 10.1017/S0950268812001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin SA, Link MA, Sauder CJ, Zhang C, Ngo L, Rima BK, et al. Recent mumps outbreaks in vaccinated populations: no evidence of immune escape. Journal of Virology. 2012 Jan;86(1):615–20. doi: 10.1128/JVI.06125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu XX, Plotkin SA, Edwards KM, Sette A, Mills KHG, Levy O, et al. Waning Immunity and Microbial Vaccines-Workshop of the National Institute of Allergy and Infectious Diseases. Clinical and vaccine immunology: CVI. 2017 Jul;24(7) doi: 10.1128/CVI.00034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller E, Hill A, Morgan-Capner P, Forsey T, Rush M. Antibodies to measles, mumps and rubella in UK children 4 years after vaccination with different MMR vaccines. Vaccine. 1995;13:799–802. doi: 10.1016/0264-410x(94)00086-3. [DOI] [PubMed] [Google Scholar]
- 42.Davidkin I, Valle M. Vaccine-induced measles virus antibodies after two doses of combined measles, mumps and rubella vaccine: a 12-year follow-up in two cohorts. Vaccine. 1998;16(20):2052–7. doi: 10.1016/s0264-410x(98)00081-4. [DOI] [PubMed] [Google Scholar]
- 43.Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, et al. Effectiveness of a Third Dose of MMR Vaccine for Mumps Outbreak Control. The New England Journal of Medicine. 2017 Sep 07;377(10):947–56. doi: 10.1056/NEJMoa1703309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis JE, Chernesky MA, Rawls ML, Rawls WE. Epidemic of mumps in a partially immune population. Canadian Medical Association Journal. 1979 Sep 22;121(6):751–4. [PMC free article] [PubMed] [Google Scholar]
- 45.Weibel RE, Buynak EB, McLean AA, Roehm RR, Hilleman MR. Persistence of antibody in human subjects for 7 to 10 years following administration of combined live attenuated measles, mumps, and rubella virus vaccines. Proceedings of the Society for Experimental Biology and Medicine. 1980;165:260–3. doi: 10.3181/00379727-165-40967. [DOI] [PubMed] [Google Scholar]
- 46.Hanna-Wakim R, Yasukawa LL, Sung P, Arvin AM, Gans HA. Immune responses to mumps vaccine in adults who were vaccinated in childhood. J Infect Dis. 2008;197(12):1669–75. doi: 10.1086/588195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narita M, Matsuzono Y, Takekoshi Y, Yamada S, Itakura O, Kubota M, et al. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clinical and Diagnostic Laboratory Immunology. 1998;5(6):799–803. doi: 10.1128/cdli.5.6.799-803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atrasheuskaya AV, Blatun EM, Kulak MV, Atrasheuskaya A, Karpov IA, Rubin S, et al. Investigation of mumps vaccine failures in Minsk, Belarus, 2001–2003. Vaccine. 2007 Jun 11;25(24):4651–8. doi: 10.1016/j.vaccine.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Fiebelkorn AP, Coleman LA, Belongia EA, Freeman SK, York D, Bi D, et al. Measles Virus Neutralizing Antibody Response, Cell-Mediated Immunity, and Immunoglobulin G Antibody Avidity Before and After Receipt of a Third Dose of Measles, Mumps, and Rubella Vaccine in Young Adults. The Journal of Infectious Diseases. 2016 Apr 01;213(7):1115–23. doi: 10.1093/infdis/jiv555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galazka AM, Robertson SE, Kraigher A. Mumps and mumps vaccine: a global review. Bulletin of the World Health Organization. 1999;77:3–14. [PMC free article] [PubMed] [Google Scholar]
- 51.Vandermeulen C, Clement F, Roelants M, Van DP, Hoppenbrouwers K, Leroux-Roels G. Evaluation of cellular immunity to mumps in vaccinated individuals with or without circulating antibodies up to 16 years after their last vaccination. J Infect Dis. 2009;199(10):1457–60. doi: 10.1086/598482. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Notifiable Diseases and Mortality Tables. [Date accessed: May 7, 2018];MMWR-Morbidity & Mortality Weekly Report. 2015 64(22):ND381–ND98. http://wwwcdcgov/mmwr/preview/mmwrhtml/mm6422mdhtm?s_cid=mm6422md_w. [Google Scholar]


