Abstract
Landmark trials on diabetes control have shown variable results in terms of cardiovascular benefits, with the majority showing a favorable effect of glycemic control on microvascular and, more recently, macrovascular complications. However, some trials pointed out a CV hazard with tight diabetes mellitus (DM) control. Most of those trials were assessing the impact of glycemic control, more than evaluating the effect of a certain medication. In the last decade, food and drugs administration (FDA) has mandated that all new hypoglycemic agents run a CV outcome trial (CVOT) for safety in order to grant and sustain approval. The most stunning results came from relatively new agents in the field of diabetes management, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and the glucagon-like peptide-1 agonists (GLP-1 agonists), details of these CVOTs will be addressed later in this document. SGLT2i effect on the cardiovascular system remains an area of extensive research. We aimed in this review to summarize what is the current evidence of cardiovascular protection upon using SGLT2i. Moreover, we wanted to raise a point that may be strongly adopted in the future, combining SGLT2i plus GLP-1 agonists, having a cardiovascular privilege in both molecules.
Keywords: SGLT2, Diabetes, Cardiovascular protection, GLP-1 agonist
Introduction
The burden of diabetes mellitus (DM) has been increasing since it was early diagnosed, DM prevalence is expected to reach an estimate of 629 million in the year 2045 [1]. Economic cost of diabetes in the US economy in 2007 was 174 billion USD, whilst the cost of the DM complications management was as high as 58 billion USD [2]. Meta-analysis on 102 prospective studies published in 2010 has shown that “diabetes confers about a twofold excess risk for coronary heart disease, major stroke subtypes, and deaths attributed to other vascular causes” [3]. Additionally, earlier supporting Framingham studies that dating back to 1974 specified DM as a risk factor for cardiovascular diseases (CVD) [4].
Landmark trials on diabetes control have shown variable results in terms of cardiovascular benefits; majority showing a favorable effect of glycemic control of microvascular and, more recently, macrovascular complications [5, 6]. However, some trials pointed out a CV hazard with tight DM control [7]. Most of those trials were assessing the impact of glycemic control, more than evaluating the effect of a certain medication. In the last decade, food and drugs administration (FDA) has mandated that all new hypoglycemic agents run a CV outcome trial (CVOT) for safety in order to grant and sustain approval. The most stunning results came from relatively new agents in the field of diabetes management, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and the glucagon-like peptide-1 agonists (GLP-1 agonists), details of these CVOTs will be addressed later in this document.
The story of the renal tubular excretion of glucose has come to clinical attention in the 1970s, where the phlorizin (old antimalarial agent, has a side effect of glycosuria at high doses) was tried to induce glycosuria in patients with diabetes [8]. A few years later, SGLT1 and 2 receptors were identified (SGLT2 has higher capacity and lower affinity than SGLT1 receptor), phlorizin was tested in high doses in animals, aiming for SGLT receptors inhibition, but high side effects profile decline further progress into human trials [9]. First dedicated selective SGLT2 inhibitor developed in the mid-1990s [10], but it had a short half-life and limited bioavailability [10]. Pharmaceutical developments and clinical trials have evolved slowly afterward, and, currently, we have numerous options of SGLT2 inhibitors agents [11].
Is the Effect of SGLT2 Inhibitors Glycemic Driven?
The current SGLT2i CVOTs are EMPA-REG [12], CANVAS (Canagliflozin Cardiovascular Assessment Study) [13], and DECLARE (Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) [14], which had some similarities in design, objectives, and outcomes. They were all randomized, placebo-controlled, event-driven studies that looked at the cardiovascular outcomes of a certain class of anti-diabetes medicines. The primary outcome was the composite of three major cardiovascular events (3-point major adverse cardiovascular event (MACE)) that is defined as death from cardiovascular causes, nonfatal myocardial infarction (excluding silent myocardial infarction), or nonfatal stroke. The key secondary outcome was a composite of the primary outcome plus hospitalization for unstable angina (4-point MACE). Other endpoints included hospitalization for heart failure. The CVD real [4] was a retrospective trial that assessed hospitalization for heart failure (HHF) as a primary outcome and all-cause death; and a composite of HHF or all-cause death (time-to-first-event), as secondary endpoints.
The EMPA-REG trial was the first to be published. It is a secondary prevention trial, included patients with an established cardiovascular disease. This is compared to CANVAS trial which included a percentage of patients with risk factors but no previous vascular events. The DECLARE is an ongoing trial to assess the cardiovascular outcomes with dapagliflozin has also included primary and secondary prevention cohorts (n = 10,189 vs. 5,023 respectively) [14].
In the EMPA-REG trial [12], the primary outcome occurred in a significantly lower percentage of patients in the empagliflozin group (hazard ratio (HR) in the empagliflozin group, 0.86; 95.02% confidence interval (CI), 0.74 to 0.99; P < 0.001 for non-inferiority and P = 0.04 for superiority). Empagliflozin resulted in a significantly lower risk of death from cardiovascular causes (HR, 0.62; 95% CI, 0.49 to 0.77; P < 0.001), death from any cause (HR, 0.68; 95% CI, 0.57 to 0.82, P < 0.001), and HHF (HR, 0.65; 95% CI, 0.50 to 0.85; P = 0.002). These findings have not been seen in any of the previous cardiovascular trials and seemed not to be related to glycemic effects despite the excellent glycemic benefits seen with empagliflozin. In CANVAS trial [13] the primary endpoint was reduced with canagliflozin compared with placebo (26.9 versus 31.5/1000 patient-years; HR, 0.86; 95% CI, 0.75 - 0.97; P < 0.001 for non-inferiority, P = 0.02 for superiority) in the total cohort, with no statistical evidence of heterogeneity (P = 0.18) between the primary (HR, 0.98; 95% CI, 0.74 - 1.30) and secondary (HR, 0.82; 95% CI, 0.72 - 0.95) prevention groups [13-15].
In the CVD real study, the use of SGLT-2i, versus oral glucose-lowering drugs, was associated with lower rates of HHF (HR 0.61; 95% CI 0.51 - 0.73; P < 0.001); death (HR 0.49; 95% CI 0.41 - 0.57; P < 0.001); and HHF or death (HR 0.54; 95% CI 0.48 - 0.60, P < 0.001) with no significant heterogeneity by country [16, 17].
These unique cardiovascular benefits seemed not to be related to glycemic effects despite the decent glycemic benefits seen with empagliflozin. After 12 weeks, during which glucose-lowering therapy was to remain unchanged, the adjusted mean differences in the glycated hemoglobin level between patients receiving empagliflozin and those receiving placebo were -0.54% (95% CI, -0.58 to -0.49) in the 10-mg group and -0.60% (95% CI, -0.64 to -0.55) in the 25-mg group. These differences have diminished as the study progressed as glucose-lowering therapy was allowed to be adjusted to reach (at week 206) to -0.24% (95% CI, -0.40 to -0.08) and -0.36 percentage points (95% CI, -0.51 to -0.20) in the 10-mg and 25-mg groups respectively.
Non-Glycemic Benefits of SGLT2i
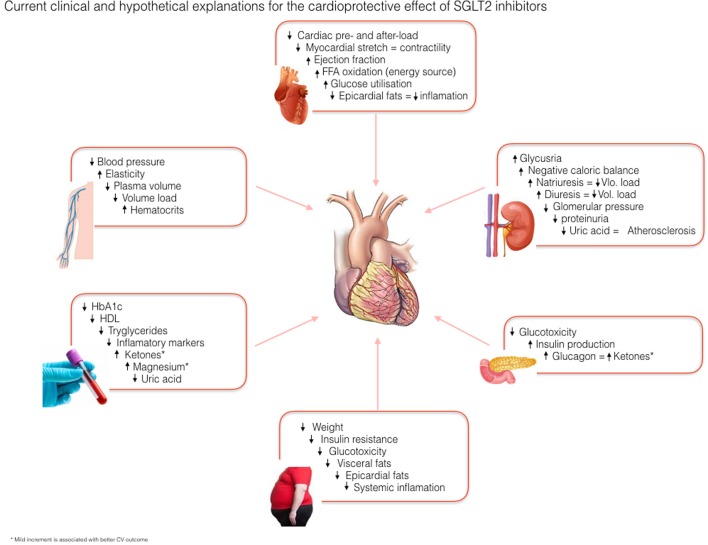
Since the clinical use of the SGLT2i and especially after the results of landmark cardiovascular outcome trials, the researchers are working on finding the probable mechanisms behind the consistent cardiovascular benefits caused by this group of antidiabetic agents. Many mechanistic analyses of clinical and experimental studies have suggested the following non-glycemic multifactorial CV protective mechanisms of SGLT2i. Summary of the possible explanations of the SGLT2i is illustrated in Figure 1.
Figure 1.
Current clinical and experimental hypothesis on cardioprotective effect of SGLT2 inhibitors.
Effect on body weight
The clinical efficacy and metabolic benefits of many SGLT2i that are out of proportion to their glycemic improvement are proven in multiple trials in comparison with other antidiabetic agents. In a prolonged trial of over 104 weeks, weight loss of 4.5 kg by empagliflozin, but the HbA1c reduction was comparable to glimepiride [18]. Studies on dapagliflozin showed an average of weight reduction of 2.3 and 2.4 kg over 24 and 48 months, respectively [19, 20]. Notably, weight reducing privilege of SGLT-2i is evident even in combination therapy with other agents known to cause weight gain like pioglitazone. In a study with empagliflozin 10 mg and 25 mg versus placebo as an add-on to pioglitazone ± metformin showed a mean weight loss of -1.62 ± 0.21 kg and -1.47 ± 0.21 kg; P < 0.001) with empagliflozin 10 mg and 25 mg, respectively, compared to +0.34 ± 0.21 kg with the placebo group [21].
Effect on body fat
Weight loss is a consistent finding in all the studies involving SGLT-2 inhibitors and associated with the loss of calories and water through glycosuria caused by these agents. Initial rapid weight loss seen with SGLT-2 is attributed secondary to fluid loss. Usually, a reduction of 2 - 3 kg is seen over 24 - 52 weeks in most of the studies [18-22]. Moreover, total body fat mass (visceral and subcutaneous adipose tissues) may also be reduced. The loss of total body fat was found to be significantly associated with increased loss of urine glucose, but no significant improvement was reported with adiponectin, hepatic lipid or leptin level in this subgroup of patients [22]. Noteworthy, studies on glucagon-like peptide-1 (GLP-1) agonists, like liraglutide, have shown a similar visceral fat reduction [23]. This may support the combination of SGLT2i and GLP-1 agonist theory that we shall discuss it at the end of this document.
Insulin sensitivity and glucose perturbation
Insulin resistance is well-known factor linked to increased cardiovascular mortality and atherosclerosis. The salutary effect of SGLT-2 inhibitors on glycemic control is its ability to work independently of insulin secretion and action. This ability translates into the introduction of this drug at any stage of T2DM and along with any other antidiabetic agents [24]. The chronic glycosuria caused by these agents, with resultant loss of calories, adipose tissues and weight increases insulin sensitivity [25], which in turn, reduces glucotoxicity, a risk of beta cell failure, and even decreases the daily requirement of exogenous insulin and other secretagogues [22].
Ferrannini and colleagues conducted a study to assess the metabolic response during fasting, and postprandial hours after a single dose and long-term use of empagliflozin on T2DM. Their findings revealed that due to significant glycosuria there is an enhanced endogenous glucose production due to counteracting phenomena of exaggerated pre-hepatic insulin to glucagon ratio. The overall blood glucose was still significantly lower than the baseline (0.9 ± 0.7 mmol/L, P < 0.0001 vs. baseline) after 3 h of fasting. During the postprandial period serum glucagon and endogenous glucose production were increased, while the area under the curve for both glucose and insulin were decreased. There is also a significant reduction in tissue glucose disposal. After chronic empagliflozin administration, there is an increase in lipid oxidation and reduced oxidative and non-oxidative glucose utilization. The study showed a numerically significant improvement in insulin and β cell glucose sensitivity [26].
Other investigators also drew the same inference about the SGLT-2 inhibitors that their use results in the reduction of glucotoxicity by inducing the renal glycosuria. They used the euglycemic hyperinsulinemic clamp technique to monitor the whole-body glucose uptake and endogenous glucose production in 18 patients with T2DM after 2 weeks of dapagliflozin use. It caused a substantial improvement in muscle insulin sensitivity [27].
Effect on blood pressure
Natriuresis and direct effect of glomerular hemodynamics may play a role in cardiovascular and extra glycemic benefits observed with this class of agents. The sustained natriuresis and increased urine output of around 107 and 470 mL per day result in decreased in plasma volume and reduction in blood pressure [28]. Many studies before large outcome trials have demonstrated this blood pressure (BP) reducing effect [29-35]. Reduction in systolic BP was more pronounced, and interestingly, the reduction in BP was independent of weight loss and glycemic control [28].
One long-term study by Kohan and colleague to monitor the effect of dapagliflozin on the patient with moderate chronic renal disease (CKD) showed significant improvement of BP and weight but with minimal HbA1c improvement. This is an important observation as usually BP control is a challenge in patients with renal impairment and considering the mode of action in renal tubules the ability of SGLT-2i to still efficiently reduce BP without worsening of renal function is a call for future studies. It is thought that patients with CKD are more salt sensitive and the natriuresis caused by SGLT2i results in more pronounced BP reduction in these patients [30].
SGLT2i-induced weight reduction is an additional factor in lowering BP; reduces visceral fats, ameliorates inflammatory markers and therefore, reduces endothelial dysfunction [28, 31, 32]. However, a study using canagliflozin showed a drop in BP of 10.4 mm Hg with a weight loss of the only 1.7 kg [33]. This again applies to patients with CKD, the combination of natriuresis, weight, and visceral fats may contribute to BP reduction [34, 35]. The controversial role of neurohormones like the sympathetic nervous system, RAAS, and vasodilators like nitric mono oxide (NO) has been also been postulated as an important mechanism for the antihypertensive effect seen with SGLT-2i [36].
Effect on arterial stiffness
Metabolic syndrome and T2DM are known risk factors for the endothelial dysfunction, arterial stiffness and end-organ damage including micro and macrovascular consequences [37, 38]. The data from SGLT-2i CVOTs showed a reduction in CV mortality beyond it’s glycemic and antihypertensive effects [12, 13]. This has initiated a new debate on potential reasons behind these new revolutionary findings; reduction in arterial stiffness by SGLT-2i is one of them. Based on available data, natriuresis and effect on arterial stiffness are probably the most significant mediators responsible for cardiovascular benefits with SGLT2 inhibitors, since the effect on 3-point MACE has started after 12 weeks in EMPA-REG which is too short to work on atherosclerosis pathology [12]. Interestingly, a study with empagliflozin in T1DM, while using clamped hyperglycemia technique showed a reduction in arterial stiffness without a change in heart rate, norepinephrine, and epinephrine [39].
On another hand, a recent study assessing the effect of dapagliflozin versus hydrochlorothiazide (HCZ) on systemic arterial stiffness and renal resistive index by monitoring the 24-h urinary glucose, sodium, isoprostanes, pulse wave velocity, and flow-mediated dilation [40]. Both agents exerted a similar amount of diuresis and equal antihypertensive effect as hydrochlorothiazide; dapagliflozin was associated with significantly reduced pulse wave velocity, renal resistive index and a decline in urinary isoprostanes level, which is an indicator of declining oxidative stress [40]. This may explain the SGLT-2i positive effect on arterial stiffness, endothelial dysfunction plausibly by reduced oxidative stress, independent of the BP effect and natriuresis. Those collectively may play a crucial role in reducing vasculopathy burden on the heart [41, 12, 13].
Effects on proteinuria and kidney function
Impact of SGLT-2 inhibitors on renal function has been a focus of primary concern since these agents came under use. As their primary mode of action is on kidney tubules and consequent mechanistic and clinical outcome associated with this activity was also a primary outcome of many studies. Most of the studies were showed favorable SGLT2i kidney function preservation, despite the infrequent mild initial reversible decline in eGFR of 5mL/min/1.73m2 is seen in patients with T2DM with or without CKD [42].
Renal glomerular hyperfiltration is a known complication of T2DM, which may induce glomerular inflammation and sclerosis, and may progress to chronic diabetic nephropathy. This jeopardy is thought to be due to increased intra-glomerular pressure caused by glomerular efferent arteriolar constriction that is caused by over-activation of macula densa [43-45], a mechanism that is in a way similar to the effect of RAAS blockers in renal function preservation [46].
In a study by Rajasekeran in T1DM showed that after empagliflozin, urinary adenosine level increased that was probably the result of increased sodium load to macula densa and increased adenosine secretion, causing afferent vasoconstriction and reduce hyper-filtration due to tubuloglomerular feedback [47].
Other than intrinsic renal hemodynamic modulation, extra-renal factors were also suspected to play a role in the anti-proteinuric action of SGLT-2 inhibitors. Minor contribution in the reduction of albuminuria could be attributed to the reduction on weight and BP [48-49]. Moreover, an interesting indicator of kidney function replenishment, rise in erythropoietin level, has been indirectly attributed to SGLT2i use [50-51]. It has been postulated that increased in hematocrit after SGLT-2i therapy is due to the recovery of renal tubulointerstitial function in diabetic patients. As per Sano and colleagues, the proximal renal tubules in diabetic patients have a compromised oxygenation due to excessive glucose reabsorption and increased oxygen consumption by the tubular cells. Prolonged hypoxia may lead to ischemic changes and excessive fibroblast formation that may negatively affect erythropoietin production. Introduction of SGLT-2i reduces nephrons’ workload and therefore recovery of above erythropoietin-producing cells. Hence, the erythropoietin-induced increment in hematocrit is taken as a marker for reversal of hyperglycemia-induced tubulointerstitial damage [51]. Nonetheless, renoprotective effect of erythropoietin has been seen in few animal studies where erythropoietin ameliorates and prevents the podocytes’ injury in advanced diabetic nephropathy [52, 53].
SGLT-2 inhibitors and atherosclerosis
There is accumulative evidence of the proved direct proportional relation between insulin resistance with atherosclerosis and the fact that improvement in insulin sensitivity would improve glucose control as well as micro and to a lesser extent macrovascular complications [54]. SGLT2i may not have a direct effect on the insulin sensitivity but have many indirect ways of doing so, this includes weight reduction, improving the glycemic state and glucotoxicity, improving the lipids panel [55].
The recent prospective, DEFENCE study, randomized newly diagnosed patients with T2DM receiving either dapagliflozin 5 mg in combination with metformin versus 1,500 mg of metformin. The primary aim was monitoring the change in vascular flow-mediated dilation (FMD) from baseline to end of the study and to follow the other parameters like HbA1c, lipids, body composition, and markers of oxidative stress. The results showed significant improvement in FMD and decease in urine 8-hydroxy-2’-deoxyguanosine; a biomarker of oxidative stress in patients receiving dapagliflozin. Rest of other parameter showed equally substantial improvement in both groups [56]. Improvement in endothelial function by SGLT-2 inhibitors (discussed earlier) may have contributed to a change in FMD in dapagliflozin group [56].
Effect on systolic and diastolic cardiac functions
Many theoretical explanations and few experimental animal studies took place to understand the pathophysiological changes responsible for the improvement in myocardial function after use of the SGLT-2 inhibitors. Diuresis and natriuresis-induced reduction in intravascular volume, and improvement in arterial elasticity decreased the cardiac preload and afterload, and hence, the myocardial strain and consequently less heart failure and CV mortality [57]. However, still, the magnitude of CV benefits still outweighs the evident current cardiac changes [34].
The only one observational study available on cardiac function by using 2D echocardiography in T2DM and established cardiovascular disease on treatment by empagliflozin showed reverse remodeling and improvement in diastolic dysfunction [58]. Though experimental studies in animals suggested the role of SGLT-2 inhibitors inhibiting Na/H exchangers (NHE-1) in cardiomyocytes, in turn, increasing the mitochondrial Ca level and decreasing the cytoplasmic Na and Ca level [59]. Animals’ studies have documented that animals with heart failure have increased activity of this NHE-1 and increased cytoplasmic Na level and thus subsequent disturbed Ca level. This disturbance in cardiomyocytes electrolyte concentration is typically induced by chronic hyperglycemia and potentiates the heart failure and cardiac death. The mitochondrial Ca level in cardiomyocytes is of high significance as it is a primary activator of the antioxidant enzyme cascade and ATP synthesis [60]. The inhibition of NHE-1 by cariporide that is an inhibitor of NHE-1 resulted in prevention and improvement in heart failure and LV remodeling [61]. Another experimental study on obese rats assessing the impact of empagliflozin on heart failure over 10 weeks, showed a reduction in interstitial cardiac fibrosis, macrophage infiltration, cardiac superoxide concentration and also diminished coronary artery thickening and peri-arterial fibrosis [49].
The fact whether these findings in animal studies will mirror human studies will require some robust mechanistic studies. Some of the trials like REFORMS are underway and will help in some of the answers [62].
Effect on hepatic steatosis
Animal studies showed significant improvement in lipid profile and hepatic steatosis by ipragliflozin in rats with T1DM [63]. Another recent study on obese and diabetic rats treated with tofogliflozin showed marked suppression of hepatic inflammation, steatosis, non- alcoholic fatty liver disease activity score, mRNA expression level of hepatic pro-inflammatory markers and pre-neoplastic lesion compared to controls. The investigator concluded that SGLT-2 inhibitors might have a cancer protective role in obesity-associated hepatic neoplasm [64].
SGLT-2 inhibitors and uric acid
The uric acid level is recently recognized as an inflammatory marker that enhances oxidative stress and promotes activation of RAAS. Uric acid is believed to be directly associated with progression of the diabetic renal disease and considered as a biomarker of cardiovascular disease [65]. There is 10-15% reduction in uric acid level after the use of SGLT-2i in exchange for glycosuria, which may also play an important role in SGLT2i cardio-protection.
SGLT-2 inhibitors and ketone body
The constant glycosuria-induced calorie loss may lead to a metabolic-fuel shift in patients with diabetes. This negative caloric balance induces endogenous glucose production through overproduction of glucagon, an altered hepatic ratio of insulin to glucagon results in increased metabolism of the free fatty acid (FFA) and ketone bodies, and lead to a more dependence on fat oxidation as an energy source [56]. Some investigators considered excessive ketone bodies as a “super fuel” for a failing heart [66].
Safety and Side Effects of SGLT2 Inhibitors
In the early days of SGLT2i clinical use, most of the physicians were quite skeptical and the adverse effects were thought to be serious and difficult to deal with. This has been slowly clearing out and raising the confidence in using them. Most of SGLT2is’ adverse events are treatable and probably short living. In fact, some biochemical changes, which thought to be a side effect of the drugs, turned out to be a possible factor in CV protection process [66-74]. The adverse effects can be summarized in the following section.
Electrolytes imbalance
Electrolytes imbalance was a concern since early year of clinical use of SGLT2i. This includes magnesium, phosphorus, and potassium. In fact, magnesium replenishment is currently considered as an additive factor for CVD protection, since hypomagnesemia is associated with fast progression of diabetes and its cardio-metabolic complications including myocytes hypertrophy, rhythm alteration, vascular stiffness [67-71]. Moreover, hypomagnesemia may expedite the beta cells apoptosis and downgrades the insulin sensitivity in the peripheral tissues [68-71]. The modest increment in magnesium has been reported in many of the SGLT2i agents (dapagliflozin, canagliflozin, empagliflozin and ipragliflozin) where magnesium has leveled up by an average of 0.05 to 0.1 mmol/L; regardless the kidney function status [71]. The exact mechanism of magnesium increment is not very clear, but it may be attributed to regulation of distal convoluted tube electrolytes exchange via SGLT2 receptors [72-74].
Mild hyperkalemia was observed in SGLT2i trials, but it was basically with those who had an acute kidney injury and/or those on a concomitant renin-aldosterone blocking agent [32]. Likewise, a metanalysis of 2,215 patients on canagliflozin showed infrequent episodes of hyperkalemia, but it was basically with reduced eGFR [75].
Diabetic ketoacidosis (DKA)
DKA was reported in all marketed SGLT2i, however the incidences of DKA across different clinical trials were as low as < 0.2% in canagliflozin studies [76-77]; handful of cases were reported to the FDA, majority of those cases were T1DM and majority were euglycemic DKA [78], hence the FDA has published a warning about use of SGLT2i in T1DM [78].
Bone fractures
Bone fracture has been a concern since the interim data of CANAVUS trial was unveiled in 2015, where there was significant increase in distal bone fracture incidences in canagliflozin group (2.7%) and 1.9% in non-canagliflozin patients [13], though there were no fracture reports in the spine or hip bones [13]. However, some studies have shown canagliflozin group having lower bone mineral density. Moreover, bone turnover markers like collagen type 1 beta-carboxy telopeptide (beta-CTX), osteocalcin and were higher in canagliflozin cohort in multiple studies [78-81].
Interestingly, comparing CANAVUS cohort versus eight non-CANAVUS studies showed significant fracture risk only in CANAVUS patients, while there was no significant difference against non-canagliflozin patients in other studies [82]. The exact mechanism behind the canagliflozin and bone health is yet to be clarified. Since there are no SGLT1 or 2 receptors identified in bone cells so far [82]. Based on the above-mentioned trials and others, US FDA has published a warning on canagliflozin and bone health [83]. FDA stated that fracture risk in other SGLT2i (other than canagliflozin) is yet to be linked, but to date, the fracture incidences in other SGLT2i agents did not vary from the control cohorts.
Thromboembolic events
Osmotic diuresis may lead to hypovolemia, especially in elderly population. This may lead to relative hemoconcentration which may increase the risk of stroke or peripheral vascular diseases [57]. This has been observed in CANAVUS and CANAVUS-R studies, where canagliflozin was associated with higher incidences of limb amputations compared to control group [13]. Once again, this was not observed in EMPA-REG trial [12]. However, in EMPA-REG, there were higher incidences of stroke compared to control population [84]. Those concerns were not observed in many phases 3 and 4 clinical trials, which raises the question about the inclusion criteria for the CANAVUS cohort, and hence avoiding initiation of canagliflozin in patients at high risk of peripheral vascular disease. Notably, the FDA has published a warning on the risk of amputation with canagliflozin [85]. FDA advised to assess amputation risk factors before initiation of canagliflozin; those factors include any history of prior amputation, peripheral vascular disease, peripheral neuropathy, and diabetic foot ulcers [85].
LDL elevation
Many SGLT2i clinical trials have shown an elevation of LDL by the end of the study [55, 86], however, the marvelous positive CV outcome results have refuted clinical significance of this LDL increment. However most of the clinical trials are intermediate-term studies [12, 13]; the long-term impact of this finding needs to be observed in the follow-up analysis of those trials. On the other hand, studies have reported improvement of LDL with SGLT2i [87, 88].
Genital infections
Mild to moderate genital mycotic infection was reported in various trials and case series [25, 89, 90]. However, the cases were usually not severe in nature and responded to standard medical treatment [25]. Females and those with a history of genital infections were at higher risk of recurrence upon using SGLT2i. Noteworthy, urinary tract infections and serious pyelonephritis have also been reported on very few occasions, which led to another FDA warning about the possibility of having serious UTI with SGLT2i [91].
Other Positive CVOTs (GLP-1 Receptor Agonists)
Apart from the SGLT2i, there were three positive CVOT in the 2 years, which are liraglutide, semaglutide, and pioglitazone [1-3]. LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) randomized 9,340 patients over 3.8 years and showed a significant reduction in stroke, CV mortality, and all-cause mortality, and a nonsignificant reduction in nonfatal stroke, MI, and HHF [92]. Similarly, SUSTAIN-6 trial (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes), has randomized 3,297 patients between the standard of care versus adding once weekly semaglutide over 104 weeks. Semaglutide showed a significant reduction in primary composite endpoint (first occurrence of CV death, non-fatal MI or non-fatal stroke), and nonfatal stroke. While nonfatal MI and CV death were the same between the two groups. Notably, semaglutide group developed more new or worsening retinopathy than placebo [93]. Interestingly, the Kaplan Meier curve showed CV benefit at 12 weeks (hemodynamic benefits) [12, 13] and 12 months in case of GLP-1 agonists’ trials (antiatherogenic benefits) [1, 2], respectively.
An interesting trial evaluated the effect of pioglitazone in insulin-resistant patients who had history of stroke or TIA, insulin resistance intervention after stroke (IRIS), showed very significant reduction in primary endpoint (fatal and nonfatal MI or fatal and nonfatal stroke), but no difference in terms of total stroke incidences, HHF and all-cause mortality [94]. All recent positive CVOTs are summarized in Table 1.
Table 1. Recent Randomized, Controlled Trials (Except for Dapagliflozin Was Real World Evidence) With Noninsulin Glucose-Lowering Drugs Showing Improvement of Cardiovascular Outcomes.
| Clinical variable | Empagliflozin | Canagliflozin | Dapagliflozin | Liraglutide | Semaglutide | Pioglitazone |
|---|---|---|---|---|---|---|
| CV death | Reduced | Reduced | Neutral* | Reduced | Neutral | NA |
| Nonfatal MI | Neutral | Reduced | Neutral | Neutral | Neutral | Neutral |
| Nonfatal stroke | Neutral | Reduced | NA | Neutral | Reduced | Neutral |
| 3-point MACE | Reduced | Reduced | Reduced* | Reduced | Neutral | Reduced |
| All-cause mortality | Reduced | Reduced | Reduced* | Reduced | Neutral | Neutral |
| Hospitalization for heart failure | Reduced | Reduced | Reduced* | Neutral | Neutral | Neutral |
| New or worsening nephropathy | Reduced | Reduced | NA | Reduced | NA | NA |
| Retinopathy | NA | NA | NA | NA | Increased | NA |
| Adverse events | ||||||
| Bone fractures | Neutral | Increased | Neutral | NA | NA | Increased |
| Hypophosphatemia | Neutral | Increased | Increased | NA | NA | NA |
| Bone density changes | Neutral | Increased | Neutral | NA | NA | Reduced |
| Limp amputation | Neutral | Increased | Neutral | NA | NA | NA |
| DKA | Increased | Increased | Increased | NA | NA | NA |
| Genital infections | Increased | Increased | Increased | NA | NA | NA |
*Evidence from CVD real studies and not from CVOT-directed RCTs.
From the current SGLT2is and GLP-1 agonists’ RCTs, it is evident that they exert their CV protective privilege through an entirely different pathway (Table 2). A fact that may call for future studies on the effect of combining those two agents, which may show superiority from a different perspective.
Table 2. Cardiovascular Benefits Upon Combining SGLT2i and the GLP-1 Agonists.
| Clinical variable | SGLT2i | GLP-1 agonists | CV risk improvement with combination SGLT2i and GLP-1 agonists |
|---|---|---|---|
| 3-point MACE | Reduced | Reduced | ++++ |
| BP | Reduced | Reduced | ++++ |
| Heart rate | Neutral | Increased | +/- |
| Fasting plasma glucose | Reduced | Reduced/neutral | ++ |
| Postprandial plasma glucose | Reduced | Reduced | ++++ |
| Dyslipidemia | Increased/reduced | Reduced | ++ |
| Insulin resistance | Reduced | Reduced | +++ |
| Systemic inflammation | Reduced | Reduced | ++++ |
| Vascular elasticity | Improved | Improved | ++++ |
| Heart muscle hypertrophy | Reduced | Reduced | ++ |
| Diuresis | Increased | Increased/neutral | +++ |
| Proteinuria | Reduced | Reduced | +++ |
| Hypermagnesemia | Increased | Neutral | ++ |
| Hyperketonemia | Increased | Neutral | ++ |
| CV effect appreciation | Started after 12 weeks only | Started at 12 months | Exert much more CV benefit at a more prolonged and sustained timeline |
Conclusions in SGLT2is and GLP-1 Agonists Combination
Having mentioned the extraordinary CV effect of SGLT2i and GLP-1 agonists, and the time difference in attaining the CV benefits, the question that arises is that why should not the SGLT2i and the GLP-1 agonists be the favored second and third antidiabetic options over all other conventional agents. The argument that all current CVOT had a vast majority of high CV risk patients will be answered, when DECLARE study is out by 2019, which assesses both primary and secondary prevention effect of dapagliflozin [17]. However, the primary subset of CANVA cohort is a preliminary answer to that question. Yet, some guidelines like American Diabetes Association clinical guidelines suggested the use of the SGLT2i (empagliflozin and canagliflozin) and the GLP-1 agonists (liraglutide) as the second line in case there is a history of CV insult, secondary prevention [95]. Moreover, adverse effects of SGLT2i are mostly minor and treatable (with exception of amputation in case of canagliflozin). Putting all of these facts together, we do not see any justification for not using those agents very early in the T2DM treatment paradigm.
References
- 1. IDF Diabetes Atlas, 8th edn. Brussels, Belgium: International Diabetes Federation, 2017. Available at: http://www.diabetesatlas.org.
- 2.American Diabetes A. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23(2):105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 7.Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB. et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31–38. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 9.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardman TC, Dubrey SW. Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther. 2011;2(3):133–145. doi: 10.1007/s13300-011-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foote C, Perkovic V, Neal B. Effects of SGLT2 inhibitors on cardiovascular outcomes. Diab Vasc Dis Res. 2012;9(2):117–123. doi: 10.1177/1479164112441190. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M. et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 14. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed on 05 Jan. 2018.
- 15.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W. et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW. et al. Lower risk of heart failure and death in patients initiated on SGLT-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL Study. Circulation. 2017;136(3):249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarafidis PA, Tsapas A. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2016;374(11):1092. doi: 10.1056/NEJMc1600827. [DOI] [PubMed] [Google Scholar]
- 18.Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC, investigators E-RHHSt. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 19.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156(6):405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 20.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13(10):928–938. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC. et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 22.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J. et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 23.Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, During M, Zdravkovic M. et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11(12):1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 24.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S. et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2016 executive summary. Endocr Pract. 2016;22(1):84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 25.Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol. 2014;70(10):1149–1158. doi: 10.1007/s00228-014-1730-x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imprialos KP, Sarafidis PA, Karagiannis AI. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33(11):2185–2197. doi: 10.1097/HJH.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 29.Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, Carbone S. et al. Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(5):e005686. doi: 10.1161/JAHA.117.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34(2):201–206. doi: 10.1161/01.HYP.34.2.201. [DOI] [PubMed] [Google Scholar]
- 32.Sjostrom CD, Hashemi M, Sugg J, Ptaszynska A, Johnsson E. Dapagliflozin-induced weight loss affects 24-week glycated haemoglobin and blood pressure levels. Diabetes Obes Metab. 2015;17(8):809–812. doi: 10.1111/dom.12500. [DOI] [PubMed] [Google Scholar]
- 33.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D. et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16(11):1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- 34.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 35.Cefalu WT, Stenlof K, Leiter LA, Wilding JP, Blonde L, Polidori D, Xie J. et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183–1187. doi: 10.1007/s00125-015-3547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muskiet MHA, van Bommel EJ, van Raalte DH. Antihypertensive effects of SGLT2 inhibitors in type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4(3):188–189. doi: 10.1016/S2213-8587(15)00457-X. [DOI] [PubMed] [Google Scholar]
- 37.Zimlichman R. Treatment of hypertension and metabolic syndrome: lowering blood pressure is not enough for organ protection, new approach-arterial destiffening. Curr Hypertens Rep. 2014;16(10):479. doi: 10.1007/s11906-014-0479-z. [DOI] [PubMed] [Google Scholar]
- 38.Jung CH, Jung SH, Kim KJ, Kim BY, Kim CH, Kang SK, Mok JO. Differential associations of central and brachial blood pressure with carotid atherosclerosis and microvascular complications in patients with type 2 diabetes. BMC Cardiovasc Disord. 2014;14:23. doi: 10.1186/1471-2261-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E. et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860–1870. doi: 10.1007/s00125-016-4008-2. [DOI] [PubMed] [Google Scholar]
- 40.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi: 10.1186/s12933-017-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 42.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 43.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 44.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75–83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH. et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 47.Rajasekeran H, Lytvyn Y, Hladunewich M, Cattran D, Bozovic A, Perkins B. et al. The effect of SGLT2 inhibition on urinary adenosine excretion in patients with type 1 diabetes. Can J Diabetes. 2016;40(5):S64. doi: 10.1016/j.jcjd.2016.08.182. [DOI] [Google Scholar]
- 48.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI, Cherney D. doi: 10.1161/CIRCULATIONAHA.116.021887. Sodium-glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications heerspink et al: cardiovascular risk and SGLT2 inhibition. 2017; Available from: http://circ.ahajournals.org/ [DOI] [PubMed]
- 49.Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M. et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanai H, Katsuyayama H. A possible mechanism for renoprotective effect of sodium-glucose cotransporter 2 inhibitor: elevation of erythropoietin production. J Clin Med Res. 2017;9(2):178–179. doi: 10.14740/jocmr2857w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844–847. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loeffler I, Ruster C, Franke S, Liebisch M, Wolf G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. Am J Physiol Renal Physiol. 2013;305(6):F911–918. doi: 10.1152/ajprenal.00643.2012. [DOI] [PubMed] [Google Scholar]
- 53.Ruester C, Franke S, Bondeva T, Wolf G. Erythropoietin protects podocytes from damage by advanced glycation end-products. Nephron Exp Nephrol. 2011;117(1):e21–30. doi: 10.1159/000319653. [DOI] [PubMed] [Google Scholar]
- 54.Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV. et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93(10):1809–1817. doi: 10.1161/01.CIR.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 55.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM. et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84. doi: 10.1186/s12933-017-0564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. 2016;39(7):1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 58.Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, Teoh H. et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39(12):e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 59.Baartscheer A, Schumacher CA, Wust RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–573. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M, O'Rourke B. et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121(14):1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baartscheer A, Hardziyenka M, Schumacher CA, Belterman CN, van Borren MM, Verkerk AO, Coronel R. et al. Chronic inhibition of the Na+/H+ - exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br J Pharmacol. 2008;154(6):1266–1275. doi: 10.1038/bjp.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovasc Diabetol. 2017;16(1):130. doi: 10.1186/s12933-017-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T. et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66(7):975–987. doi: 10.1111/jphp.12223. [DOI] [PubMed] [Google Scholar]
- 64.Obara K, Shirakami Y, Maruta A, Ideta T, Miyazaki T, Kochi T, Sakai H. et al. Preventive effects of the sodium glucose cotransporter 2 inhibitor tofogliflozin on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic mice. Oncotarget. 2017;8(35):58353–58363. doi: 10.18632/oncotarget.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lytvyn Y, Perkins BA, Cherney DZ. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes. 2015;39(3):239–246. doi: 10.1016/j.jcjd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 67.Barbagallo M, Dominguez LJ. Magnesium and type 2 diabetes. World J Diabetes. 2015;6(10):1152–1157. doi: 10.4239/wjd.v6.i10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes. 2016;65(1):3–13. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- 69.Yanagawa T. Is an increase in serum magnesium one of the causes of cardiovascular events reduction in the EMPA-REG OUTCOME study? J Clin Med Res. 2017;9(5):449–450. doi: 10.14740/jocmr3014w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2(2):366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 71.Pham PC, Pham PM, Pham PA, Pham SV, Pham HV, Miller JM, Yanagawa N. et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol. 2005;63(6):429–436. doi: 10.5414/CNP63429. [DOI] [PubMed] [Google Scholar]
- 72.Tang H, Zhang X, Zhang J, Li Y, Del Gobbo LC, Zhai S, Song Y. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546–2551. doi: 10.1007/s00125-016-4101-6. [DOI] [PubMed] [Google Scholar]
- 73.Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7 - Gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772(8):813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Dai LJ, Ritchie G, Kerstan D, Kang HS, Cole DE, Quamme GA. Magnesium transport in the renal distal convoluted tubule. Physiol Rev. 2001;81(1):51–84. doi: 10.1152/physrev.2001.81.1.51. [DOI] [PubMed] [Google Scholar]
- 75.Weir MR, Kline I, Xie J, Edwards R, Usiskin K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR) Curr Med Res Opin. 2014;30(9):1759–1768. doi: 10.1185/03007995.2014.919907. [DOI] [PubMed] [Google Scholar]
- 76.Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017;34(7):1707–1726. doi: 10.1007/s12325-017-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680–1686. doi: 10.2337/dc15-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alba M, Xie J, Fung A, Desai M. The effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on mineral metabolism and bone in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(8):1375–1385. doi: 10.1080/03007995.2016.1174841. [DOI] [PubMed] [Google Scholar]
- 80.Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G. et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35(6):1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K. et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(1):157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. https://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Accessed Dec 25, 2017.
- 84.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(1):e4–e5. doi: 10.2337/dc17-1551. [DOI] [PubMed] [Google Scholar]
- 85. S Food and Drug Administration. FDA Drug Safety Communication: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm. Accessed December 29, 2017.
- 86.Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, Qiu R. et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17(1):23–31. doi: 10.1111/dom.12385. [DOI] [PubMed] [Google Scholar]
- 87.Bashier A, Khalifa AA, Rashid F, Abdelgadir EI, Al Qaysi AA, Ali R, Eltinay A. et al. Efficacy and safety of SGLT2 inhibitors in reducing glycated hemoglobin and weight in emirati patients with type 2 diabetes. J Clin Med Res. 2017;9(6):499–507. doi: 10.14740/jocmr2976w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013;125(3):181–189. doi: 10.3810/pgm.2013.05.2667. [DOI] [PubMed] [Google Scholar]
- 89.Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2014;30(3):204–221. doi: 10.1002/dmrr.2479. [DOI] [PubMed] [Google Scholar]
- 90.Liakos A, Karagiannis T, Athanasiadou E, Sarigianni M, Mainou M, Papatheodorou K, Bekiari E. et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(10):984–993. doi: 10.1111/dom.12307. [DOI] [PubMed] [Google Scholar]
- 91. U.S. Food and Drug Administration. Drug safety communication (December 4, 2015): FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections, Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM475487.pdf. Accessed December 29, 2017.
- 92.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 94.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD. et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. AACE/ACE comprehensive type 2 diabetes management algorithm. 2018. https://www.aace.com/publications/algorithm.