Abstract
Background
Most patients with path_MMR gene variants (Lynch syndrome (LS)) now survive both their first and subsequent cancers, resulting in a growing number of older patients with LS for whom limited information exists with respect to cancer risk and survival.
Objective and design
This observational, international, multicentre study aimed to determine prospectively observed incidences of cancers and survival in path_MMR carriers up to 75 years of age.
Results
3119 patients were followed for a total of 24 475 years. Cumulative incidences at 75 years (risks) for colorectal cancer were 46%, 43% and 15% in path_MLH1, path_MSH2 and path_MSH6 carriers; for endometrial cancer 43%, 57% and 46%; for ovarian cancer 10%, 17% and 13%; for upper gastrointestinal (gastric, duodenal, bile duct or pancreatic) cancers 21%, 10% and 7%; for urinary tract cancers 8%, 25% and 11%; for prostate cancer 17%, 32% and 18%; and for brain tumours 1%, 5% and 1%, respectively. Ovarian cancer occurred mainly premenopausally. By contrast, upper gastrointestinal, urinary tract and prostate cancers occurred predominantly at older ages. Overall 5-year survival for prostate cancer was 100%, urinary bladder 93%, ureter 85%, duodenum 67%, stomach 61%, bile duct 29%, brain 22% and pancreas 0%. Path_PMS2 carriers had lower risk for cancer.
Conclusion
Carriers of different path_MMR variants exhibit distinct patterns of cancer risk and survival as they age. Risk estimates for counselling and planning of surveillance and treatment should be tailored to each patient’s age, gender and path_MMR variant. We have updated our open-access website www.lscarisk.org to facilitate this.
Keywords: cancer prevention, clinical trials, colorectal cancer screening, inherited cancers, HNPCC syndrome
Significance of this study.
What is already known on this subject?
Inherited predisposition to colorectal, gynaecological, urinary tract, upper gastrointestinal and other cancers may be caused by pathogenic variants of mismatch repair (path_MMR) genes and is then commonly referred to as Lynch syndrome (LS).
Most patients with LS undergoing surveillance nonetheless develop colorectal and/or other cancers but often survive both their first cancer and their subsequent cancers.
Penetrance and expressions of the different path_MMR genes in older age are uncertain.
What are the new findings?
Prostate, urinary tract, gastric, duodenal, bile duct and pancreatic cancers occurred frequently in older patients with LS.
Prostate and urinary tract cancers predominantly occurred in path_MSH2 carriers.
Upper gastrointestinal tract cancers predominantly occurred in path_MLH1 carriers.
Ovarian cancer occurred predominantly in younger women, which is in contrast to ovarian cancers in path_BRCA1/2 carriers and in the general population.
Survival was very poor for bile duct and pancreatic cancers, intermediate for gastric, duodenal and urinary tract cancers and good for prostate cancer.
Except for endometrial and prostate cancer, typical LS cancers were not diagnosed in path_PMS2 carriers.
How might it impact on clinical practice in the foreseeable future?
Knowledge of path_MMR gene-specific incidence and survival of cancers affecting different organs will inform genetic counselling and clinical management in the ageing LS population.
Risk assessment and surveillance in LS should be based on age, gender and the path_MMR gene involved.
Subgroups of patients with LS defined by age and path_MMR gene have high risks for different cancers, some with serious prognoses in older age.
Prevention and cure of these late onset cancers is a growing clinical challenge in LS.
Introduction
Lynch syndrome (LS) is associated with a high probability of gastrointestinal, gynaecological and other cancers.1 2 It is caused by germline pathogenic variants in any of four DNA MMR genes referred to here as path_MSH2, path_MLH1, path_PMS2 or path_MSH6 and collectively as path_MMR. Deletions in the EPCAM gene, which lead to inherited methylation of the adjacent MSH2 promoter, are also referred to as path_MSH2. To date, most patients with LS have been identified following investigation because of their family or personal history of multiple and/or early onset cancers.
Carriers of path_MMR variants require reliable information about their future cancer risks so that they can be offered appropriately targeted surveillance. However, a paucity of prospectively obtained information has led current clinical guidelines to rely heavily upon retrospective data from patient cohorts whose selection for molecular testing has been subject to bias. The efficacy of effects of interventions to prevent cancers in path_MMR carriers is debated.1–4
Most patients with LS receiving surveillance for LS-associated tumours develop colorectal, gynaecological or other cancers and now survive both their first and subsequent cancers.1 2 The consequence is a growing and ageing population of patients with LS. There is limited information on their cancer risks and survivals. One reason for this is that in former generations most patients with LS died from their first cancer, and the risks for survivors are therefore difficult to determine from retrospective family studies.5 A second reason is that measures to prevent or cure early onset cancers in LS are recent developments that have benefited mainly younger patients with LS. A third reason is the possibility that patients with LS affected by cancer at younger ages might have path_MMR variants of higher penetrance (as in path_BRCA1 6) and/or carry genetic modifiers that increase penetrance7 and/or were exposed to a more carcinogenic environment (as for path_RB 8).
Indeed, it was unknown whether LS patients with and without early onset cancer had similar risks for cancer later in life. If the risks differed substantially, these groups could not be pooled when assessing risks in older patients. The first report from the Prospective Lynch Syndrome Database (PLSD) described LS patients without cancer before their inclusion and showed that the different path_MMR genes had different penetrance and/or expression.1 Few previous prospective reports had stratified results this way and the question of whether LS patients with early onset cancer have an increased risk for subsequent cancer could be confounded by differences related to which path_MMR gene the patients carried. We examined this issue further in a second report from PLSD and found similar future cancer incidence in patients with LS who had a previous cancer compared with those who did not, when stratifying by path_MMR gene.2 These findings allowed us to combine the two groups in the present report.
No single centre has sufficient patient numbers to estimate risks in older individuals and international multicentre collaboration is needed to obtain results within a reasonable timeframe. The PLSD provides sufficient numbers for calculation of cumulative incidences of cancer by organ, path_MMR variant and gender up to 75 years of age with reasonable precision. For some groups, we also present some figures for cumulative incidences up to 80 years of age.
In our two former reports1 2, we demonstrated differences in the penetrance and/or expression between pathogenic variants of the four MMR genes MLH1, MSH2, MSH6 and PMS2, and we demonstrated that within each group of path_MMR carriers, the annual incidences of cancers were age dependent. We also demonstrated that for carriers of pathogenic variants of a given MMR gene, annual incidence rates of cancers were similar in those who had a previous (early) cancer and those who did not. That is, cancers appeared to be age-dependent stochastic events and any putative differences in penetrance between carriers who had or did nor have an early cancer, including influences of inherited genetic modifiers and environmental factors could be ignored when compiling the current report based on the combined series of both sets of patients. We emphasise that while such factors may be important determinants of penetrance, it is nonetheless legitimate to combine the data from the previous reports and calculate results for the current report without them being subject to major confounders because of these factors.
This report together with the two former reports now provide information relevant to personalised medical care for patients with LS of any age, gender, with or without previous cancer and with any path_MMR variant.
Methods
Recruitment and follow-up
PLSD database design and inclusion criteria have been described previously.1 2 This study is an open observational study without a control group. Path_MMR carriers aged 25 years or older on the day of their first prospectively planned and completed surveillance colonoscopy were included whether they had previous cancer. We have reported previously time to first cancer1 and time to any subsequent cancer2 up to 70 years in these patients. Any cancers that were diagnosed before or at the same age as the first prospectively planned and completed colonoscopy were scored as previous cancers, and all incident cancers reported here were detected at an older age than the age at inclusion. All patients received follow-up surveillance as reported previously (1-2, see onlineSupplementary table S1) until the last update of information when they were scored as alive or dead. All patients were followed up according to local clinical guidelines. Follow-up times were censored if a patient was lost to follow-up. Intervals between colonoscopies were not considered. Each patient was censored at the age at which the last information was available, which might have been a colonoscopy, any other clinical examination, a report from an examination done by others, or information that the patient had died, whichever came last. In this report, we consider only incidences and survival of cancers in patients with LS undergoing surveillance. As we found that cancers continued to occur, we are planning future studies to investigate the relationships between factors such as colonoscopy interval, adenoma detection and removal and the incidence and stage at diagnoses of colorectal cancer.
gutjnl-2017-314057supp001.pdf (212.2KB, pdf)
All patients were demonstrated to be carriers of path_MMR variants (as determined by reporting centres) by gene testing or were obligate carriers of familial path_MMR variants by pedigree structure. All reported path_MMR variants were checked against the Leiden Open Variation Database (LOVD) for LS (http://chromium.lovd.nl/LOVD2/colon_cancer/) in October 2015. Deletions in the EPCAM gene that silence MSH2 were scored as path_MSH2. In sum 1865 (59.7%) of the patients had pathogenic (class 5) variants, 42 (1.3%) patients had probably pathogenic (class 4) variants and the remaining 1212 (38.9%) patients had variants that were not found in LOVD.
The following information was used for analyses: gender, path_MMR variant, age at inclusion, age at last update, age at any cancer, cancers scored by the first three positions in the International Classification of Diseases version 9 (ICD9) diagnostic system and age at death. Cancer stage at diagnosis was not included.
Some centres have reported prospective findings independently9–11 and their previously reported cases are included in the present series.
Statistical methods
The statistical methods were described in detail previously.1 2 In short, annual incidence rates (AIRs) by age were calculated in 5-year cohorts from 25 to 75 years of age. Cumulative incidence, denoted by Q, was computed starting at age 25, assuming zero incidence before age 25, using the formula Q(age) = Q(age−1)+[1−Q(age−1)]·AIR(age) where AIR(age) is the annual incidence rate as estimated from the corresponding 5-year interval. The 95% CIs were estimated as appropriate.
When calculating the AIR for a given cancer, only patients without previous cancer in that organ were included, and observation time was censored at first cancer in the organ, while cancers in other organs were ignored. This was considered appropriate based on our reported findings that LS patients with previous cancer have approximately the same incidence of subsequent cancers as those with no previous cancer.1 2 This way of calculating organ-specific cumulative incidences is in line with the established method for reporting cumulative incidence of breast and ovarian cancer separately in carriers of path_BRCA1/2.6 12
Lifetime cumulative incidence of cancer was calculated as cumulative incidence from 25 to 75 years of age and compared with the population average from the reporting countries as extracted from http://globocan.iarc.fr in December 2015. Specific lifetime cumulative incidences for duodenal, colon and rectal cancers were extracted from http://www-dep.iarc.fr/NORDCAN/NO/frame.asp in December 2015 for the year 2013. Relative cumulative lifetime risk was calculated as observed cumulative lifetime incidence divided by population cumulative incidence at 75 years. Crude 5-year and 10-year survival following cancer was assessed by the Kaplan-Meier survivor function for patients aged 65 years or less at diagnoses.
Ethics
All reporting centres exported only deidentified data to the joint database used for this report. All genetic tests were performed with appropriate informed consent according to local and national requirements for health care and/or research. No named data was exported from participating centres.
Results
A total of 3119 patients including 1723 females, and 1396 males met the inclusion criteria. The total number of observation years was 24 475. The numbers of patients and observation years were 1473 and 13 846 for path_MLH1 carriers; 1060 and 7492 for path_MSH2 carriers; 462 and 2613 for path_MSH6 carriers; and 124 and 524 for path_PMS2 carriers. Mean age at inclusion by gene and gender varied from 41.5 to 53.3; carriers of path_MSH6 and path_PMS2 were significantly older than carriers of path_MLH1 and path_MSH2 (table 1). Results for path_PMS2 carriers are given, although numbers are generally insufficient to draw firm conclusions. In all, 813 cancers were diagnosed during follow-up. They affected 618 patients (20% of all patients with a mean of 1.3 cancers per affected patient) (details are given in online supplementary table S2). The observed incidences and cumulative incidences may be considered as risks of developing cancers and are discussed as such. Time from last ccolonoscopyto ccolorectalcancer in patients without prior cancer is given in our first report,1 and time from last colonoscopy to colorectal cancer in patients having had a prior cancer is given in our second report2
Table 1.
Patients included, follow-up years and age at inclusion stratified on country, gender and path_MMR variants
| Group | Sex | Cases | Follow-up years | Age at inclusion | |||||||
| Number | Mean | Min | Max | 95% CI | Mean | Min | Max | 95% CI | |||
| All | 3119 | 24 475 | 7.8 | 1 | 33 | ±0.2 | 43.8 | 25 | 79 | ±0.46 | |
| F | 1723 | 13 534 | 7.9 | 1 | 33 | ±0.26 | 44.6 | 25 | 79 | ±0.62 | |
| M | 1396 | 10 941 | 7.8 | 1 | 30 | ±0.29 | 42.9 | 25 | 79 | ±0.67 | |
| Country of origin | |||||||||||
| Finland | 952 | 9212 | 9.7 | 1 | 30 | ±0.38 | 43.2 | 25 | 79 | ±0.88 | |
| Denmark | 515 | 3084 | 6.0 | 1 | 17 | ±0.31 | 44.4 | 25 | 77 | ±1.09 | |
| UK | 386 | 2229 | 5.8 | 1 | 17 | ±0.4 | 45.7 | 25 | 79 | ±1.29 | |
| Norway | 294 | 2217 | 7.5 | 1 | 22 | ±0.61 | 44.7 | 25 | 79 | ±1.47 | |
| Spain | 202 | 1167 | 5.8 | 1 | 14 | ±0.51 | 47.6 | 25 | 75 | ±1.75 | |
| Germany | 179 | 1276 | 7.1 | 1 | 29 | ±0.76 | 43.5 | 25 | 72 | ±1.61 | |
| Holland | 165 | 898 | 5.4 | 1 | 28 | ±0.88 | 45.5 | 25 | 78 | ±2.00 | |
| Italy | 163 | 1773 | 10.9 | 1 | 33 | ±1.04 | 35.9 | 25 | 65 | ±1.42 | |
| Sweden | 159 | 1568 | 9.9 | 1 | 27 | ±0.88 | 44.7 | 25 | 77 | ±2.07 | |
| Australia | 104 | 1051 | 10.1 | 1 | 30 | ±1.35 | 39.2 | 25 | 70 | ±1.99 | |
| Gene | |||||||||||
| MLH1 | F | 781 | 7365 | 9.4 | 1 | 33 | ±0.41 | 42.9 | 25 | 79 | ±0.93 |
| M | 692 | 6481 | 9.4 | 1 | 30 | ±0.44 | 41.5 | 25 | 79 | ±0.96 | |
| MSH2 | F | 599 | 4233 | 7.1 | 1 | 30 | ±0.41 | 44.6 | 25 | 77 | ±1.02 |
| M | 461 | 3259 | 7.1 | 1 | 27 | ±0.45 | 42.2 | 25 | 73 | ±1.05 | |
| MSH6 | F | 276 | 1633 | 5.9 | 1 | 21 | ±0.49 | 48.7 | 25 | 79 | ±1.60 |
| M | 186 | 980 | 5.3 | 1 | 28 | ±0.61 | 46.8 | 25 | 79 | ±1.85 | |
| PMS2 | F | 67 | 303 | 4.5 | 1 | 20 | ±1.07 | 47.7 | 25 | 78 | ±2.73 |
| M | 57 | 221 | 3.9 | 1 | 22 | ±0.97 | 53.3 | 26 | 77 | ±3.59 | |
gutjnl-2017-314057supp002.pdf (196.1KB, pdf)
Cumulative incidences of cancer by organ and age
The cumulative incidences of cancers in individual organs or groups of organs from 25 years of age are shown in tables 2 and 3. Cumulative incidence at 75 years for any cancer was 76% (95% CI 68.5% to 83.2%), 80% (95% CI 69.8% to 90.9%) and 61% (95% CI 42.7% to 79.0%) for path_MLH1, path_MSH2 and path_MSH6 carriers, respectively (table 2). A higher lifetime incidence for colorectal cancer was seen in path_MLH1 and path_MSH2 carriers compared with path_MSH6 carriers (46% (95% CI 37.8% to 53.9%), 43% (95% CI 33.2% to 52.8%) and 15% (95% CI 3.3% to 26.6%), respectively). Colorectal cancer was not observed in the small number of path_PMS2 carriers studied. Most colorectal cancer affected the colon rather than the sigmoid or rectum (table 3). Lifetime incidences for endometrial cancer were 43% (95% CI 33.1% to 52.3%); 57% (95% CI 41.8% to 71.6%) and 46% (95% CI 27.3% to 65.0%) for path_MLH1, path_MSH2 and path_MSH6 carriers, respectively. The corresponding incidences for ovarian cancer were: 10% (95% CI 4.8% to 15.4%), 17% (95% CI 5.7% to 28.0%) and 13% (95% CI 0.1% to 31.2%), respectively. Most gynaecological cancers occurred before the age of 60 years (tables 2 and 3). There was a significantly higher incidence of colon cancer in middle-aged path_MLH1 males compared with females, but no significant gender differences were observed for colon cancer in path_MSH2 and path_MSH6 carriers (table 4).
Table 2.
Cumulative incidence of cancers in groups of organs from 25 years of age up to the age indicated in the column Age, stratified on carriers of path_MMR variants in the different genes and 95% CIs in parentheses. Gynaecological cancer include endometrial and ovarian cancer. Upper gastrointestinal cancer include stomach, duodenum, bile duct, gall bladder or pancreas cancer. Genitorurinary tract cancer include urinary bladder, ureter or kidney
| ICD9 | Organ | Age | Cumulative incidence at age (% (95% CI)) | |||
| path_MLH1 | path_MSH2 | path_MSH6 | path_PMS2 | |||
| Any cancer | 40 | 16.8 (12.2 to 21.3) | 14.3 (8.0 to 20.3) | 0 | 0 | |
| 50 | 40.2 (34.7 to 45.7) | 37.2 (29.4 to 45.1) | 18.1 (8.0 to 28.3) | 0 | ||
| 60 | 58.7 (52.8 to 64.6) | 57.6 (49.3 to 65.9) | 39.0 (25.8 to 52.2) | 18.2 (0.0 to 41.0) | ||
| 70 | 71.7 (64.7 to 78.7) | 71.6 (61.9 to 81.2) | 53.6 (38.6 to 68.6) | 18.2 (0.0 to 41.0) | ||
| 75 | 75.8 (68.5 to 83.2) | 80.4 (69.8 to 90.9) | 60.9 (42.7 to 79.0) | 52.1 (0.1 to 100.0) | ||
| 153, 154 | Colorectal cancer | 40 | 12.7 (8.6 to 16.9) | 8.9 (4.0 to 13.7) | 0 | 0 |
| 50 | 25.0 (20.0 to 30.0) | 19.4 (13.0 to 25.8) | 1.8 (0.0 to 5.4) | 0 | ||
| 60 | 34.6 (28.9 to 40.3) | 27.1 (19.9 to 34.3) | 5.6 (0.0 to 11.9) | 0 | ||
| 70 | 40.1 (33.5 to 46.7) | 40.8 (31.6 to 50.1) | 15.0% (3.3 to 26.6) | 0 | ||
| 75 | 45.8 (37.8 to 53.9) | 43.0 (33.2 to 52.8) | 15.0 (3.3 to 26.6) | 0 | ||
| 182, 183 | Gynaecological cancer | 40 | 5.7 (2.1 to 9.3) | 5.2 (0.2 to 10.2) | 4.2 (0.0 to 12.3) | 0 |
| 50 | 24.6 (18.2 to 30.9) | 25.3 (16.1 to 34.5) | 19.2 (6.2 to 32.1) | 0 | ||
| 60 | 41.4 (33.6 to 49.2) | 49.2 (37.9 to 60.4) | 36.1 (19.2 to 53.1) | 26.4 (0.8 to 51.9) | ||
| 70 | 47.6 (38.9 to 56.4) | 62.1 (49.1 to 75.0) | 54.0 (34.6 to 73.4) | 26.4 (0.8 to 51.9) | ||
| 75 | 49.8 (40.5 to 59.0) | 65.7 (52.2 to 79.1) | 54.0 (34.6 to 73.4) | 26.4 (0.8 to 51.9) | ||
| 151, 152, 156, 157 | Upper gastrointestinal tract cancer | 40 | 1.0 (0.0 to 2.1) | 0 | 0 | 0 |
| 50 | 3.3 (1.4 to 5.1) | 1.5 (0.0 to 3.1) | 0 | 0 | ||
| 60 | 7.0 (4.3 to 9.7) | 4.1 (1.3 to 6.9) | 2.8 (0.0 to 6.7) | 0 | ||
| 70 | 17.1 (12.1 to 22.0) | 6.7 (2.7 to 10.6) | 2.8 (0.0 to 6.7) | 0 | ||
| 75 | 21.4 (15.6 to 27.1) | 10.2 (4.1 to 16.3) | 6.6 (0.0 to 14.8) | 0 | ||
| 188, 189 | Urinary tract | 40 | 0.3 (0.0 to 0.9) | 0 | 0 | 0 |
| 50 | 1.3 (0.2 to 2.5) | 3.4 (0.9 to 5.9) | 3.0 (0.0 to 7.0) | 0 | ||
| 60 | 3.0 (1.1 to 4.8) | 9.8 (5.6 to 14.0) | 5.7 (0.3 to 11.1) | 0 | ||
| 70 | 7.2 (3.8 to 10.6) | 21.3 (14.2 to 28.3) | 7.2 (1.1 to 13.3) | 0 | ||
| 75 | 8.0 (4.3 to 11.7) | 24.9 (16.6 to 33.2) | 11.0 (1.7 to 20.3) | 0 | ||
Table 3.
Cumulative incidences of individual cancers in carriers of path_MMR variants in the different genes from 25 years of age up to the age of 75 are shown. Incidence of breast, endometrial and ovarian cancers were calculated in females and incidence of prostate cancers calculated in men; all others in both genders combined
| ICD9 | Organ | Age | Cumulative incidence by age (% (95% CI)) | |||
| path_MLH1 | path_MSH2 | path_MSH6 | path_PMS2 | |||
| Colorectal cancer | ||||||
| 153 | Colon | 40 | 12.9 (8.8 to 17.1) | 8.6 (3.9 to 13.3) | 0 | 0 |
| 50 | 25.7 (20.7 to 30.7) | 20.2 (13.9 to 26.4) | 1.8 (0.0 to 5.2) | 0 | ||
| 60 | 36.0 (30.4 to 41.6) | 27.3 (20.3 to 34.3) | 5.3 (0.0 to 11.2) | 0 | ||
| 70 | 41.6 (35.2 to 48.0) | 40.2 (31.3 to 49.1) | 14.2 (3.1 to 25.4) | 0 | ||
| 75 | 46.7 (39.2 to 54.3) | 42.4 (32.9 to 51.9) | 14.2 (3.1 to 25.4) | 0 | ||
| 154 | Sigmoid and rectum | 40 | 1.6 (0.2 to 3.0) | 0.6 (0.0 to 1.8) | 0 | 0 |
| 50 | 3.2 (1.3 to 5.0) | 4.7 (1.7 to 7.7) | 0 | 0 | ||
| 60 | 6.3 (3.6 to 9.0) | 9.8 (5.5 to 14.1) | 3.0 (0.0 to 7.1) | 0 | ||
| 70 | 9.2 (5.6 to 12.9) | 13.5 (8.0 to 19.0) | 4.6 (0.0 to 9.7) | 0 | ||
| 75 | 11.8 (7.2 to 16.4) | 18.3 (10.9 to 25.6) | 4.6 (0.0 to 9.7) | 0 | ||
| Endometrial and ovarian cancer | ||||||
| 182 | Endometrium | 40 | 3.1 (0.4 to 5.8) | 1.5 (0.0 to 4.4) | 0 | 0 |
| 50 | 18.7 (12.9 to 24.5) | 15.9 (7.9 to 23.8) | 15.4 (4.1 to 26.8) | 0 | ||
| 60 | 34.8 (27.1 to 42.5) | 40.9 (29.2 to 52.6) | 33.2 (16.5 to 49.9) | 26.4 (0.8 to 51.9) | ||
| 70 | 40.3 (31.5 to 49.1) | 52.7 (38.7 to 66.8) | 46.2 (27.3 to 65.0) | 26.4 (0.8 to 51.9) | ||
| 75 | 42.7 (33.1 to 52.3) | 56.7 (41.8 to 71.6) | 46.2 (27.3 to 65.0) | 26.4 (0.8 to 51.9) | ||
| 183 | Ovaries | 40 | 2.6 (0.1 to 5.2) | 3.8 (0.0 to 8.0) | 4.2 (0.0 to 12.3) | 0 |
| 50 | 6.8 (2.9 to 10.7) | 10.7 (4.1 to 17.4) | 4.2 (0.0 to 12.3) | 0 | ||
| 60 | 8.6 (4.0 to 13.2) | 12.3 (5.1 to 19.5) | 4.2 (0.0 to 12.3) | 0 | ||
| 70 | 10.1 (4.8 to 15.4) | 16.9 (5.7 to 28.0) | 13.1 (0.0 to 31.2) | 0 | ||
| 75 | 10.1 (4.8 to 15.4) | 16.9 (5.7 to 28.0) | 13.1 (0.0 to 31.2) | 0 | ||
| Upper gastrointestinal cancer | ||||||
| 151 | Stomach | 40 | 0.3 (0.0 to 0.9) | 0 | 0 | 0 |
| 50 | 0.8 (0.0 to 1.7) | 0.5 (0.0 to 1.4) | 0 | 0 | ||
| 60 | 2.4 (0.7 to 4.0) | 1.6 (0.0 to 3.4) | 1.4 (0.0 to 4.2) | 0 | ||
| 70 | 6.3 (3.0 to 9.7) | 4.1 (0.8 to 7.5) | 1.4 (0.0 to 4.2) | 0 | ||
| 75 | 7.1 (3.5 to 10.8) | 7.7 (1.9 to 13.6) | 5.3 (0.0 to 13.1) | 0 | ||
| 152 | Duodenum | 40 | 0.4 (0.0 to 1.1) | 0 | 0 | 0 |
| 50 | 1.1 (0.0 to 2.3) | 1.0 (0.0 to 2.3) | 0 | 0 | ||
| 60 | 2.1 (0.5 to 3.7) | 2.0 (0.1 to 4.0) | 0 | 0 | ||
| 70 | 4.1 (1.4 to 6.8) | 2.0 (0.1 to 4.0) | 0 | 0 | ||
| 75 | 6.5 (2.7 to 10.2) | 2.0 (0.1 to 4.0) | 0 | 0 | ||
| 156 | Bile duct and gall bladder | 40 | 0 | 0 | 0 | 0 |
| 50 | 0.3 (0.0 to 0.8) | 0 | 0 | 0 | ||
| 60 | 1.3 (0.0 to 2.5) | 0 | 0 | 0 | ||
| 70 | 3.7 (1.3 to 6.2) | 0 | 0 | 0 | ||
| 75 | 3.7 (1.3 to 6.2) | 1.7 (0.0 to 5.1) | 0 | 0 | ||
| 157 | Pancreas | 40 | 0.3 (0.0 to 0.9) | 0 | 0 | 0 |
| 50 | 1.1 (0.0 to 2.1) | 0 | 0 | 0 | ||
| 60 | 1.7 (0.3 to 3.1) | 0.5 (0.0 to 1.5) | 1.4 (0.0 to 4.2) | 0 | ||
| 70 | 3.9 (1.4 to 6.4) | 0.5 (0.0 to 1.5) | 1.4 (0.0 to 4.2) | 0 | ||
| 75 | 6.2 (2.6 to 9.8) | 0.5 (0.0 to 1.5) | 1.4 (0.0 to 4.2) | 0 | ||
| Urinary tract cancer | ||||||
| 188 | Urinary bladder | 40 | 0 | 0 | 0 | 0 |
| 50 | 0.5 (0.0 to 1.2) | 1.0 (0.0 to 2.3) | 2.9 (0.0 to 7.0) | 0 | ||
| 60 | 1.9 (0.4 to 3.4) | 3.6 (1.0 to 6.3) | 4.3 (0.0 to 9.1) | 0 | ||
| 70 | 4.1 (1.5 to 6.7) | 6.2 (2.3 to 10.2) | 4.3 (0.0 to 9.1) | 0 | ||
| 75 | 4.1 (1.5 to 6.7) | 8.1 (2.8 to 13.3) | 8.2 (0.0 to 16.9) | 0 | ||
| 189 | Ureter and kidney | 40 | 0.3 (0.0 to 0.9) | 0 | 0 | 0 |
| 50 | 0.8 (0.0 to 1.8) | 2.4 (0.3 to 4.5) | 0 | 0 | ||
| 60 | 1.1 (0.0 to 2.2) | 6.9 (3.3 to 10.5) | 1.4 (0.0 to 4.2) | 0 | ||
| 70 | 3.8 (1.2 to 6.4) | 16.0 (9.6 to 22.4) | 3.0 (0.0 to 7.0) | 0 | ||
| 75 | 4.6 (1.6 to 7.6) | 17.8 (10.6 to 25.0) | 3.0 (0.0 to 7.0) | 0 | ||
| Other LS or possible LS cancers | ||||||
| 174 | Breast | 40 | 0 | 2.6 (0.0 to 6.2) | 0 | 0 |
| 50 | 2.9 (0.6 to 5.2) | 5.2 (0.7 to 9.8) | 0 | 0 | ||
| 60 | 8.3 (4.2 to 12.4) | 6.1 (1.3 to 11.0) | 4.7 (0.0 to 11.0) | 8.6 (0.0 to 24.8) | ||
| 70 | 12.0 (6.7 to 17.3) | 11.5 (4.6 to 18.4) | 13.3 (2.2 to 24.4) | 8.6 (0.0 to 24.8) | ||
| 75 | 12.0 (6.7 to 17.3) | 11.5 (4.6 to 18.4) | 13.3 (2.2 to 24.4) | 55.9 (0.0 to 100.0) | ||
| 185 | Prostate | 40 | 0 | 0 | 0 | 0 |
| 50 | 0.6 (0.0 to 1.6) | 0 | 0 | 37.9 (0.0 to 95.9) | ||
| 60 | 5.3 (1.4 to 9.1) | 4.2 (0.0 to 8.8) | 0 | 37.9 (0.0 to 95.9) | ||
| 70 | 13.3 (6.1 to 20.4) | 13.2 (3.7 to 6.1) | 4.4 (0.0 to 12.9) | 37.9 (0.0 to 95.9) | ||
| 75 | 16.9 (8.5 to 25.3) | 31.6 (11.7 to 51.5) | 18.3 (0.0 to 44.4) | 37.9 (0.0 to 95.9) | ||
| 191 | Brain | 40 | 0.3 (0.0 to 0.9) | 0 | 0 | 0 |
| 50 | 0.3 (0.0 to 0.9) | 0 | 0 | 0 | ||
| 60 | 0.3 (0.0 to 0.9) | 1.1 (0.0 to 2.6) | 1.4 (0.0 to 4.2) | 0 | ||
| 70 | 1.0 (0.0 to 2.4) | 1.9 (0.0 to 4.0) | 1.4 (0.0 to 4.2) | 0 | ||
| 75 | 1.0 (0.0 to 2.4) | 5.3 (0.2 to 10.3) | 1.4 (0.0 to 4.2) | 0 | ||
LS, Lynch syndrome
Table 4.
Cumulative incidence colon and sigmoidal-rectal cancer stratified by age, path_MMR variant and gender. There was no observed colon or sigmoid-rectal cancer in path_PMS2 carriers in any age group (see table 3)
| Age | Cumulative incidence by age (% (95% CI)) | ||||||
| path_MLH1 | path_MSH2 | path_MSH6 | |||||
| Females | Males | Females | Males | Females | Males | ||
| Colon cancer (ICD9=153) | 40 | 10.4 (5.2 to 15.6) | 15.4 (9.0 to 21.9) | 10.1 (2.9 to 17.3) | 7.4 (1.2 to 13.6) | 0 | 0 |
| 50 | 18.8 (12.6to 25.0) | 33.3 (25.5 to 41.1) | 23.8 (14.8 to 32.8) | 15.8 (7.2 to 24.3) | 0 | ||
| 60 | 28.1 (20.9 to 35.2) | 45.2 (36.6 to 53.8)* | 31.4 (21.9 to 41.0) | 21.5 (11.3 to 31.6) | |||
| 70 | 37.3 (28.5 to 46.1) | 45.2 (36.6 to 53.8) | 44.7 (33.7 to 55.7) | 32.4 (17.7 to 47.2) | |||
| 75 | 42.1 (31.8 to 52.5) | 50.8 (40.1 to 61.5) | 44.7 (33.7 to 55.7) | 42.6 (20.4 to 64.9) | |||
| Sigmoid- rectal cancer(ICD9=154) | 40 | 1.4 (0.0 to 3.3) | 1.9 (0.0 to 4.1) | 0 | 1.3 (0.0 to 3.8) | 0 | 0 |
| 50 | 2.9 (0.3 to 5.4) | 3.6 (0.8 to 6.4) | 1.0 (0.0 to 3.0) | 8.9 (3.0 to 14.7) | 0 | 0 | |
| 60 | 6.1 (2.4 to 9.8) | 6.5 (2.6 to 10.5) | 4.0 (0.2 to 7.8) | 17.9 (9.2 to 26.5) | 4.6 (0.0 to 10.7) | 0 | |
| 70 | 10.2 (4.9 to 15.6) | 7.6 (3.2 to 12.1) | 8.2 (2.2 to 14.3) | 20.7 (10.7 to 30.7) | 7.0to (0.0 to 14.6) | 0 | |
| 75 | 11.7 (5.7 to 17.7) | 11.7 (4.7 to 18.7) | 12.9 (4.4 to 21.3) | 26.0 (12.3 to 39.7) | 7.0 (0.0 to 14.6) | 0 | |
*Increased (p<0.05) compared with same-aged females.
**Lower (p<0.05) than in path_MLH1 or path_MSH2 carrier with same gender and age.
Upper gastrointestinal cancers including those affecting the stomach, duodenum, bile duct, gall bladder or pancreas were diagnosed predominantly in old age. The cumulative lifetime incidence of upper gastrointestinal tumours was particularly high for path_MLH1 carriers (21% (95% CI 15.6% to 27.1%) compared with path_MHS2 carriers (10% (95% CI 4.1% to 16.3%)) and path_MSH6 carriers (7% (95% CI 0% to 14.8%)) (table 2). Among upper gastrointestinal cancers, the cumulative risk for stomach cancer was greatest: 7% (95% CI 3.5% to 10.8%) and 8% (95% CI 1.9% to 13.6%) for path_MLH1 and path_MSH2 carriers, respectively. Urinary tract cancers also occurred predominantly in older age. Their incidence was elevated particularly in path_MSH2 carriers: 25% (95% CI 16.6% to 33.2%) compared with path_MLH1 carriers (8% (95% CI 4.3% to 11.7%)) and path_MSH6 carriers (11% (95% CI 1.7% to 20.3%)). Ureteric cancer was most prevalent. Cumulative incidences up to 75 years of age by path_MMR category for any cancer, upper gastrointestinal cancer and urinary tract cancer are shown in figure 1.
Figure 1.
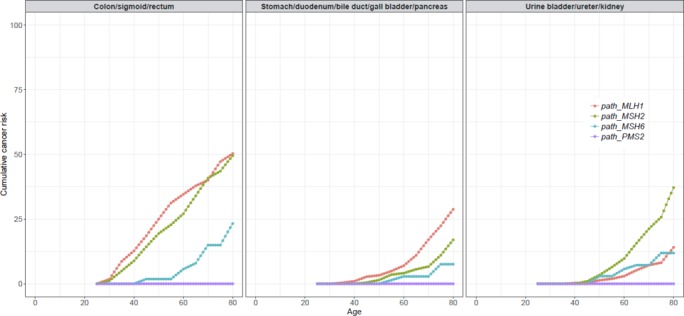
Cumulative incidences of colorectal cancer, upper gastrointestinal cancer (including stomach, duodenum, bile duct, gall bladder and pancreas) and urinary tract cancer (not including prostate).
The cumulative incidences at 75 years of age for prostate cancer were 17% (95% CI 8.5% to 25.3%), 32% (95% CI 11.7% to 51.5%) and 18% (95% CI 0% to 44.4%) for path_MLH1, path_MSH2 and path_MSH6 carriers, respectively. For detailed description of all observation years and incident cancer cases in each 5-year age group, see online supplementary table S3.
gutjnl-2017-314057supp003.pdf (399.6KB, pdf)
Relative cumulative incidences
Cumulative incidences at 75 years of age were compared with population incidences to arrive at cumulative relative risk (RR) (see table 5). Path_MLH1, path_MSH2 and path_MSH6 carriers showed an RR of any cancer of 3.1 (95% CI 2.8 to 3.4), 3.3 (95% CI 2.9 to 3.7) and 2.5 (95% CI 1.7 to 3.2), respectively. By genotype, path_MLH1 carriers had the highest RR for duodenal (RR 64.7), colon (RR 22.3), bile duct/gall bladder (RR 18.7) and pancreatic cancers (RR 7.8); path_MSH2 carriers had the highest RR for endometrial (RR 35.5), ovarian (RR 16.9), urinary tract (RR 10.8) and prostate cancers (RR 3.2). Path_MSH6 carriers had similar RR for endometrial and ovarian cancer as path_MLH1 and path_MSH2 carriers (RR 28.8 and 13.0, respectively) but less frequent cancer in other organs. The few path_PMS2 carriers included had endometrial prostate and breast cancers, but none of the other cancers typically seen in LS were diagnosed (table 5).
Table 5.
Relative cumulative incidence (RR) cancer at 75 years in carriers of path_MMR genes stratified by gene. 95% CIs are shown in parentheses. Significantly increased (p<0.05) RRs are highlighted in bold. Maximum RR by gene underlined
| ICD9 | Organ | Population incidence (%) | Relative cumulative incidence (95% CI) | |||
| path_MLH1 | path_MSH2 | path_MSH6 | path_PMS2 | |||
| Any cancer | 24.4 | 3.1 (2.8 to 3.4) | 3.3 (2.9 to 3.7) | 2.5 (1.7 to 3.2) | 2.1 (0 to 4.1) | |
| In separate organs order by RR | ||||||
| 152 | Duodenum | 0.1 | 64.7 (27.4 to 102.1) | 20.1 (0.6 to 39.6) | 0 | 0 |
| 182 | Endometrium | 1.6 | 26.7 (20.7 to 32.7) | 35.5 (26.1 to 44.8) | 28.9 (17.1 to 40.6) | 16.5 (0.5 to 32.4) |
| 153 | Colon | 2.1 | 22.3 (18.7 to 25.9) | 20.2 (15.6 to 24.7) | 6.8 (1.5 to 12.1) | 0 |
| 156 | Bile duct and gall bladder | 0.2 | 18.7 (6.3 to 31.1) | 8.6 (0 to 25.4) | 0 | 0 |
| 183 | Ovary | 1.0 | 10.1 (4.8 to 15.4) | 16.9 (5.7 to 28.0) | 13.1 (0 to 31.2) | 0 |
| 189 | Ureter and kidney | 1.3 | 3.5 (1.2 to 5.9) | 13.7 (8.2 to 19.2) | 2.3 (0 to 5.4) | 0 |
| 154 | Sigmoid and rectum | 1.4 | 8.4 (5.2 to 11.7) | 13.0 (7.8 to 18.3) | 3.3 (0 to 6.9) | 0 |
| 191 | Brain | 0.5 | 1.9 (0 to 4.8) | 10.5 (0.4 to 20.6) | 2.9 (0 to 8.4) | 0 |
| 151 | Stomach | 0.8 | 8.9 (4.4 to 13.4) | 9.7 (2.3 to 17.0) | 6.6 (0 to 16.4) | 0 |
| 188 | Urine bladder | 1.0 | 4.1 (1.5 to 6.7) | 8.1 (2.8 to 13.3) | 8.2 (0 to 16.9) | 0 |
| 157 | Pancreas | 0.8 | 7.8 (3.3 to 12.3) | 0.6 (0 to 1.9) | 1.8 (0 to 5.2) | 0 |
| 185 | Prostate | 10 | 1.7 (0.9 to 2.7) | 3.2 (1.2 to 5.1) | 1.8 (0 to 4.4) | 3.8 (0 to 9.6) |
| 174 | Breast | 9.4 | 1.3 (0.7 to 1.8) | 1.2 (0.5 to 2.0) | 1.4 (0.2 to 2.6) | 6.0 (0 to 10.6) |
| In anatomical regions ordered by RR | ||||||
| Gynaecological | 2.6 | 19.1 (15.6 to 22.7) | 25.3 (20.1 to 30.4) | 20.8 (13.3 to 28.2) | 10.1 (0.3 to 20) | |
| Colorectal | 3.8 | 12.1 (10 to 14.2) | 11.3 (8.7 to 13.9) | 3.9 (0.9 to 7.0) | 0 | |
| Upper gastrointestinal cancer | 1.9 | 11.2 (8.2 to 14.3) | 5.4 (2.1 to 8.6) | 3.5 (0 to 7.8) | 0 | |
| Urinary tract cancer | 2.3 | 3.5 (1.9 to 5.1) | 10.8 (7.2 to 14.4) | 4.8 (0.7 to 8.8) | 0 | |
Survival following cancer
Five-year and 10-year crude survival by affected organ for all cases diagnosed with cancer before 65 years of age are shown in table 6. Five-year survival was 96% for colon cancer and 75% for recto-sigmoid cancer. Corresponding 10-year survival was 88% and 70%, respectively. Five-year survival for endometrial cancer was 93% and for ovarian cancer 83%, and corresponding 10-year survival was 93% and 74%, respectively. Among other extracolonic cancers, 5-year survival was also very good for prostate (100%), urine bladder (93%) and ureter and kidney (85%) cancers and remained good at 10 years. Five-year survival for stomach and duodenal cancer was lower at 61% and 67%, respectively, while 5-year survival was poor following bile duct or gallbladder cancer (29%) or pancreatic cancer (0%).
Table 6.
Five-year and 10-year overall survival calculated by the Kaplan-Meier algorithm stratified on diagnoses for patients prospectively diagnosed <65 years of age
| ICD9 diagnosis | Organ | n | 5-year survival | 10-year survival | ||
| Survival (%) | 95% CI | Survival (%) | 95% CI | |||
| Colorectal cancer | ||||||
| 153 | Colon | 159 | 96 | 91% to 98% | 88 | 77% to 94% |
| 154 | Sigmoid rectum | 47 | 75 | 56% to 87% | 70 | 49% to 84% |
| Gynaecological cancer | ||||||
| 182 | Endometrium | 104 | 93 | 85% to 97% | 93 | 85% to 97% |
| 183 | Ovary | 25 | 83 | 55% to 94% | 74 | 44% to 90% |
| Upper gastrointestinal tract cancer | ||||||
| 151 | Stomach | 17 | 61 | 33% to 81% | 61 | 33% to 81% |
| 152 | Duodenum | 11 | 67 | 28% to 88% | 67 | 28% to 88% |
| 157 | Pancreas | 10 | 0 | |||
| 156 | Bile duct and gall bladder | 8 | 29 | 04% to 61% | 14 | 1% to 47% |
| Urinary tract cancer | ||||||
| 189 | Ureter kidney | 28 | 85 | 64% to 94% | 71 | 34% to 89% |
| 188 | Urine bladder | 20 | 93 | 59% to 99% | 81 | 42% to 95% |
| Other | ||||||
| 185 | Prostate | 17 | 100 | 80 | 20% to 97% | |
| 174 | Breast | 31 | 95 | 70% to 99% | 89 | 61% to 97% |
| 191 | Brain | 9 | 22 | 3% to 51% | 22 | 3% to 59% |
Given the high mortality of some late-onset cancers and the probability that individuals affected by early-onset cancers with good prognoses might have developed and died from such cancers later, we recalculated survival after censoring patients as lost to follow-up when they developed a cancer with <70% 5-years survival. This did not result in any significant differences. The results of these calculations are illustrated in figure 2.
Figure 2.
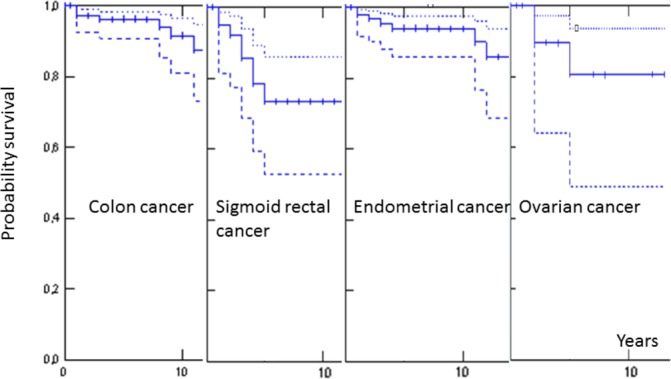
Kaplan-Meier estimates of 10-year crude survival following colon cancer, sigmoid-rectal cancer, endometrial cancer or ovarian cancer that was diagnosed before 65 years of age after censoring for upper gastrointestinal or brain tumours. Solid line indicates point values; dotted lines indicate 95% CIs.
Combining risk for cancer and survival following cancer
Data on the risk of cancer affecting an organ and the chance of survival after that cancer occurs may be combined to calculate the risk of dying from a cancer in that organ. This combined risk may be calculated for a patient based on age, gender and path_MMR variant. As an example, the cumulative risk for colon cancer in a path_MSH6 carrier at 60 years is 5%, 10-year survival is 88% and the risk of developing and dying of colorectal cancer before 70 years of age (subject to follow-up according to guidelines) is (5% · 12% =) 0.6%. Other examples may be: the combined risk for a path_MSH6 carrier of developing and dying from an ovarian cancer before 70 years would be (4% · 26% =) 1%. The combined risk for a path_MLH1 carrier of developing and dying from bile duct cancer before 80 years would be (4% · 86% =) 3%. It follows from the CIs in tables 3 and 6 that such calculated combined risks for contracting and dying from specific cancers have wide confidence intervals.
Path_MSH2 carriers had a substantial risk of urinary tract and prostate cancers in older ages and most but not all survived these cancers. In this report, the risk of cancer is presented for some ages only. Using our interactive website www.lscarisk.org, the risks of cancers may be calculated for patients with LS of any age.
Discussion
This study included both LS patients without a previous cancer and patients with LS who had survived early cancer(s), thereby providing numbers that were sufficient to calculate cancer risks stratified by path_MMR variant for each organ involved up to 75 years of age. The majority of patients included in the study were path_MLH1 or path_MSH2 carriers, and the majority of older patients in these groups had survived previous cancer(s).1 2 The tables describing cumulative cancer incidences are the results of the study and are discussed below. An interactive website (www.lscarisk.org) based on online supplementary table S3 allows cancer risks to be calculated for any patients with LS simply by providing the patient’s age and gender and represents the applied outcome of the study.
Older path_MSH2 carriers had a particularly high incidence of urinary tract and prostate cancers, while older path_MLH1 carriers had the highest incidence of upper gastrointestinal cancers. Survival was very poor for bile duct and pancreatic cancer, intermediate for gastric and urinary tract cancers and good for prostate cancer. These findings demonstrate that genetic subgroups in LS are at risk for different cancers in old age and that these cancers have very different prognoses. Compared with path_MLH1 and path_MSH2 carriers, we show that path_MSH6 carriers are at lower risk for early onset cancer and, except for an intermediate risk for urinary tract or prostate cancer, at lower risk for late onset cancers too.
The current study refines estimates of cancer risk for all patients with LS but in particular provides data that will better inform counselling and clinical management of older patients with LS. Previous reports9–11 13–17 did not include sufficient numbers of patients to stratify cancer risk in older age by path_MMR variant, as has been possible here.
Despite its international multicentre design, this study still identified too few path_PMS2 carriers to draw firm conclusions about their cancer risks. Apart from endometrial and prostate cancer, however, none of the cancers considered to be part of LS were diagnosed in path_PMS2 carriers.
We calculated the incidence of ovarian or endometrial cancer in female LS patients with censoring at prophylactic oophorectomy or hysterectomy that was undertaken in some patients. In contrast to path_BRCA1/2 carriers12 and the general population, most ovarian cancers in LS were diagnosed before the menopause, and their prognosis was good. This finding brings into question the practice of recommending prophylactic oophorectomy at the menopause in females with LS.4
Risks for breast cancer in patients with LS were not increased significantly, and therefore we could not confirm previous reports that have suggested that breast cancer is part of the LS cancer spectrum17
The structure of the database (PLSD) established for our studies enables us to add any information of interest later for all patients. The results obtained so far raise further questions that we should now investigate, such as the relationships between colonoscopy interval, stage at prospectively detected CRC and its treatment and survival. We have a lot more to do, and we invite further centres to join PLSD so that we can answer these clinically important questions as quickly and as definitively as possible.
The novelty of this report is that we provide for the first time the empirical prospectively observed outcome of healthcare into old ages for patients with LS including stratification by path_MMR variant. Before management guidelines are revised to take account of this stratification, validation of the findings of this study should be achieved in at least one other similar but independent study. We are in collaboration with the European Hereditary Tumour Group (www.mallorca-group.org) and InSiGHT (www.insight-group.org) with the aim of establishing an independent series to validate the findings presented here. If our findings are confirmed, we may have a larger combined cohort to narrow the CIs of the observed point estimates before discussing conclusions with respect to clinical guidelines. To this end, we invite other centres with compatible cohorts of patients with LS to contribute. In particular, there is a need for much more data on path_PMS2 and path_MSH6 patients.
This is the first comprehensive study of cancer risk and prognosis in older LS patients stratified by path_MMR variant. We have updated our website www.lscarisk.org to include the results presented here. Using the website, the age, gender and path_MMR variant in any given patient may now be entered to calculate the remaining lifetime risk for cancer in individual organs. This is likely to be of interest both to patients with LS and their healthcare providers
For further information on the collaborating activities, please visit http://insight-group.org/ and http://mallorca-group.org/.
Acknowledgments
This work was supported by The Finnish Cancer Foundation; The Sigrid Juselius Foundation; The Finnish Medical Foundation; Jane and Aatos Erkko Foundation; Finnish State Research Funding; the Swedish Cancer Society; the Swedish Research Council; the Stockholm Cancer Society; the Norwegian Radium Hospital Foundation; the Wales Gene Park funded by Health and Care Research Wales; and the Spanish Ministry of Economy and Competitiveness and co-funded by FEDER funds- a way to build Europe- (SAF2012-33636 and SAF2015-68016); the Carlos III Health Institute; RTICC (RD12/0036/0031); the Scientific Foundation Asociación Española Contra el Cáncer; and the Government of Catalonia (2014 SGR 338). D Gareth Evans is an NIHR senior investigator. Mark Jenkins has a fellowship from the National Health and Medical Research Council of Australia.
Footnotes
Contributors: PåMø: Designed the study, managed the database and computed the results.PåMø, Julian Sampson and GC wrote the manuscript.EH, SN, EARø and KT calculated the confidence intervals to the cumulative incidences.EH and SN constructed the website calculating individual risks.All: Participated in study design, interpreting of results, commenting the manuscript and approved the final manuscript.
Competing interests: John Burn has a patent for high speed low cost tumour profiling pending to John Burn and QuantuMDx.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The report is based on The Prospective Lynch Syndrome Database which is a joint venture by the contribuotrs and endorsed by EHTG (mallorca-gropu.org) Group and InSiGTH (insight-group.org). Formal ownerships and development of the activities will be discussed at this year joint meeting for EHTG and InSiGHT in Florence July 5th-8th this year. All interested are wellcomed to join.
References
- 1. Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2017;66:464–72. 10.1136/gutjnl-2015-309675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Møller P, Seppälä T, Bernstein I, et al. Mallorca Group (http://mallorca-group.org). Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut 2017;66:1657–64. 10.1136/gutjnl-2016-311403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812–23. 10.1136/gutjnl-2012-304356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 2014;109:1159–79. 10.1038/ajg.2014.186 [DOI] [PubMed] [Google Scholar]
- 5. Warthin AS. Heredity with reference to carcinoma as shown by the study of the cases examined in the pathological laboratory of the University of Michigan, 1895-1913. CA: A Cancer J Clin 1985;35:348–59. 10.3322/canjclin.35.6.348 [DOI] [PubMed] [Google Scholar]
- 6. Møller P, Maehle L, Vabø A, et al. Age-specific incidence rates for breast cancer in carriers of BRCA1 mutations from Norway. Clin Genet 2013;83:88–91. 10.1111/j.1399-0004.2012.01855.x [DOI] [PubMed] [Google Scholar]
- 7. Talseth-Palmer BA, Wijnen JT, Grice DM, et al. Genetic modifiers of cancer risk in Lynch syndrome: a review. Fam Cancer 2013;12:207–16. 10.1007/s10689-013-9614-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong FL, Boice JD, Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA 1997;278:1262–7. [DOI] [PubMed] [Google Scholar]
- 9. Grindedal EM, Blanco I, Stormorken A, et al. High risk of endometrial Cancer in colorectal Cancer kindred is pathognomonic for MMR-mutation carriers. Fam Cancer 2009;8:145–51. 10.1007/s10689-008-9219-3 [DOI] [PubMed] [Google Scholar]
- 10. Pylvänäinen K, Lehtinen T, Kellokumpu I, et al. Causes of death of mutation carriers in Finnish Lynch syndrome families. Fam Cancer 2012;11:467–71. 10.1007/s10689-012-9537-3 [DOI] [PubMed] [Google Scholar]
- 11. Barrow PJ, Ingham S, O’Hara C, et al. The spectrum of urological malignancy in Lynch syndrome. Fam Cancer 2013;12:57–63. 10.1007/s10689-012-9573-z [DOI] [PubMed] [Google Scholar]
- 12. Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on Cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 2014;32:1547–53. 10.1200/JCO.2013.53.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grindedal EM, Møller P, Eeles R, et al. Germ-line mutations in mismatch repair genes associated with prostate Cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2460–7. 10.1158/1055-9965.EPI-09-0058 [DOI] [PubMed] [Google Scholar]
- 14. Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol 2012;30:4409–15. 10.1200/JCO.2012.43.2278 [DOI] [PubMed] [Google Scholar]
- 15. Grindedal EM, Aarset H, Bjørnevoll I, et al. The Norwegian PMS2 founder mutation c.989-1G > T shows high penetrance of microsatellite instable cancers with normal immunohistochemistry. Hered Cancer Clin Pract. 2014. April 21;12(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Win AK, Lindor NM, Young JP, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 2012;104:1363–72. 10.1093/jnci/djs351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harkness EF, Barrow E, Newton K, et al. Lynch syndrome caused by MLH1 mutations is associated with an increased risk of breast cancer: a cohort study. J Med Genet 2015;52:553–6. 10.1136/jmedgenet-2015-103216 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2017-314057supp001.pdf (212.2KB, pdf)
gutjnl-2017-314057supp002.pdf (196.1KB, pdf)
gutjnl-2017-314057supp003.pdf (399.6KB, pdf)


