Abstract
Microglia are the resident macrophage and immune cell of the brain and are critically involved in combatting disease and assaults on the brain. Virtually all brain pathologies are accompanied by acidosis of the interstitial fluid, meaning that microglia are exposed to an acidic environment. However, little is known about how extracellular acidosis impacts on microglial function. The activity of microglia is tightly controlled by ‘on’ and ‘off’ signals, the presence or absence of which results in generation of distinct phenotypes in microglia. Activation of G protein coupled purinergic (P2Y) receptors triggers a number of distinct behaviours in microglia, including activation, migration, and phagocytosis. Using pharmacological tools and fluorescence imaging of the murine cerebellar microglia cell line C8B4, we show that extracellular acidosis interferes with P2Y receptor-mediated Ca2+ signalling in these cells. Distinct P2Y receptors give rise to signature intracellular Ca2+ signals, and Ca2+ release from stores and Ca2+ influx are differentially affected by acidotic conditions: Ca2+ release is virtually unaffected, whereas Ca2+ influx, mediated at least in part by store-operated Ca2+ channels, is profoundly inhibited. Furthermore, P2Y1 and P2Y6-mediated stimulation of migration is inhibited under conditions of extracellular acidosis, whereas basal migration independent of P2Y receptor activation is not.
Taken together, our results demonstrate that an acidic microenvironment impacts on P2Y receptor-mediated Ca2+ signalling, thereby influencing microglial responses and responsiveness to extracellular signals. This may result in altered behaviour of microglia under pathological conditions compared with microglial responses in healthy tissue.
Keywords: P2Y receptors, microglia, acidosis, store-operated Ca2+ entry, migration
1. Introduction
Microglia are the resident macrophage of the brain and play a number of diverse and important functions during development, in health and disease [1, 2]. As the only resident immune cell, microglia are also the first response unit to react to injury and assault, and initiate pro- and anti-inflammatory responses [3, 4]. Under resting conditions, microglia are in a surveillance mode, in which they monitor the brain environment and enable proper brain function [5, 6]. In response to an assault, however, they become activated and can assume a number of distinct phenotypes [4, 7]. Many different extracellular chemicals come together to control the functional state of microglia, and it is thought that microglial activity is controlled by “on” and “off” signals [7]. “On” signals are extracellular messengers, which are normally not present, and whose presence triggers activation of microglia; these can be further divided into “eat me” and “help me” signals [3]. “Off” signals, on the other hand, are extrinsic factors that are present under resting conditions and promote the surveying microglial phenotype; they can be divided into “resting” and “do not eat me” signals [3].
Purines such as ATP, its break-down product ADP, as well as UTP and UDP, are key signalling molecules for microglia that are released from injured and dying cells and are seen as “on” signals by microglia. Activation of different types of purinergic G protein coupled (P2Y) receptors stimulates distinct signalling cascades, including Gq-mediated Ca2+ release from intracellular Ca2+ stores and Ca2+ influx into microglia (P2Y1, 2, 4, 6, 11 receptor subtypes), as well as Gs/Gi-induced increases/decreases in cAMP (Gs: P2Y11 receptor subtype; Gi: P2Y12, 13 and 14 receptor subtype) [8], which in turn trigger distinct responses in microglia, including phagocytosis and migration [7].
Acidification of extracellular pH occurs in the brain under physiological and pathological conditions. Activity-dependent pH changes in brain interstitial fluid were first reported over 60 years ago and occur over varying time courses [9, 10]. Pathologically, extracellular acidosis occurs as a direct consequence of oxygen deprivation (e.g. in stroke/ischemia, neurodegenerative diseases), cell death (e.g. stroke, neurodegeneration, trauma) and (altered) cell metabolism (e.g. cancer, immune cell activity) [11–14]. Hence, microglia are exposed to changes in extracellular pH, yet very little is known about how this affects microglial function.
This study addresses the impact that extracellular acidosis has on P2Y receptor-mediated intracellular Ca2+ signalling in microglia and functional consequence of extracellular acidosis on P2Y receptor-mediated migration of these cells. We find that extracellular acidosis impairs P2Y receptor mediated Ca2+ signalling and migration, providing a mechanistic explanation how microglia can be concentrated at sites of cell injury and death.
2. Materials and Methods
2.1. Cell culture
C8B4 cells were obtained from ATCC and cultured in DMEM (2 mM L-glutamine), 10% fetal calf serum and 20 U/ml penicillin + 100μg/ml streptomycin in 75 mm3 cell culture flasks at 37°C in a humidified atmosphere of 5% CO2. Medium was changed every 3-4 days and cells were subcultured when confluency reached 70%. For imaging experiments, cells were plated at 30 – 50% confluency on glass coverslips (13 mm diameter; four coverslips per 3.5 cm cell culture dish). C8B4 cells are murine cerebellar microglia that retained microglial markers and behaviour and are therefore an excellent model for studying microglial physiology (http://www.lgcstandards-atcc.org/products/all/CRL-2540.aspx?geo_country=gb#characteristics).
2.2. Fluorescence experiments
Solutions and fluorescence experimental setup were as described in [15]. Experiments in the absence of extracellular Ca2+ were performed in the additional presence of 0.1 mM EGTA. Fluorescence ratios were measured every 2 s.
MRS 2365, MRS 2693, MRS 4062, PSB 1114, MRS 2768 and NF 546 were purchased from R&D Systems, dissolved/diluted as required and stored in aliquots at -20°C. Fresh aliquots were used for each new experimental day. Fura-2 AM was bought from Molecular Probes, Invitrogene. ATP (Mg2+ salt) was bought from Sigma Aldrich, dissolved in water and buffered with HEPES to pH7.2. BTP2 and synta66 were kindly provided by Prof. A. Parekh’s lab; cells were exposed to these drugs following Fura 2 AM incubation for at least 30 min prior to the experiment and the drug of interest was present throughout the experiment. To test impact of P2Y receptor agonists at a given extracellular pH, agonists were dissolved in buffer at the required pH value and applied to cells following 100 s baseline recording to ensure stable read-out of baseline fluorescence levels. Control and test experiments were carried out on the same day and individual graphs only contain data obtained on the same experimental days.
2.3. Migration assay
Radius™ 24-well cell migration assay kit (Cell Biolabs, Inc.) was used as manufacturer’s protocol. Extracellular pH was adjusted by adding 22, 5.5 or 2.2 mM sodium bicarbonate to culturing medium, yielding pH values of 7.4, 6.8 or 6.4 in 5% CO2, respectively. Cells were allowed to migrate for 22 hours before images were taken. Areas of migrated cells were analysed by Image J software.
To assess impact of store-operated calcium channel blocker BTP2 on P2Y receptor mediated cell migration, cells were plated into 6-well cell culture plate and allowed to grow to confluence. A scratch was made across the cell layer using a sterile pipette tip. After washing with PBS twice, DMEM medium containing 1 μM P2Y1 agonist MRS 2365 or 1 μM P2Y6 agonist MRS 2693 in the presence or absence of 10 μM BTP2 was added. Plates were photographed at 0h and 24h at the identical location of initial image. Results were analysed with software Image J.
2.4. RT-PCR experiments
Total RNA from C8B4 cells was extracted using RNeasy MiniKit (Qiagen) according to manufacturer’s protocols; three distinct C8B4 preparations were used. First-strand complementary DNA (cDNA) was prepared from 1 μg of total RNA with the Superscript III Kit (Invitrogen, Carlsbad, California) in the presence of 1 μg of Oligo(dT). PCR was performed with primers (forward and reverse) specific for the mRNA encoding each of the following: P2Y1, P2Y2, P2Y4, and P2Y6 receptors and GAPDH.
For murine P2Y1, forward (5’-TTATGTGCAAGCTGCAGAGG-3’) and reverse (5’-CGGAGAGGAGAGTTGTCCAG-3’) primers were used to amplify a 381-bp fragment.
For murine P2Y2, forward (5’-TCCTCTTCCTCACCTGCATC-3’) and reverse (5’-GCGAAGACGGCCAGTACTAA-3’) primers were used to amplify a 400-bp fragment.
For murine P2Y4, forward (5’-CATCAACCTGGTGGTGACTG-3’) and reverse (5’-ACACATGATACGGCCTGTGA-3’) primers were used to amplify a 391-bp fragment.
For murine P2Y6, forward (5’-AGCATCCTGTTCCTCACCTG-3’) and reverse (5’-CTGCTACCACGACAGCCATA-3’) primers were used to amplify a 400-bp fragment.
For murine GAPDH, forward (5’-GTGCAGTGCCAGCCTCGTCC-3’) and reverse (5’-TTCAAGTGGGCCCCGGCCTT-3’) primers were used to amplify a 362-bp fragment.
The following protocol was used: (1) 95° C for 5 minutes. (2) 20 cycles for GADPH and 30 cycles for the other genes using the following settings for each cycle: 95° C for 30 seconds, 56° C for 30 seconds and then 72° C for 30 seconds. (3) 72° C for 5 minutes. The RT-PCR products were then electrophoresed through a 8% polyacrylamide gel and subsequently visualised by ethidium bromide staining.
2.5. Data analysis
Waves containing fluorescence ratios (356 nm/380 nm) were analysed offline using Igor Pro and Microsoft Excel (2010). Cells were divided into responders (those with increases in fluorescence following exposure to agonist) and non-responders; these groups were used to calculate percentage of responding cells. Fluorescence signals in responding cells were analysed in terms of peak response (peak fluorescence ratio – basal fluorescence ratio just before application of drug) and time to peak (time lapse between application of drug and peak fluorescence response); in some cases, time to peak was further divided into time to respond (time lapse between drug application and start of fluorescence change following drug application) and rise time of peak (time lapse between start of fluorescence change and peak fluorescence change following drug application). Error bars represent SEM; n represents number of cells. Statistical analysis was carried out using InStat 2.03 (Macintosh) and Excel (Windows), and either ANOVA (for comparison of more than two mean values) or unpaired Student t test (for comparison of two mean values) were used.
3. Results
3.1. Extracellular acidosis impairs P2Y receptor mediated Ca2+ signalling in microglial cells
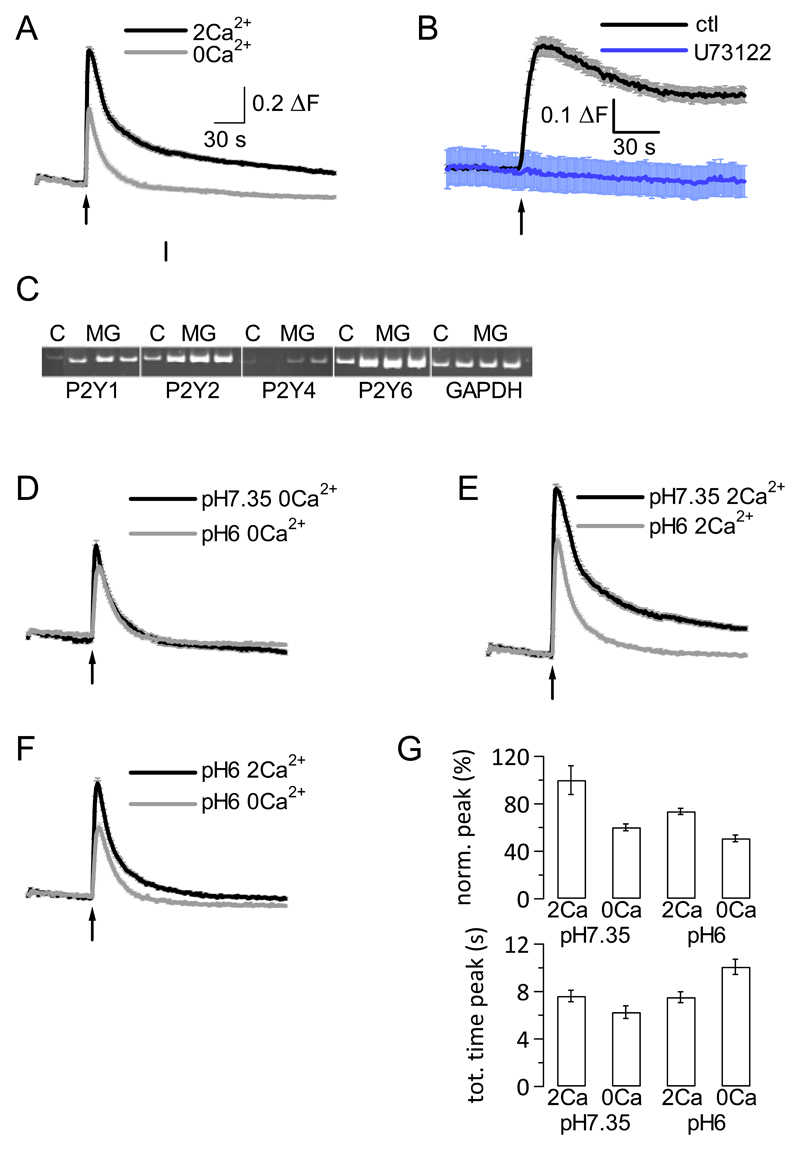
Application of 100 μM ATP to C8B4 microglia cells led to increases in intracellular Ca2+ concentration in the presence and absence of extracellular Ca2+ in these cells (Fig. 1A). The fluorescence signal obtained in the presence of 2 mM extracellular Ca2+ displayed a larger peak and sustained plateau phase, indicative of Ca2+ influx under these conditions, compared with fluorescence signals obtained in the absence of extracellular Ca2+. To confirm that phospholipase C-coupled receptors were responsible for the observed changes in intracellular Ca2+ concentration, we repeated ATP application in the presence of extracellular Ca2+ (to monitor Ca2+ release and Ca2+ influx) under control conditions and following preincubation of microglia cells with the phospholipase C inhibitor U73122 (10 μM). Cells pretreated with this inhibitor failed to exhibit changes in intracellular Ca2+ signalling upon ATP application, demonstrating that phospholipase C-coupled receptors were responsible for the observed changes in intracellular Ca2+ concentration (Fig. 1B).
Figure 1. C8B4 microglial cells functionally express Gq-coupled P2Y receptors, activation of which is reduced by extracellular acidosis.
A, Application of 100 μM ATP (application time point indicated by arrow) at extracellular pH7.35 in the presence (2Ca2+, black line) and absence (0Ca2+, grey line) of extracellular Ca2+. Traces show averages (n=97 and 67 cells, respectively) ± SEM.
B, Application of 100 μM ATP at extracellular pH7.35 under control conditions (in extracellular Ca2+) and following preincubation with 10 μM U73122 (present throughout experiment; purple line). Traces show averages (n=17 and 21 cells, respectively) ± SEM.
C, Gel showing RT-PCR products for P2Y1, 2, and 6 receptor; GAPDH was positive control. ‘C’ is positive control (mouse brain); ‘MG’ stands for C8B4 microglia. 3 different C8B4 preparations were used in 3 separate PCR reactions, generating 3 lanes on the gel.
D, Application of 100 μM ATP at extracellular pH7.35 (black line) and pH6 (grey line) in the absence of extracellular Ca2+ (0Ca2+). Traces show averages (n=67 and 72 cells, respectively) ± SEM.
E, Application of 100 μM ATP at extracellular pH7.35 (black line) and pH6 (grey line) in the presence of extracellular Ca2+ (2Ca2+). Traces show averages (n=97 and 93 cells, respectively) ± SEM.
F, Application of 100 μM ATP at extracellular pH6 in the presence (black line; 2Ca2+) and absence (grey line; 0Ca2+) of extracellular Ca2+. Traces show averages (n=93 and 72 cells, respectively) ± SEM.
G, Aggregate data depicting average peak fluorescence signal normalised (norm.) to average peak fluorescence signal (= peak fluorescence – basal fluorescence) measured at pH7.35 in the presence of 2 mM extracellular Ca2+, which corresponded to 100% (top), and average total (tot.) time to peak (= time lapse in seconds (s) between application of 100 μM ATP and peak fluorescence signal; bottom) for the different conditions (n between 67 and 97).
We next carried out RT-PCR experiments on three separate microglial preparations to confirm mRNA expression of Gq-coupled P2Y receptors in these cells. We found that P2Y1, 2, 4 and 6 were all expressed at mRNA level (Fig. 1C).
To test for impact of extracellular acidosis on ATP-mediated intracellular Ca2+ signals in microglia, we repeated ATP applications under 4 distinct conditions: in the absence or presence of extracellular Ca2+ (2 mM) at extracellular pH7.35 or pH6 (Fig. 1D-F). There was a reduction in the peak fluorescence signal as well as in the Ca2+ influx component at extracellular pH6 compared with pH7.35 (Fig. 1E); however, some Ca2+ influx still took place even at extracellular pH6 (Fig. 1F). Analysis of individual traces revealed that there was a highly significant difference in peak amplitude between the different conditions (P < 0.0001, ANOVA), the peak fluorescence Ca2+ signal (peak – basal fluorescence signal) being largest in the presence of 2 mM extracellular Ca2+ at extracellular pH7.35 and smallest in the absence of extracellular Ca2+ at extracellular pH6 (Fig. 1G top panel). The peak signal in the presence of extracellular Ca2+ at extracellular pH6 was larger than that in the absence of extracellular Ca2+ at extracellular pH7.35 (73.7 ± 2.6% versus 60.2 ± 2.9%, respectively), suggesting that even at extracellular pH6, there was still Ca2+ influx occurring. Moreover, we found a significant delay in the time it took for the fluorescence signal to peak following ATP application (P < 0.0001, ANOVA). The fluorescence peak response was clearly delayed in the absence of extracellular Ca2+ at pH6 (10.08 ± 0.64 s) compared with the other conditions (Fig. 1G bottom panel).
Taken together, these results suggest that P2Y receptor-mediated intracellular Ca2+ signals, comprised of Ca2+ release from stores and Ca2+ influx through plasma membrane channels, were significantly reduced under acidotic conditions in microglia.
3.2. Extracellular acidosis profoundly impairs P2Y receptor mediated Ca2+ influx at extracellular pH below pH7.1
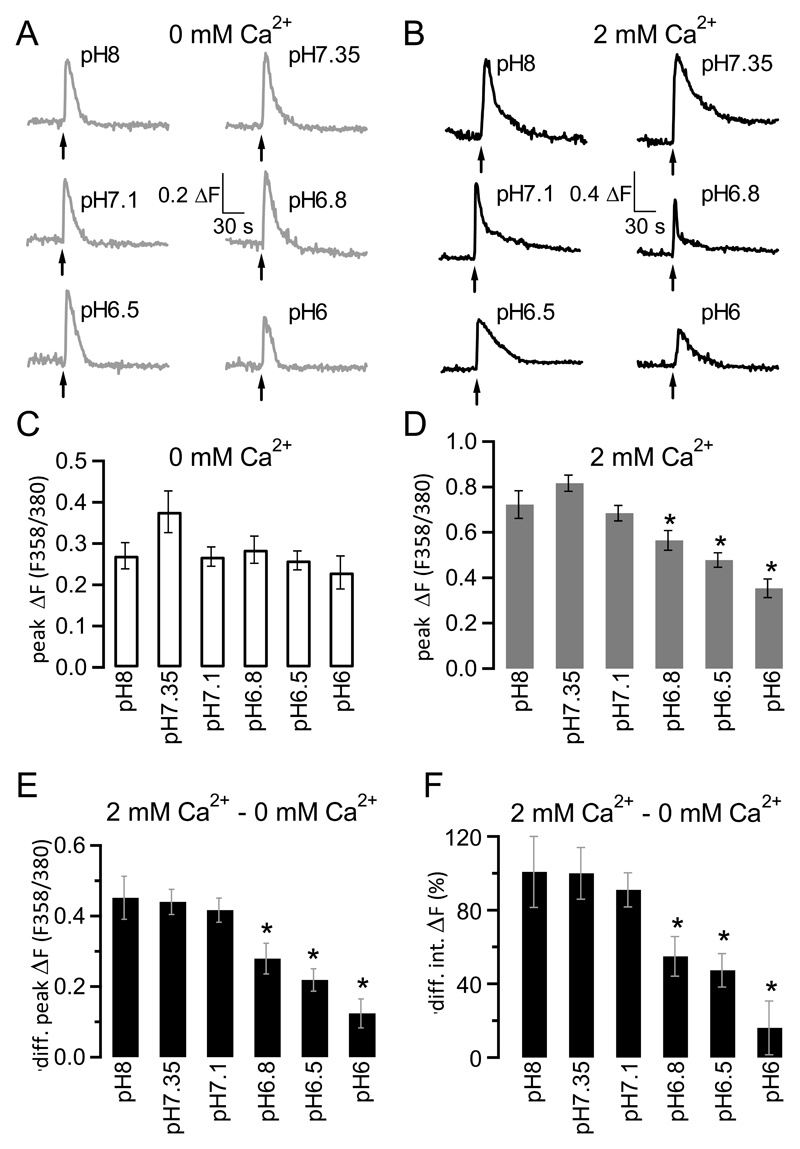
In order to understand better the impact of extracellular acidosis on ATP-mediated intracellular Ca2+ signalling in microglia, we compared intracellular Ca2+ signals in response to ATP application at different extracellular pH values to establish a pH profile for ATP responses. ATP applications (100 μM) were carried out at different extracellular pH values (pH8, pH7.35, pH7.1, pH6.8, pH6.5 and pH6) in either the absence (Fig. 2A) or presence (Figl 2B) of 2 mM extracellular Ca2+. Analysis of individual traces showed that ATP-mediated Ca2+ release from intracellular stores was largest at extracellular pH7.35 (though not quite statistically significant with P = 0.0779, ANOVA) and that there was no difference in extent of Ca2+ release from stores between extracellular pH8, pH7.1, pH6.8, pH6.5 and pH6 (P = 0.7868, ANOVA; Fig. 2C). In the presence of extracellular Ca2+, however, there was a significant decrease in intracellular Ca2+ signalling at extracellular pH values below pH7.1 (Fig. 2D; P < 0.0001, ANOVA; star indicates statistical difference between starred graph and graphs for pH8 as well as pH7.35 using unpaired Student’s t test). To study the impact of extracellular acidosis on ATP-mediated Ca2+ influx only, we subtracted peak fluorescence values obtained in the absence of extracellular Ca2+ (i.e. ATP-mediated Ca2+ release only) from that obtained in the presence of extracellular Ca2+ (i.e. ATP-mediated Ca2+ influx and release) for each given extracellular pH value to reveal the peak Ca2+ influx component (Fig. 2E). Interestingly, we found that Ca2+ influx was virtually identical between extracellular pH8 - 7.1, and that below extracellular pH7.1, it was increasingly reduced with increasing extracellular acidosis. To confirm that this was true not only for peak Ca2+ influx but for Ca2+ influx on the whole, we looked at impact of extracellular acidosis on the Ca2+ influx integral (rather than just peak) and found that the pattern was identical (Fig. 2F): There was no difference in the overall Ca2+ influx signal between pH8 and pH7.1, but at pH values below pH7.1, there was increasingly less Ca2+ influx with decreasing extracellular pH (P < 0.0001 for Fig. 2E and F, ANOVA).
Figure 2. pH-dependence of ATP-mediated intracellular Ca2+ signalling in microglia cells.
A, Raw traces depicting changes in fluorescence (and hence intracellular Ca2+) following application of 100 μM ATP at extracellular pH8 (top left), pH7.35 (top right), pH7.1 (middle left), pH6.8 (middle right), pH6.5 (bottom left) and pH6 (bottom right) in the absence of extracellular Ca2+. ΔF = fluorescence emission following excitation at 356 nm/fluorescence emission following excitation at 380 nm.
B, As A but in presence of 2 mM extracellular Ca2+.
C, Aggregate data of experiments looking at average peak fluorescence increases (peak ΔF; fluorescence ratio after excitation at 356 and 380 nm, F356/380) in response to application of 100 μM ATP at increasingly acidic extracellular pH (pH8 – pH6) in the absence of extracellular Ca2+. Error bars represent SEM; n = 44 - 83 cells. The difference in amplitude was statistically just not significant (P = 0.0779, ANOVA).
D, As C, but in presence of 2 mM extracellular Ca2+; n = 44 – 100 cells. The differences in amplitude were highly significant (P < 0.0001, ANOVA). Stars indicate values that differ significantly from both pH8 and pH7.35 values using an unpaired Student’s t test.
E, Difference (diff.) between peak fluorescence value obtained in absence (cells from C) of extracellular Ca2+ from that obtained in presence of 2 mM extracellular Ca2+(cells from D), reflecting peak fluorescence signal caused by Ca2+ influx. The differences in amplitude were highly significant (P < 0.0001, ANOVA). Error bars are SEM. Stars indicate values that differ significantly from both pH8 and pH7.35 values using an unpaired Student’s t test.
F, Same as E, but looking at difference in integral (int.) fluorescence signal, i.e. total Ca2+ influx rather than peak Ca2+ influx. All integrals were normalised to average integral difference at pH7.35. The differences in integral signals were highly significant (P < 0.0001, ANOVA). Stars indicate values that differ significantly from both pH8 and pH7.35 values using an unpaired Student’s t test.
When comparing responsiveness of microglia to ATP at the distinct extracellular pH values, we found that in the presence of extracellular Ca2+, all (or almost all) cells responded to ATP with changes in intracellular Ca2+ signalling at all pH values tested (Table 1), whereas responsiveness of cells in the absence of extracellular Ca2+ decreased below extracellular pH6.8; however, even at extracellular pH6, 73.7% of cells still responded to ATP application.
Table 1. Percentage of cells responding to application of 100 μM ATP with changes in intracellular Ca2+ signalling at increasingly acidic extracellular pH in the absence and presence of 2 mM extracellular Ca2+.
Total number of cells tested ranged between 47 and 100 for the varying conditions.
| 0 mM Ca2+ | 2 mM Ca2+ | |
|---|---|---|
| pH8 | 93.9% | 98.7% |
| pH7.35 | 98.3% | 100% |
| pH7.1 | 94.3% | 100% |
| pH6.8 | 95.7% | 94.4% |
| pH6.5 | 86.7% | 100% |
| pH6 | 73.7% | 95.7% |
Taken together, these results show that ATP triggered Ca2+ release from intracellular Ca2+ stores tended to be largest for physiological pH and was identical for all other pH values, and that ATP-mediated Ca2+ influx was significantly inhibited at extracellular pH below pH7.1.
3.3. P2Y1 and P2Y6 receptors give rise to distinct intracellular Ca2+ signals
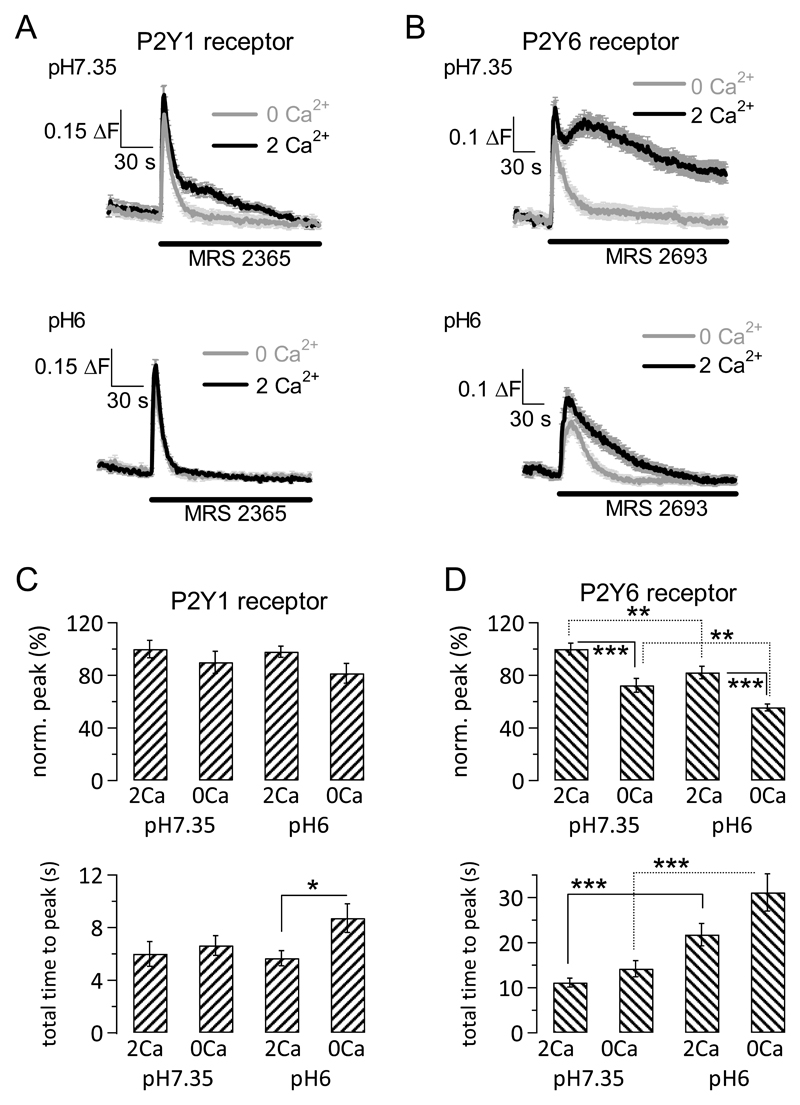
Our RT-PCR results revealed mRNA expression of four Gq-coupled P2Y receptors, P2Y1, 2, 4 and 6 (Fig. 1C), all of which are activated by ATP or ADP in mice [8]. To assess functional expression of these receptors, we used selective pharmacological agonists for the different P2Y receptors, including P2Y11, which is thought to signal through both Gs and Gq [16, 17]. Of all agonists tested, only two (MRS 2365 and MRS 2693) consistently produced responses in the presence of 2 mM extracellular Ca2+ at extracellular pH7.35 (i.e. under conditions in which all cells responded to ATP applications with intracellular Ca2+ signalling). MRS 2354 is a selective agonist for P2Y1 receptors (100% of cells responded), and MRS 2693 is a selective agonist for P2Y6 receptors (87.8% of cells responded) (Fig. 3A and B, respectively). PSB 1114 and MRS 2768 (P2Y2 receptor agonists, 10 μM and 100 – 300 μM, respectively), MRS 4062 (P2Y4 receptor agonist, 0.3 μM), and NF 546 (P2Y11 receptor agonist, 200 μM) either did not elicit convincing responses (MRS 2768) or gave rise to small responses (less than 25% of the ATP response under equivalent conditions) in only a subset of cells (between 10-40% of cells tested). We therefore decided to focus on P2Y1 and 6 receptors because these were the two receptors that generated reliable responses (Fig. 3A and B).
Figure 3. Ca2+ signalling through distinct P2Y receptors is differentially affected by extracellular acidosis.
A, Fluorescence Ca2+ signals elicited by application of P2Y1 receptor selective agonist MRS 2365 (1 μM). Top, Average time course of fluorescence Ca2+ signals at extracellular pH7.35 in presence (2 Ca2+, black line) and absence (0 Ca2+, grey line) of extracellular Ca2+ (2 mM) following application of MRS 2365 (application start and duration indicated by line), demonstrating a small Ca2+ influx component. Bottom, as top but at extracellular pH6. The small Ca2+ influx component is completely blocked by extracellular acidosis. Error bars are SEM; n = 31 – 38 cells.
B, As A but using the P2Y6 receptor selective agonist MRS 2693 (1 μM). There is a large Ca2+ influx component that is only partially inhibited by extracellular acidosis. Error bars are SEM; n = 31 – 39 cells.
C, Aggregate data showing peak responses (top) and total time to peak (defined as time lapse between application of agonist and peak fluorescence response; bottom) following application of 1 μM P2Y1 receptor agonist MRS 2365 for different experimental conditions: in presence (2Ca) or absence (0Ca) of 2 mM extracellular Ca2+, at pH7.35 or pH6; values were normalised (norm.) to average peak in presence of extracellular Ca2+ at pH7.35 (100%). Same cells as in A; error bars are SEM.
D, As C but using the P2Y6 receptor agonist MRS 2693 (1 μM). Same cells as in B; error bars are SEM. Two stars indicated P values between 0.0082 and 0.0099, three stars indicate P values between 0.0003 and smaller than 0.0001 using an unpaired Student’s t test.
Stimulation of P2Y1 receptors (1 μM MRS 2365) resulted in a tall Ca2+ release peak followed by a small Ca2+ plateau that was absent in the absence of extracellular Ca2+ (Fig. 3A top panel). This Ca2+ influx component was completely inhibited by extracellular acidosis (pH6), whereas the peak Ca2+ release was unaffected (Fig. 3A bottom panel). Analysis of individual fluorescence signals showed that the average peak fluorescence signal following activation of P2Y1 receptors did not differ significantly between the distinct conditions (presence or absence of 2 mM extracellular Ca2+, extracellular pH7.35 or 6; Fig. 3C top panel; P = 0.2069 ANOVA). However, there was an increase in time lapse between drug application and peak response (total time to peak; Fig. 3C bottom panel) at extracellular pH6 in the absence of extracellular Ca2+ compared with that in the presence of extracellular pH (P = 0.0225; unpaired Student’s t test). Importantly, whilst there was no difference in peak fluorescence signal between the different conditions, the percentage of cells responding to MRS 2365 application with intracellular Ca2+ signalling depended on the experimental conditions: 100% of cells responded at extracellular pH7.35 in the presence of 2 mM extracellular Ca2+, 95% responded at the same pH but in the absence of extracellular Ca2+, 76.2% of cells responded at extracellular pH6 in the presence of extracellular Ca2+, and 51.7% of cells responded at the same pH but in the absence of extracellular Ca2+. Hence, extracellular acidosis reduced the number of cells responsive to P2Y1 receptor activation as well as Ca2+ influx in response to P2Y1 receptor stimulation.
Stimulation of P2Y6 receptors (1 μM MRS 2693) at extracellular pH7.35 gave rise to a Ca2+ release peak followed by a large Ca2+ plateau that was only observed in the presence of extracellular Ca2+ (Fig. 3B top panel). This Ca2+ influx was reduced but not completely inhibited at extracellular pH6 (Fig. 3B bottom panel). Analysis of the individual fluorescence traces revealed that there was a significant change in average peak fluorescence response between the four different conditions (P < 0.0001, ANOVA; Fig. 3D top panel); specifically, there was a significant decrease in fluorescence upon omission of extracellular Ca2+ for a given pH value (unbroken lines) as well as a significant decrease in fluorescence when pH was acidified (but Ca2+ concentration remained unchanged; dotted lines). The largest average peak was observed for pH7.35 in the presence of 2 mM extracellular Ca2+; in the absence of extracellular Ca2+ at pH6, this was reduced to 55.6 ± 2.6% of the 2 Ca2+ pH7.35 signal. Furthermore, there was a significant increase in time lapse between drug application and peak fluorescence response (Fig. 3D bottom panel; P < 0.0001, ANOVA). Intriguingly, when we broke down the total time to peak response into two components, time lapse between application of drug and first sign of change in fluorescence signal (= time to respond) and time lapse between first sign of change in fluorescence signal to peak fluorescence signal (= rise time of peak), there was a striking pattern: At pH6, both time to respond and rise time of peak were significantly slowed compared with pH7.35 (P < 0.0001 for time to respond and P = 0.0005 for rise time, ANOVA) (Table 2).
Table 2. Statistics for P2Y6 receptor activation mediated fluorescence Ca2+ signals.
Time to respond (= time lapse between application of drug and first observed change in fluorescence signal) and rise time of peak (= time lapse between first observed change in fluorescence signal and peak fluorescence signal) in the absence and presence of 2 mM extracellular Ca2+ at extracellular pH7.35 and pH6.
| Time to respond (s) | Rise time of peak (s) | |
|---|---|---|
| 2 Ca2+ pH7.35 | 5.36 ± 0.8 | 5.79 ± 0.64 |
| 0 Ca2+ pH7.35 | 5.86 ± 1.12 | 8.34 ± 1.15 |
| 2 Ca2+ pH6 | 11.59 ±1.89 | 10.21 ± 1.08 |
| 2 Ca2+ pH6 | 15.15 ± 2.99 | 15.93 ± 2.2 |
3.4. Ca2+ influx in response to P2Y1 and P2Y6 receptor stimulation is mediated by store-operated Ca2+ channels
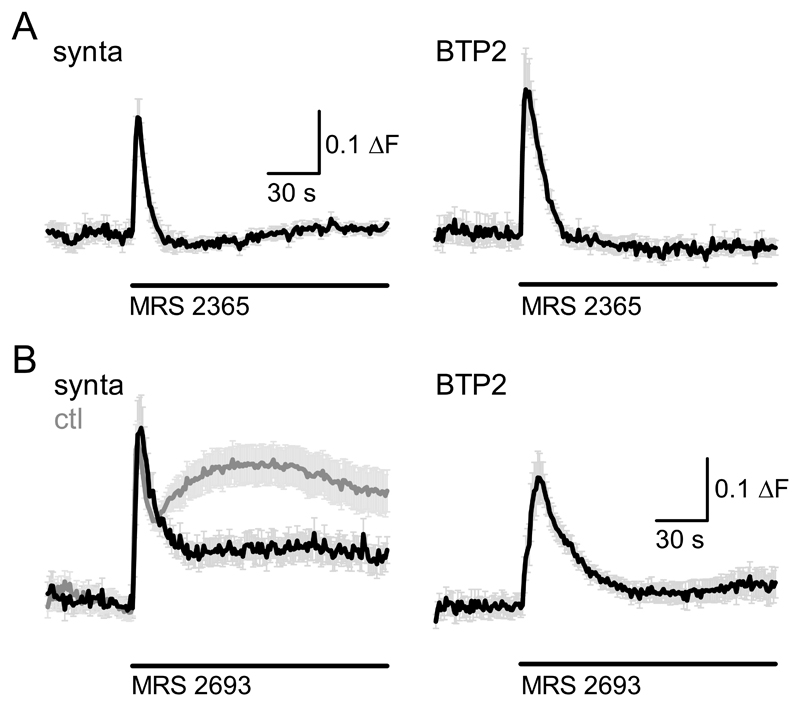
One obvious channel candidate for mediating P2Y receptor-dependent Ca2+ influx into microglia are store-operated Ca2+ channels, which are functionally expressed in microglia and have been demonstrated to be activated following stimulation of purinergic receptors [7, 18–21]. To investigate whether Ca2+ influx observed subsequent to P2Y1 and P2Y6 receptor activation in C8B4 cells depended on store-operated Ca2+ entry, we repeated P2Y1 and P2Y6 receptor activation following preincubation with two distinct pharmacological inhibitors of store-operated Ca2+ channels, BTP2 and synta66 [22]. The small Ca2+ influx component observed in response to P2Y1 receptor stimulation (in extracellular pH7.35) was not observed in cells pretreated with either 10 μM synta66 or 10 μM BTP2 (Fig. 4A left and right panel, respectively), suggesting block of Ca2+ entry under these conditions. Importantly, there was no difference in peak fluorescence response in the presence of synta66 and BTP2 compared with control conditions (P=0.4494, ANOVA), demonstrating that BTP2 did not inhibit Ca2+ release from stores.
Figure 4. Store-operated Ca2+ entry is at least in part responsible for P2Y1 and P2Y6-mediated Ca2+ signals.
A, Fluorescence Ca2+ signals elicited by application of P2Y1 receptor selective agonist MRS 2365 (1 μM) following preincubation with and in the continued presence of CRAC channel blockers syntax 66 (10 μM) and 10 μM BTP2 (10 μM); left and right graph, respectively. Error bars are SEM; n = 9 (syntax 66) and 12 (BTP2) cells.
B, As A but using the P2Y6 receptor selective agonist MRS 2693 (1 μM). The left graph contains control P2Y6 response at extracellular pH7.35 (grey) for the same experimental days to demonstrate extent of reduction of Ca2+ influx signal by syntax 66. Error bars are SEM; n = 16 - 20 cells.
Exposure of cells to synta66 and BTP2 also led to a dramatic reduction of the Ca2+ influx component subsequent to P2Y6 receptor activation. The Ca2+ influx phase was profoundly reduced following exposure of cells to synta66 compared with control treatment (Fig. 4B, left panel, black and grey graph, respectively) and virtually absent from cells treated with BTP2 (Fig. 4B right panel). Taken together, these results strongly suggest that in C8B4 cells, store-operated Ca2+ entry is the main source of Ca2+ influx following P2Y1 and P2Y6 receptor stimulation.
3.5. Extracellular acidosis impairs P2Y receptor-triggered but not basal migration of microglia cells
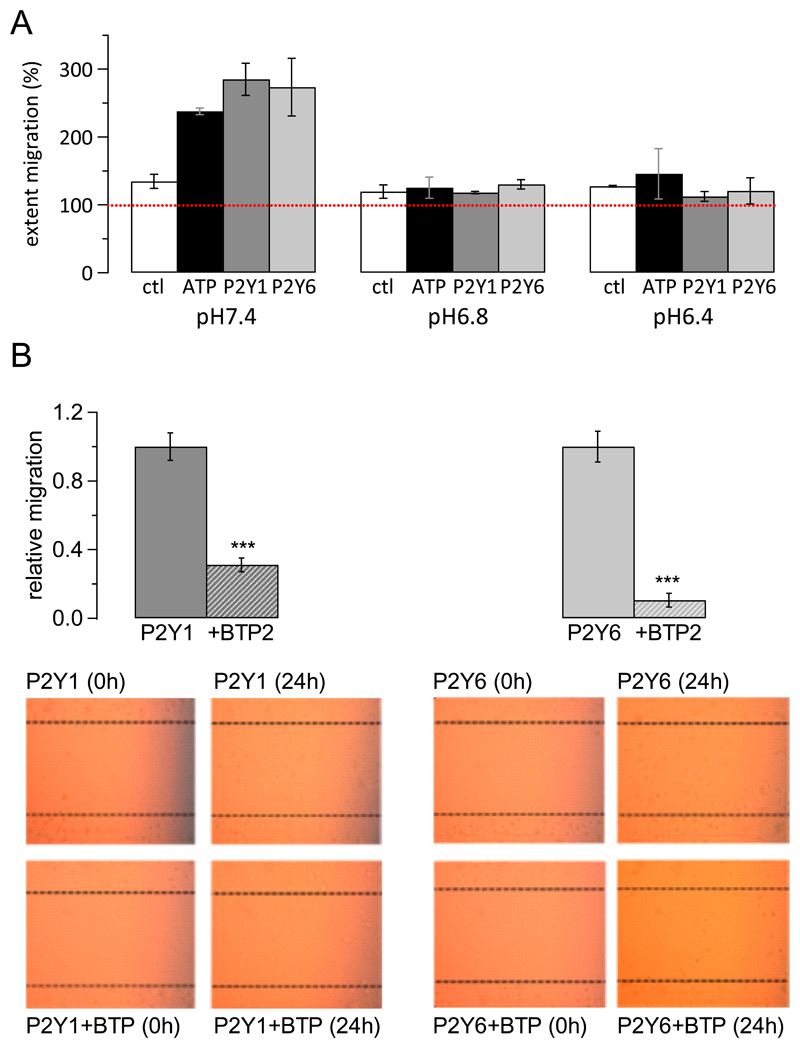
Activation of P2Y receptors has been shown to trigger a variety of responses in microglia, including migration [7]. We therefore tested the impact of extracellular acidosis on P2Y receptor-mediated migration of microglia cells. In the absence of any added agonist, extracellular acidosis (pH6.8 or pH6.4) did not impact significantly on basal migration (Fig. 5A white columns; P = 0.8297, ANOVA). Addition of ATP (100 μM, black columns), MRS 2365 (1 μM, dark grey columns) or MRS 2693 (1 μM, light grey columns) led to a significant increase in migration (P = 0.041, ANOVA; Fig. 5A) at extracellular pH7.4. Intriguingly, however, at extracellular pH6.8 and pH6.4, there was no difference in the extent of migration between control conditions and in the presence of ATP, MRS 2365 or MRS 2693 (P=0.8996, ANOVA), suggesting that extracellular acidosis interfered with P2Y receptor-mediated but not basal migration of microglia.
Figure 5. Extracellular acidification interferes with P2Y receptor mediated migration but does not affect basal migration of microglia.
A, Bar charts show average migration of microglia under distinct experimental conditions; dotted red line at 100% depicts level indicating absence of migration. White bars, control (ctl) conditions with no added agonist; black bars, migration in presence of 100 μ ATP; dark grey, migration in presence of P2Y1 (Y1) receptor agonist MRS 23651 μM ; light grey, migration in presence of P2Y6 (Y6) receptor agonist MRS 2693 (1 μM). All experiments were carried out at extracellular pH7.4, 6.8 and 6.4 in duplicates.
B, Top panel shows average normalised migration rate (normalised to average migration rate in presence of P2Y1 receptor agonist or P2Y6 receptor agonist, respectively) when stimulating migration using 1 μM MRS 23651 (P2Y1 receptor; dark grey) or MRS 2693 (P2Y6 receptor, light grey) in the absence (solid bar) or presence (striped bar) of 10 μM BTP2. Experiments were repeated 8 times for each condition.
Bottom panels, representative images for migration experiments. Note that C8B4 cells do not grow fully confluent. Left, P2Y1 receptor activation (1 μM MRS 2365) in absence (top) and presence (bottom) of 10 μM BTP2 (BTP); right, P2Y6 receptor activation (1 μM MRS 2693) in absence (top) and presence (bottom) of 10 μM BTP2 (BTP).
Store-operated Ca2+ entry subsequent to P2Y2 receptor activation was shown to be involved in microglial migration [19]. We confirmed that store-operated Ca2+ channels were also involved in P2Y1 and P2Y6 receptor dependent migration of C8B4 microglial cells by repeating microglial migration in response to P2Y1 and P2Y6 receptor stimulation in the absence and presence of the store-operated Ca2+ channel inhibitor BTP2 (Fig. 5B). There was a highly significant reduction in P2Y1 and P2Y6 receptor dependent migration when BTP2 was present (P < 0.0001, unpaired Student’s t test), which was almost complete for P2Y6 receptor dependent migration.
4. Discussion
P2Y receptor activation on microglia has been shown to initiate a number of distinct processes, including phagocytosis of neurons [23], migration [24, 25], chemokine expression [26], pinocytosis [27], and neuropathic pain [28, 29]. Importantly, the same receptor may be involved in more than one process; e.g. the P2Y12 receptor has been shown to be involved in migration [24] and neuropathic pain sensing [28–30], and equally more than one P2Y receptor type is involved in the same physiological process (e.g. migration: P2Y1 and P2Y12; [25]). This suggests that activation of individual P2Y receptors is contextualised by microglia and that other signals and factors influence which of the possible functions are going to be executed.
One contextual factor for microglia is the microenvironment in which they operate. The microenvironment of a given cell can be defined as the sum of all biophysical factors that immediately surround the cell, and extracellular H+ concentration, i.e. extracellular pH, is one key component of the microenvironment. It is becoming increasingly clear that the pH of the interstitial fluid in the brain changes in a manner dependent on surrounding cells and their physiological or pathological state [31]. Neuronal activity leads to fluctuations in extracellular pH in the ms to min time scale [9, 10, 32, 33], as does immune cell activity [11, 13] including microglia activity [34], leading to microenvironmental acidosis in inflammatory foci [35–38]. Moreover, oxidative stress and extracellular acidosis occur in neurodegenerative disease [39–41], and a drop in extracellular pH is a common feature of acute neurological conditions such as ischemic stroke, brain trauma, and epileptic seizures [12]. Finally, acidosis of the interstitial fluid is commonly encountered in solid tumours [14], which are infiltrated with immune cells [42]; brain tumours contain up to 30% microglia [43], the resident immune cell. Hence, microglia have to operate in a microenvironment that can be acidotic, yet very little is known about the impact of extracellular acidosis on microglial cell function.
We show that Ca2+ influx into microglia following stimulation of P2Y receptors and P2Y-dependent migration of microglia are inhibited by extracellular acidosis. ATP application to microglia at varying extracellular pH resulted in suppression of Ca2+ influx at extracellular pH below pH7.1, whereas Ca2+ release from stores was not significantly different between extracellular pH8 and pH6 (though enhanced at physiological pH compared with acidotic or alkaline conditions), suggesting that extracellular pH did not impact on the ability of P2Y receptor to respond to ligand binding with initiating intracellular signalling cascades. ATP is not necessarily the most potent agonist for all P2Y receptors, but it can be metabolised extracellularly to ADP and AMP [44], and at high concentrations, application of ATP results in activation of all murine Gq-coupled P2Y receptors [45]. Hence, changes in intracellular Ca2+ concentration following application of ATP to C8B4 microglial cells reflect activation of all functionally expressed Gq-coupled receptors in these cells, and the overall response is a reduction in Ca2+ influx at extracellular pH below pH7.1.
Investigating impact of P2Y1 receptor activation at different extracellular pH revealed that P2Y1-mediated Ca2+ release was not affected by extracellular pH6 compared with physiological pH, demonstrating that the signalling ability of P2Y1 receptors was not affected by changes in extracellular pH. Strikingly, however, we found that P2Y1 receptor-dependent Ca2+ influx was completely inhibited at extracellular pH6, suggesting that the ion channel underlying Ca2+ influx itself was subject to inhibition by extracellular acidosis. Moreover, we found that activation of P2Y1 receptors enhances microglial migration, and that P2Y1 receptor-dependent, but not basal, migration is inhibited by extracellular acidosis. Since P2Y1 receptor-mediated Ca2+ influx but not release was inhibited by extracellular acidosis, our data suggest that P2Y1 receptor-dependent migration was inhibited by extracellular acidosis because of acidosis-mediated inhibition of P2Y receptor-activated Ca2+ influx.
When investigating impact of extracellular acidosis on P2Y6 receptor-mediated intracellular Ca2+ signalling, we found that extracellular acidosis resulted in a significant slowing of the P2Y6 receptor-dependent signalling as well as a reduction in Ca2+ release from intracellular Ca2+ stores and in Ca2+ influx. This may be caused by a number of distinct factors, including altered metabolism of ATP by ectonucleotidase under acidotic conditions [46], impaired binding of agonist to receptor, and/or reduced coupling to the G protein due to altered conformation in the acidic environment [47].
P2Y1 and P2Y6 receptor mediated Ca2+ influx were inhibited following treatment of cells with store-operated Ca2+ channel blockers, suggesting a key role for this Ca2+ influx pathway. Consistent with our finding that Ca2+ entry into C8B4 microglial cells was inhibited under conditions of extracellular acidosis, store-operated Ca2+ channels have been shown to be inhibited by extracellular acidosis [48–50], and several amino acids (Glu106, D110 and D112) have been demonstrated to contribute to extracellular proton sensing in, and acidosis-dependent inhibition of, Orai1 (51, 52). Our data strongly suggest that this acidosis-dependent inhibition of store-operated Ca2+ influx is at least in part responsible for the observed reduction in microglial migration under acidotic extracellular conditions
Importantly, we observed full inhibition of P2Y receptor-mediated migration already at extracellular pH6.8, at which ATP-dependent Ca2+ influx was reduced by around 37%, suggesting high sensitivity of the migratory machinery to changes in Ca2+ influx. Acidosis-mediated inhibition of migration means that microglia can be concentrated at sites of cell injury and death, which may allow microglia to help contain the site of injury by enabling them to stay on the fringes of assault foci to confine it.
Conclusions
P2Y receptor mediated Ca2+ signalling is affected by extracellular acidosis, which accompanies virtually all brain pathologies. P2Y receptor-dependent Ca2+ release from stores is largely unaffected under acidotic conditions, though P2Y6 receptor dependent release may be slightly reduced. In contrast, Ca2+ influx, mediated at least in part through store-operated Ca2+ channels, is strongly inhibited by extracellular acidosis. Finally, migration of microglia is inhibited under conditions of extracellular acidosis, likely reflecting reduced Ca2+ influx under these conditions.
Acknowledgement
This work was supported by the John Fell Fund and Wellcome Trust (AL holds Oxion Wellcome Trust funded studentship). The funders had neither involvement in the design, collection, analysis and interpretation of data, nor in the writing of the report or in the decision to submit.
References
- 1.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Ueno M, Yamashita T. Bidirectional tuning of microglia in the developing brain: from neurogenesis to neural circuit formation. Curr Opin Neurobiol. 2014;27C:8–15. doi: 10.1016/j.conb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Kierdorf K, Prinz M. Factors regulating microglia activation. Front Cell Neurosci. 2013;7:44. doi: 10.3389/fncel.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gertig U, Hanisch UK. Microglial diversity by responses and responders. Front Cell Neurosci. 2014;8:101. doi: 10.3389/fncel.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–1609. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Kettenmann H, Hanisch UK, Noda A, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 8.Von Kügelgen I, Harden TK. Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol. 2011;61:373–415. doi: 10.1016/B978-0-12-385526-8.00012-6. [DOI] [PubMed] [Google Scholar]
- 9.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sinning A, Hübner CA. Minireview: pH and synaptic transmission. FEBS Let. 2013;587:1923–1928. doi: 10.1016/j.febslet.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522–530. [PubMed] [Google Scholar]
- 12.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013;4:370. doi: 10.3389/fphys.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang WC, Swietach P, Vaughan-Jones RD, Ansorge O, Glitsch MD. Extracellular acidification elicits spatially and temporally distinct Ca2+ signals. Curr Biol. 2008;18:781–785. doi: 10.1016/j.cub.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Zambon AC, Brunton LL, Barrett KE, Hughes RJ, Torres B, Insel PA. Cloning, expression, signalling mechanisms, and membrane targeting of P2Y11 receptors in madin darby canine kidney cells. Mol Pharmacol. 2001;60:26–35. doi: 10.1124/mol.60.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Nakata H, Suzuki T, Namba K, Oyanagi K. Dimerisation of G protein coupled purinergic receptors: increasing the diversity of purinergic receptor signal responses and receptor functions. J Recept Signal Transduct. 2010;30:337–346. doi: 10.3109/10799893.2010.509729. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Kim SU, van Breemen C, McLarnon JG. Activation of purinergic P2X receptors inhibits P2Y-mediated Ca2+ influx in human microglia. Cell Calcium. 2000;27:205–212. doi: 10.1054/ceca.2000.0110. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira R, Schlichter LC. Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca2+ signalling, store refilling and migration of rat microglial cells. PLoS One. 2013;8:e62345. doi: 10.1371/journal.pone.0062345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda M, Tsuno S, Sugiyama T, Hashimoto A, Yamoto K, Takeuchi K, Kishi H, Mizuguchi H, Kohsaka S, Yoshioka T. Ca2+ spiking activity caused by the activation of store-operated Ca2+ channels mediates TNF-α release from microglial cells under chronic purinergic stimulation. Biochim Biophys Acta. 2013;1833:2573–2585. doi: 10.1016/j.bbamcr.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Verkhratsky A, Parpura V. Store-operated calcium entry in neuroglia. Neurosci Bull. 2014;30:125–133. doi: 10.1007/s12264-013-1343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putney Pharmacology of store-operated calcium channels. Mol Interv. 2010;10:209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 25.De Simone R, Niturad CE, De Nuccio C, Ajmone-Cat MA, Visentin S, Minghetti L. TGF-β and LPS modulate ADP-induced migration of microglial cells through P2Y1 and P2Y12 receptor expression. J Neurochem. 2010;155:450–459. doi: 10.1111/j.1471-4159.2010.06937.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim B, Jeong HK, Kim JH, Lee SY, Jou I, Joe EH. Uridine 5’-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol. 2011;186:3701–3709. doi: 10.4049/jimmunol.1000212. [DOI] [PubMed] [Google Scholar]
- 27.Li HQ, Chen C, Dou Y, Wu HJ, Liu YJ, Lou HF, Zhang JM, Li XM, Wang H, Duan S. P2Y4 receptor-mediated pinocytosis contributes to amyloid beta-induced self-uptake by microglia. Mol Cell Biol. 2013;33:4282–4293. doi: 10.1128/MCB.00544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60:1529–1539. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- 31.Stock C, Mueller M, Kraehling H, Mally S, Noël J, et al. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem. 2007;20:679–686. doi: 10.1159/000107550. [DOI] [PubMed] [Google Scholar]
- 32.Hosoi N, Arai I, Tachibana M. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J Neurosci. 2005;25:4062–4039. doi: 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki H, Eguchi S, Miyashita S, Chan S, Hirai K, Hobara N, Yokomizo A, Fujiwara H, Zamami Y, Koyama T, Jin X, et al. Proton acts as a neurotransmitter for nicotine-induced adrenergic and calcitonin gene-related peptide-containing nerve-mediated vasodilation in the rat mesenteric artery. J Pharmacol Exp Ther. 2009;330:745–755. doi: 10.1124/jpet.108.149435. [DOI] [PubMed] [Google Scholar]
- 34.Wu LJ. Voltage-gated proton channel HV1 in microglia. Neuroscientist. 2014 doi: 10.1177/1073858413519864. [DOI] [PubMed] [Google Scholar]
- 35.Dubos RJ. The micro-environment of inflammation or Metchnikoff revisited. Lancet. 1955;269:1–5. doi: 10.1016/s0140-6736(55)93374-2. [DOI] [PubMed] [Google Scholar]
- 36.Edlow DW, Sheldon WH. The pH of inflammatory exudates. Proc Soc Exp Biol Med. 1971;137:1328–1332. doi: 10.3181/00379727-137-35782. [DOI] [PubMed] [Google Scholar]
- 37.Kopaniak M, Issekutz AC, Movat HZ. Kinetics of acute inflammation induced by E. coli in rabbits. Quantification of blood flow, enhanced vascular permeability, haemorrhage, and leukocyte accumulation. Am J Pathol. 1980;90:485–498. [PMC free article] [PubMed] [Google Scholar]
- 38.Van Zwieten R, Wever R, Hamers MN, Weening RS, Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981;68:310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yates CM, Butterworth J, Tennant MC, Gordon A. Enzyme activities in relation to pH and lactate in post-mortem brain in Alzheimer-type and other dementias. J Neurochem. 1990;55:1624–1630. doi: 10.1111/j.1471-4159.1990.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 40.Simonian NA, Colye JT. Oxydative stress in neurodegenerative diseases. Annue Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 41.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 42.Hannah D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumour microenvironment. Glia. 2012;60:502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 44.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Sowa NA, Vadakkan KI, Zylka MJ. Recombinant mouse PAP has pH-dependent ectonucleotidase activity and acts through A1-adenosine receptors to mediate antinociception. PLoS One. 2009;4:e4248. doi: 10.1371/journal.pone.0004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreira IS. Structural features of the G-protein/GPCR interactions. Biochim Biophys Acta. 2014;1840:16–33. doi: 10.1016/j.bbagen.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 48.Marumo M, Suehiro A, Kakishita E, Groschner K, Wakabayashi I. Extracellular pH affects platelet aggregation associated with modulation of store-operated Ca2+ entry. Thromb Res. 2001;104:353–360. doi: 10.1016/s0049-3848(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 49.Asai M, Takeuchi K, Saotome M, Urushida T, Katoh H, Satoh H, Hayashi H, Watanabe H. Extracellular acidosis suppresses endothelial function by inhibiting store-operated Ca2+ entry via non-selective cation channels. Cardiovasc Res. 2009;83:97–105. doi: 10.1093/cvr/cvp105. [DOI] [PubMed] [Google Scholar]
- 50.Mancarella S, Wang Y, Deng X, Landesberg G, Scalia R, Panettieri RA, Mallilankaraman K, Tankg XD, Madesh M, Gill DL. Hypoxia-induced acidosis uncouples the STIM-Orai calcium signalling complex. J Biol Chem. 2011;286:44788–44798. doi: 10.1074/jbc.M111.303081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck A, Fleig A, Penner R, Peinelt C. Regulation of endogenous and heterologuous Ca2+ release-activated Ca2+ currents by pH. Cell Calcium. 2014;56:235–243. doi: 10.1016/j.ceca.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scrimgeour NR, Wilson DP, Rychkov GY. Glu106 in the Orai1 pore contributes to fast Ca2+ dependent inactivation and pH dependence of Ca2+ release-activated Ca2+ (CRAC) cirremt. Biochem J. 2012;441:743–753. doi: 10.1042/BJ20110558. [DOI] [PubMed] [Google Scholar]