Abstract
In addition to its well-recognized role in neurodegeneration, tau participates in maintenance of genome stability and chromosome integrity. In particular, peripheral cells from patients affected by frontotemporal lobar degeneration carrying a mutation in tau gene (genetic tauopathies), as well as cells from animal models, show chromosome numerical and structural aberrations, chromatin anomalies, and a propensity toward abnormal recombination. As genome instability is tightly linked to cancer development, we hypothesized that mutated tau may be a susceptibility factor for cancer. Here we conducted a retrospective cohort study comparing cancer incidence in families affected by genetic tauopathies to control families. Additionally, we carried out a bioinformatics analysis to highlight pathways associated with the tau protein interactome. We report that the risk of developing cancer is significantly higher in families affected by genetic tauopathies, and a high proportion of tau protein interactors are involved in cellular processes particularly relevant to cancer. These findings disclose a novel role of tau as a risk factor for cancer, providing new insights in the various pathological roles of mutated tau.
Keywords: neurodegeneration, cancer, tau, MAPT, mutation, risk factor, epidemiology, bioinformatics
Introduction
Cancer arises from sequential accumulation of genomic alterations, including mutations in oncogenes and tumour suppressor genes (1). Over the years different models of oncogenesis have been proposed (2), in which an initiating event often caused by a mutation leads to genomic instability, comprising: (i) subtle sequence instabilities (base pair substitutions, deletions or insertions of few nucleotides), favoured by mutations in genes involved in the DNA nucleotide-excision and mismatch repair systems; (ii) chromosome instability (CIN), in particular aneuploidy, defined as loss or gain of whole chromosomes or large fragments thereof, that occurs following mutations in genes involved in cellular processes affecting correct chromosome segregation, such as chromosome condensation, chromatid cohesion, kinetocore assembly, centrosome replication/microtubule dynamics, DNA repair and cell cycle checkpoints (3–5). Overall, these types of genomic instability can cause changes in sequence, structure or allelic number of tumour genes, leading cancer cells to acquire functional capabilities that allow them to survive, proliferate and metastasize.
Microtubule-associated proteins (MAPs) are defined as proteins promoting in vivo tubulin self-association into microtubules (MT). Other proteins interacting with MT have different functions such as MT destabilization, linking of various structures and motor properties. Most of these MT-related proteins play a role in mitotic spindle formation, ensuring correct chromosome segregation (6,7). Among MAPs, tau is the most relevant to the nervous system, being abundantly expressed in neurons. Deposition of insoluble filamentous forms of tau gives rise to tauopathy, a neuronal pathology that leads to dementia and atypical parkinsonian syndromes (8). Tau binds to interphasic cytoskeleton MT as well as to mitotic spindle MT (9–11). A mutated tau usually exhibits a reduced ability to bind to MT and to promote their assembly (12), altering MT dynamics (13,14). This can lead to an unstable mitotic spindle, from which chromosome mis-segregation can arise, causing aneuploidy.
In this regard, we demonstrated the consistent presence of aneuploidy in peripheral cells of patients affected by frontotemporal lobar degeneration (FTLD) due to autosomal dominant mutations in microtubule-associated protein tau (MAPT) gene, suggesting a role of tau in chromosome and genome stability (11,15). In addition, it has been reported that tau knock-out mice show chromosome mis-segregation and aneuploidy (16). Based on these studies and as previously suggested (3), we hypothesized that mutated tau can cause CIN resulting in aneuploidy. Aneuploidy is frequently observed in cancer cells, where it can lead to loss of heterozygosity of tumour suppressor genes or amplification of oncogenes, thereby contributing to tumorigenesis.
Moreover, a different type of CIN was observed in cells of our patients carrying mutated tau, i.e. structural chromosome aberrations (11,15), that may be ascribed to defects in DNA single- or double-strand break repair or in DNA-damage checkpoint (3), to telomeric DNA loss or telomerase defects (17), or to alterations in proteins contributing to chromatin stability, predisposing to a higher rate of DNA damage. Whereas there is no evidence of the involvement of tau in DNA repair systems (15) or in telomere preservation, a chaperone role of tau in protecting DNA from free radicals and heat stress damage has recently emerged (18,19), as well as a structural role of tau in chromatin stabilization (15).
Based on these findings, we hypothesized that CIN associated with tau mutations can lead to cancer. Thus, we propose a dual pathogenic role of tau mutations, in cancer and neurodegeneration.
Cancer and neurodegeneration have been proven to share alteration of some biological pathways such as cell cycle, apoptosis, ubiquitin-proteasome system (20,21). These pathways are usually differentially regulated in cancer and neurodegeneration due to the different nature of the impacted cells, that is, proliferating or post-mitotic, respectively. Cell cycle is dysregulated in cancer, where control over cell proliferation is inhibited, and in some forms of neurodegeneration, where abnormal re-entry of post-mitotic cells into the proliferating phase eventually leads to neuronal death via apoptosis (21,22). The tumor suppressor protein p53 plays a pro-apoptotic role and, while protecting the body from cancer, it promotes the aging phenotype through cellular loss. Its deficiency by mutation can lead to higher cancer risk on one hand, and lower degree of neurodegeneration on the other (21). The ubiquitin-proteasome system, while impaired in neurodegenerative diseases and leading to misfolded protein accumulation, is upregulated in several types of cancer (21). A recent study showed that transcripts up-regulated in cancer are down-regulated in central nervous system diseases and vice versa. In line with this finding, a reduced risk for developing some types of cancer has been observed in patients affected by Parkinson’s disease (PD) and Alzheimer’s disease (AD) (23).
Metabolic dysregulation, oxidative stress, DNA damage and inflammation have been shown to be initiating events for both cancer and neurodegeneration (21). In addition, a number of genes (i.e. ATM, PARK2 and LRRK2) are known to confer risk for both cancer and neurodegeneration. ATM plays a central role in cell division and DNA repair: homozygous mutations cause ataxia-teleangectasia, with degeneration of some cerebellar neurons, and predispose to high frequency to cancer, especially to lymphomas (24). PARK2, the most commonly mutated gene in autosomal recessive PD (25), is a well-known tumor-suppressor gene, whose loss of function mutations are associated with cancer (26). Similarly LRRK2, the most frequently mutated gene in late-onset PD has been linked to increased risk of some types of cancers (27).
Similarly to what happens for ATM, PARK2 and LRRK2 mutations, we here suggest that tau mutations may lead to both neurodegeneration and cancer. In fact, if tau is altered by a mutation, its role as MT-binding protein can lead to cytoskeleton disruption and tau deposits in neurodegeneration, and chromosome missegregation and aneuploidy in cancer; furthermore, its role as DNA-chaperone can lead to DNA damage and apoptosis in neurodegeneration and structural chromosome aberrations in cancer.
To verify the hypothesis that CIN associated with tau mutations can lead to cancer, we conducted a retrospective cohort study comparing cancer incidence in families affected by FTLD carrying tau mutations and reference families with superimposable pedigrees, and carried out a bioinformatics analysis of pathways associated with the tau protein interactome. We found that (i) members of FTLD families have a significantly higher risk of developing cancer than members of control families, and (ii) 1/3rd of the tau interactors are involved in DNA damage recognition and repair, cell cycle checkpoints and phase transition, chromatin and telomere maintenance processes, and response to radiation stressors, supporting a role for tau in DNA protection/repair systems.
Materials and Methods
All the subjects participating in the study gave their written informed consent for using their clinical and genetic data for research purposes. All the procedures involving human subjects were done in accordance with the Helsinki Declaration of 1975. As this is a retrospective study, approval by an Ethics Committee was not required.
Pedigrees
We considered FTLD kindreds with a family history of disease and a mutation in the MAPT gene (detected by sequencing of exons 1, 9-13). We analysed 15 families bearing 7 different tau mutations. We designed this study as a retrospective cohort study, where tau-mutated families represented the “exposed cohort”. For each tau-mutated family we collected data from 3 reference families with superimposable pedigrees, with a member born in the same year of the proband, of the same gender and native Italian region. To retrieve comparable informative data for all the tau-mutated families, the pedigrees were accurately investigated for the presence of cancer in: 1) the proband, 2) his/her siblings, 3) the parent whose family is affected by FTLD, 4) his/her siblings, 5) the grandparent affected by FTLD.
Clinical data were obtained from interviews with relatives and family doctors or directly from available clinical charts. For the reference families, the same methods were applied, except both paternal and maternal lines were investigated. Follow up was assessed by interviews for both tau-mutated families and reference families. Where it was not possible to obtain an answer we used social security list to update vital status (2%).
Statistical methods
We compared cancer incidence for the tau-mutated families versus the reference families. In the analysis of tau-mutated families, we did not include the spouses and all the grandparents for which the information about presence of FTLD was not available (subjects in gray in Figure 1). We did not include 2 subjects affected by cancer whose genotype was wild-type (see legend of Figure 1).
Figure 1. Pedigrees of FTLD families.
Black symbols indicate subjects affected by FTLD, red symbols subjects affected by cancer and blue symbols subjects affected by both diseases. Gray symbols represent the spouse parents or grandparents (who do not belong to the family carrying FTLD); note that both grandparents were considered spouses in families 3, 6, 11 and 14, because information about which grandparent carried FTLD was not available. Diagonal lines indicate the deceased, arrows the probands. Smaller symbols represent subjects deceased within few days from birth, stillborn or aborted. Asterisks indicate subjects whose MAPT genotype was determined as wild-type (not included in the statistical analysis).
The statistical analyses are based on the Cox proportional hazard model which specifies the hazard as λ (t)=λ0(t)exp (βX), where λ(t) is the hazard function for the event in question (cancer incidence). X is a vector of covariates, and β is a vector of coefficients to be estimated. The hazards for two participants with fixed covariate vectors Xi and Xj are λi (t)=λ0(t)exp(βXi) and λj (t)=λ0(t)exp (βXj), respectively. The hazard ratio (HR) is λi(t)/λj(t)=exp (β(Xi−Xj)). To test the null hypothesis H0 that β=0, we used the likelihood ratio test. Since the Cox model assumes proportional hazards, this was tested by analysis of scaled Schoenfeld residuals, with associated p-values. When the hazard was suspected to be non-proportional over time, we performed additional analyses, substituting the conventional Cox β coefficient (for a given variable) with a time-dependent function β(t) obtained by adding the smoothed scaled Schoenfeld residuals to the conventional β coefficient.
Factors known or thought to influence cancer incidence in the tau-mutated families were initially analysed by univariate Cox proportional hazard modelling to verify the effect on incidence in our retrospective cohort. Factors analysed were gender, year of birth and region of origin. We next ran multivariate Cox proportional hazard models to estimate HRs with 95% CIs of cancer events. The multivariate model was stratified (separate baseline hazard functions for each variable category within the model) by the same factors above to control for the possible confounding effects of these variables on incidence. Time to event or end of follow-up was calculated from date of birth to date of cancer diagnosis or death or end of follow-up.
Data analysis was carried out using R-language.
Bioinformatics analysis of tau protein interactome
We built the tau weighted protein-protein interactome by extracting all the currently known tau’s protein-protein interactors (PPIs), obtained from peer reviewed literature filtered and scored by our in house pipeline in a supervised manner (28). Briefly, we downloaded PPIs (in June-2017) from the following databases within the IMEX consortium: APID Interactomes, BioGrid, bhf-ucl, InnateDB, InnateDB-All, IntAct, mentha, MINT, InnateDB-IMEx, UniProt, and MBInfo by means of the “PSICQUIC” R package (version 1.15.0 by Paul Shannon, http://code.google.com/p/psicquic/). We converted Protein IDs to Swiss-Prot and Entrez gene ID; we removed TrEMBL, non-protein interactors (e.g. chemicals), obsolete Entrez and Entrez matching to multiple Swiss-Prot identifiers. All PPIs underwent quality control (QC) and filtering leading to the removal of: i) all the non-human taxid annotations; ii) all the annotations with multiple or none PubMed identifiers or no description of Interaction Detection Method. We then scored the interactions taking into consideration the following parameters: i) the number of different publications reporting the interaction; ii) the number of different methods reporting the interaction. We discarded all the interactors with a final score ≤ 2 because (still) not replicated. We performed Gene Ontology (GO) biological processes (BPs) enrichment analyses in g:Profiler (g:GOSt, http://biit.cs.ut.ee/gprofiler/; (29)) for the complete tau’s interactome. Fisher's one-tailed test was used as statistical method for enrichment; SCS-threshold was applied as multiple testing correction; statistical domain size was only annotated genes; no hierarchical filtering was included. We grouped enriched GO-BP terms into custom-made “semantic classes”. Data was handled, filtered and scored through in-house R scripts (https://www.r-project.org/) as described before (28). The final network was visualized through the freely available Cytoscape 2.8.2 (30) software and analyzed through the network analysis plug-in.
Results
Epidemiology
The study population comprised 15 families bearing 7 different tau mutations (Fig. 1), whose FTLD-related pathogenic mechanisms are reported in Table 1. For four of these mutations, we demonstrated chromosome or genomic instability (11;15; Supplementary Table S1). As controls, for each tau-mutated family we selected three reference families with a member born in the same year of the proband, being of the same gender and native of the same Italian region. Demographics of both tau-mutated and reference families is shown in Table 2, while further details for reference families and regions of origin are reported in Supplementary Table S2.
Table 1.
Pathogenetic mechanisms of tau mutations
| Number of families in this study | Tau mutation | Reported pathogenetic mechanismsa | Chromosome aberrations |
|---|---|---|---|
| 1 | N279K | tau isoform imbalance; increase of tau self-aggregation. | n.a. |
| 1 | delN296 | reduction of MT polymerization; slower kinesin translocation along MT | yesb |
| 8 | P301L | reduction of MT polymerization; increase of tau self-aggregation; slower kinesin translocation along MT | yesb,c |
| 2 | IVS10+16C>T | tau isoform imbalance | yesb |
| 1 | V337M | reduction of MT polymerization; increase of tau self-aggregation | n.a. |
| 1 | V363A | reduction of MT polymerization; oligomer production | yesd |
| 1 | T427M | n.a. | n.a. |
Table 2. Cohort demographic.
| N° of subjects of the whole cohort |
N° of subjects of tau-mutated families |
N° of subjects of reference families |
|
|---|---|---|---|
| Total subjects | 879 | 162 | 717 |
| Subjects with dementia | 68 | ||
| Subjects with cancer | 16 | 68 | |
| Subjects with dementia and cancer | 8 | ||
| Gender | |||
| M | 473 | 94 | 379 |
| F | 406 | 68 | 338 |
| Period | |||
| 1877-1916 | 107 | 5 | 102 |
| 1917-1936 | 210 | 24 | 186 |
| 1937-1956 | 232 | 46 | 186 |
| 1957-1996 | 219 | 50 | 169 |
| 1997-2013 | 111 | 37 | 74 |
All families were accurately investigated for the presence of cancer. Within the tau-mutated families, 24 subjects (15%) had cancer, while within the reference families 68 subjects (9%) had cancer. The mean age at diagnosis of subjects with cancer was 58 years, while the average age of dementia onset was 50 years. The types of cancer detected in tau-mutated and reference families are reported in Supplementary Table S3. A great variability was observed in both cohorts, showing no recurrence of a particular type of cancer even in tau-mutated families.
Factors known or thought to influence cancer incidence such as gender, year of birth and region of origin were initially analysed by univariate Cox proportional hazard model (Table 3A). The study showed that the tau-mutated families had significantly greater risk of cancer than the reference families (HR = 3.11), and, when the gender variable was assessed, the risk in females appeared to be greater than in males, though not significant (HR = 1.16). We then performed multivariate Cox proportional hazard model to estimate HRs with 95% CIs of cancer events. The model was stratified by gender, year of birth and region of origin to control for potential confounding effects on incidence of cancer. The likelihood ratio test resulted in p = 0.0005, supporting robust association between the presence of a tau mutation and the development of cancer with HR = 3.72 (CI 95% 2.07 – 6.67), thus indicating a nearly 4-fold risk of developing cancer in tau-mutated families (Table 3B).
Table 3A. Univariate analysis HRs and 95% CI for cancer risk.
| HR (95% CI) | |
|---|---|
| univariate | |
| Tau exposure | 3.11 (1.93 – 5.31) |
| Gender | 1.16 (0.76 - 1.74) |
Table 3B. Multivariate analysis HRs and 95% CI for cancer risk.
| HR (95% CI) |
|---|
| multivariate |
| 3.72 (2.07 – 6.67)* |
Multivariate stratified by gender, year of birth and region of origin
Bioinformatics analysis of tau protein interactome
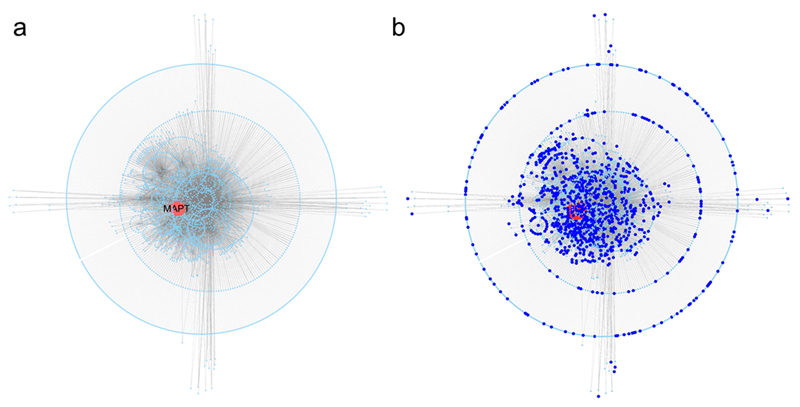
We generated a two layers interactome for tau (Fig. 2). The first layer interactors (65 nodes) are directly connected to tau, whilst the second layer interactors (3132 nodes) represent the interactors of each first layer node thus diluting the seed centrality bias as previously reported (28). The global tau’s interactome comprised a total of 3197 nodes and 5711 edges, with characteristic path length of 3.407 and average number of neighbors of 3.3 (Fig. 2A). To gather insight into the biological functions associated with tau’s entire interactome we performed functional annotation analysis evaluating Gene Ontology-Biological Processes (GO-BPs) enrichment (Supplementary Table S4). Some of the biological functions enriched within tau’s interactome were expected (e.g. cytoskeleton dynamics and transport). Nevertheless, nearly 1/3rd of the proteins contributing to the entire tau’s interactome (989/3197) was directly involved in the enrichment of GO-BPs terms globally pointing to DNA METABOLISM and, particularly, to DNA damage, stress response to radiation, DNA damage checkpoint and repair, and cell death after DNA damage; additionally, we found terms related to CELL CYCLE, particularly, indicating cell cycle checkpoints and chromosome segregation, and CHROMATIN, the latter pointing to processes such as histone and telomere maintenance (Table 4; Fig. 2B). Importantly, to assess the specificity of the enrichment reported above, we generated 25 random protein sets (by extracting a series of numbers using random permutation without replacement in R) with similar size to the tau’s interactome and processed them through functional enrichment. Out of the 25 protein sets, only 8 (32%) led to a significant functional enrichment. Only 1/8 random protein set revealed 0.8% enriched terms that were similar to those reported for tau. Considering the former (0.8%) against the latter (30.9%) (Supplementary Table S5), it follows that the specificity of tau’s interactome is strong and unbiased.
Figure 2. Tau protein interactome.
a Tau (MAPT), in red, is used as seed to download direct protein interactors (first layer tau’s interactome); direct interactors of tau are used to download direct protein interactors of the first layer nodes generating the second layer tau’s interactome. b Updated version of A where all the first and second layer interactors (989 nodes) of tau associated with GO-BPs related to DNA damage, cell cycle checkpoints and chromatin/telomere maintenance are highlighted in dark blue.
Table 4.
Summary of Gene Ontology - Biological Processes (GO-BPs) terms enriched within the tau interactome and generally relevant to cancer.
| p-value | term ID | t name | t depth | Semantic class |
|---|---|---|---|---|
| 5.88E-09 | GO:0071156 | regulation of cell cycle arrest | 6 | cell cycle |
| 3.14E-08 | GO:0071158 | positive regulation of cell cycle arrest | 6 | cell cycle |
| 1.49E-07 | GO:0044819 | mitotic G1/S transition checkpoint | 9 | cell cycle - checkpoint |
| 5.53E-07 | GO:0072413 | signal transduction involved in mitotic cell cycle checkpoint | 7 | cell cycle - checkpoint |
| 9.28E-08 | GO:0072395 | signal transduction involved in cell cycle checkpoint | 6 | cell cycle - checkpoint |
| 7.13E-12 | GO:0007093 | mitotic cell cycle checkpoint | 6 | cell cycle - checkpoint |
| 1.85E-15 | GO:0000075 | cell cycle checkpoint | 5 | cell cycle - checkpoint |
| 5.58E-06 | GO:0000079 | regulation of cyclin-dependent protein serine/threonine kinase activity | 6 | cell cycle - enzyme |
| 1.37E-13 | GO:2000045 | regulation of G1/S transition of mitotic cell cycle | 8 | cell cycle - phase transition |
| 3.28E-08 | GO:2000134 | negative regulation of G1/S transition of mitotic cell cycle | 8 | cell cycle - phase transition |
| 7.20E-06 | GO:0010389 | regulation of G2/M transition of mitotic cell cycle | 8 | cell cycle - phase transition |
| 2.37E-03 | GO:0010972 | negative regulation of G2/M transition of mitotic cell cycle | 8 | cell cycle - phase transition |
| 4.68E-02 | GO:0010824 | regulation of centrosome duplication | 7 | cell cycle - segregation/cytokinesis |
| 6.12E-12 | GO:0000280 | nuclear division | 6 | cell cycle - segregation/cytokinesis |
| 3.78E-10 | GO:0007088 | regulation of mitotic nuclear division | 6 | cell cycle - segregation/cytokinesis |
| 2.11E-09 | GO:0051783 | regulation of nuclear division | 6 | cell cycle - segregation/cytokinesis |
| 7.23E-03 | GO:0033045 | regulation of sister chromatid segregation | 6 | cell cycle - segregation/cytokinesis |
| 3.37E-02 | GO:0051988 | regulation of attachment of spindle microtubules to kinetochore | 6 | cell cycle - segregation/cytokinesis |
| 3.58E-02 | GO:0032465 | regulation of cytokinesis | 6 | cell cycle - segregation/cytokinesis |
| 6.04E-04 | GO:0046605 | regulation of centrosome cycle | 6 | cell cycle-cytoskeleton |
| 6.51E-03 | GO:0090307 | mitotic spindle assembly | 6 | cell cycle-cytoskeleton |
| 3.93E-10 | GO:0032206 | positive regulation of telomere maintenance | 7 | chomatin - telomere |
| 1.54E-08 | GO:0032212 | positive regulation of telomere maintenance via telomerase | 7 | chomatin - telomere |
| 6.17E-03 | GO:0032205 | negative regulation of telomere maintenance | 7 | chomatin - telomere |
| 8.52E-14 | GO:0032200 | telomere organization | 6 | chomatin - telomere |
| 1.04E-03 | GO:0090671 | telomerase RNA localization to Cajal body | 6 | chomatin - telomere |
| 7.16E-03 | GO:1904814 | regulation of protein localization to chromosome, telomeric region | 6 | chomatin - telomere |
| 1.04E-03 | GO:0090672 | telomerase RNA localization | 5 | chomatin - telomere |
| 3.02E-09 | GO:1905269 | positive regulation of chromatin organization | 7 | chromatin - organisation |
| 1.99E-07 | GO:0031056 | regulation of histone modification | 7 | histone |
| 2.79E-07 | GO:0031058 | positive regulation of histone modification | 7 | histone |
| 4.40E-03 | GO:0070932 | histone H3 deacetylation | 8 | histone - acetylation |
| 3.25E-02 | GO:0035065 | regulation of histone acetylation | 8 | histone - acetylation |
| 1.12E-07 | GO:0016572 | histone phosphorylation | 7 | histone - phosphorylation |
| 5.64E-16 | GO:0042770 | signal transduction in response to DNA damage | 6 | DNA metabolism - damage |
| 1.50E-03 | GO:0006975 | DNA damage induced protein phosphorylation | 6 | DNA metabolism - damage |
| 6.84E-06 | GO:0042771 | intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator | 8 | DNA metabolism - damage - cell death |
| 2.82E-04 | GO:1902229 | regulation of intrinsic apoptotic signaling pathway in response to DNA damage | 8 | DNA metabolism - damage - cell death |
| 2.48E-14 | GO:0008630 | intrinsic apoptotic signaling pathway in response to DNA damage | 7 | DNA metabolism - damage - cell death |
| 3.27E-03 | GO:1902230 | negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage | 7 | DNA metabolism - damage - cell death |
| 1.49E-07 | GO:0031571 | mitotic G1 DNA damage checkpoint | 10 | DNA metabolism - damage - checkpoint |
| 5.48E-06 | GO:0006977 | DNA damage response, signal transduction by p53 class mediator resulting in cell cycle arrest | 10 | DNA metabolism - damage - checkpoint |
| 5.53E-07 | GO:1902402 | signal transduction involved in mitotic DNA damage checkpoint | 9 | DNA metabolism - damage - checkpoint |
| 1.75E-06 | GO:0072431 | signal transduction involved in mitotic G1 DNA damage checkpoint | 9 | DNA metabolism - damage - checkpoint |
| 4.56E-05 | GO:0006289 | nucleotide-excision repair | 7 | DNA metabolism - repair |
| 2.38E-02 | GO:0006302 | double-strand break repair | 7 | DNA metabolism - repair |
| 1.43E-06 | GO:0034644 | cellular response to UV | 7 | response to stimulus - stress - radiation |
| 2.51E-03 | GO:0071480 | cellular response to gamma radiation | 7 | response to stimulus - stress - radiation |
For each semantic class the top GO terms are reported (based on t-depth).
Discussion
It is well established that tau, as a MT-binding protein, is a major player in neurodegenerative diseases also known as tauopathies, such as FTLD and AD. Cytoplasmic abnormal tau deposits represent a burden to neurons and glial cells, whilst toxic soluble tau oligomers are now being envisaged as responsible for the neuronal dysfunction and death (8,31).
However, other lines of evidence suggest that tau may be also involved in other functions. Nuclear and nucleolar localizations of tau were first described several years ago (32–34) and, more recently, a role of tau in ribosome biogenesis was suggested (35). We confirmed the nuclear and peri-chromosomal localization of tau and, in addition, discovered that FTLD patients bearing the P301L tau mutation had several numerical and structural chromosome aberrations and chromatin defects in their peripheral blood lymphocytes and fibroblasts (11).
A number of observations argued for a link between mutated tau and chromosome aberrations: 1) tau’s physical association with mitotic spindle, thus possibly regulating correct chromosome segregation; 2) tau’s nuclear localization and its physical interaction with the chromatin (36); 3) tau’s ability to protect DNA in vitro (37). Additionally, a link between tau and genome integrity was evidenced when tau was shown to translocate from the cytoplasm to the nucleus during cellular stress and to protect DNA from stress-induced DNA breaks (19). Furthermore, in an in vivo mouse model of heat stress, tau was shown to protect genomic DNA and also RNA from oxidative damage (38), whilst in a tau knock-out mouse model, the absence of tau caused disruption of the neuronal peri-centromeric heterochromatin, which showed an abnormal accumulation of DNA breaks (39).
Our group examined peripheral cells of FTLD patients carrying different tau mutations and demonstrated chromosome aberrations, as well as a tendency to abnormal recombination events, indicating genome instability (15). All the more, in two mouse models of genetic tauopathy, we detected a higher level of aneuploidy than in control mice (40). More recently, in a Drosophila melanogaster model of tauopathy, mitotic spindle anomalies and aneuploidy were observed after overexpressing wt tau (41).
Aneuploidy is a condition often associated with cancer. An aneuploid karyotype can or cannot promote cancer depending on its inherent imbalance of oncogenes or tumor suppressor genes, and other genes controlling cell viability and fitness. It has been proposed that in the harsh cell environment experimented by cancer cells, aneuploidy may conceivably confer an advantage and promote cancer cells’ survival and proliferation (42). Similar considerations apply to structural chromosome aberrations.
We therefore hypothesized that tau mutations might predispose to cancer. We had access to a retrospective cohort of families affected by genetic tauopathies (tau-mutated families) and investigated the potential link between tau mutations and cancer. We surveyed the presence of any type of cancer in subjects within tau-mutated families. In parallel, we collected reference families to compare cancer incidence. For the period of time when most of the tau-mutated families’ subjects had lived there was not any national or regional cancer registry available, therefore, in the setting of retrospective studies, the most appropriate analysis model we could choose was the cohort model. Multivariate analysis correcting for the possible confounding factors showed that the presence of tau mutation raises the risk of developing cancer by 3.72 times, assigning to tau mutation a prominent role as a risk factor for cancer. If we accept the definition of moderate risk (in terms of disease incidence) as a risk two to four times as high as in the general population, we can affirm that MAPT mutations, as in the case of other genes such as ATM or CHEK2 (43), represent a moderate risk factor for cancer.
This finding was further supported by our computational analysis where we applied a systems biology approach focused on the tau protein interactome on the basis of the guilt by association principle (i.e. the unknown function of protein A can be inferred via the known function of protein B if A and B interact) (44). Functional annotation analysis of the in silico model of tau’s interactome showed that over 1/3rd of tau interactors was directly involved in functions such as DNA damage, response to radiation stressors, DNA damage checkpoint, repair and cell death, cell cycle checkpoints and chromatin maintenance, processes that are arguably associated with cancer; this is cross-supportive with previous computational work when considering tau’s co-expression or PPI-networks analyses in FTLD (28,45).
Bridging the in silico and functional data, and considering in particular the cell cycle checkpoints, it has in fact been shown, in a Drosophila model of tauopathy, that human mutations cause neurodegeneration by abnormally activating the cell cycle in post-mitotic neurons (46), or can induce heterochromatin relaxation, DNA damage and widely altered gene expression with cell cycle activation (47,48). While abnormal neuronal cell-cycle reentry is now accepted as a phenomenon associated with neurodegeneration (22,49), the ability of mutated tau to activate the cell cycle in different tissues should be taken into account as a possible risk factor for abnormal cell proliferation, linking cancer and neurodegeneration. On the other hand, the DNA damage checkpoint mediated by ATM and p53 appears to be protective in mouse and Drosophila models of tauopathy, again linking neurodegeneration and cancer (50).
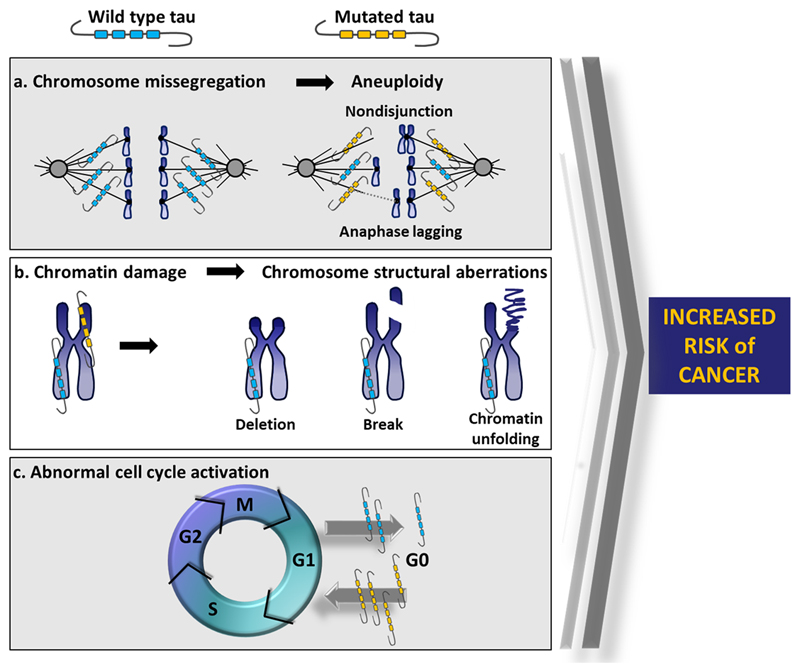
Figure 3 synthetically illustrates the possible mechanisms through which mutated tau can increase the risk of developing cancer. In particular, chromosome missegregation, leading to aneuploidy (Fig. 3A), chromatin damage, causing structural chromosome aberrations (Fig. 3B), and abnormal cell cycle activation (Fig. 3C) are depicted.
Figure 3. Tau mutations increase the risk of cancer.
a Mutated tau alters microtubule dynamics producing an unstable mitotic spindle, which in turn leads to chromosome missegregation. b Mutated tau fails to protect chromatin structure and DNA integrity, producing chromosome structural aberrations. c Mutated tau induces an aberrant cell cycle activation. All these pathological events can increase the risk of developing cancer.
The types of tumors that we detected in tau-mutated families were variable, ranging from haematological (e.g. leukemias) to solid, from common (lung, breast, colorectal cancer) to rare, from benign (e.g. leiomyoma) to malignant, from strictly epithelial to teratomas (e.g., ovarian teratoma). This spectrum suggests that tau mutations may represent a risk factor predisposing to genomic instability with no tissue specificity, as previously suggested by the presence of different and not recurrent types of chromatin and chromosome aberrations in FTLD patients (15).
By interrogating publicly available RNA/protein expression databases, we verified that tau was detected in almost all evaluated tissues (in both normal as well as cancerous tissues/cell lines) (Supplementary Table S6). It may be also worth considering that, due to the metastatic nature of many cancers, the tissue where a cancer is detected – as indicated in Supplementary Table S2 –may not be the primary tumor site and may thus be independent of tau’s site-specific expression.
As shown in Figure 1, as well as in Supplementary Table S2, there are some families without cancer-affected subjects. As tau mutations do not represent a causative but a risk factor for cancer, it may be that not every subject carrying the mutation will develop cancer, and by pure chance it is possible that in a tau-mutated family there should not be cancer-affected subjects. This is the case of Fam11, Fam15 and Fam8, whereas other families carrying the same mutation show cancer-affected subjects; this may also be the case of the V337M mutation. However we do not exclude that some mutations may be less cancer-predisposing than others, depending on their position in the protein or their amino-acid change, which may affect to a minor degree the microtubule-binding capacity or the DNA-chaperone ability, involved in the cancer development. This may for example be the case of the V337M mutation.
In summary, we here show that tau’s functions go beyond assuring MT stability, as we demonstrate its nuclear involvement and association with genome stability and increased risk for cancer. This is a novel concept for tau’s biology and, in line with other reports tying cancer and neurodegeneration (20,21), it might prove critical for a better understanding of both cancer and neurodegeneration (i.e. tauopathies) etiologies. As such it is warranted to further explore tau-associated molecular mechanisms as a mean for untangling manifold disorders.
Supplementary Material
Significance.
This study reveals a novel role for tau as a risk factor for cancer providing new insights beyond its role in neurodegeneration
Acknowledgements
Giacomina Rossi was supported by funding from Ministero della Salute (Ministry of Health, Italy). Raffaele Ferrari was supported by funding from Alzheimer’s Society (grant number 284). Claudia Manzoni was supported by funding from the MRC Programme grant MR/N026004/1 (to John Hardy and Patrick A. Lewis) and the MRC New Investigator Research Grant MR/L010933/1 (to Patrick A. Lewis).
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest
References
- 1.Michor F, Iwasa Y, Vogelstein B, Lengauer C, Nowak MA. Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol. 2005;15:43–9. doi: 10.1016/j.semcancer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer. 2016;16:35–42. doi: 10.1038/nrc.2015.4. [DOI] [PubMed] [Google Scholar]
- 3.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–41. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995;75:835–64. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- 7.Maiato H, Sampaio P, Sunkel CE. Microtubule-associated proteins and their essential roles during mitosis. Int Rev Cytol. 2004;241:53–153. doi: 10.1016/S0074-7696(04)41002-X. [DOI] [PubMed] [Google Scholar]
- 8.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12:609–22. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- 9.Preuss U, Mandelkow EM. Mitotic phosphorylation of tau protein in neuronal cell lines resembles phosphorylation in Alzheimer's disease. Eur J Cell Biol. 1998;76:176–84. doi: 10.1016/S0171-9335(98)80032-0. [DOI] [PubMed] [Google Scholar]
- 10.Cross DC, Munoz JP, Hernandez P, Maccioni RB. Nuclear and cytoplasmic tau proteins from human nonneuronal cells share common structural and functional features with brain tau. J Cell Biochem. 2000;78:305–17. [PubMed] [Google Scholar]
- 11.Rossi G, Dalprà L, Crosti F, Lissoni S, Sciacca FL, Catania M, et al. A new function of microtubule-associated protein tau: involvement in chromosome stability. Cell Cycle. 2008;7:1788–94. doi: 10.4161/cc.7.12.6012. [DOI] [PubMed] [Google Scholar]
- 12.Rossi G, Tagliavini F. Frontotemporal lobar degeneration: old knowledge and new insight into the pathogenetic mechanisms of tau mutations. Front Aging Neurosci. 2015;7:192. doi: 10.3389/fnagi.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunker JM, Wilson L, Jordan MA, Feinstein SC. Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration. Mol Biol Cell. 2004;15:2720–8. doi: 10.1091/mbc.E04-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunker JM, Kamath K, Wilson L, Jordan MA, Feinstein SC. FTDP-17 mutations compromise the ability of tau to regulate microtubule dynamics in cells. J Biol Chem. 2006;281:11856–63. doi: 10.1074/jbc.M509420200. [DOI] [PubMed] [Google Scholar]
- 15.Rossi G, Conconi D, Panzeri E, Redaelli S, Piccoli E, Paoletta, et al. Mutations in MAPT gene cause chromosome instability and introduce copy number variations widely in the genome. J Alzheimers Dis. 2013;33:969–82. doi: 10.3233/JAD-2012-121633. [DOI] [PubMed] [Google Scholar]
- 16.Granic A, Padmanabhan J, Norden M, Potter H. Alzheimer Abeta peptide induces chromosome mis-segregation and aneuploidy, including trisomy 21: requirement for tau and APP. Mol Biol Cell. 2010;21:511–20. doi: 10.1091/mbc.E09-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y, Qu MH, Wang XS, Chen L, Wang DL, Liu Y, et al. Binding to the minor groove of the double-strand, tau protein prevents DNA from damage by peroxidation. PLoS One. 2008;3:e2600. doi: 10.1371/journal.pone.0002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultan A, Nesslany F, Violet M, Bégard S, Loyens A, Talahari S, et al. Nuclear tau, a key player in neuronal DNA protection. Biol Chem. 2011;286:4566–75. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plun-Favreau H, Lewis PA, Hardy J, Martins LM, Wood NW. Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet. 2010;6:e1001257. doi: 10.1371/journal.pgen.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driver JA. Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology. 2014;15:547–57. doi: 10.1007/s10522-014-9523-2. [DOI] [PubMed] [Google Scholar]
- 22.Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull. 2016;126:238–92. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 2014;10:e1004173. doi: 10.1371/journal.pgen.1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavrou A, Tsangaris GT, Roma E, Kolialexi A. The ATM gene and ataxia telangiectasia. Anticancer Res. 2008;28:401–5. [PubMed] [Google Scholar]
- 25.Hernandez DG, Reed X, Singleton AB. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem. 2016;139(Suppl 1):59–74. doi: 10.1111/jnc.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Natl Acad Sci U S A. 2003;100:5956–61. doi: 10.1073/pnas.0931262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agalliu I, San Luciano M, Mirelman A, Giladi N, Waro B, Aasly J, et al. Higher frequency of certain cancers in LRRK2 G2019S mutation carriers with Parkinson disease: a pooled analysis. JAMA Neurol. 2015;72:58–65. doi: 10.1001/jamaneurol.2014.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari R, Lovering RC, Hardy J, Lewis PA, Manzoni C. Weighted Protein Interaction Network Analysis of Frontotemporal Dementia. J Proteome Res. 2017;16:999–1013. doi: 10.1021/acs.jproteome.6b00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–9. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafiei SS, Guerrero-Munoz MJ, Castillo-Carranza DL. Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front Aging Neurosci. 2017;9:83. doi: 10.3389/fnagi.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loomis PA, Howard TH, Castleberry RP, Binder LI. Identification of nuclear tau isoforms in human neuroblastoma cells. Proc Natl Acad Sci U S A. 1990;87:8422–6. doi: 10.1073/pnas.87.21.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brady RM, Zinkowski RP, Binder LI. Presence of tau in isolated nuclei from human brain. Neurobiol Aging. 1995;16:479–86. doi: 10.1016/0197-4580(95)00023-8. [DOI] [PubMed] [Google Scholar]
- 34.Thurston VC, Zinkowski RP, Binder LI. Tau as a nucleolar protein in human nonneural cells in vitro and in vivo. Chromosoma. 1996;105:20–30. doi: 10.1007/BF02510035. [DOI] [PubMed] [Google Scholar]
- 35.Sjoberg MK, Shestakova E, Mansuroglu Z, Maccioni RB, Bonnefoy E. Tau protein binds to pericentromeric DNA: a putative role for nuclear tau in nucleolar organization. J Cell Sci. 2006;119:2025–34. doi: 10.1242/jcs.02907. [DOI] [PubMed] [Google Scholar]
- 36.Greenwood JA, Johnson GV. Localization and in situ phosphorylation state of nuclear tau. Exp Cell Res. 1995;220:332–7. doi: 10.1006/excr.1995.1323. [DOI] [PubMed] [Google Scholar]
- 37.Hua Q, He RQ. Tau could protect DNA double helix structure. Biochim Biophys Acta. 2003;1645:205–11. doi: 10.1016/s1570-9639(02)00538-1. [DOI] [PubMed] [Google Scholar]
- 38.Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci. 2014;8:84. doi: 10.3389/fncel.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansuroglu Z, Benhelli-Mokrani H, Marcato V, Sultan A, Violet M, Chauderlier A, et al. Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci Rep. 2016;6:33047. doi: 10.1038/srep33047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi G, Conconi D, Panzeri E, Paoletta L, Piccoli E, Ferretti MG, et al. Mutations in MAPT give rise to aneuploidy in animal models of tauopathy. Neurogenetics. 2014;15:31–40. doi: 10.1007/s10048-013-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malmanche N, Dourlen P, Gistelinck M, Demiautte F, Link N, Dupont C, et al. Developmental Expression of 4-Repeat-Tau Induces Neuronal Aneuploidy in Drosophila Tauopathy Models. Sci Rep. 2017;7:40764. doi: 10.1038/srep40764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giam M, Rancati G. Aneuploidy and chromosomal instability in cancer: a jackpot to chaos. Cell Div. 2015;10:3. doi: 10.1186/s13008-015-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–57. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Chen L, He W, Li W, Qu X, Liang B, et al. Prioritizing disease candidate proteins in cardiomyopathy-specific protein-protein interaction networks based on "guilt by association" analysis. PLoS One. 2013;8:e71191. doi: 10.1371/journal.pone.0071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari R, Forabosco P, Vandrovcova J, Botía J, Guelfi S, Warren JD, et al. Frontotemporal dementia: insights into the biological underpinnings of disease through gene co-expression network analysis. Mol Neurodegener. 2016;11:21. doi: 10.1186/s13024-016-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16:230–41. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 47.Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014;17:357–66. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frost B, Bardai FH, Feany MB. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr Biol. 2016;26:129–36. doi: 10.1016/j.cub.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arendt T. Cell cycle activation and aneuploid neurons in Alzheimer's disease. Mol Neurobiol. 2012;46:125–35. doi: 10.1007/s12035-012-8262-0. [DOI] [PubMed] [Google Scholar]
- 50.Khurana V, Merlo P, DuBoff B, Fulga TA, Sharp KA, Campbell SD, et al. A neuroprotective role for the DNA damage checkpoint in tauopathy. Aging Cell. 2012;11:360–2. doi: 10.1111/j.1474-9726.2011.00778.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.