ABSTRACT
Environmental resources are proposed to fine-tune the timing of breeding, yet how they may do so remains unclear. In female European starlings (Sturnus vulgaris), nest cavities are limited resources that are necessary for breeding. Females that explore nest cavities, compared with those that do not, readily perform sexually motivated behaviors. We assigned female starlings to aviaries with: (1) no nest boxes, (2) nest boxes, or (3) nest boxes, plants, flowing water, insects and berries to test the hypothesis that environmental resources alter neural systems to stimulate mating behavior. Compared with other females, females that were housed with and explored nest boxes had higher estradiol, higher preproenkephalin (PENK) mRNA and lower levels of D1 and D2 dopamine receptor mRNA in the medial preoptic area (mPOA); a region in which opioids and dopamine modify female sexual behaviors and sexual motivation. Additionally, in the mPOA, PENK and tyrosine hydroxylase mRNA positively predicted, whereas estrogen receptor beta mRNA negatively predicted, nest box exploration. In the ventromedial hypothalamus (a region in which estradiol acts to stimulate sexual behavior), estrogen receptor alpha mRNA was highest in females that had access to but did not explore nest cavities. It is likely that seasonal increases in estradiol modify mRNA in the mPOA to facilitate nest cavity exploration. It is also possible that nest cavity exploration further alters gene expression in the mPOA, functioning to coordinate mating with resource availability. Thus, nest cavity exploration may be a form of self-stimulation that alters neural systems to fine-tune sexual behavior.
KEY WORDS: Nest cavity, Limited resource, Opioid, Dopamine, Estradiol, Avian
Summary: Environmental resources crucial for breeding may alter breeding physiology and circuits controlling sexual and sexually motivated behaviors to coordinate breeding with resource availability.
INTRODUCTION
To optimize breeding success, animals must coordinate breeding behavior with the availability of environmental resources such as breeding territories, rainfall, food and mate availability. Advances have been made in understanding the roles of photoperiod, the hypothalamic–pituitary–gonadal (HPG) axis and steroid hormones in timing sexual behaviors to match the availability of these crucial resources (Calisi et al., 2011; Davies and Deviche, 2014; Small et al., 2008; Watts and Hahn, 2012; Williams, 2012; Wingfield and Kenagy, 1991). Studies also suggest that such resources may alter activity in brain regions underlying sexual motivation (Kelm et al., 2011; Riters et al., 2000); however, mechanisms by which this occurs have not been well studied. The goal of this study was to identify neural mechanisms connecting the availability of limited resources to mating behaviors by studying the resource-dependent mating behavior of female European starlings (Sturnus vulgaris).
Secondary cavity-nesting birds (i.e. those that cannot create their own nest sites), such as starlings, cannot breed without access to cavities (Feare, 1984; Kessel, 1957; Newton, 1994; Scott et al., 1977). In starlings, studies using a conditioned place preference test of reward show that only females with nest cavities find hearing male courtship song to be rewarding (Riters et al., 2013). Furthermore, the reward value of hearing male song correlated positively with the number of times a female was observed entering and exiting a nest cavity (Riters et al., 2013). It is likely that physiological changes that occur at the onset of the breeding season (e.g. increases in estradiol; Dawson, 1983) in female starlings cause females to explore nest cavities. However, it is also possible that exploration of nest cavities further stimulates changes in estradiol and the brain to stimulate reproductive physiology and facilitate mating behavior. The idea that a female's own behavior could stimulate her reproductive development is supported by past studies. For example, nest building in female canaries induces development of a brood patch and eggs (Warren and Hinde, 1961), and in ring doves, females ‘self-stimulate’ development of their ovarian follicles by producing ‘nest-coos’ (Cheng, 1986; Cheng and Peng, 1997). Similarly, in female starlings, entering and exiting a nest cavity may act to self-stimulate reproductive development and to alter activity in brain regions involved in sexual behavior and sexual motivation.
Both the medial preoptic area (mPOA) and the ventromedial hypothalamus (VMH) are candidate brain regions for coordinating environmental information (e.g. nest cavity access) with breeding physiology and behavior. Across vertebrates, the mPOA integrates hormonal and environmental signals to alter sexually motivated behaviors (Alward et al., 2013; Ball, 1993; Hull et al., 1995; Schulz et al., 2003; Simerly and Swanson, 1986). Projections from the mPOA modulate activity in the ventromedial hypothalamic nucleus (VMN; a nucleus within the VMH) to regulate female sexual behavior (reviewed in Micevych and Meisel, 2017). The mPOA and VMH are sensitive to estradiol (i.e. both contain large populations of estrogen receptors), and estradiol acting in the mPOA and VMH modulates sexual behavior (Shughrue et al., 1997; Snoeren et al., 2015; Spiteri et al., 2010, 2012; Veney and Rissman, 2000).
Neuromodulators such as opioids are also implicated in the control of female sexual behavior (Acosta-Martinez and Etgen, 2002; García-Horsman et al., 2008; Maney and Wingfield, 1998; Nicot et al., 1997). In female starlings, measurements of immunolabeling for met-enkephalin were higher in mPOA and VMH in females that explored compared with those that did not explore nest cavities (Riters et al., 2013). Met-enkephalin labeling measurements in VMH also correlated positively with the number of times females entered nest cavities and with reward induced by hearing male song (measured using conditioned place preference; Riters et al., 2013). Opioids in both the mPOA and VMH underlie reward induced by paced mating behavior in female rats (García-Horsman et al., 2008). Furthermore, opioids (e.g. enkephalins and μ-opioid receptors) in the mPOA and VMH are upregulated in female rats treated with estradiol compared with ovariectomized females (Holland et al., 1998; Watson et al., 1986). Together these findings suggest that seasonal increases in estradiol, or increases in estradiol caused by entering and exiting a nest cavity, may increase opioid expression and activity in mPOA and VMH to increase the reward value of male song, which may alter the expression of sexual behavior.
Dopamine also plays a role in regulating female sexually motivated behaviors, including starling responses to nest cavities broadcasting male song (Pawlisch and Riters, 2010; Riters et al., 2007). In rodents, dopamine receptor D1 antagonists injected into the female mPOA decreased, whereas dopamine receptor D2 antagonists increased, sexually motivated behaviors in female rats (Graham and Pfaus, 2012). Dopamine release in the female mPOA is also elevated during sexual interactions with a male (Matuszewich et al., 2000). Although immunolabeling for tyrosine hydroxylase (TH; a rate-limiting enzyme for both dopamine and noradrenaline synthesis) in the mPOA of female starlings did not differ in females that did or did not explore boxes, other dopamine markers such as D1 and D2 receptors have not been examined. In female rats, increases in estradiol that facilitate sexual behavior alter dopamine D1 and D2 receptors in the mPOA, which may facilitate sexual motivation (Graham et al., 2015; Hull et al., 1989). Thus, it is possible that seasonal, or behaviorally induced, increases in estradiol may alter D1 and D2 receptors in mPOA to facilitate sexually motivated behaviors.
This study was designed to provide insight into the hypothesis that in female starlings entering and exiting nest cavities acts to self-stimulate reproductive development and to alter opioid and dopamine activity in brain regions involved in sexual behavior and sexual motivation. We also examined the effect of other resources, e.g. females of many songbird species use the presence of flowing water, abundant food and warming temperatures to time breeding such that chicks hatch when environmental conditions are most favorable for chick survival (reviewed in Ball and Ketterson, 2008). These resources can increase measurements of HPG axis activity such as plasma luteinizing hormone and the size of ovarian follicles (Ettinger and King, 1981; Watts and Hahn, 2012; Wingfield et al., 2003, 2012); however, the degree to which they modify neural systems involved in sexual behavior and sexual motivation is not known. To explore the effects of resources on physiology and behavior, we assigned females to either: (1) standard aviaries, (2) aviaries containing nest boxes, or (3) aviaries containing nest boxes, plants, flowing water and supplementary food. We observed behavior in response to conspecific males, collected measurements of breeding physiology, and measured opioid- and dopamine-related mRNA, and estrogen receptor mRNA in the mPOA and VMH.
MATERIALS AND METHODS
Animals and housing
Thirty-six female starlings (Sturnus vulgaris Linnaeus 1758) were captured on a farm in Madison, WI, USA, and housed on an indoor photoperiod of 18 h:6 h light (L):dark (D) for 6 weeks, followed by 8 h:16 h L:D for at least 6 weeks before the study. This pattern of light exposure induces photosensitivity; a state in which increasing photoperiod stimulates the onset of breeding-typical physiology (Dawson et al., 2001). Once photosensitive, females were given colored leg bands for identification, moved to outdoor aviaries measuring 2.13 m×2.4 m×1.98 m and then randomly assigned to one of three conditions: (1) control aviaries that contained artificial perches; (2) aviaries that contained artificial perches, a tub for bathing and three nest boxes (human-made nest cavities); and (3) semi-natural aviaries that contained natural tree branches, five hanging flowering plants, three shrubs, a bubbling water fountain and three nest boxes. All aviaries were provided with standard laboratory bird feed and drinking water ad libitum. Semi-natural aviaries (condition 3) received supplementary food in the form of insect larvae (mealworms), berries (a mixture of raspberries, blackberries and blueberries) and spinach. After 1 week, 29.2 m of artificial ivy was added to semi-natural aviaries to mimic the gradual appearance of spring vegetation. All outdoor aviaries were exposed to limited visual and acoustic stimuli from neighboring aviaries and the surrounding outdoor environment, which may include wild starlings. The difference between aviaries, therefore, was direct access to nest cavities and/or semi-natural conditions.
Each aviary housed three females. Eighteen females were tested during the first 3 weeks of July 2015 and 18 females were tested during the first 3 weeks of August 2015, when natural photoperiods were 14.5 h:9.5 h L:D and 13.25 h:10.75 h L:D, respectively. Experimental conditions were equally represented in both the July and August cohorts. Birds remained in their respective aviaries for 3 weeks. Age of wild-caught female starlings in this study is unknown, although all females were at least 1 year of age at the start of the study. All procedures adhered to methods approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Behavioral observations
An unfamiliar photosensitive male was released into the aviaries once per week during the first 2 weeks of the study to mimic potential mate availability. During the third week, a single observer stationed behind a screen recorded behavioral observations for 30 min, once after introducing a photosensitive male and once after introducing a photosensitive female into the aviaries with the order counterbalanced across observation days. We introduced a female during this final week because previous data show that females may respond to unfamiliar females by singing in defense of nest cavities and we reasoned that nest cavity defense may differ across our conditions (Ellis and Riters, 2013). However, females did not sing during the observation period and thus behavioral data in response to the introduction of a female will not be discussed further. Half of the aviaries were observed on Monday and Wednesday and the other half were observed on Tuesday and Thursday. Fresh nest material was placed on the floor of all aviaries prior to observations. Experimental conditions were equally represented across observation days. The same male or female was presented to all aviaries on a given day. Observations took place between 09:00 h and 10:30 h. The observer recorded the number of times females perched on top of a box, perched in front of a nest box cavity, looked into a box and entered a box, as well as each time a bird approached a stimulus bird (within ∼4 cm), bouts of feeding and bouts of beak wiping. Bouts of behavior were counted separately when they occurred with a gap of at least 2 s. Behavior for each individual bird was recorded by a single observer by hand in separate columns based on the unique and easily identifiable combination of colored leg bands on each individual.
Physiological measurements and tissue collection
Immediately prior to placing birds into aviaries, we took a blood sample from the wing vein to assay estradiol concentrations and measured the width (in mm) of the cloacal protuberance (CP). CP width in male birds is an indicator of circulating sex steroid hormones and is typically not measured in females. However, in a previous study, female starlings with high levels of estradiol compared with females with undetectable levels of estradiol had large, swollen cloacal glands and thus we measured CP width in the present study (Riters et al., 2007). Within 2 days of the final observation period, females were killed by rapid decapitation and a final blood sample was taken from the trunk. Brains were immediately removed from the skull and frozen for ∼30 s in isopentane (Catalog No. 277258; Sigma-Aldrich, St Louis, MO, USA) on dry ice. The frozen brains were transferred to −80°C until sectioning. The diameter (in mm) of the largest ovarian follicle and the final width of the CP were measured using calipers.
Estradiol assay
Blood samples were centrifuged at 1476 g for 20 min at 4°C on the day they were collected. Plasma was aspirated and stored at −20°C. Plasma estradiol concentrations were measured using a commercial grade competitive immunoassay (Cayman Chemical, Ann Arbor, MI, USA). This kit has been previously used to validate that estradiol increases when female starlings are subcutaneously implanted with estradiol (Pawlisch et al., 2012). Diluted plasma samples beginning at a 1:6.25 dilution in assay buffer were run alongside a standard curve, and demonstrated parallelism in this range (test of equal slopes; P=0.27). Samples were run in duplicate at a dilution of 1:10 in buffer solution as per the manufacturer's protocol and visualized at 405 nm with a BioTek 800 plate reader (ELv800™, BioTek Instruments, Inc., Winooski, VT, USA). Sensitivities of the commercial enzyme immunoassay according to the manufacturer's specifications indicate the limits of detection [80% bound/maximum binding (B/B0): 19 pg ml−1] and sensitivity (50% B/B0: 129 pg ml−1). The manufacturer reports cross-reactivity to estradiol-3-glucuronide 14%, 5α-dihydrotestosterone 0.06% and testosterone <0.01%. All samples were run on three plates and inter-plate variability was 11.78%. Intra-plate coefficients of variability were 8.24%, 11.41% and 18.37%. We distributed experimental conditions equally across plates, and distributed females that did or did not explore nest boxes equally across plates.
Tissue preparation, quantitative real-time PCR (qPCR) and Pfaffl data transformation
Brains were sectioned coronally at 200 µm using a cryostat at −15°C. Sections were laid onto slides and moved to dry ice, where a Fine Science Tools Sample Corer (Item No. 18035-02; Foster City, CA, USA) was used to dissect the mPOA and the VMH on consecutive sections (Fig. 1). For each bird, punches on each section were 2 mm in diameter and punches for all sections for a given region were stored together in capped 1.8 ml centrifuge tubes at −80°C. Four samples were lost during this process. Of the remaining 32 samples, 26 were randomly selected for qPCR in order to analyse samples on a single standard 96-well plate. RNA from tissue punches was extracted using a Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Catalog No. 73206830; Bio-Rad, Hercules, CA, USA), following instructions by the manufacturer. After isolation, the resulting RNA concentration and integrity were measured with a Nanodrop system (Catalog No. ND-2000; Thermo-Scientific, Wilmington, DE, USA). Samples of extracted RNA were then converted into single-stranded cDNA with an Invitrogen SuperScript III First-Strand Synthesis System (catalog #18080-051; Life Technologies, Carlsbad, CA, USA).
Fig. 1.
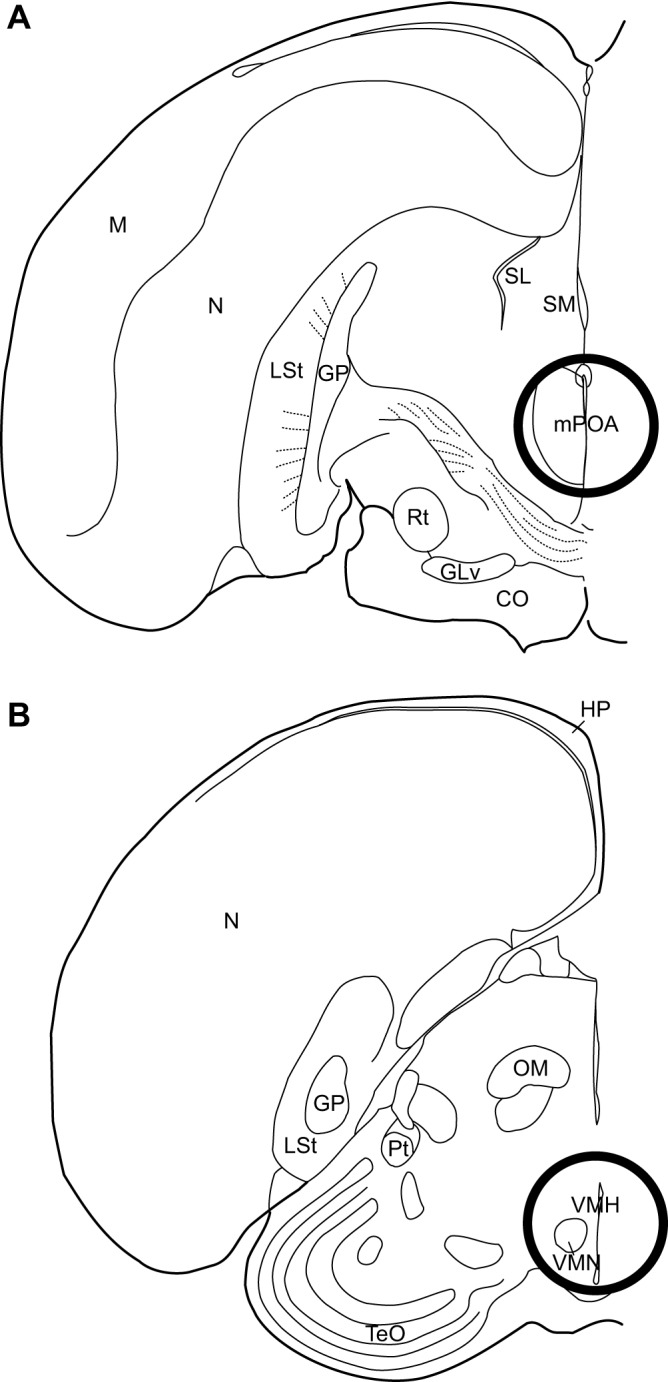
Location of tissue punches used for mRNA analysis. Location of intermediate (A) mPOA tissue punch and (B) VMH tissue punch used for mRNA analysis. The approximate punch size for mPOA was 2 mm diameter. One 2 mm punch directly rostral and one 2 mm punch directly caudal of this coronal section were also obtained. With respect to the VMH, punch size was also 2 mm in diameter. Punches were taken throughout the VMH, beginning at the appearance of anterior commissure and continuing for 800 µm, just rostral to the ventral tegmental area. Diagram represents the middle of this range. CO, optic chiasm; GLv, ventral lateral geniculate nucleus, pars ventralis; GP, globus pallidus; HP, hippocampus; LSt, lateral striatum; M, mesopallium; mPOA, medial preoptic area; N, nidopallium; OM, occipitomesencephalic tract; Pt, pretectal nucleus; Rt, nucleus rotundus; SL, lateral septum; SM, medial septum; TeO, optic tectum; VMH, ventromedial hypothalamus; VMN, ventromedial nucleus of the hypothalamus.
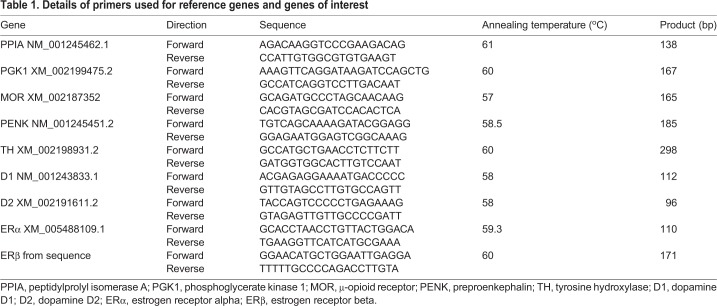
Following cDNA conversion, qPCR was used to measure relative mRNA expression (i.e. relative to two reference genes stated below) for estrogen receptors alpha (ERα) and beta (ERβ), dopamine-related genes (dopamine receptor D1 and D2), a marker for catecholamine synthesis, i.e. TH, and opioid-related genes [μ-opioid receptor (MOR) and preproenkephalin (the mRNA precursor of enkephalin; PENK)] in the mPOA and the VMH. Two reference genes, peptidylprolyl isomerase A (PPIA) and phosphoglycerate kinase 1 (PGK1), were run to normalize the variation in endogenous mRNA expression between animals (Table 1 and Table 2).
Table 1.
Details of primers used for reference genes and genes of interest
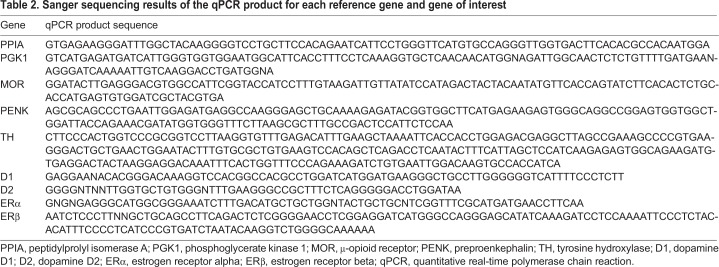
Table 2.
Sanger sequencing results of the qPCR product for each reference gene and gene of interest
Primers for each gene were designed using the NCBI Gene Database Primer-Blast. Netprimer (Premier Biosoft, Palo Alto, CA, USA) was then used to examine any secondary structures of primers. Prior to running primers with sample tissue, primers were run with standard tissue, and products of successful runs were sent for Sanger sequencing with both forward and reverse primers at the University of Wisconsin – Madison Biotechnology Center. NCBI BLAST shows that all sequences match their intended targets (Table 2).
Samples were prepared for qPCR as previously described (Spool et al., 2016). Five standards were prepared in a 1:10 dilution series using nuclease-free water, as well as one negative template control (nuclease-free water only). Samples, standards and controls were plated and run in triplicate.
Plates were run in a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Catalog No. 185-5195; Bio-Rad). Runs consisted of a 30 s initiation step at 95°C, 40 cycles at 95°C for 5 s, a 30 s annealing step set at a melting temperature specific to the primer set (retrieved from NCBI Gene Database Primer-Blast) and a 20 s elongation step at 72°C, followed by a 60–88°C melt curve (5 s for each 0.5°C). Runs were only used that met the listed MIQE guidelines (Bustin et al., 2009). qPCR raw data were transformed according to the Pfaffl Method to obtain expression level values relative to the two reference genes as in previous studies (Cordes et al., 2014; Pfaffl, 2001).
Statistics
Data were analysed using R v. 3.4.1 with RStudio v. 1.0.143 (http://www.R-project.org/; http://www.rstudio.org/) and Statistica (version 6.0, StatSoft, Tulsa, OK, USA). For analyses restricted to behavior and measurements of physiology, data from all 36 females were used. The subset of 26 birds used for qPCR (described above) was used for any analyses involving mRNA expression in the mPOA and VMH. Linear mixed models using restricted maximum likelihood were used to analyse differences across experimental conditions to account for multiple measurements on the same animals and to account for non-independence of females housed in the same aviary. Thus, individual aviary was included as a random effect in all models, and female identification (ID) was included as a second random effect in any model that included more than one measurement per female. Following significant main effects and/or interactions, least-squared means pairwise comparisons were performed and corrected using the sequential Bonferroni method to reduce Type 1 error. With respect to measurements of physiology, we calculated the change in estradiol and change in CP width over the course of the study by subtracting the initial from the final measurement. Using mixed models with aviary entered as a random effect, initial measurements of plasma estradiol and CP width did not differ significantly between groups (F2,13.4=0.20, P=0.82 and F2,20.7=1.53, P=0.24, respectively).
When change in estradiol, change in CP width and largest follicle diameter were considered in the same model, the model did not meet assumptions of normality or homogeneity of variances and transformations did not correct violations. Thus, these measurements were considered in three separate models, in which aviary condition was entered as a fixed effect and aviary in which birds were housed was entered as a random effect. Three models for each brain region (mPOA and VMH) were also constructed for mRNA expression of estrogen receptors (ERα, ERβ), dopamine-related genes (D1, D2) and TH (hereafter run in dopamine-related gene models although TH may also represent noradrenaline synthesis), and opioid-related genes (MOR, PENK). For each of these three models, experimental condition, gene and their interaction were entered as fixed effects, and aviary in which birds were housed and female ID were entered as random effects. We repeated the above analyses, but rather than aviary condition, we compared females in aviaries with no nest boxes available (control females) with females with access to boxes that did or did not enter the box during the third week of observation (boxYES and boxNO females, respectively).
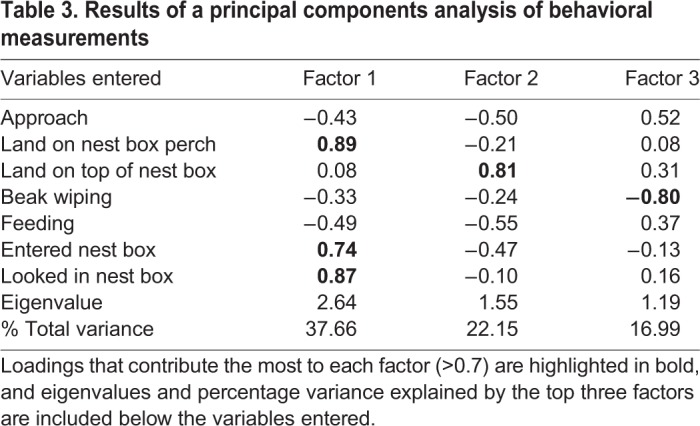
Due to the large number of behavioral variables measured in this study, we conducted a principal components analysis to identify factors that explain observed behavioral variance. We restricted this analysis to the observation period in which females were exposed to a stimulus male due to our focus on sexually motivated responses. We entered all behavioral variables but we included only birds that were given access to a nest cavity because it would not be appropriate to include birds that had no opportunity to display nest box-related behaviors. The analysis was run twice including all available birds (to examine relationships between behavior and physiology) and the subset of birds for which qPCR was conducted (to examine relationships between behavior and gene expression). Both analyses returned near-identical results; to avoid redundancy, we report principal components analysis results from the subset of birds used to perform qPCR in Table 3.
Table 3.
Results of a principal components analysis of behavioral measurements
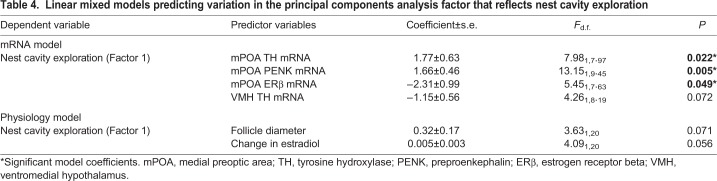
We used two linear mixed models with Factor 1 from the principal components analysis (which reflected nest box exploration, as detailed in the Results section) as a dependent variable, mRNA expression and measurements of physiology as predictor variables, and aviary entered as a random effect. For mRNA expression, we performed an exhaustive comparison of all possible models from 14 predictor variables (seven genes in each brain region), ranked models by Akaike information criterion for small sample sizes (AICc) and examined Akaike weights of models within two points of the top model (Anderson, 2008; Burnham and Anderson, 2002). For measurements of physiology, we performed an exhaustive comparison of all possible models from three predictor variables and selected a model using the same criteria described above. Normality assumptions for all models were tested using Shapiro–Wilk tests and visual inspection of normal QQ plots of residuals, and homogeneity of variance assumptions were tested using Levene's test and visual inspection of residuals versus fitted values plots. Models that did not meet assumptions were log10 transformed. Any violations of assumptions were corrected with log10 transformations.
For all genes measured in the mPOA, one sample did not amplify during qPCR, leaving a starting sample size of N=25. Additionally, one sample did not amplify during qPCR for ERα expression in the VMH. Outliers were identified as highly influential points greater than two standard deviations away from the mean of their respective experimental groups, using Cook's distance, and confirmed visually with side-by-side boxplots or scatterplots. This resulted in the removal of two birds from analysis of physiology markers (one no box available: log10 change in plasma estradiol=2.99, log10 largest follicle diameter=0.78; and one boxNO: log10 change in plasma estradiol=0), two birds from analysis of dopamine markers (one boxNO: mPOA D1 mRNA=1.96, mPOA D2 mRNA=2.05; and one boxYES: mPOA D1 mRNA=1.54), one bird from analysis of estrogen receptors (one boxNO: VMH ERα=3.85) and one bird from analysis of behavior and mRNA expression (nest cavity exploration factor=2.64, mPOA ERβ=0.52).
R packages used in analysis include reshape2 (Wickham, 2007), lme4 (Bates et al., 2015), lmerTest (https://CRAN.R-project.org/package=lmerTest), lsmeans (Lenth, 2016), leaps (https://CRAN.R-project.org/package=leaps), glmulti (https://CRAN.R-project.org/package=glmulti), car (Fox and Weisberg, 2011), ggplot2 (Wickham, 2009), plyr (Wickham, 2011), Rmisc (https://CRAN.R-project.org/package=Rmisc) and cowplot (https://CRAN.R-project.org/package=cowplot).
RESULTS
Experimental condition did not affect physiology, gene expression or behavior
The aviary condition of females did not statistically affect measurements of physiology, behavior or any gene measured in the mPOA or VMH. Additionally, a χ2 test showed that females were not more likely to explore available nest boxes (i.e. not more likely to be boxYES females) in semi-natural aviaries with nest boxes than if they had been placed in aviaries with nest boxes alone (χ21=1.6, P=0.20).
Exploration of nest boxes relates to measurements of physiology
In contrast to aviary condition, a linear mixed model with log10 change in estradiol as the dependent variable and nest box status (i.e. no box access, boxNO and boxYES females) as a grouping variable returned a significant main effect of nest box status (F2,31=4.62, P=0.018; Fig. 2). Sequential Bonferroni-corrected post hoc comparisons showed that boxYES females had a larger change in estradiol compared with birds with no box access (t31=−3.00, P=0.016) and a trend for a larger change in estradiol compared with boxNO females (t31=−2.15, P=0.078; Fig. 2). With respect to log10 follicle diameter, a linear mixed model revealed a trend for the diameter to be different among the three groups (F2,17.7=3.42, P=0.055; Fig. 2). A model with change in CP width as the dependent variable did not return a significant effect of nest box status (F2,17.8=2.19, P=0.14; Fig. 2).
Fig. 2.
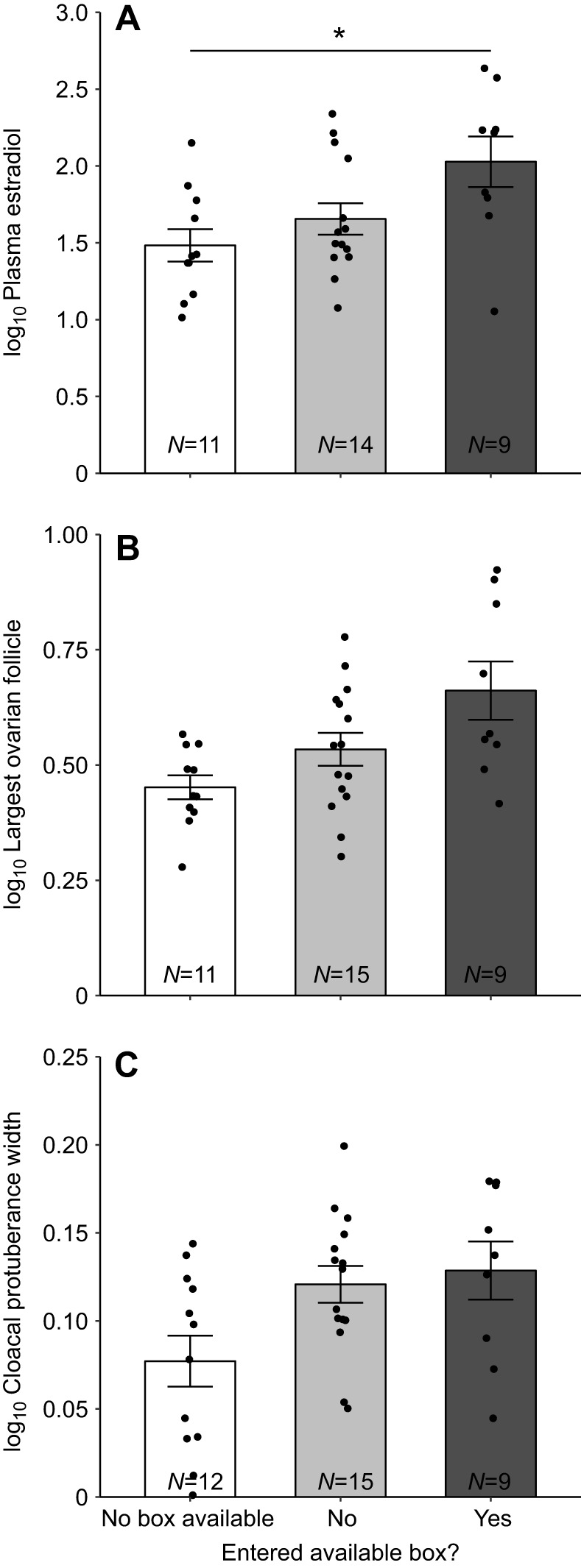
Physiology measurements in females that had no nest box available, and in females that had access to and did or did not enter a nest box during behavioral observations in the third week in response to a novel male conspecific. Mean change in (A) plasma estradiol (pg ml−1), (B) largest ovarian follicle diameter (mm) and (C) change in cloacal protuberance width (mm). All measurements are shown as log10 transformed as they were in analyses. Change in plasma estradiol and cloacal protuberance were calculated by subtracting the measurement taken at the end of the study from the measurement taken at the beginning of the study. Error bars represent ±1 s.e.m. Sample sizes for each group are indicated at the bottom of each bar. Individual data points are plotted over bars. *P<0.05 by sequential Bonferroni-corrected post hoc tests.
Exploration of nest boxes relates to relative mRNA expression in the mPOA
A linear mixed model with opioid mRNA expression in the mPOA entered as the dependent variable, and nest box status and opioid gene entered as predictor variables returned a significant main effect of nest box status (F2,22=6.09, P=0.0079) and a nest box status by opioid gene interaction (F2,22=4.07, P=0.031). Sequential Bonferroni-corrected pairwise tests showed that boxYES females had higher relative PENK mRNA expression compared with boxNO females (t43.9=−3.74, P=0.0025) and with females with no box access (t43.9=−4.16, P=0.0006; Fig. 3). There was no significant difference between boxNO females and females with no box access (P>0.50) for PENK or between any groups for MOR mRNA expression (all P>0.10; Fig. 3).
Fig. 3.
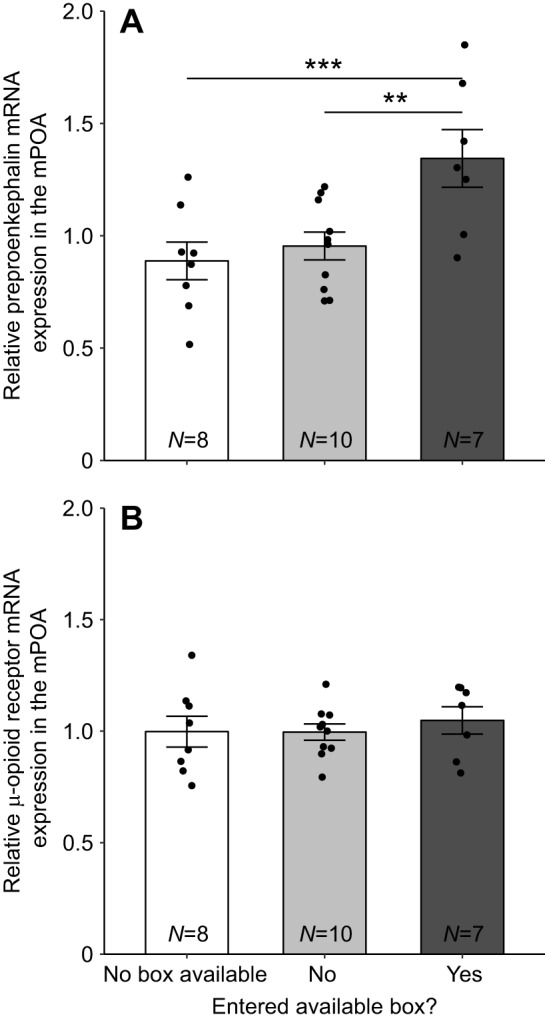
Mean relative mRNA expression in the medial preoptic area (mPOA) in females that did not have access to a nest box, females that had access to and did or did not enter a nest box during behavioral observations in the third week in response to a novel male conspecific. Mean relative mRNA expression measurements of (A) preproenkephalin and (B) μ-opioid receptor in the mPOA. Error bars represent ±1 s.e.m. Sample sizes for each group are indicated at the bottom of each bar. Individual data points are plotted over bars. **P<0.01 and ***P<0.001 by sequential Bonferroni-corrected post hoc tests.
A similar linear mixed model with dopamine-related gene expression in the mPOA entered as the dependent variable returned a significant nest box status by dopamine gene interaction (F4,42.4=6.71, P=0.0003). Sequential Bonferroni-corrected pairwise tests showed that D1 and D2 mRNA expression was lower in boxYES females compared with females with no box access (D1, t13.4=3.5, P=0.028; D2, t11.6=3.68, P=0.030; Fig. 4). No other pairwise differences were significant for D2, D1 or TH mRNA expression (all P>0.10; Fig. 4).
Fig. 4.
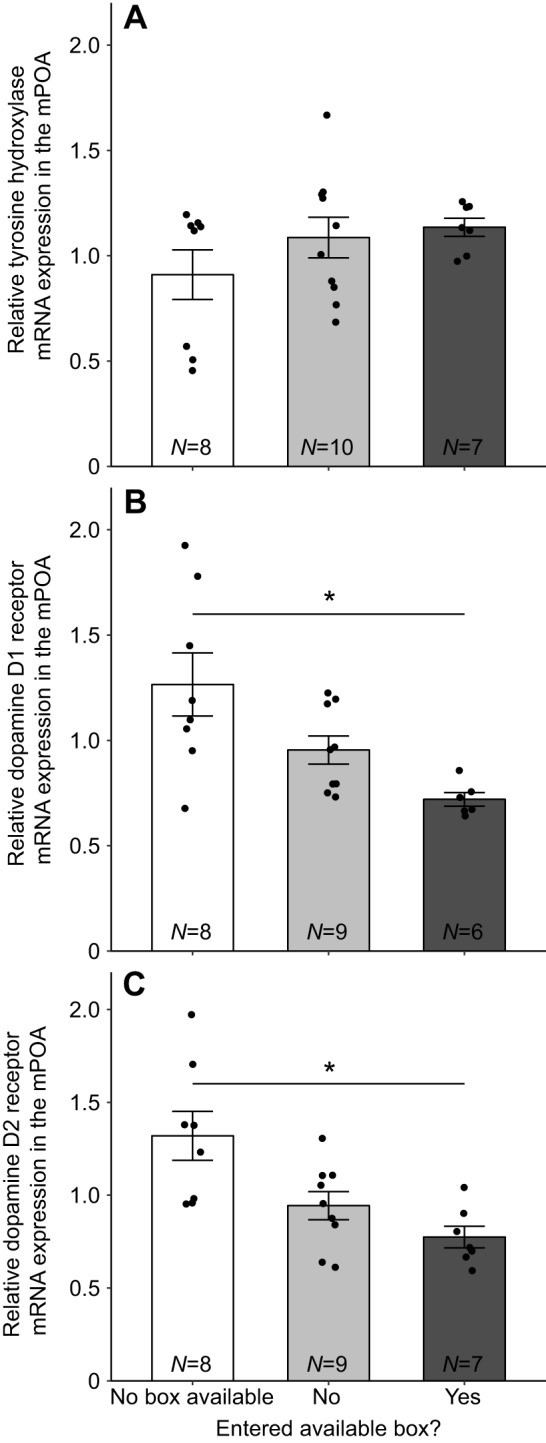
Mean relative mRNA expression in the medial preoptic area (mPOA) in females that did not have access to a nest box, females that had access to and did or did not enter a nest box during behavioral observations in the third week in response to a novel male conspecific. Mean relative mRNA expression measurements of (A) tyrosine hydroxylase, (B) dopamine D1 and (C) dopamine D2 in the mPOA. Error bars represent ±1 s.e.m. Sample sizes for each group are indicated at the bottom of each bar. Individual data points are plotted over bars. *P<0.05 by sequential Bonferroni-corrected post hoc tests.
A linear mixed model with estrogen receptor-related expression in the mPOA entered as the dependent variable returned no significant main effects or interactions (Fig. 5).
Fig. 5.
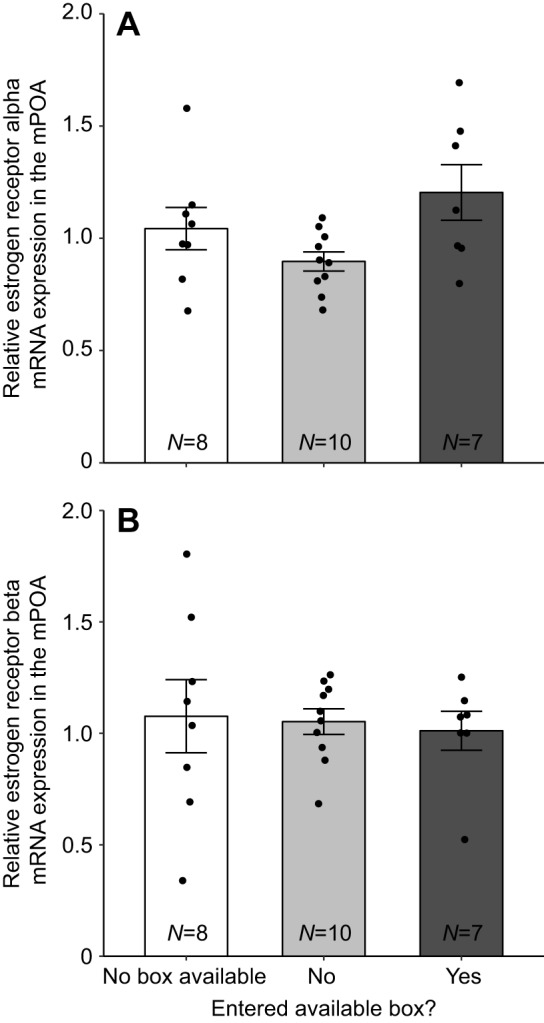
Mean relative mRNA expression in the medial preoptic area (mPOA) in females that did not have access to a nest box, females that had access to and did or did not enter a nest box during behavioral observations in the third week in response to a novel male conspecific. Mean relative mRNA expression measurements of (A) estrogen receptor alpha and (B) estrogen receptor beta in the mPOA. Error bars represent ±1 s.e.m. Sample sizes for each group are indicated at the bottom of each bar. Individual data points are plotted over bars.
Exploration of nest boxes relates to relative mRNA expression in the VMH
Mixed models for opioid and dopamine mRNA expression in the VMH returned no significant effects or interactions for nest box status (all P>0.1). However, a model with estrogen receptor mRNA in the VMH returned a significant nest box status by estrogen receptor gene interaction (F2,20.6=4.85, P=0.019; Fig. 6). Sequential Bonferroni-corrected pairwise tests showed that ERα mRNA expression was higher in boxNO females compared with females without box access (t34.9=−3.02, P=0.028) but no other group differences were significant (all P>0.25; Fig. 6).
Fig. 6.
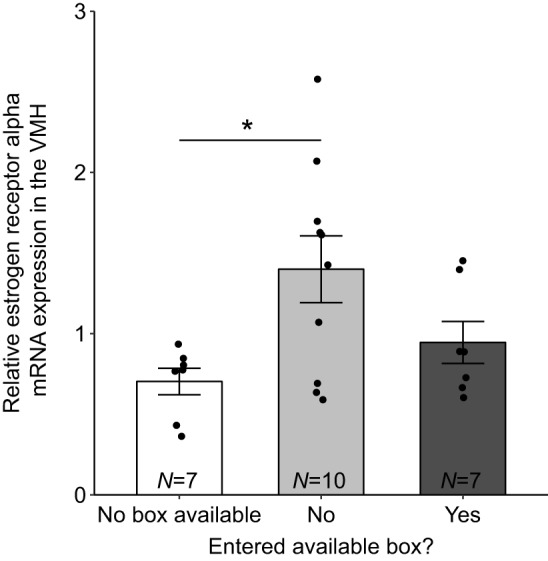
Mean relative mRNA expression measurements of estrogen receptor alpha in the ventromedial hypothalamus (VMH) in females that did not have access to a nest box, females that had access to and did or did not enter a nest box during behavioral observations in the third week in response to a novel male conspecific. Error bars represent ±1 s.e.m. Sample sizes for each group are indicated at the bottom of each bar. Individual data points are plotted over bars. *P<0.05 by sequential Bonferroni-corrected post hoc tests.
Principal components analysis
Results of the principal components analysis on the subset of birds used for qPCR are shown in Table 3, highlighting factors with Eigenvalues above 1.00. To determine whether variables significantly loaded onto a factor (i.e. had high Eigenvector coefficients), we used a cut-off of |0.7| or higher, which is a common cut-off for sample sizes <100 (Budaev, 2010). For factor 1, use of the box perch, entering a nest box and looking in a nest box loaded significantly (Table 3). Perching on the nest box top (which all females use as a general perch in aviaries) loaded independently onto a second factor. A third factor loaded only beak wiping behavior. We focus analyses on the first factor, the ‘nest cavity exploration’ factor, because our aim was to gain insight into relationships between female nest cavity exploration, physiology and the brain. Positive loadings for all nest box-directed behaviors mean that individuals with high ‘nest cavity exploration’ scores performed more of these behaviors.
Degree of nest box exploration is statistically explained by mRNA expression in the mPOA
For the analysis of mRNA relationships to the nest cavity exploration factor, the top model (Akaike weight=0.11 versus the second-best model=0.069) showed TH, ERβ and PENK mRNA expression in the mPOA to significantly predict variance in the nest cavity exploration factor. TH and PENK related positively whereas ERβ related negatively to the nest cavity exploration factor (Table 4). There was also a non-significant trend for TH mRNA expression in the VMH to negatively predict variance in the nest cavity exploration factor (Table 4). With respect to relationships between the nest cavity exploration factor and physiological measurements, the top model (Akaike weight of this model: 0.32 versus the second-best model containing only change in estradiol: 0.21) revealed non-significant trends for change in estradiol (P=0.056) and follicle diameter (P=0.071) to positively predict the nest cavity exploration factor (Table 4).
Table 4.
Linear mixed models predicting variation in the principal components analysis factor that reflects nest cavity exploration
To gain further insight into possible functional relationships between opioid- and dopamine-related mRNA expression in the mPOA and estradiol, we performed correlation analyses between ERα and ERβ, and PENK, TH, D1 and D2 relative mRNA expression in the mPOA. Following sequential Bonferroni corrections for eight tests (four genes correlated to each of two estrogen receptors), there was a positive correlation between PENK and ERα (r2=0.65, P<0.0001) and between TH and ERβ (r2=0.29, P=0.040).
DISCUSSION
Our data demonstrate close relationships between female starling exploration of a crucial breeding resource, circulating estradiol concentrations and the expression of opioid- and dopamine-related mRNA in the mPOA. It is likely that increases in circulating estradiol that occur at the onset of the breeding season in female starlings (Dawson, 1983) modify opioid- and dopamine-related gene expression in the mPOA to facilitate nest cavity exploration. However, it is also possible that the exploration of a nest cavity further alters gene expression in the mPOA associated with sexual behavior and sexual motivation, thus functioning to coordinate mating with resource availability.
Circulating estradiol related to nest cavity exploration but not aviary condition
In this study, females that explored nest cavities had a higher increase in circulating estradiol over the course of the study (with a similar trend for increased size of ovarian follicles over the course of the study) compared with females that did not have access to a nest cavity. The presence of extra food, flowing water or green plants had no statistical effect on these physiological measurements, which was somewhat surprising given past studies showing that such supplementary cues stimulate female reproductive physiology (Ettinger and King, 1981; Watts and Hahn, 2012; Wingfield et al., 2003, 2012). Still, it is worth noting that the only females in the present study with yolky, well-developed follicles were in the semi-natural aviary condition, which in the past has rarely been observed in our captive population.
It is possible that differences between females in different aviary conditions were masked because birds had a limited view of neighboring aviaries and were exposed to stimuli from the outdoor environment. For example, it is possible that visual and acoustic stimuli from conditions outside the immediate aviary caused females to explore nest cavities when they were available, which then led to changes in estradiol and mRNA expression (see below for discussion of mRNA). Complete acoustic and visual isolation between semi-natural aviaries and others is now needed to test this possibility. Alternatively, it may be that exploring an available nest cavity is a crucial prerequisite for physiological development regardless of what other resources are present. This is true for male starlings, which will not sing to court females unless they take ownership of a nest cavity (Spool et al., 2016). The mechanisms by which females time breeding using environmental cues are still poorly understood and should be examined in future studies (Davies and Deviche, 2014).
Exploring a nest cavity related to mRNA expression in the mPOA
In addition to differences in estradiol concentrations, females that explored nest cavities had the highest relative PENK mRNA expression in the mPOA. There was also a positive relationship between PENK expression and repeated nest cavity exploration (i.e. the nest cavity exploration principal components factor). These findings are consistent with a previous study in starlings demonstrating greater immunolabeling measurements for the protein product of PENK, met-enkephalin, in the mPOA of female starlings that explored nest boxes compared with those that did not (Riters et al., 2013). We also found that PENK mRNA correlated positively with ERα mRNA expression in the mPOA. Previous work in rats shows that estradiol treatment upregulates PENK gene expression and that ERα binds to response elements in the PENK promotor (Watson et al., 1986; Zhu et al., 2001). Thus, the correlation reported here could reflect a functional relationship between ERα and PENK expression. However, whereas PENK was highest in females that explored nest cavities, ERα mRNA expression in the mPOA was not different among our groups (Fig. 5) and did not have any relationship to nest cavity exploration. It is noteworthy that in a past study in female starlings, even when estradiol concentrations were experimentally elevated in all females, met-enkephalin labeling in the mPOA was highest in females that explored nest boxes, and only these females found sexual stimuli (i.e. male song) to be rewarding (Riters et al., 2013). This suggests that nest cavity exploration can relate to met-enkephalin in the mPOA independent of estradiol. Thus, our findings in the present study indicate that increased PENK mRNA expression in females that explore nest cavities may occur through an estradiol-independent mechanism.
With respect to dopamine-related mRNA, both D1 and D2 expression were similarly depressed in females that explored a nest cavity (and had relatively high estradiol concentrations) compared with females with no box available (and relatively low estradiol concentrations). It is possible that increases in estradiol caused the reduction of D1 and D2 receptor mRNA in females that explored nest boxes. However, neither D1 nor D2 correlated with ERα or ERβ mRNA expression in the mPOA, and past studies in rats show that estradiol alters ratios of D1 and D2 receptors in the mPOA by elevating D2 receptor expression, which is inconsistent with our present findings (Graham et al., 2015). In contrast, TH mRNA related positively to the nest cavity exploration principal components factor but did not differ with respect to nest cavity exploration groups. This suggests that observed decreases in D1 and D2 receptors mRNA in the mPOA are not due to altered catecholamine synthesis. Our finding may instead reflect negative feedback due to increased dopamine signaling or a third unknown mechanism by which nest cavity exploration decreases sensitivity to dopamine signaling in the mPOA (i.e. independent of changes in dopamine signaling). Finally, we identified a positive relationship between TH and ERβ expression in the mPOA, suggesting potential functional relationships between estradiol, TH and nest cavity exploration. Somewhat difficult to interpret is the finding that although TH related positively to both nest cavity exploration and ERβ, ERβ related negatively to the nest cavity exploration factor. Future studies are needed to gain insight into the relationships between these measurements.
Nest cavity exploration may be a form of reproductive self-stimulation
It is likely that increases in circulating estradiol that occur at the onset of the breeding season in female starlings (Dawson, 1983) modify opioid- and dopamine-related gene expression in the mPOA, which facilitates nest cavity exploration. However, if a priori differences in physiology and gene expression were the sole cause of whether females explored or did not explore nest cavities, then differences should be apparent before females have the opportunity to explore nest cavities. That is, females without access to nest boxes should consist of some females with low estradiol, low PENK and high D1 and D2 mRNA (i.e. those that would not explore boxes if given the opportunity) and some females with high estradiol, high PENK and low D1 and D2 mRNA (i.e. those that would explore boxes if given the opportunity) such that on average they would fall between boxNO and boxYES females. This was not the case. Instead, females without nest cavity access more closely resembled boxNO females, lending support to the hypothesis that the exploration of nest cavities further stimulates changes in estradiol and gene expression to stimulate mating behavior such as the extent to which females respond positively to male courtship song (Spool and Riters, 2017).
In this way, repeated nest cavity exploration may be considered another form of reproductive self-stimulation, similar to nest building in female canaries and nest-coos in female ring doves (described in the Introduction section). It is noteworthy that in female ring doves nest-coo self-stimulation alters neural activity in the preoptic area (Cheng et al., 1998) and met-enkephalin axonal projections (Cheng and Zuo, 1994), suggesting potential common pathways (i.e. various met-enkephalin projections converging on a common nucleus) by which different forms of self-stimulation prepare females for reproduction.
ERα mRNA in the VMH was highest in females that had access to but did not explore a nest cavity
In the VMH, boxNO females had higher ERα mRNA expression compared with females with no box access, despite similar concentrations of circulating estradiol. One possibility is that the presence of nest boxes increases sensitivity of the VMH to estradiol, which may allow females not currently prepared to breed (i.e. females without possession of a nest cavity) to make rapid transitions to breeding readiness (i.e. able to engage in sexual behaviors as soon as a nest cavity is acquired). The same pattern is seen in male cichlid fish during social transitions, in which ERα mRNA is highest in the anterior tuberal nucleus (the homologous region in teleost fish to the VMH in other vertebrates; O'Connell and Hofmann, 2011) only in males that have not yet taken the opportunity to obtain an available territory (Maruska et al., 2013).
Consistent with past studies in female starlings (Pawlisch et al., 2012), we observed no differences in dopamine measurements in VMH in females that did or did not explore nest cavities. However, given past studies in starlings showing met-enkephalin measurements in VMH (specifically in the VMN) to be highest in females that explored nest boxes (Riters et al., 2013), we were surprised to find no relationships between nest cavity exploration and opioid-related mRNA measurements in VMH. This may reflect the fact that our micropunches extended beyond the borders of VMN. Thus, future studies using smaller punches specifically targeting this portion of VMH are needed.
Study limitations
Measurements of mRNA may not reflect levels of translated or active protein. However, similar to the mRNA measurements reported here, in past studies, the measurements of immunolabeling for TH and met-enkephalin protein in the female starling mPOA correlated with nest box exploration (Pawlisch et al., 2012; Riters et al., 2013). Thus, it is likely that mRNA levels of these genes are reflective of translated protein in this brain region.
Concluding remarks
Data from this study support the hypothesis that access to a crucial breeding resource (in this case a nest cavity) alters breeding physiology and opioid and dopamine systems in the mPOA to fine-tune sexual behaviors and sexual motivation. Furthermore, the extent to which females explore nest cavities may self-stimulate changes in opioid and dopamine systems in the mPOA. Relationships between nest cavity exploration, opioid- and dopamine-related mRNA expression in the mPOA, and estrogen receptors in the mPOA suggest that these changes may occur in part through estrogen-dependent mechanisms. The presence of nest cavities may also alter estrogen sensitivity in the VMH to prepare females to make rapid transitions from non-breeding typical behavior to sexual behavior. Testing hypotheses generated from this study will be necessary to understand the mechanisms by which the presence and physical exploration of critical breeding resources fine-tunes the expression of breeding behaviors.
Acknowledgements
The authors are grateful to Sharon Stevenson, Caroline Angyal and Alice Piepenburg for assisting with data collection, Chris Elliott and Kate Skogen for animal care in our outdoor aviaries, and Devin Merullo for feedback on drafts of the manuscript. We also thank Dr Kathleen Grogan for providing primers for ERα. Finally, we thank the Science Research Internship program through the Madison Metropolitan School District for facilitating this scientific collaboration between J.A.S., M.D.J. and L.V.R.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.A.S., M.D.J., L.V.R.; Methodology: J.A.S., M.D.J.; Software: J.A.S.; Validation: J.A.S.; Formal analysis: J.A.S.; Investigation: J.A.S., M.D.J.; Resources: L.V.R.; Data curation: J.A.S., M.D.J., L.V.R.; Writing - original draft: J.A.S., M.D.J., L.V.R.; Writing - review & editing: J.A.S., L.V.R.; Visualization: J.A.S.; Supervision: J.A.S., L.V.R.; Project administration: L.V.R.; Funding acquisition: L.V.R.
Funding
This work was supported by National Institutes of Health grant R01 MH080225 to L.V.R. Deposited in PMC for release after 12 months.
References
- Acosta-Martinez M. and Etgen A. M. (2002). Activation of mu-opioid receptors inhibits lordosis behavior in estrogen and progesterone-primed female rats. Horm. Behav. 41, 88-100. 10.1006/hbeh.2001.1741 [DOI] [PubMed] [Google Scholar]
- Alward B. A., Balthazart J. and Ball G. F. (2013). Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc. Natl. Acad. Sci. USA 110, 19573-19578. 10.1073/pnas.1311371110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. R. (2008). Model Based Inference in the Life Sciences: A Primer on Evidence. New York: Springer-Verlag. [Google Scholar]
- Bálint F., Liposits Z. and Farkas I. (2016). Estrogen receptor beta and 2-arachidonoylglycerol mediate the suppressive effects of estradiol on frequency of postsynaptic currents in gonadotropin-releasing hormone neurons of metestrous mice: an acute slice electrophysiological study. Front. Cell. Neurosci. 10, 1-14. 10.3389/fncel.2016.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G. F. (1993). The neural integration of environmental information by seasonally breeding birds. Am. Zool. 33, 185-199. 10.1093/icb/33.2.185 [DOI] [Google Scholar]
- Ball G. F. and Ketterson E. D. (2008). Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 231-246. 10.1098/rstb.2007.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B. and Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Budaev S. V. (2010). Using principal components and factor analysis in animal behaviour research: caveats and guidelines. Ethology 116, 472-480. 10.1111/j.1439-0310.2010.01758.x [DOI] [Google Scholar]
- Burnham K. P. and Anderson D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer. [Google Scholar]
- Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L. et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611-622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- Calisi R. M., Díaz-Muñoz S. L., Wingfield J. C. and Bentley G. E. (2011). Social and breeding status are associated with the expression of GnIH. Genes Brain Behav. 10, 557-564. 10.1111/j.1601-183X.2011.00693.x [DOI] [PubMed] [Google Scholar]
- Cheng M.-F. (1986). Female cooing promotes ovarian development in ring doves. Physiol. Behav. 37, 371-374. 10.1016/0031-9384(86)90248-9 [DOI] [PubMed] [Google Scholar]
- Cheng M.-F. and Peng J. P. (1997). Reciprocal talk between the auditory thalamus and the hypothalamus: an antidromic study. Neuroreport 8, 653-658. 10.1097/00001756-199702100-00015 [DOI] [PubMed] [Google Scholar]
- Cheng M.-F. and Zuo M. (1994). Proposed pathways for vocal self-stimulation: met-Enkephalinergic projections linking the midbrain vocal nucleus, auditory-responsive thalamic regions and neurosecretory hypothalamus. J. Neurobiol. 25, 361-379. 10.1002/neu.480250403 [DOI] [PubMed] [Google Scholar]
- Cheng M.-F., Peng J. P. and Johnson P. (1998). Hypothalamic neurons preferentially respond to female nest coo stimulation: demonstration of direct acoustic stimulation of luteinizing hormone release. J. Neurosci. 18, 5477-5489. 10.1523/JNEUROSCI.18-14-05477.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes M. A., Stevenson S. A. and Riters L. V. (2014). Status-appropriate singing behavior, testosterone and androgen receptor immunolabeling in male European starlings ( Sturnus vulgaris). Horm. Behav. 65, 329-339. 10.1016/j.yhbeh.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. and Deviche P. (2014). At the crossroads of physiology and ecology: food supply and the timing of avian reproduction. Horm. Behav. 66, 41-55. 10.1016/j.yhbeh.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Dawson A. (1983). Plasma gonadal steroid levels in wild starlings ( Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen. Comp. Endocrinol. 49, 286-294. 10.1016/0016-6480(83)90146-6 [DOI] [PubMed] [Google Scholar]
- Dawson A., King V. M., Bentley G. E. and Ball G. F. (2001). Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365-380. 10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Ellis J. M. S. and Riters L. V. (2013). Patterns of FOS protein induction in singing female starlings. Behav. Brain Res. 237, 148-156. 10.1016/j.bbr.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger A. O. and King J. R. (1981). Consumption of green wheat enhances photostimulated ovarian growth in white-crowned sparrows. Auk 98, 832-834. [Google Scholar]
- Feare C. (1984). The Starling. Oxford: Oxford University Press. [Google Scholar]
- Fox J. and Weisberg S. (2011). An {R} Companion to Applied Regression, 2nd edn Thousand Oaks, CA: Sage Publications. [Google Scholar]
- García-Horsman S. P., Ǻgmo A. and Paredes R. G. (2008). Infusions of naloxone into the medial preoptic area, ventromedial nucleus of the hypothalamus, and amygdala block conditioned place preference induced by paced mating behavior. Horm. Behav. 54, 709-716. 10.1016/j.yhbeh.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Graham M. D. and Pfaus J. G. (2012). Differential effects of dopamine antagonists infused to the medial preoptic area on the sexual behavior of female rats primed with estrogen and progesterone. Pharmacol. Biochem. Behav. 102, 532-539. 10.1016/j.pbb.2012.06.020 [DOI] [PubMed] [Google Scholar]
- Graham M. D., Gardner Gregory J., Hussain D., Brake W. G. and Pfaus J. G. (2015). Ovarian steroids alter dopamine receptor populations in the medial preoptic area of female rats: implications for sexual motivation, desire, and behaviour. Eur. J. Neurosci. 42, 3138-3148. 10.1111/ejn.13121 [DOI] [PubMed] [Google Scholar]
- Holland K., Norell A. and Micevych P. (1998). Interaction of thyroxine and estrogen on the expression of estrogen receptor alpha, cholecystokinin, and preproenkephalin messenger ribonucleic acid in the limbic-hypothalamic circuit. Endocrinology 139, 1221-1228. 10.1210/endo.139.3.5842 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E., Steinhauser A., Barabás K., Shughrue P. J., Petersen S. L., Merchenthaler I. and Liposits Z. (2001). Estrogen receptor beta immunoreactivity in luteinizing hormone-releasing hormone neurons in the rat brain. Endocrinology 142, 3261-3264. 10.1210/endo.142.7.8176 [DOI] [PubMed] [Google Scholar]
- Hull E. M., Warner R. K., Bazzett T. J., Eaton R. C., Thompson J. T. and Scaletta L. L. (1989). D2/D1 ration in the medial preoptic area affects copulation of male rats. J. Pharmacol. Exp. Therapeutics 251, 422-427. [PubMed] [Google Scholar]
- Hull E. M., Du J., Lorrain D. S. and Matuszewich L. (1995). Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J. Neurosci. 15, 7465-7471. 10.1523/JNEUROSCI.15-11-07465.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm C. A., Forbes-Lorman R. M., Auger C. J. and Riters L. V. (2011). Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings ( Sturnus vulgaris) defending nest sites and courting females. Behav. Brain Res. 219, 15-22. 10.1016/j.bbr.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel B. (1957). A Study of the breeding biology of the European Starling in North America. Am. Midl. Nat. 58, 257-331. 10.2307/2422615 [DOI] [Google Scholar]
- Lenth R. V. (2016). Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1-33. 10.18637/jss.v069.i01 [DOI] [Google Scholar]
- Maney D. L. and Wingfield J. C. (1998). Neuroendocrine suppression of female courtship in a wild passerine: corticotropin-releasing factor and endogenous opioids. J. Neuroendocrinol. 10, 593-599. 10.1046/j.1365-2826.1998.00238.x [DOI] [PubMed] [Google Scholar]
- Maruska K. P., Zhang A., Neboori A. and Fernald R. D. (2013). Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 25, 145-157. 10.1111/j.1365-2826.2012.02382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L., Lorrain D. S. and Hull E. M. (2000). Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behav. Neurosci. 114, 772-782. 10.1037/0735-7044.114.4.772 [DOI] [PubMed] [Google Scholar]
- Micevych P. E. and Meisel R. L. (2017). Integrating neural circuits controlling female sexual behavior. Front. Syst. Neurosci. 11, 1-12. 10.3389/fnsys.2017.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton I. (1994). Experiments on the limitation of bird breeding densities: a review. Ibis 136, 397-411. 10.1111/j.1474-919X.1994.tb01115.x [DOI] [Google Scholar]
- Nicot A., Ogawa S., Berman Y., Carr K. D. and Pfaff D. W. (1997). Effects of an intrahypothalamic injection of antisense oligonucleotides for preproenkephalin mRNA in female rats: Evidence for opioid involvement in lordosis reflex. Brain Res. 777, 60-68. 10.1016/S0006-8993(97)00967-0 [DOI] [PubMed] [Google Scholar]
- O'Connell L. A. and Hofmann H. A. (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599-3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- Pawlisch B. A. and Riters L. V. (2010). Selective behavioral responses to male song are affected by the dopamine agonist GBR-12909 in female European starlings ( Sturnus vulgaris). Brain Res. 1353, 113-124. 10.1016/j.brainres.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Pawlisch B. A., Kelm-Nelson C. A., Stevenson S. A. and Riters L. V. (2012). Behavioral indices of breeding readiness in female European starlings correlate with immunolabeling for catecholamine markers in brain areas involved in sexual motivation. Gen. Comp. Endocrinol. 179, 359-368. 10.1016/j.ygcen.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters L. V., Eens M., Pinxten R., Duffy D. L., Balthazart J. and Ball G. F. (2000). Seasonal changes in courtship song and the medial preoptic area in male European starlings ( Sturnus vulgaris). Horm. Behav. 38, 250-261. 10.1006/hbeh.2000.1623 [DOI] [PubMed] [Google Scholar]
- Riters L. V., Olesen K. M. and Auger C. J. (2007). Evidence that female endocrine state influences catecholamine responses to male courtship song in European starlings. Gen. Comp. Endocrinol. 154, 137-149. 10.1016/j.ygcen.2007.05.029 [DOI] [PubMed] [Google Scholar]
- Riters L. V., Ellis J. M. S., Angyal C. S., Borkowski V. J., Cordes M. A. and Stevenson S. A. (2013). Links between breeding readiness, opioid immunolabeling, and the affective state induced by hearing male courtship song in female European starlings ( Sturnus vulgaris). Behav. Brain Res. 247, 117-124. 10.1016/j.bbr.2013.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K. M., Richardson H. N., Romeo R. D., Morris J. A., Lookingland K. J. and Sisk C. L. (2003). Medial preoptic area dopaminergic responses to female pheromones develop during puberty in the male Syrian hamster. Brain Res. 988, 139-145. 10.1016/S0006-8993(03)03358-4 [DOI] [PubMed] [Google Scholar]
- Scott V. E., Evans K. E., Patton D. R. and Stone C. P. (1977). Cavity-Nesting Birds of North American Forests. Washington DC: US Department of Agriculture. [Google Scholar]
- Shughrue P. J., Lane M. V. and Merchenthaler I. (1997). Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. 388, 507-525. [DOI] [PubMed] [Google Scholar]
- Simerly R. B. and Swanson L. W. (1986). The organization of neural inputs to the medial preoptic nucleus of the rat. J. Comp. Neurol. 246, 312-342. 10.1002/cne.902460304 [DOI] [PubMed] [Google Scholar]
- Small T., Sharp P. J., Bentley G. E., Millar R. P., Tsutsui K., Mura E. and Deviche P. (2008). Photoperiod-independent hypothalamic regulation of luteinizing hormone secretion in a free-living sonoran desert bird, the rufous-winged sparrow ( Aimophila carpalis). Brain. Behav. Evol. 71, 127-142. 10.1159/000111459 [DOI] [PubMed] [Google Scholar]
- Snoeren E. M. S., Antonio-Cabrera E., Spiteri T., Musatov S., Ogawa S., Pfaff D. W. and Ågmo A. (2015). Role of oestrogen alpha receptors in sociosexual behaviour in female rats housed in a seminatural environment. J. Neuroendocrinol. 27, 803-818. 10.1111/jne.12321 [DOI] [PubMed] [Google Scholar]
- Spiteri T., Musatov S., Ogawa S., Ribeiro A., Pfaff D. W. and Ågmo A. (2010). Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology 91, 142-154. 10.1159/000255766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T., Ogawa S., Musatov S., Pfaff D. W. and Ågmo A. (2012). The role of the estrogen receptor alpha in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav. Brain Res. 230, 11-20. 10.1016/j.bbr.2012.01.048 [DOI] [PubMed] [Google Scholar]
- Spool J. A. and Riters L. V. (2017). Associations between environmental resources and the ‘wanting’ and ‘liking’ of male song in female songbirds. Integr. Comp. Biol. 57, 835-845. 10.1093/icb/icx117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spool J. A., Stevenson S. A., Angyal C. S. and Riters L. V. (2016). Contributions of testosterone and territory ownership to sexually-motivated behaviors and mRNA expression in the medial preoptic area of male European starlings. Horm. Behav. 86, 36-44. 10.1016/j.yhbeh.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veney S. L. and Rissman E. F. (2000). Steroid implants in the medial preoptic area or ventromedial nucleus of the hypothalamus activate female sexual behaviour in the musk shrew. J. Neuroendocrinol. 12, 1124-1132. 10.1046/j.1365-2826.2000.00567.x [DOI] [PubMed] [Google Scholar]
- Warren R. P. and Hinde R. A. (1961). Roles of the male and the nest-cup in controlling the reproduction of female canaries. Anim. Behav. 9, 64-67. 10.1016/0003-3472(61)90050-1 [DOI] [Google Scholar]
- Watson R. E. Jr, Hoffmann G. E. and Wiegand S. J. (1986). Sexually dimorphic opioid distribution in the preoptic area: manipulation by gonadal steroids. Brain Res. 398, 157-163. 10.1016/0006-8993(86)91261-8 [DOI] [PubMed] [Google Scholar]
- Watts H. E. and Hahn T. P. (2012). Non-photoperiodic regulation of reproductive physiology in the flexibly breeding pine siskin ( Spinus pinus). Gen. Comp. Endocrinol. 178, 259-264. 10.1016/j.ygcen.2012.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2007). Reshaping data with the reshape package. J. Stat. Softw. 21, 1-20. 10.18637/jss.v021.i12 [DOI] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Wickham H. (2011). The split-apply-combine strategy for data analysis. J. Stat. Softw. 40, 1-29. 10.18637/jss.v040.i01 [DOI] [Google Scholar]
- Williams T. D. (2012). Physiological Adaptations for Breeding in Birds. Princeton: Princeton University Press. [Google Scholar]
- Wingfield J. and Kenagy G. (1991). Neural regulation of reproductive cycles. In Vertebrate endocrinology: fundamentals and biomedical implications (ed. Schreibman M. and Jones R.), pp. 181-241. New York: Academic Press. [Google Scholar]
- Wingfield J. C., Hahn T. P., Maney D. L., Schoech S. J., Wada M. and Morton M. L. (2003). Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows, Zonotrichia leucophrys oriantha Gen. Comp. Endocrinol. 131, 143-158. 10.1016/S0016-6480(02)00648-2 [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Sullivan K., Jaxion-Harm J. and Meddle S. L. (2012). The presence of water influences reproductive function in the song sparrow ( Melospiza melodia morphna). Gen. Comp. Endocrinol. 178, 485-493. 10.1016/j.ygcen.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Zhu Y.-S., Cai L.-Q., You X., Duan Y., Imperato-McGinley J., Chin W. W. and Pfaff D. W. (2001). Molecular analysis of estrogen induction of preproenkephalin gene expression and its modulation by thyroid hormones. Mol. Brain Res. 91, 23-33. 10.1016/S0169-328X(01)00109-7 [DOI] [PubMed] [Google Scholar]