ABSTRACT
Macroglial cells in the central nervous system exhibit regional specialization and carry out region-specific functions. Diverse glial cells arise from specific progenitors in specific spatiotemporal patterns. This raises an interesting possibility that glial precursors with distinct developmental fates exist that govern region-specific gliogenesis. Here, we have mapped the glial progeny produced by the Drosophila type II neuroblasts, which, like vertebrate radial glia cells, yield both neurons and glia via intermediate neural progenitors (INPs). Distinct type II neuroblasts produce different characteristic sets of glia. A single INP can make both astrocyte-like and ensheathing glia, which co-occupy a relatively restrictive subdomain. Blocking apoptosis uncovers further lineage distinctions in the specification, proliferation and survival of glial precursors. Both the switch from neurogenesis to gliogenesis and the subsequent glial expansion depend on Notch signaling. Taken together, lineage origins preconfigure the development of individual glial precursors with involvement of serial Notch actions in promoting gliogenesis.
KEY WORDS: Gliogenesis, Astrocyte, Ensheathing glia, Apoptosis, Proliferation, Notch
Summary: Drosophila multi-potent progenitors produce brain region-specific glial populations, including astrocyte-like and ensheathing glia, with Notch signaling promoting the switch to glial fate and subsequent expansion.
INTRODUCTION
Converging evidence suggests that mammalian macroglial cells are highly regionally specialized to support local neural circuits (Tomassy and Fossati, 2014; Zhang and Barres, 2010). However, it is controversial whether glial heterogeneity stems from extrinsic environmental differences or is intrinsically patterned by lineage and temporal birth order (Bayraktar et al., 2015). Emerging studies support the notion that astrocyte diversity comes from distinct glial precursors located in specific domains of the nervous system (García-Marqués and López-Mascaraque, 2013; Hochstim et al., 2008; Magavi et al., 2012; Tsai et al., 2012).
Contrasting the extensive glial complexity in vertebrate brains, the glial network is relatively simple in the Drosophila brain. Based on location, morphology and function, Drosophila glia have been divided into at least three subclasses (Awasaki et al., 2008; Edwards and Meinertzhagen, 2010; Stork and Freeman, 2013; Ito et al., 1995; Hartenstein, 2011; Kato et al., 2017; Stork et al., 2012): surface glia make up the blood-brain barrier on the brain surface; cortex glia provide gases and nutrients to neuronal cell bodies through close contact; and neuropil glia, including astrocyte-like and ensheathing glia, have cell bodies in the synaptic neuropil. Although the processes of ensheathing glia surround and subdivide distinct neuropil regions, the processes of astrocyte-like glia infiltrate the neuropil profusely and interact dynamically with synapses (Awasaki et al., 2008; Doherty et al., 2009).
There are two types of neural stem cells present in Drosophila central brain. Type I neuroblasts (NBs) produce a series of ganglion mother cells (GMCs) that can each generate a pair of neurons or a neuron and a glia. Type II NBs make more complex lineages by generating intermediate neural progenitors (INPs) (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). One type II NB can produce about 40 INPs, which can each generate around five GMCs and thus about 10 neurons. Recent studies have shown that type II NBs give rise to both neurons and glia through INPs (Bayraktar and Doe, 2013; Izergina et al., 2009; Omoto et al., 2015; Viktorin et al., 2011, 2013). Only early born INPs give rise to glial progeny, suggesting loss of gliogenic potential as the NBs age (Bayraktar and Doe, 2013). Within an INP sublineage, neurogenesis most likely precedes gliogenesis (Bayraktar and Doe, 2013; Viktorin et al., 2011). Similar to other glia, the newly born type II-derived glial cells are positive for glial cells missing (gcm), a transcription factor that is necessary and sufficient for glial fate determination (Jones, 2005). Before its expression ends, gcm induces downstream genes, including reversed polarity (repo), a homeodomain transcription factor. After this initial glial specification at larval stage, there is a phase of glial amplification by clonal expansion of Repo-positive glial cells at pupal stage, resulting in a fourfold increase in glial cell numbers (Omoto et al., 2015; Viktorin et al., 2011).
The above studies have made significant progress in understanding gliogenesis from type II lineages. However, owing to technical limitations, the number, location, morphology and diversity of glia from individual type II lineages are poorly characterized, especially at the adult stage. Thus, little is known about whether lineage origin can specify distinct fates of glial precursors and generate regionally specialized glial phenotypes in type II lineages. Moreover, the molecular mechanisms of glial specification and proliferation remain poorly understood for type II lineage-derived glia (Awasaki et al., 2008; Omoto et al., 2015).
In the present study, we used the newly developed cell class-lineage intersection (CLIn) system (Ren et al., 2016) to map glial cells onto their lineages of origin. We were able to trace specific glial cells to their precursors using CLIn by taking advantage of the characteristic innervation patterns of the neuronal progeny of the precursors. We found that six of the eight type II lineages are gliogenic and that most of the gliogenic NBs/INPs are multipotent, generating neurons, astrocyte-like glia and ensheathing glia. We detected lineage-characteristic glial distribution, where individual INP glial clones occupy a restricted space that could vary in position. Contrasting with the variable spatial distribution, sibling INPs from a NB lineage independently make specific subsets of adult glia. We further demonstrate that the specification, survival and proliferative potential of glial precursors are patterned by lineage identity. Finally, we show that Notch signaling plays multiple essential roles in the generation and expansion of these diverse glial cells.
RESULTS
Mapping glial offspring of type II NBs by CLIn
We recently developed an intersectional system to subdivide cells of a particular class based on their distinct lineage origins (Ren et al., 2016). Briefly, the cell class of interest is labeled by the GAL4-UAS system, whereas the lineage(s) of interest are marked by the LexAp65-lexAop system. The lineage labeling is achieved through a cascade of transgenes and recombinases. First, we use a promoter that drives expression in, but is not necessarily exclusive to, the NB(s) of interest. With this promoter, we drive KD recombinase, which turns on Cre recombinase expression only in NBs via a NB-specific deadpan (dpn) promoter. Finally, in the NBs and their progeny, Cre recombinase triggers LexAp65 transcriptional activator expression, therefore labeling all the cells of the lineage of interest. Cre also causes loss of the GAL4 inhibitor GAL80, allowing GAL4 to be exclusively expressed in the cells that are both of the GAL4 cell class and the targeted lineage. The patterns of GAL4 and LexA::p65 activity are revealed by driving GFP and RFP expression (via UAS and lexAop), respectively. Moreover, various effectors can be incorporated into CLIn to manipulate the targeted lineages using lexAop transgenes and/or the cell class of interest within the targeted lineages using UAS transgenes. One can further conduct clonal analysis within CLIn by adding an intermediate step between the lineage-specific KD and the dpn promoter-driven Cre recombinase – a Flippase (FLP), under the control of heat-shock promoter. Stage-specific induction of FLP would permit stochastic labeling of single lineages expressing KD.
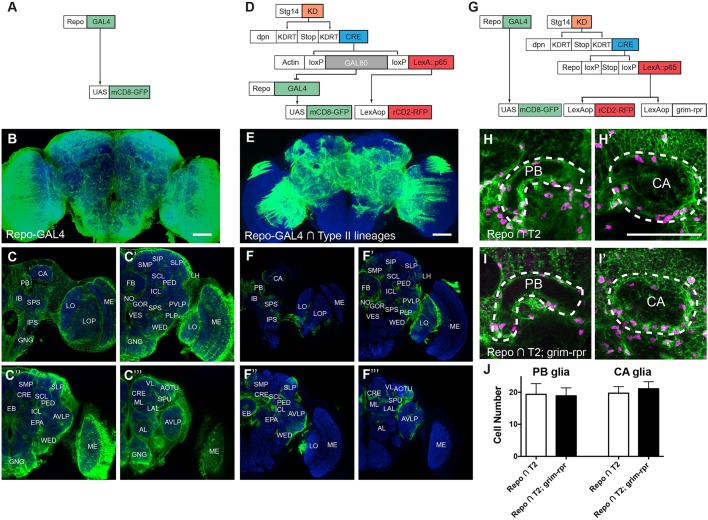
We used CLIn to reveal most, if not all, of the glia produced by type II NBs. We intersected a pan-glial driver, repo-GAL4 (Fig. 1A-C‴) with the stg14-patterned type II NB lineage-restricted line (Fig. 1D) (Ren et al., 2016). The majority of the uncovered glial population occupies the neuropil region, consistent with a neuropil glia identity (Fig. 1E-F‴). The type II NB-derived glia occupy the core of the brain, but largely spare the periphery (Fig. 1F-F‴, compare with Fig. 1C-C‴). In the core regions, the glial cells extensively wrap and infiltrate individual neuropils (Fig. 1F-F‴). This distribution hints that type II NBs produce glia for specific adult brain neuropils.
Fig. 1.
CLIn reveals glial cells made by type II NBs. Representative images of anti-mCD8 immunostained (green) and nc82-counterstained (blue) adult fly brains. (A) Genetics for labeling all brain glia (repo-GAL4 driving UAS-mCD8::GFP expression). (B-C‴) Complete expression pattern of brain glia (n=5). (B) Maximum projection image of a z-stack. (C-C‴) Single confocal sections showing glia distribution from posterior (C) to anterior (C‴). MB calyx (CA), protocerebral bridge (PB), inferior bridge (IB), superior posterior slope (SPS), inferior posterior slope (IPS), lobula (LO), lobula plate (LOP), medulla (ME), superior medial procerebrum (SMP), superior intermediate protocerebrum (SIP), superior lateral protocerebrum (SLP), superior clamp (SCL), inferior clamp (ICL), pedunculus (PED), lateral horn (LH), fan-shaped body (FB), noduli (NO), gorget (GOR), posteriorlateral protocerebrum (PLP), vest (VES), wedge (WED), posterior ventrolateral protocerebrum (PVLP), anterior ventrolateral protocerebrum (AVLP), ellipsoid body (EB), MB medial and vertical lobes (ML and VL), spur (SPU), crepine (CRE), epaulette (EPA), lateral accessory lobe (LAL), anterior optic tubercle (AOTU), antennal lobe (AL), and gnathal ganglia (GNG). We follow the naming nomenclature of Ito et al. (2014). (D) CLIn technique marks only the repo-GAL4-positive glia within type II NB lineages targeted with stg14-KD. The∩represents an intersection. (E) Glia innervating only subsets of brain neuropils, particular the CX, are visible after intersection. n=7. (F-F‴) Single confocal sections showing the major neuropils outlined and covered by glia of type II lineages. (G) Independent labeling of type II glia and all brain glia via dual genetic systems (GAL4-UAS and LexAp65-lexAop). (H,H′) Glial cells (green) in PB and CA regions. Magenta, Repo staining. Repo∩T2 indicates the presence of type II lineage glia. (I,I′) Glial cell distributions after inducing apoptosis in all type II lineage glia (Repo∩T2; grim). (J) Quantification of glial cell numbers. n=14 and 10, respectively. Scale bars: 50 μm.
We expected that type II lineage-derived glia are essential for fly survival, given their widespread elaborations in brain neuropils. In particular, they surround and extend dense processes into the central complex (CX) at the brain center (Fig. 1F-F″). The CX comprises the protocerebral bridge (PB), fan-shaped body (FB), ellipsoid body (EB) and noduli (NO). It functions as the locomotor control center integrating past experience and present behavioral context (Turner-Evans and Jayaraman, 2016). We ablated type II lineage-derived glia by adding a UAS-grim transgene into the CLIn genetic regime. Ectopic expression of grim, a cell death gene that activates apoptosis, eliminated all type II lineage-derived glia (data not shown). However, such flies were able to survive into adult stage and no obvious locomotor defect was observed.
We hypothesized that glial cells from non-type II lineage sources could effectively compensate for the loss of type II lineage-derived glia (type II glia). To examine this possibility, we specifically manipulated type II lineage-derived glia using the LexAp65-lexAop system and simultaneously marked all brain glia using GAL4-UAS (Fig. 1G). The specific accessing of type II glia was achieved by reconstituting a functional repo-LexA::p65 transgene in the stg14/dpn-targeted type II lineages via removal of a simple stop cassette rather than GAL80. The independent GAL4-UAS system then allows examination of all brain glia. Using this dual genetic system, we ablated type II lineage-derived glia by ectopically expressing grim and reaper (rpr) via a lexAop-grim-rpr transgene (Fig. 1G). Both grim and rpr activate apoptosis. We found that the various brain regions normally infiltrated by type II glia still possess extensive glial processes after removal of all type II glia (compare Fig. 1I,I′ with 1H,H′). Moreover, quantification of cell numbers revealed comparable numbers of glial cells in PB and mushroom body (MB) calyx (CA) regions before and after inducing apoptosis (P=0.72 and 0.13 respectively, unpaired t-test, Fig. 1H-J). Together, these data imply plastic proliferation potential for glial precursors outside of type II lineages.
Lineage-characteristic glial distribution and diversity
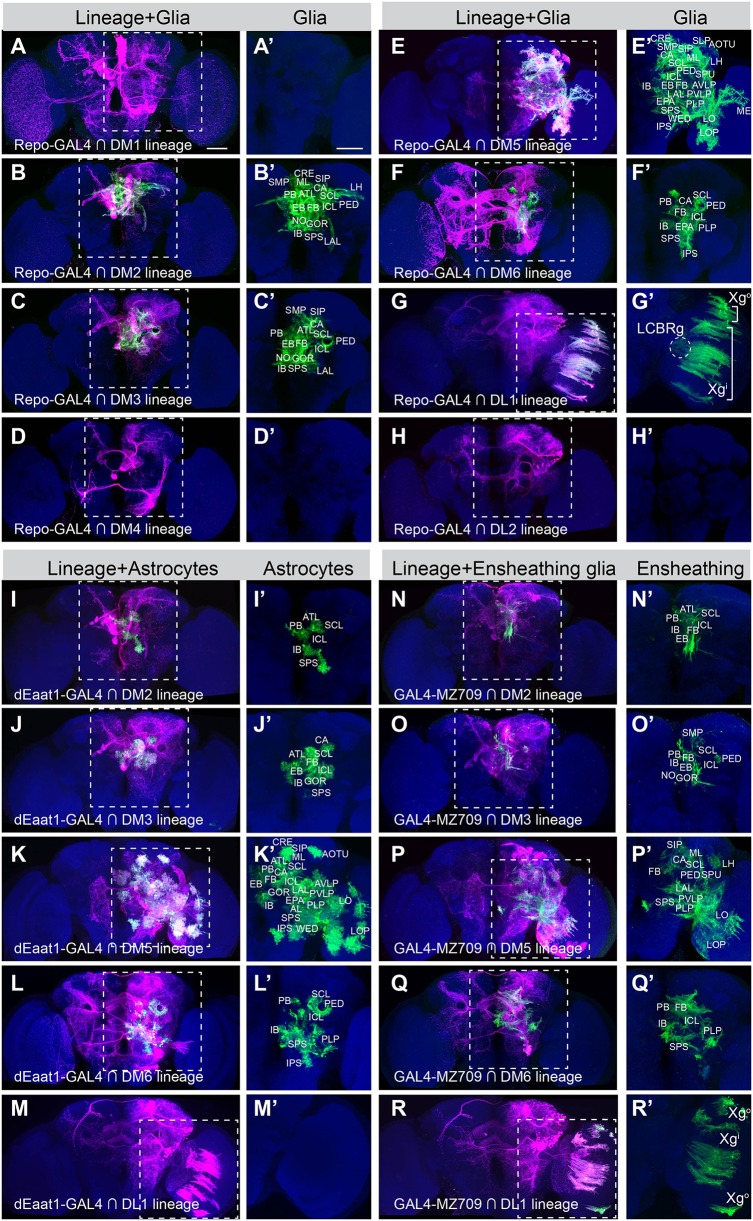
It is unclear whether the mature glial networks show any clonal organization. The glial elaborations in the above experiments conceal lineage boundaries. We thus mapped the glial progeny of single type II NB using the single-lineage CLIn technique. Stochastic clonal labeling revealed that different type II NB clones make distinct contributions to the glial networks of adult fly brains. First, the DM1-DM4 NBs, which each produce one quarter of the isomorphic CX neurons (Yang et al., 2013), produce either many (DM2 and DM3) or no adult glia (DM1 and DM4) (Fig. 2A-D′). In addition, unlike their neuronal siblings with distinct lineage-characteristic trajectories, the glial offspring of DM2 and DM3 NBs show similar dense distributions around the CX (PB, FB, EB, NO), MB (CA, PED), lateral complex (LAL), superior neuropils (SMP, SIP), inferior neuropils (SCL, ICL, IB, ATL) and ventromedial neuropils (GOR, SPS) (Fig. 2B-C′, see Fig. 1 legend for the full names of neuropils). Second, the DM5 NB clones exhibit modest neurite elaboration but have extensive glial coverage, spreading from the central brain into the OL (Fig. 2E,E′). These DM5 glial cells cover the lobula complex (lobula and lobula plate, LO and LOP), MB (CA, PED, SPU, ML), CX (PB, FB, EB, NO), lateral complex (LAL), ventrolateral neuropils (AOTU, VLP, PLP, WED), lateral horn, superior neuropils (SMP, SIP, SLP), inferior neuropils (CRE, SCL, ICL, IB, ATL) and ventrolateral neuropils (EPA, PS). By contrast, the neighboring DM6 NB clones show limited glial distribution despite very exuberant neurite elaborations (Fig. 2F,F′). These DM6 glial cells form sheaths around and extend processes into MB (CA), CX (FB, EB), ventrolateral neuropil (PLP), inferior neuropils (SCL, ICL, IB) and ventromedial neuropils (EPA, SPS, IPS). Third, despite the similarities between DL1 and DL2 clones in the neuronal cell body position and neurite trajectory, the DL1 lineage, but not the DL2 lineage, yields mature glia that reside in the ipsilateral optic lobe (OL) separated from their neuronal siblings (Fig. 2G-H′). As reported previously (Viktorin et al., 2013), the mature DL1 glia comprise outer chiasm glia (Xgo) in the medulla, inner chiasm glia (Xgi) between the medulla and the LO, and cortex glia-like cells of the lateral cell body rind (LCBRg) that wrap neuronal cell bodies in the cortex (Fig. 2G,G′).
Fig. 2.
Glial cells originating from individual type II NBs. (A-H′) Composite confocal images of adult fly brains with the offspring of the type II NB labeled in magenta, in which the repo-GAL4-positive glia, if any, are further marked in green. The magenta/green and green-only views of the same brains, counterstained with nc82 mAb (blue), are shown side by side. Note absence of detectable glia in the DM1 (A′), DM4 (D′) and DL2 (H′) lineages, and presence of three distinct glial subsets, including outer chiasm glia (Xgo), inner chiasm glia (Xgi), lateral cell body rind glia (LCBRg) of the OL, in the DL1 clone (G′). DM1, n=17; DM2, n=10; DM3, n=8; DM4, n=3; DM5, n=15; DM6, n=12; DL1, n=7; DL2, n=8. The LCBRg, located between ME and PVLP, was present in 86% of DL1 samples. (I-M′) CLIn with dEaat-GAL4 marks the astrocyte-like glia (green) in gliogenic type II lineages (magenta). No astrocyte-like glia exist in the DL1 lineage (M′). DM2, n=4; DM3, n=8; DM5, n=10; DM6, n=10; DL1, n=2. (N-R′) CLIn with GAL4-MZ709 marks the ensheathing glia (green) in gliogenic type II lineages (magenta). Xgo and Xgi, but not LCBRg, express the ensheathing glial marker (compare R′ with G′). DM2, n=6; DM3, n=5; DM5, n=7; DM6, n=4; DL1, n=5. Scale bars: 50 μm. See also Fig. S2.
We wondered whether the individual type II NBs are capable of producing multiple glial subtypes. We examined this possibility by marking the glial progeny for each type II NB lineage using GAL4 drivers that label either ensheathing (GAL4-MZ709) or astrocyte-like glia (dEaat1-GAL4) (Doherty et al., 2009). We detected both ensheathing and astrocyte-like glia in the DM2, DM3, DM5 and DM6 NB clones, and confirmed the presence of ensheathing, but not astrocyte-like glia in the DL1 NB clones (Fig. 2I-R′ and Fig. S1). Ensheathing and astrocyte-like glia from a common NB occupy comparable domains, roughly reflecting the relative spatial positions of the NB clones (Fig. 2I-L′,N-Q′). The ensheathing glia originating from the laterally positioned DL1 NB migrate into the OL and spread between the medulla and lobula complex in a regular array (Fig. 2R,R′). Interestingly, DL1 OL glia are spatially separate from DM5 OL glia, which remain within the lobula complex (Fig. 2K,K′).
Recent studies have identified more specific drivers for ensheathing glia (Kremer et al., 2017; Peco et al., 2016; Richier et al., 2017; Wu et al., 2017). Among them, we selected the R56F03-GAL4, which predominantly labels ensheathing glia associated with neuropil regions, and intersected it with type II lineages using CLIn at single-lineage resolution. In general, R56F03-GAL4 labels more ensheathing glia processes than GAL4-MZ709 (compare Fig. S2 with Fig. 2N-R′). For the DM5 lineage, both drivers uncovered glial processes surrounding and extending into the medulla and LO/LOP neuropils in the OL (Fig. 2P,P′ and Fig. S2C,C′). However, in the DL1 lineage, R56F03-GAL4 revealed inner chiasm glia (Xgi), but not outer chiasm glia (Xgo) or cortex glia-like cells of the lateral cell body rind (LCBRg) (Fig. S2E,E′). In summary, five out of the eight type II NBs yield mature neuropil glia that show lineage-characteristic elaboration domains and glial composition.
INP glia diversity and location variability
Previous studies suggest that glial offspring, together with their neuronal sisters, come from INPs in type II lineages (Bayraktar and Doe, 2013; Omoto et al., 2015; Viktorin et al., 2011). However, it is unclear whether there are specific INPs for each of the cell types, or actually individual INPs are multi-potent and can produce neurons, astrocyte-like and ensheathing glia. In order to map the glial progeny (if any) of a particular INP, we used CLIn to mark the glial offspring in isolated INP clones. We can isolate individual INPs by stochastically activating the dpn-recombinase transgene using transient FLP induction. The identity of distinct INP clones can be unambiguously determined based on the neurite trajectory of their neuronal constituents, given the production of a distinct neuron series by INPs (Wang et al., 2014).
Although at a lower frequency than the derivation of NB clones, the first larval-born INP clone, denoted as INP1, could be recovered by clone induction at the beginning of larval neurogenesis (Ren et al., 2016). Marking glia with repo-GAL4 revealed the existence of glia in the INP1 sublineage of DM2, DM3, DM5 and DL1 lineages (Fig. S3A-C′,E,E′). However, in the DM6 lineage, the INP1 clones never contained glia (Fig. S2D,D′). The glia made by a single INP account for only a subset of the total glial elaborations observed in NB clones. Together, these results confirm that individual INPs are capable of producing both neurons and glia.
Next we explored whether a single INP can generate both ensheathing and astrocyte-like glia. Judging from the elaboration of Repo-positive cells, the glia made by DM5 INP1 are limited in coverage but still maintain both laminar and multi-ramified types of glial processes characteristic of the ensheathing and astrocyte-like glia, respectively (Fig. S3C′). Using CLIn to mark the astrocyte-like (dEaat1-GAL4) and ensheathing glia (GAL4-MZ709 and R56F03-GAL4) in isolated INP clones substantiated their co-existence in the DM5 INP1 sublineage (Fig. 3A-C′ and Fig. S2F,F′). Similar results were obtained for the INP1 clones of DM2 and DM3 lineages (data not shown). These findings support the notion that individual INPs are multi-potent precursors that can generate neurons as well as astrocyte-like and ensheathing glia.
Fig. 3.
Diversity and location variability of DM5 INP1 glia. (A-C′) Composite confocal images of nc82-counterstained (blue) adult fly brains in which the DM5-INP1 sublineage is labeled in magenta (A-C) and the glial offspring positive for dEaat1-GAL4 (A-B′, n=3 samples) or for GAL4-MZ709 (C,C′, n=6 samples) are marked in green. Insets in A-C′ show single confocal sections of the corresponding dashed box areas in A-C. Magenta-only labels the neurons as well as additional types of glia. We can identify ensheathing glia morphology (arrowheads) in A-B′ and astrocyte-like glial morphology (arrow) in C,C′. The clone shown in B (five neurons and glia) is a sub-clone of DM5 INP1 (eight neurons and glia). Scale bars: 50 μm. See also Figs S2 and S3.
Notably, the glial progeny of an INP could elaborate in different subregions in different brains, which is in great contrast with the stereotyped neurite projections of their neuronal siblings (Fig. 3A-C′). Nonetheless, the ensheathing and astrocyte-like glia made by a given INP tend to mingle extensively in space, suggesting either co-migration or a rather late fate segregation (Fig. 3A-C′, also see Fig. 5B″-C″). These results hint at the presence of likely equivalent multi-potent glial precursors derived from sibling INPs, which jointly establish the lineage-characteristic glial distributions.
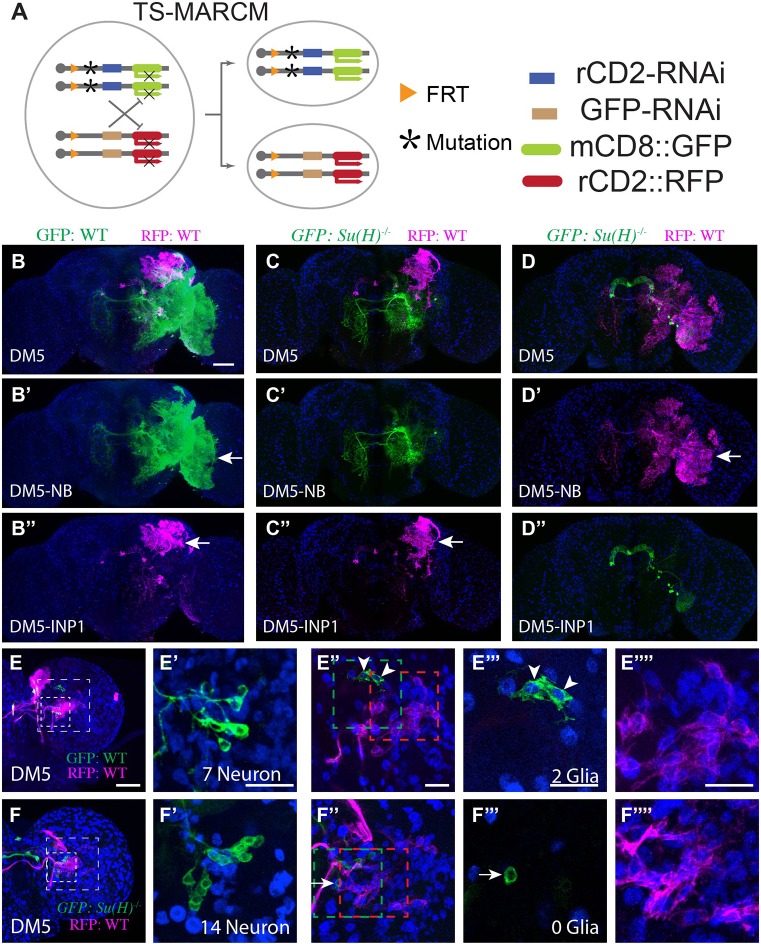
Fig. 5.
Notch is required for gliogenic switch. (A) Illustration of twin-spot MARCM, which marks the homozygous mutant clone with mCD8::GFP and its paired wild-type sister clone with rCD2::RFP. (B-D″) Su(H) mutant clones lack glia. Twin-spot MARCM clones of the DM5 lineage induced shortly after larval hatching and examined in adult fly brains counterstained with anti-Repo Ab (blue). (B-D) Merged views of B′-D′ and B″-D″. The glia-like processes (arrow) are present on both green and magenta sides of the control DM5 twin-spot clones (B-B″, n=3). By contrast, the glia-like processes are not seen on the mutant green side of the twin-spot MARCM clones derived from Su(H) heterozygous precursors (C′,D″, n=3 and 2, respectively). (E-F″″) Su(H) mutant clones have excess neurons at the expense of glia. Composite confocal images of the late larval brain carrying twin-spot MARCM clones. Partial projection of the INP cell body region (small box in E,F) and the glia region (big box in E,F) are shown in E′,F′ and E″,F″, respectively. Single confocal sections of INP glia (green box in E″,F″) and NB glia (E″″,F″; red box in E″,F″) are shown in E‴,F‴ and E″″,F″″, respectively. (E-E″″) Wild-type DM5 and DM5-INP1 twin-spot clone. The INP1 example shown has seven neurons (E′) and two glial cells (E‴). On average, DM5-INP1 has 7.7±0.5 neurons and 1.1±0.4 glial cells (n=7). Arrowheads indicate glial cells. (F-F″″) Wild-type DM5 and mutant DM5-INP1 twin-spot clone. The INP1 example shown has 14 neurons (F′) and no glia (F‴). The cell in F‴ is negative for Repo (arrow). On average, mutant INP1 has 13±2 neurons and 0±0 glial cells (n=4). Scale bars: 50μm in B-D″; 20 μm in E-F″″. Data are mean±s.d.
Lineage-patterned glial precursor specification, proliferation and survival
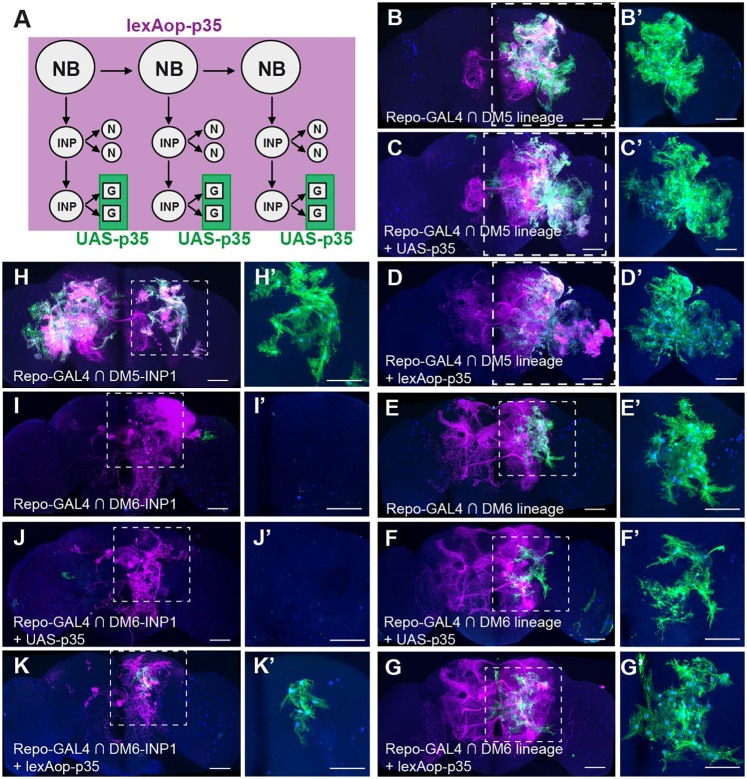
Given the lineage-specific glial phenotypes, we hypothesized that distinct glial precursors are produced by different type II lineages. We define glial precursors as cells with the potential to differentiate into glial cells. The specification, proliferation and survival of these precursors may vary based on developmental origin. To better assess the proliferation and apoptosis of glial precursors from different NB/INP lineages, we inhibited cell death by expressing the anti-apoptotic p35 viral gene (Hay et al., 1994) in the repo-GAL4-positive cells (UAS-p35, Fig. 4A). However, given that repo-GAL4 might be turned on too late for effective rescue, we also expressed p35 throughout the development of the entire lineage (lexAop-p35 driven by actin-LexAp65, Fig. 4A). When apoptosis was blocked in all eight type II lineages, we observed more glial elaborations among the regions covered by type II glia, and a few additional regions mostly spared by type II glia (Fig. S4). These results suggest that some type II glial precursors or mature glia normally undergo apoptosis but could be rescued by p35 expression.
Fig. 4.
Blocking cell death in Repo-positive cells or entire lineages reveals lineage-characteristic phenotypes. (A) Genetics for blocking apoptosis in type II lineages. (B-K′) Composite confocal images of adult fly brains carrying various CLIn clones of type II lineages. All the offspring of the CLIn clones are labeled in magenta, and the offspring positive for repo-GAL4 are also marked in green for morphology and in blue for nuclei (lacZ). The clone identity and the transgene used to block apoptosis in Repo-positive offspring (UAS-p35) or entire progeny (lexAop-p35) are indicated. The dashed boxes in D-K are shown at higher magnification on the right (D′-K′). (B-D′) Increase of DM5 glia (B,B′) by either UAS-p35 (C,C′) or lexAop-p35 expression (D,D′). (E-G′) The DM6 glial cells (E,E′) are increased after lexAop-p35 (G,G′), but not UAS-p35, expression (F,F′). (H,H′) Glial cells from DM5-INP1 (right hemisphere). (I-K′) The DM6-INP1 do not contain glial cells (I,I′). Blocking apoptosis by lexAop-p35 rescued some glia (K,K′), while blocking apoptosis by UAS-p35 fails to rescue any glia (J,J′). In H,I, brains were contaminated with a DM5 NB clone and a type I lineage clone, respectively. Scale bars: 50 μm. See also Figs S4, S5 and S6.
To characterize the proliferation and apoptosis of glial precursors from individual type II NB/INP lineages, we quantified glial cell number before and after apoptosis induction. We marked the glial offspring using two UAS-reporters that label both glial morphology (UAS-mCD8GFP; Lee and Luo, 1999) and nuclei (UAS-nls-LacZ; Doherty et al., 1996). The glial numbers in specific type II NB/INP lineages are summarized in Table 1.
Table 1.
Glial cell number in type II lineages before (wild type) and after (UAS-p35 and lexAop-p35) blocking cell death
Previous studies have shown that the DM1 lineage contains Gcm/Repo-positive glial cells at larval stage (Izergina et al., 2009; Viktorin et al., 2011). Thus, the lack of adult glia in the DM1 lineage could be due to glial apoptosis at a later stage of development. To test this possibility, we blocked apoptosis in DM1 lineage using either repo-driven p35 or actin-driven p35. Preventing apoptosis recovered glial cells in the normally glia-lacking DM1 NB clones (Fig. S5A-C′, 6±0 cells by UAS-p35, n=5; 9±2 cells by lexAop-p35, n=10). These ectopic glial cells consistently reside along the median bundle (MBDL) and around the DM1 cell body region (Fig. S5B′-C′). Next, we extended similar analysis to the DL2 lineage, which do not contain glia at larval stage (Viktorin et al., 2013). We failed to restore any glia in the DL2 lineages by transgenic p35 driven either by repo or actin promoter (data not shown), suggesting that glial precursors are not specified. These DM1 and DL2 lineage results suggest that lineage identity determines the specification and production of glial precursors.
We next examined the extent of proliferation and survival of glial precursors in the DM5 and DM6 lineages. Counting the wild-type glia cell numbers revealed the presence of many more glia in DM5 NB clones (147±23, n=15, Fig. 4B,B′) than in DM6 NB clones (23±9, n=12, Fig. 4E,E′). We detected a striking increase in the total number of DM5 glia when using either repo (206±24, n=13, P<0.001 versus wild type, Fig. 4C,C′) or actin (193±34, n=10, P<0.001 versus wild type, Fig. 4D,D′) promoters to inhibit apoptosis. The p35-expressing DM5 clones that possess many more glia than wild-type controls showed enlarged glial coverage but retained lineage-characteristic glial distribution patterns (Fig. 4C-D′). The excessive DM5 glia could extend deep into the OL (Fig. 4C′-D′) but never developed into the DL1-characteristic interface glia that lie between the medulla and lobula complex. In the DM6 lineage, blocking cell death in Repo-positive cells did not alter the number of glia (24±8, n=10, P=0.71 versus wild type, Fig. 4F,F′), but blocking apoptosis in the entire lineage led to a significant rescue of glia (62±13, n=8, P<0.001 versus wild type, Fig. 4G,G′). The glial cells of DM6 NB clones with actin-driven p35 were almost tripled in number but remained localized to the posterior brain areas (Fig. 4G,G′).
Compared with actin-driven p35, repo-driven p35 is equally potent at increasing the DM5 adult glial numbers. This indicates no premature loss of Repo-negative glial precursors, but significant loss of Repo-positive glial precursors and/or differentiated glia. Preventing the premature loss of Repo-negative glial precursors (and thus making additional INP sublineages ‘gliogenic’, as shown below) probably underlies the ability of expressing p35 in an entire clone to rescue more glia in DM6 NB clones.
Increasing the number of glial progeny in the DM2 lineage also requires the blockade of apoptosis throughout lineage development. Only actin-driven p35, but not repo-driven p35, could significantly expand the DM2 glial population by almost double (wild type: 58±9, n=12; repo-driven p35: 67±8, n=9, P=0.09 versus wild type; actin-driven p35: 107±4, n=4, P<0.001 versus wild type, Fig. S5D-F′). By contrast, neither actin- nor repo-driven p35 could increase the DM3-derived glia (wild type: 56±10, n=6; UAS-p35: 55±13, n=5; lexAop-p35: 56±12, n=5, Fig. S5G-I′) that are otherwise indistinguishable from the DM2-derived glia in terms of adult glia number and distribution (Fig. 2B′-C′). For the DM4 lineage (wild type: 0±0, n=7), we detected one glia in 50% of clone samples with actin-driven p35 (0.5±0.5, n=10), but no glia in any samples with repo-driven p35 (0±0, n=7) (Fig. S5J-L′). For the DL1 lineage, both repo- and actin-driven p35 led to an increase of OL glia (wild type: 17±2, n=6; UAS-p35: 21±3, n=6, P<0.05 versus wild type; lexAop-p35: 22±4, n=4, P<0.05 versus wild type; Fig. S5M-O′). Qualitatively similar phenotypes were obtained by blocking cell death via targeted RNAi of dronc, an essential component of the cell death pathway (Xu et al., 2009), assuring no p35 transgene-specific side effects (Fig. S6). Taken together, our results suggest that different lineages exhibit distinct gliogenic potentials, partly shaped by differential apoptosis at various stages of development.
INP sublineage identity determines glial precursor proliferation and survival
It is unclear whether INP sublineage identity determines the proliferation and survival of glial precursors, as it does in the specification of diverse neurons. We induced CLIn clones that label the first INP sublineage of larval neurogenesis, as their neurite trajectories have been reported (Wang et al., 2014). In this set of experiments, we selectively focused on DM5 and DM6 INP1 due to the low frequencies of INP1 clones from other lineages. Marking glia with repo-GAL4 revealed the production of glia from the INP1 sublineage of DM5 (32±11 cells, n=7, Fig. 4H,H′). As seen in DM5 NB clones, blocking apoptosis in DM5 INP1 clones using either repo-driven p35 (54±15, n=4) or actin-driven p35 (51±18, n=4) rescued comparable numbers of glia.
By contrast, the adult DM6 INP1 clones never contained glia (0±0, n=5, Fig. 4I,I′), even after blocking cell death in Repo-positive cells (0±0, n=4, Fig. 4J,J′). Consistent with the above DM6 NB clone data, the DM6 INP1 sublineage required an earlier induction of p35 to unmask its gliogenic potential. Driven by an actin promoter, p35 could restore glial cells in the normally neuron-only INP sublineage (11±4, n=4, Fig. 3K,K′). This phenomenon implies that some INPs may give rise to Repo-negative glial precursors but produce no glia because of premature loss of the glial precursors prior to the onset of repo. We identified three later derived (at 24 h or 48 h ALH) DM6 INP clones that all possess some glia (Fig. S3F,F′). Together with the previous finding that late INPs (after 52 h ALH) cannot generate glia (Bayraktar and Doe, 2013; Izergina et al., 2009), these observations suggest that the specification, proliferation and survival of glial precursors are pre-patterned by INP sublineage identity and temporal fate.
Requirement of Notch signaling for the gliogenic switch
Although the molecular mechanisms specific to type II lineage gliogenesis are poorly understood (Awasaki et al., 2008; Viktorin et al., 2011), gliogenesis has been widely studied in Drosophila and vertebrate nervous systems (Jones, 2005; Rowitch and Kriegstein, 2010). It has been shown that Notch signaling could either promote or repress gliogenesis, depending on the specific developmental context (Cattenoz and Giangrande, 2013; Chen et al., 2016; Gaiano and Fishell, 2002; Gho et al., 1999; Morrison et al., 2000; Udolph et al., 2001; Umesono et al., 2002; Van De Bor and Giangrande, 2001).
To examine the potential requirement of Notch for the gliogenic switch, we created clones homozygous for mutations in Su(H), an essential gene for Notch signaling (Bray, 2006). We selectively labeled type II lineage clones using twin-spot MARCM (Yu et al., 2009) with an stg14/dpn-patterned actin-LexAp65 driver (Wang et al., 2014). Twin-spot MARCM labels the mutant clone and its paired wild-type sister clone in distinct colors (Fig. 5A). We focused on DM5, the largest gliogenic lineage. In great contrast to the wild-type NB clones carrying many glial cells in lineage-characteristic spatial patterns (Fig. 5B-B″), all DM5 NB clones mutant for Su(H) showed an absence of glia (Fig. 5C-C″). However, when mutant NB clones were paired with wild-type INP1 clones, the INP1 sublineage still yielded normal numbers of mature glia [Su(H) mutant: 40.6±8.5 cells, n=5 versus wild type: 39.2±5.9 cells, n=5, P=0.77, unpaired t-test] that, moreover, showed no expansion in the size of elaboration domains (compare Fig. 5B″ with C″). Complete loss of glia was also observed in all mutant DM5-INP1 clones paired with wild-type NB clones (Fig. 5D-D″). Taken together, these results indicate that Notch signaling is required within the INP sublineage for type II lineage gliogenesis.
The clone-autonomous glia-missing phenotype possibly occurred as a consequence of no gliogenic switch. Assuming that INPs (rather than GMCs) undergo a gliogenic switch, blocking this switch should prolong neurogenesis and result in the production of extra neuronal offspring by an INP. To examine this possibility, we determined whether one could detect more neuronal progeny in the Su(H) mutant DM5 INP1 sublineage. In the wild-type case, at 72 h ALH, we detect two glial cells in the DM5 INP1 clones (Fig. 5E-E″″, green) and the paired NB clones contain additional glial cells arising from later-born INPs (Fig. 5E-E″″, red). The majority of glial offspring of DM5 form a separate cluster that is distant from neuronal cell bodies (Fig. 5E-E″″). As to the Su(H) mutant DM5 INP1 clone, we could not detect any Repo-positive progeny at 72 h ALH (Fig. 5F-F″″, green). We further noticed the presence of many more neuronal cell bodies in the Su(H) mutant INP clones (Fig. 5F′). These observations argue that Notch promotes gliogenic switch within the INPs, thus ending neurogenesis in glia-producing INP sublineages.
Delta-dependent Notch activity promotes glial expansion
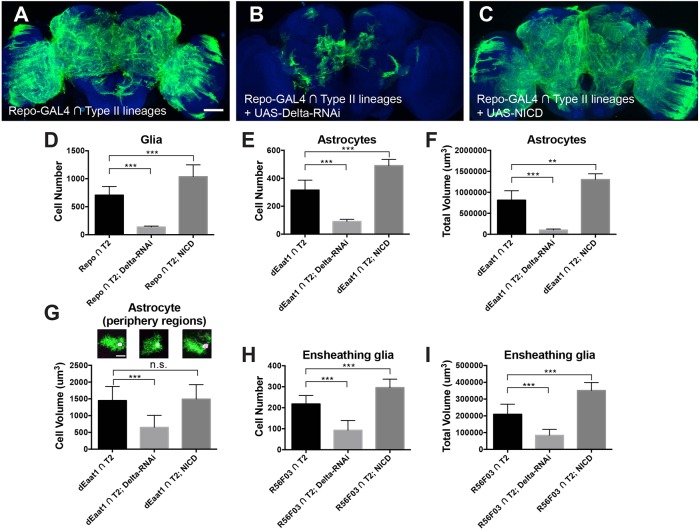
In addition to the gliogenic switch, Notch may also govern subsequent glial development, including glial expansion and the differentiation of glial subtypes (Griffiths et al., 2007; Griffiths and Hidalgo, 2004; Kato et al., 2011; Losada-Perez et al., 2016; Peco et al., 2016; Stacey et al., 2010; Thomas and van Meyel, 2007). To uncover the functions of Notch after the gliogenic switch, we next manipulated Notch activity specifically in Repo-positive cells of type II NB lineages using CLIn. Induction of UAS-miRNA transgenes against the Notch ligand delta (Bray, 2006) drastically reduced the type II glial population. This is evidenced by an extensive shrinkage of the type II glial coverage (Fig. 6A,B and Fig. S7B-C″) and significantly reduced cell number (P<0.001, Fig. 6D). Knocking down Notch by RNAi also reduced type II glia (Fig. S7A,A′ and D-D″), although to a lesser extent than delta knockdown. This is potentially due to variable gene-specific RNAi efficiency. Conversely, increasing Notch activity, via ectopic expression of the Notch intracellular domain (UAS-NICD), greatly increased cell number (P<0.001, Fig. 6D) and expanded the area of brain with glial elaboration (Fig. 6C and Fig. S7E,E′). The excessive type II glia showed dense aggregations in multiple neuropil regions (Fig. S7E-E″).
Fig. 6.
Notch promotes glia expansion. Composite confocal images of adult fly brains with the type II glial population labeled in green. Blue indicates nc82 counterstaining. (A) Glial cell distribution in wild-type type II lineages (n=6). (B) Type II glia-specific RNAi knockdown of Delta, a Notch ligand, effectively reduces the glial coverage of neuropil regions (n=6). (C) Type II glia-specific overexpression of the Notch intracellular domain (NICD) greatly increases the glial coverage of neuropil regions (n=6). (D) Quantification of glial cell number by staining nuclear localized β-galactosidase after Notch manipulations driven by repo-GAL4. n=11, 7 and 10, respectively. (E-G) Quantifications of cell number, total volume and individual cell volume of astrocyte-like glia after Notch manipulations driven by dEaat1-GAL4. (E,F) n=10, 10 and 8, left to right columns. (G) n=11, 20 and 7, left to right columns. (H,I) Quantifications of cell number and total volume of ensheathing glia after Notch manipulations driven by R56F03-GAL4. n=9, 8 and 7, left to right columns. *P<0.05, **P<0.01, ***P<0.001; n.s., not significant. Scale bars: 50 μm. See also Fig. S7.
Similar phenomena were observed when we manipulated Notch activity using astrocyte- and ensheathing glia-specific drivers. We found that induction of delta RNAi or UAS-NICD by dEaat1-GAL4 effectively reduced or increased the cell number and total volume of the type II lineage-derived astrocyte-like glia (both P<0.001, Fig. 6E,F). Similarly, induction of delta-RNAi or UAS-NICD by R56F03-GAL4 led to reduced or increased cell number and total volume of the type II lineage-derived ensheathing glia (both P<0.001, Fig. 6H,I). Based on the total volume and cell number, we calculated the average volume per astrocyte-like or ensheathing glial cell before and after Notch manipulation. For ensheathing glia, there were no significant differences among all groups (P=0.27, one-way ANOVA). For astrocytes, the average cell volume was reduced upon Notch loss of function (P<0.001) but unaffected by Notch gain of function (P>0.05, one-way ANOVA followed by Dunnett's post-hoc test). To verify this result, we measured the average cell volume of individual astrocytes located in periphery regions where they could be singly segmented out. The single-cell analysis validated the reduction in astrocyte volume following induction of delta-RNAi (P<0.001) and no significant change with NICD expression (P>0.05, Fig. 6G). Taken together, these results support the notion that Notch signaling enhances the expansion of type II lineage-derived glia, likely through a cell-autonomous mechanism.
DISCUSSION
Glial precursors are fated by cell lineage
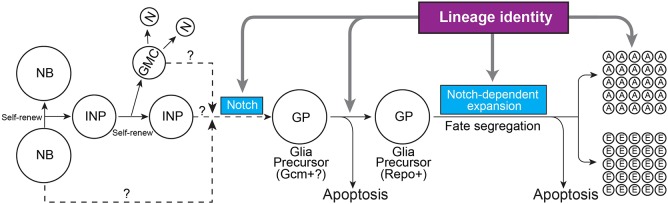
In this study, we systematically characterized gliogenesis in Drosophila type II lineages. We found that developmental origin plays an important role in the fating of glial precursors with distinct gliogenic potentials (Fig. 7). Glial precursors in the DM1 lineage generate glial cells that are temporary (Izergina et al., 2009; Viktorin et al., 2011) and undergo apoptosis before the adult stage, suggesting that they may serve transient developmental functions. The DM2 and DM3 lineages yield spatially indistinguishable sets of adult glia. Surprisingly, some glial precursors in the DM2, but not DM3, lineage undergo apoptosis. Furthermore, significant apoptosis of Repo-positive glial cells occurs only in the DM5 lineage, which generates excessive glia. By contrast, some glial precursors in the DM2 and DM6 lineages undergo apoptosis before repo is expressed. However, we cannot be sure whether there is normally a gliogenic switch in those DM2 and DM6 INPs (e.g. DM6 INP1). It is possible that those apoptotic ‘glial precursors’ are terminal INPs that become gliogenic if aberrantly prolonged by actin-driven p35. Regardless of the timing of apoptosis relative to glial precursor specification, the phenomenon is lineage specific. All these lines of evidence underscore the importance of lineage identity in the specification, survival and the extent of proliferation of glial precursors in distinct type II NB lineages (Fig. 7).
Fig. 7.
A working model of gliogenesis in Drosophila type II lineages. Type II NBs divide asymmetrically to self-renew and give rise to INPs. Each INP also undergoes multiple rounds of asymmetrical division to self-renew and generates ganglion mother cells (GMCs), which divide once to produce two neurons. Although the exact source of glial precursors remains to be determined, Notch signaling is required for gliogenic switch. The glial precursor might initially be positive for Gcm, which then turn on the downstream glial genes, including repo (Jones, 2005; Viktorin et al., 2011). The glial precursor generates both astrocyte-like and ensheathing glia. The expansion of both glial subtypes is Notch dependent. Lineage identity governs multiple stages of glial development, controlling which INPs produce glial precursors, which glial precursors undergo apoptosis before repo is expressed, the expanding potential of glial precursors, the diversity of glial progeny and their final distribution.
The temporal identity of INPs further governs the gliogenesis of type II NB lineages. The glial precursor(s) from DM6-INP1 undergoes premature apoptosis before repo is turned on. Interestingly, we observe that later INP sublineages, but not INP1 sublineage of DM6, contain glial cells. Moreover, previous studies have demonstrated that early (before ∼52 h ALH), but not late, INPs produce glial progeny (Bayraktar and Doe, 2013; Ren et al., 2017). Glial cells are increased when the early temporal window is extended, while being reduced upon shortening the early window (Ren et al., 2017; Syed et al., 2017). These results indicate that the INP birth order determines the gliogenic potential of an INP.
In the present study, our results indicate that INPs are multipotent to produce both neurons and glia, and neurogenesis most probably precedes gliogenesis (also see Bayraktar and Doe, 2013; Viktorin et al., 2011). However, a previous study reported DM5 INP sublineage that produce only glia (Viktorin et al., 2011). The exact INP(s) that generates these glial cells remains to be determined. We speculate that these glial cells arise from later-born DM5 INPs, the morphology of which remains obscure (Wang et al., 2014). It is also possible that neurons within those INPs undergo premature apoptosis. Characterization of the projection pattern of later DM5 INP lineages, in combination with manipulations that include blocking cell death, will be helpful to decipher the mode of lineage proliferation for pure glial sublineages.
Mammalian studies suggest that macroglial heterogeneity is organized by positional cues provided by homeodomain transcriptional factors such as Nkx6 and Pax6/Pax7 (Hochstim et al., 2008; Magavi et al., 2012; Tsai et al., 2012). In C. elegans, molecularly distinct cephalic sheath glial cells in the ventral and dorsal domains are specified by MLS-2 and VAB-3, respectively, which are transcriptional regulators closely related to vertebrate Nkx6 and Pax6/Pax7 (Yoshimura et al., 2008). Thus, the generation of glial diversity by positional information is likely to be evolutionarily conserved. We speculate that the distinct gliogenic potential of different type II NBs/INPs might analogously be specified by positional cues or morphogens. The genetic mechanisms of spatial patterning of NBs in the embryonic ventral nerve cord have been extensively studied in Drosophila (Skeath, 1999). Molecular characterization of cell lineage identity is crucial for elucidating the genetic basis of such distinct developmental potentials.
Diversity and location variability of INP glia
It was unclear whether astrocyte-like and ensheathing glia arise from distinct type II NBs, distinct INPs or actually common INPs that are multi-potent (Omoto et al., 2015; Viktorin et al., 2011, 2013). Using dual-expression-control MARCM, our lab previously concluded that ensheathing and astrocyte-like glia in the central brain are most likely generated from distinct precursors (Awasaki et al., 2008). However, our current results indicate that INPs are multipotent in the production of neurons, astrocyte-like and ensheathing glia. Two lines of evidence argue that the clones examined in the previous study may not come from type II origins. First, re-examination of the original data reveals that most of the previously obtained clones reside in regions not typically occupied by type II glia. Second, those glial clones were relatively small in size (mostly three to five cells), whereas type II-derived glial clones induced at newly hatched larval stage should contain around 10-30 or 30-150 cells depending on the INP or NB origin, respectively. Because of these distinctions, we now believe that the glial precursors previously examined were of non-type-II lineage origin and were likely restricted to produce either astrocyte-like or ensheathing glia by the time of larval hatching.
We observe that the location of glial cells derived from a specific INP sublineage varies from individual to individual. However, glial cells from the entire lineage are distributed in a stereotypic pattern. This is even the case for glial cells rescued from apoptosis. This phenomenon suggests that the assembly of lineage-characteristic glial distribution is actually a mosaic arrangement of glial progeny arising from successive INP lineages. Curiously, the astrocyte-like and ensheathing glial progeny of an INP tend to mingle extensively. This might stem from co-migration of astrocyte and ensheathing glial precursors from a common INP origin.
Notch signaling promotes gliogenesis and expansion
Notch has been implicated in regulating various aspects of gliogenesis. Previous studies have shown that Notch can promote or repress gliogenesis depending on the developmental context (Gaiano and Fishell, 2002; Umesono et al., 2002). In vertebrate gliogenesis, Notch promotes the generation of Müller glia, astrocytes and Schwann cells, but represses oligodendrocyte differentiation (Kato et al., 2015; Morrison et al., 2000; Pierfelice et al., 2011). Similarly, Notch can either promote glial fate in specific lineages of the fly central nervous system (Chen et al., 2016; Udolph et al., 2001; Umesono et al., 2002) or inhibit gliogenesis in the sensory organ precursors of the Drosophila periphery nervous system (Gho et al., 1999; Van De Bor and Giangrande, 2001). In Drosophila, Notch regulates the expression of gcm, which is both necessary and sufficient to specify glial cells (Udolph et al., 2001; Umesono et al., 2002; Van De Bor and Giangrande, 2001). Although the relationship with Notch/gcm remains to be characterized, the fibroblast growth factor, insulin-like receptor and Merlin-Hippo pathways also regulate gliogenesis or proliferation of Drosophila surface and cortex glia (Avet-Rochex et al., 2012, 2014; Reddy and Irvine, 2011). Here, we show that Notch promotes gliogenesis for Drosophila type II lineages in the central brain. At mid-larval stage, glial cells are just starting to appear, while neurogenesis of INP1 has apparently ended (Viktorin et al., 2011). Notch is required for the gliogenic switch. However, the genes that function in parallel or downstream of Notch in regulating gliogenesis remain to be fully uncovered.
We also examined INP1-derived glia in response to a complete loss of glia from the paired mutant NB clone. We initially hypothesized that normal apoptosis occurs, at least partly, due to competition between INP sublineages for trophic support (Hidalgo et al., 2011). Interestingly, the wild-type INP1 glia did not increase in number or coverage when the remaining glia of the DM5 lineage were eliminated. This implies that a significant number of INP1-derived glia still underwent apoptosis even in the absence of all cousin glia. Thus, the apoptotic fate of post-mitotic glia is likely pre-determined in each INP sublineage irrespectively of sibling sublineages.
In the present study, we found that Notch signaling is required for glia expansion in type II lineages. This is consistent with the role of Notch in promoting glial proliferation during the regenerative response after central nervous system injury (Kato et al., 2017). A series of studies have identified an intricate gene network, consisting of Notch, prospero, Kon-tiki, dorsal and eiger, that drives successive repair (Griffiths et al., 2007; Griffiths and Hidalgo, 2004; Kato et al., 2011; Losada-Perez et al., 2016). Interestingly, feedback interactions among Kon-tiki, Notch and prospero enable proper glial proliferation and ensuing differentiation (Losada-Perez et al., 2016). Further in-depth studies are needed to determine whether this gene network is also essential for type II lineage glial development.
Notably, silencing the expression of the Notch ligand Delta in type II glia precursors can potently suppress their expansion. This argues for a cell-autonomous mechanism in the promotion of glial expansion by Notch. It remains to be determined whether other, potentially extrinsic, mechanisms may also influence the lineage-guided type II gliogenesis (Hidalgo et al., 2011; Miguel-Aliaga and Thor, 2009). For example, fibroblast growth factor signaling has been implicated in gliogenesis in multiple systems (Avet-Rochex et al., 2012; Richier et al., 2017; Stork et al., 2014; Wu et al., 2017).
Previous studies revealed that Notch signaling is implicated in the fate separation of astrocyte-like and ensheathing glia in Drosophila VNC (Griffiths and Hidalgo, 2004; Peco et al., 2016; Stacey et al., 2010; Thomas and van Meyel, 2007). In this study, we failed to detect a drastic shift between the two glial fates following manipulation of Notch activity. Up- or downregulating Notch activity specifically in Repo-positive cells resulted in either the increase or decrease of both astrocyte-like and ensheathing glia (determined based on morphology), rather than increasing one type and decreasing the other. However, we cannot completely rule out the role of Notch in glial subclass specification as this role might be shadowed by its role in glial expansion or gliogenic switch. Direct analysis on fate segregation during development would be needed to nail down the role of Notch, if any, in the specification of astrocyte-like versus ensheathing glia.
MATERIALS AND METHODS
Fly strains
The GAL4 driver stocks include: repo-GAL4 (III) (Sepp et al., 2001), dEaat1-GAL4 (Rival et al., 2004) and GAL4-MZ709 (Ito et al., 1995) (kind gifts from Prof. Marc Freeman, Vollum Institute, OHSU, OR, USA), R56F03-GAL4 (Jenett et al., 2012) and stg14-GAL4 (Wang et al., 2014). The UAS-transgenes include: UAS-nls-LacZ (Doherty et al., 1996), UAS-NICD (Go et al., 1998) and UAS-grim (Chen et al., 1998). The UAS-Dronc-RNAi (BL#32963), UAS-Delta-RNAi (BL#34322) and UAS-Notch-RNAi (BL#33611, 33616, 28981, 27988) were requested from the Bloomington Stock Center (Ni et al., 2011). The lexAop-p35 has been reported previously (Ren et al., 2016). Mutant stock was FRT40A, Su(H)delta47/CyO (Koelzer and Klein, 2006).
For LexAp65-lexAop system-based twin-spot MARCM (Awasaki et al., 2014), we employed hsFLPop [attP18 (6C12)], lexAop-rCD2i-spacer-lexAop-mCD8::GFP [VK27 (89E11)], lexAop-GFPi-spacer-lexAop-rCD2::RFP [VK27 (89E11)] and actin^LoxP-stop-LoxP^LexA::P65 [Su(Hw)attP8(8E10), attP40 (25C7)]. For details of the genetic design of twin-spot MARCM, see Yu et al. (2009).
In order to make CLIn compatible with more drivers or effectors, we inserted a few CLIn-related transgenes into new genomic loci using site-specific integration (Markstein et al., 2008). The stg14-KD was placed into attP4 (12C6); actin^LoxP-GAL80-stop-LoxP^LexA::P65 into VK00027 (89E11); lexAop-rCD2::RFP-p10-spacer-UAS-mCD8::GFP-p10 into VK00005 (75A10). See Table S1 for the exact fly genotype of each experiment.
DNA constructs
For lexAop-Dronc-RNAi, we followed the miRNA construct design as described by Chen et al. (2007). We first generated a polycistronic transcript that encodes two miRNAs against the Dronc gene (CG8091). The miRNA targeting sequences were 5′-CCTTGCTAGGATCATCTAGTGC-3′ and 5′-ATTTCCAGACGGCCAAATGTCCT-3′. Then, a lexAop promoter was added before the above transcript through the 5′-BglII to 3′-NotI restriction enzyme sites. The final DNA construct was injected into the VK00001 (59D3) and VK00033 (65B2) landing sites.
For LexAop2-grin-Insu-lexAop-rpr (LexAop-grim-rpr), we first amplified the cDNA sequence of grim and rpr by PCR from EST clone (RE28551 and IP02530, respectively, Berkeley Drosophila Genome Project). Next, we cloned the cDNA sequence of grim into the pMLH vector at NotI/XbaI sites to make pMLH-grim, and the cDNA of rpr in XbaI/BglII sites to make pMLH-rpr. A synthetic insulated spacer cassette (Pfeiffer et al., 2010) was inserted before the lexAop promotor through the NgoMIV site in pMLH-rpr. Finally, we combined the PmeI to HindIII fragment of pMLH-grim with the insulator-pMLH-rpr through the PmeI site. The final construct was injected into the attP16 (53C4) landing site.
For Repo-loxP-stop-loxP-LexA::p65, we first combined a synthetic Flox cassette (Nern et al., 2011) with the LexA::p65 in pBPLexA::p65w (Pfeiffer et al., 2010) through the Acc65I site to generate loxP-stop-loxP-LexA::p65. Then, a Repo promoter was inserted in front of the Flox cassette through gateway cloning (Invitrogen). We injected the final construct into the VK00027 (89E11) landing site.
Clonal analysis
The detailed methods to induce MARCM and CLIn clones in type II lineages have been described previously (Ren et al., 2017; Wang et al., 2014). Briefly, we manually collected newly hatched larva over a 4 h period. Larva were put into vials with standard fly food and cultured in an incubator with constant temperature (25°C) and humidity (50% relative humidity). To induce mitotic recombination, we applied heat shock at 8-12 h ALH by submerging vials into a 37°C water bath for 15-30 min. Animals were returned to the incubator and dissected at 72 h ALH or at adult stage.
Immunohistochemistry
Male and female brains from larva (72 h ALH) or adult stage (3-6 days old) were dissected in ice-cold PBS and fixed with 2% paraformaldehyde for 30-50 min at room temperature. The primary antibodies used include: rat antibody to mCD8 (1:100, Life Technologies, MCD0800), rabbit antibody to RFP (1:1000, Clontech, 632496), mouse antibody to Bruchpilot (nc82 mAb, 1:50, Developmental Studies Hybridoma Bank), rabbit anti-GFP (1:1000, Life Technologies, A11122), mouse antibody to β-galactosidase (1:500, Promega, Z3783), mouse antibody to Repo (1:50, Developmental Studies Hybridoma Bank, 8D12). The secondary antibodies used include: Alexa-488-conjugated goat antibody to rat (1:200, Life Technologies A11006), Cy3-conjugated goat antibody to rabbit (1:500, Jackson ImmunoResearch, 111-165-144), Cy5-conjugated goat antibody to mouse (1:500, Jackson ImmunoResearch, 115-605-146) and Alexa-568-conjugated goat antibody to rabbit, 1:200 (Life Technologies, A11039). Brain specimens were whole mounted in SlowFade Gold Reagent (Molecular Probes) on charged slides to eliminate movement (Fisherbrand, 12-550-15).
Confocal microscopy and image processing
Confocal stacks were imaged on a Zeiss LSM710 confocal microscope. We employed a motorized stage and a Janelia software plug-in to sequentially image multiple brain samples within a session. Images were acquired at 1 µm intervals using a 40× C-Apochromat water objective (NA=1.2) and 0.7 zoom factor at 1024×1024 pixel resolution. The z-stack images were then processed using ImageJ and Adobe Photoshop. For comparisons between experimental conditions, image stacks were collected with the same confocal settings (laser power, pinhole size, gain, etc.). For glial volume, we performed thresholding in image stacks to identify the voxels that belonged to glial cells. The threshold value is 20 for the ‘green’ channel labeled with GFP (8-bit image). Then we calculated the total glial volume by integrating those voxels. We manually counted glial cell number using the cell counter plugin of ImageJ. Glial cell nuclei were labeled using antibodies against either Repo or nuclear localized β-galactosidase.
Statistical analysis
Cell numbers were shown as mean±s.d. For comparison between two groups, we used unpaired t-test. For comparison between multiple groups, we employed one-way ANOVA followed by planned pair-wise comparisons with Dunnett's post-hoc test. All tests were two-sided. Statistical analysis was carried out using Prism 6 (GraphPad Software).
Supplementary Material
Acknowledgements
We thank Janelia Flycore and Workstation project team for technical support. We thank Marc Freeman and Liqun Luo for providing fly stocks and antibodies. We thank Rosa Miyares, Hideo Otsuna, Masayoshi Ito, Jorge Garcia Marques, Ching-Po Yang, Ying-Jou Lee and Kent Mok for input and critical reading of the manuscript, and Crystal Di Pietro for administrative support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Q.R., T.A., T.L.; Methodology: Q.R., T.A., Y.-C.W., Y.-F.H., T.L.; Formal analysis: Q.R., T.A., Y.-C.W.; Investigation: Q.R., T.A., Y.-C.W., T.L.; Writing - original draft: Q.R., T.L.; Writing - review & editing: Q.R., T.A., T.L.; Supervision: T.L.; Project administration: T.L.; Funding acquisition: T.L.
Funding
This work was supported by the Howard Hughes Medical Institute. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.160127.supplemental
References
- Avet-Rochex A., Kaul A. K., Gatt A. P., McNeill H. and Bateman J. M. (2012). Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain. Development 139, 2763-2772. 10.1242/dev.074179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Rochex A., Maierbrugger K. T. and Bateman J. M. (2014). Glial enriched gene expression profiling identifies novel factors regulating the proliferation of specific glial subtypes in the Drosophila brain. Gene Expr. Patterns 16, 61-68. 10.1016/j.gep.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T., Lai S.-L., Ito K. and Lee T. (2008). Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 28, 13742-13753. 10.1523/JNEUROSCI.4844-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T., Kao C.-F., Lee Y.-J., Yang C.-P., Huang Y., Pfeiffer B. D., Luan H., Jing X., Huang Y.-F., He Y. et al. (2014). Making Drosophila lineage-restricted drivers via patterned recombination in neuroblasts. Nat. Neurosci. 17, 631-637. 10.1038/nn.3654 [DOI] [PubMed] [Google Scholar]
- Bayraktar O. A. and Doe C. Q. (2013). Combinatorial temporal patterning in progenitors expands neural diversity. Nature 498, 449-455. 10.1038/nature12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar O. A., Fuentealba L. C., Alvarez-Buylla A. and Rowitch D. H. (2015). Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol. 7, a020362 10.1101/cshperspect.a020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello B. C., Izergina N., Caussinus E. and Reichert H. (2008). Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev. 3, 5 10.1186/1749-8104-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J. Q. and Doe C. Q. (2008). Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev. Neurobiol. 68, 1185-1195. 10.1002/dneu.20648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman S. K., Rolland V., Betschinger J., Kinsey K. A., Emery G. and Knoblich J. A. (2008). The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev. Cell 14, 535-546. 10.1016/j.devcel.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. J. (2006). Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678-689. 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- Cattenoz P. B. and Giangrande A. (2013). Lineage specification in the fly nervous system and evolutionary implications. Cell Cycle 12, 2753-2759. 10.4161/cc.25918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Rodriguez A., Erskine R., Thach T. and Abrams J. M. (1998). Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila. Dev. Biol. 201, 202-216. 10.1006/dbio.1998.9000 [DOI] [PubMed] [Google Scholar]
- Chen C.-H., Huang H., Ward C. M., Su J. T., Schaeffer L. V., Guo M. and Hay B. A. (2007). A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science 316, 597-600. 10.1126/science.1138595 [DOI] [PubMed] [Google Scholar]
- Chen Z., Del Valle Rodriguez A., Li X., Erclik T., Fernandes V. M. and Desplan C. (2016). A unique class of neural progenitors in the Drosophila optic lobe generates both migrating neurons and glia. Cell Rep. 15, 774-786. 10.1016/j.celrep.2016.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D., Feger G., Younger-Shepherd S., Jan L. Y. and Jan Y. N. (1996). Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 10, 421-434. 10.1101/gad.10.4.421 [DOI] [PubMed] [Google Scholar]
- Doherty J., Logan M. A., Tasdemir O. E. and Freeman M. R. (2009). Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 29, 4768-4781. 10.1523/JNEUROSCI.5951-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T. N. and Meinertzhagen I. A. (2010). The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 90, 471-497. 10.1016/j.pneurobio.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N. and Fishell G. (2002). The role of notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 25, 471-490. 10.1146/annurev.neuro.25.030702.130823 [DOI] [PubMed] [Google Scholar]
- García-Marqués J. and López-Mascaraque L. (2013). Clonal identity determines astrocyte cortical heterogeneity. Cereb. Cortex 23, 1463-1472. 10.1093/cercor/bhs134 [DOI] [PubMed] [Google Scholar]
- Gho M., Bellaiche Y. and Schweisguth F. (1999). Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development 126, 3573-3584. [DOI] [PubMed] [Google Scholar]
- Go M. J., Eastman D. S. and Artavanis-Tsakonas S. (1998). Cell proliferation control by Notch signaling in Drosophila development. Development 125, 2031-2040. [DOI] [PubMed] [Google Scholar]
- Griffiths R. L. and Hidalgo A. (2004). Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. EMBO J. 23, 2440-2450. 10.1038/sj.emboj.7600258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. C., Benito-Sipos J., Fenton J. C., Torroja L. and Hidalgo A. (2007). Two distinct mechanisms segregate Prospero in the longitudinal glia underlying the timing of interactions with axons. Neuron Glia Biol. 3, 75-88. 10.1017/S1740925X07000610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V. (2011). Morphological diversity and development of glia in Drosophila. Glia 59, 1237-1252. 10.1002/glia.21162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B. A., Wolff T. and Rubin G. M. (1994). Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121-2129. [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Kato K., Sutcliffe B., McIlroy G., Bishop S. and Alahmed S. (2011). Trophic neuron-glia interactions and cell number adjustments in the fruit fly. Glia 59, 1296-1303. 10.1002/glia.21092 [DOI] [PubMed] [Google Scholar]
- Hochstim C., Deneen B., Lukaszewicz A., Zhou Q. and Anderson D. J. (2008). Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510-522. 10.1016/j.cell.2008.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Urban J. and Technau G. M. (1995). Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux's Arch. Dev. Biol. 204, 284-307. [DOI] [PubMed] [Google Scholar]
- Ito K., Shinomiya K., Ito M., Armstrong J. D., Boyan G., Hartenstein V., Harzsch S., Heisenberg M., Homberg U., Jenett A. et al. (2014). A systematic nomenclature for the insect brain. Neuron 81, 755-765. 10.1016/j.neuron.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Izergina N., Balmer J., Bello B. and Reichert H. (2009). Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Dev. 4, 44 10.1186/1749-8104-4-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A., Rubin G. M., Ngo T.-T. B., Shepherd D., Murphy C., Dionne H., Pfeiffer B. D., Cavallaro A., Hall D., Jeter J. et al. (2012). A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991-1001. 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. W. (2005). Transcriptional control of glial cell development in Drosophila. Dev. Biol. 278, 265-273. 10.1016/j.ydbio.2004.11.022 [DOI] [PubMed] [Google Scholar]
- Kato K., Forero M. G., Fenton J. C. and Hidalgo A. (2011). The glial regenerative response to central nervous system injury is enabled by pros-notch and pros-NFkappaB feedback. PLoS Biol. 9, e1001133 10.1371/journal.pbio.1001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Konno D., Berry M., Matsuzaki F., Logan A. and Hidalgo A. (2015). Prox1 inhibits proliferation and is required for differentiation of the oligodendrocyte cell lineage in the mouse. PLoS ONE 10, e0145334 10.1371/journal.pone.0145334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Losada-Perez M. and Hidalgo A. (2017). Gene network underlying the glial regenerative response to central nervous system injury. Dev. Dyn. 247, 85-93. 10.1002/dvdy.24565 [DOI] [PubMed] [Google Scholar]
- Koelzer S. and Klein T. (2006). Regulation of expression of Vg and establishment of the dorsoventral compartment boundary in the wing imaginal disc by Suppressor of Hairless. Dev. Biol. 289, 77-90. 10.1016/j.ydbio.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Kremer M. C., Jung C., Batelli S., Rubin G. M. and Gaul U. (2017). The glia of the adult Drosophila nervous system. Glia 65, 606-638. 10.1002/glia.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. and Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451-461. 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Losada-Perez M., Harrison N. and Hidalgo A. (2016). Molecular mechanism of central nervous system repair by the Drosophila NG2 homologue kon-tiki. J. Cell Biol. 214, 587-601. 10.1083/jcb.201603054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi S., Friedmann D., Banks G., Stolfi A. and Lois C. (2012). Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns. J. Neurosci. 32, 4762-4772. 10.1523/JNEUROSCI.3560-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstein M., Pitsouli C., Villalta C., Celniker S. E. and Perrimon N. (2008). Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 40, 476-483. 10.1038/ng.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Aliaga I. and Thor S. (2009). Programmed cell death in the nervous system—a programmed cell fate? Curr. Opin. Neurobiol. 19, 127-133. 10.1016/j.conb.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Perez S. E., Qiao Z., Verdi J. M., Hicks C., Weinmaster G. and Anderson D. J. (2000). Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499-510. 10.1016/S0092-8674(00)80860-0 [DOI] [PubMed] [Google Scholar]
- Nern A., Pfeiffer B. D., Svoboda K. and Rubin G. M. (2011). Multiple new site-specific recombinases for use in manipulating animal genomes. Proc. Natl. Acad. Sci. USA 108, 14198-14203. 10.1073/pnas.1111704108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J.-Q., Zhou R., Czech B., Liu L.-P., Holderbaum L., Yang-Zhou D., Shim H.-S., Tao R., Handler D., Karpowicz P. et al. (2011). A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405-407. 10.1038/nmeth.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto J. J., Yogi P. and Hartenstein V. (2015). Origin and development of neuropil glia of the Drosophila larval and adult brain: two distinct glial populations derived from separate progenitors. Dev. Biol. 404, 2-20. 10.1016/j.ydbio.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peco E., Davla S., Camp D., Stacey S. M., Landgraf M. and van Meyel D. J. (2016). Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development 143, 1170-1181. 10.1242/dev.133165 [DOI] [PubMed] [Google Scholar]
- Pfeiffer B. D., Ngo T.-T. B., Hibbard K. L., Murphy C., Jenett A., Truman J. W. and Rubin G. M. (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735-755. 10.1534/genetics.110.119917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierfelice T., Alberi L. and Gaiano N. (2011). Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840-855. 10.1016/j.neuron.2011.02.031 [DOI] [PubMed] [Google Scholar]
- Reddy B. V. V. G. and Irvine K. D. (2011). Regulation of Drosophila glial cell proliferation by Merlin-Hippo signaling. Development 138, 5201-5212. 10.1242/dev.069385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Awasaki T., Huang Y.-F., Liu Z. and Lee T. (2016). Cell class-lineage analysis reveals sexually dimorphic lineage compositions in the Drosophila brain. Curr. Biol. 26, 2583-2593. 10.1016/j.cub.2016.07.086 [DOI] [PubMed] [Google Scholar]
- Ren Q., Yang C.-P., Liu Z., Sugino K., Mok K., He Y., Ito M., Nern A., Otsuna H. and Lee T. (2017). Stem cell-intrinsic, seven-up-triggered temporal factor gradients diversify intermediate neural progenitors. Curr. Biol. 27, 1303-1313. 10.1016/j.cub.2017.03.047 [DOI] [PubMed] [Google Scholar]
- Richier B., Vijandi C. M., Mackensen S. and Salecker I. (2017). Lapsyn controls branch extension and positioning of astrocyte-like glia in the Drosophila optic lobe. Nat. Commun. 8, 317 10.1038/s41467-017-00384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T., Soustelle L., Strambi C., Besson M.-T., Iché M. and Birman S. (2004). Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 14, 599-605. 10.1016/j.cub.2004.03.039 [DOI] [PubMed] [Google Scholar]
- Rowitch D. H. and Kriegstein A. R. (2010). Developmental genetics of vertebrate glial-cell specification. Nature 468, 214-222. 10.1038/nature09611 [DOI] [PubMed] [Google Scholar]
- Sepp K. J., Schulte J. and Auld V. J. (2001). Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev. Biol. 238, 47-63. 10.1006/dbio.2001.0411 [DOI] [PubMed] [Google Scholar]
- Skeath J. B. (1999). At the nexus between pattern formation and cell-type specification: the generation of individual neuroblast fates in the Drosophila embryonic central nervous system. BioEssays 21, 922-931. [DOI] [PubMed] [Google Scholar]
- Stacey S. M., Muraro N. I., Peco E., Labbe A., Thomas G. B., Baines R. A. and van Meyel D. J. (2010). Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J. Neurosci. 30, 14446-14457. 10.1523/JNEUROSCI.1021-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T. and Freeman M. R. (2013). Invertebrate glia. In Patterning and Cell Type Specification in the Developing CNS and PNS, pp. 891-910. Cambridge, MA, USA: Academic Press. [Google Scholar]
- Stork T., Bernardos R. and Freeman M. R. (2012). Analysis of glial cell development and function in Drosophila. Cold Spring Harb. Protoc. 2012, 1-17. 10.1101/pdb.top067587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T., Sheehan A., Tasdemir-Yilmaz O. E. and Freeman M. R. (2014). Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron 83, 388-403. 10.1016/j.neuron.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed M. H., Mark B. and Doe C. Q. (2017). Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity. eLife 6, e26287 10.7554/eLife.26287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. B. and van Meyel D. J. (2007). The glycosyltransferase Fringe promotes Delta-Notch signaling between neurons and glia, and is required for subtype-specific glial gene expression. Development 134, 591-600. 10.1242/dev.02754 [DOI] [PubMed] [Google Scholar]
- Tomassy G. S. and Fossati V. (2014). How big is the myelinating orchestra? Cellular diversity within the oligodendrocyte lineage: facts and hypotheses. Front. Cell. Neurosci. 8, 201 10.3389/fncel.2014.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.-H., Li H., Fuentealba L. C., Molofsky A. V., Taveira-Marques R., Zhuang H., Tenney A., Murnen A. T., Fancy S. P. J., Merkle F. et al. (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358-362. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Evans D. B. and Jayaraman V. (2016). The insect central complex. Curr. Biol. 26, R453-R457. 10.1016/j.cub.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Udolph G., Rath P. and Chia W. (2001). A requirement for Notch in the genesis of a subset of glial cells in the Drosophila embryonic central nervous system which arise through asymmetric divisions. Development 128, 1457-1466. [DOI] [PubMed] [Google Scholar]
- Umesono Y., Hiromi Y. and Hotta Y. (2002). Context-dependent utilization of Notch activity in Drosophila glial determination. Development 129, 2391-2399. [DOI] [PubMed] [Google Scholar]
- Van De Bor V. and Giangrande A. (2001). Notch signaling represses the glial fate in fly PNS. Development 128, 1381-1390. [DOI] [PubMed] [Google Scholar]
- Viktorin G., Riebli N., Popkova A., Giangrande A. and Reichert H. (2011). Multipotent neural stem cells generate glial cells of the central complex through transit amplifying intermediate progenitors in Drosophila brain development. Dev. Biol. 356, 553-565. 10.1016/j.ydbio.2011.06.013 [DOI] [PubMed] [Google Scholar]
- Viktorin G., Riebli N. and Reichert H. (2013). A multipotent transit-amplifying neuroblast lineage in the central brain gives rise to optic lobe glial cells in Drosophila. Dev. Biol. 379, 182-194. 10.1016/j.ydbio.2013.04.020 [DOI] [PubMed] [Google Scholar]
- Wang Y.-C., Yang J. S., Johnston R., Ren Q., Lee Y.-J., Luan H., Brody T., Odenwald W. F. and Lee T. (2014). Drosophila intermediate neural progenitors produce lineage-dependent related series of diverse neurons. Development 141, 253-258. 10.1242/dev.103069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Li J., Chou Y.-H., Luginbuhl D. and Luo L. (2017). Fibroblast growth factor signaling instructs ensheathing glia wrapping of Drosophila olfactory glomeruli. Proc. Natl. Acad. Sci. USA 114, 7505-7512. 10.1073/pnas.1706533114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Woodfield S. E., Lee T. V., Fan Y., Antonio C. and Bergmann A. (2009). Genetic control of programmed cell death (apoptosis) in Drosophila. Fly (Austin) 3, 78-90. 10.4161/fly.3.1.7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. S., Awasaki T., Yu H.-H., He Y., Ding P., Kao J.-C. and Lee T. (2013). Diverse neuronal lineages make stereotyped contributions to the Drosophila locomotor control center, the central complex. J. Comp. Neurol. 521, 2645-2662. 10.1002/cne.23339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Murray J. I., Lu Y., Waterston R. H. and Shaham S. (2008). mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development 135, 2263-2275. 10.1242/dev.019547 [DOI] [PubMed] [Google Scholar]
- Yu H.-H., Chen C.-H., Shi L., Huang Y. and Lee T. (2009). Twin-spot MARCM to reveal the developmental origin and identity of neurons. Nat. Neurosci. 12, 947-953. 10.1038/nn.2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. and Barres B. A. (2010). Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588-594. 10.1016/j.conb.2010.06.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.